The Role of Oxytocin Neurons in the Paraventricular Nucleus in Chronic-Sleep-Deprivation-Mediated Abnormal Cardiovascular Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Virus Injection and In Vivo Optogenetic Stimulation
2.3. Oxytocin Receptor Antagonist
2.4. mPST Inhibitor
2.5. Sleep Deprivation
2.6. Echocardiography
2.7. Blood Pressure and Heart Rate Monitoring
2.8. Histology
2.9. RNA Isolation and RNA-Seq Library Construction
2.10. Western Blot Assay (WB)
2.11. Electrophysiological Analysis
2.12. Data Analysis
3. Results
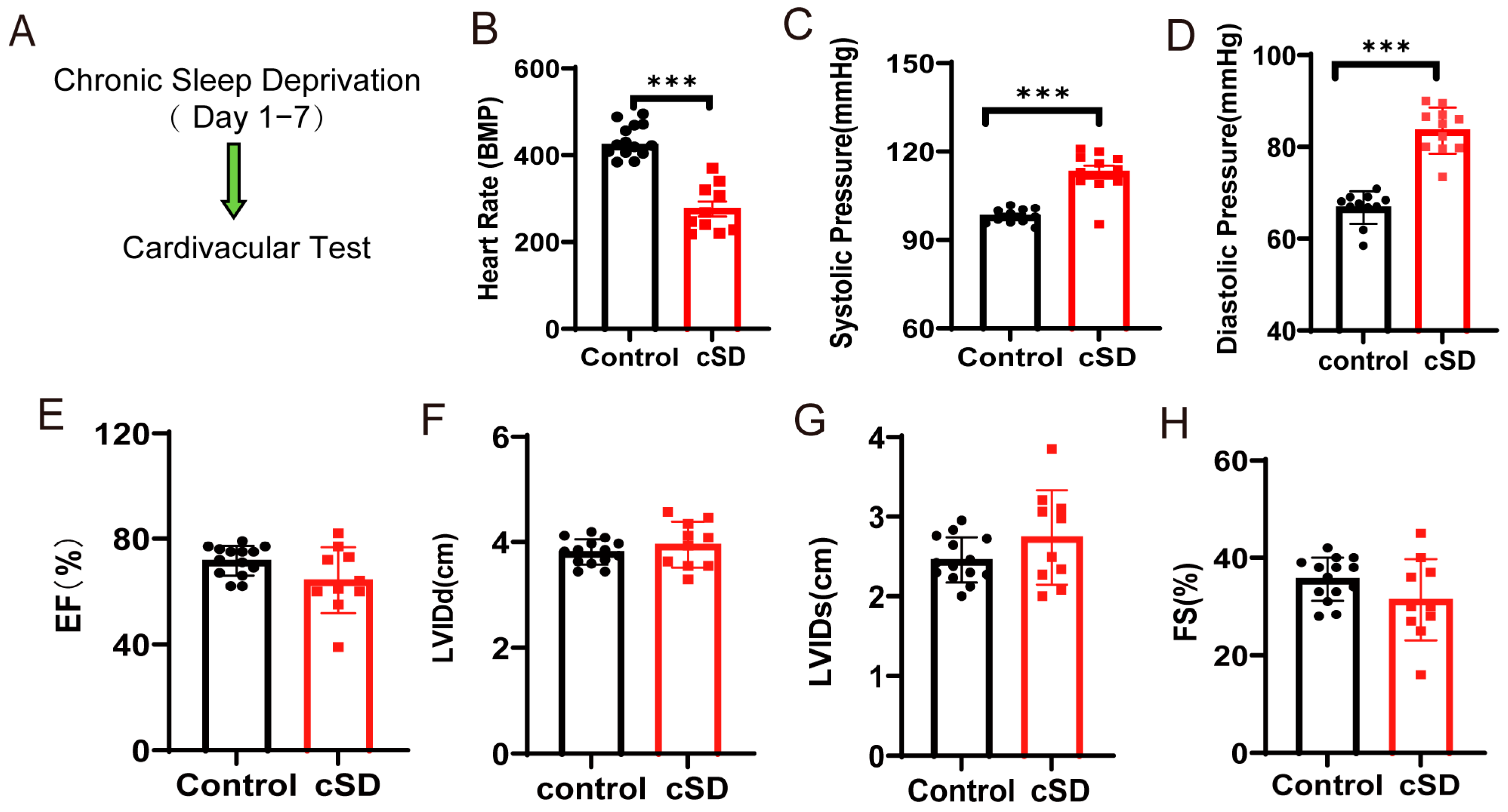
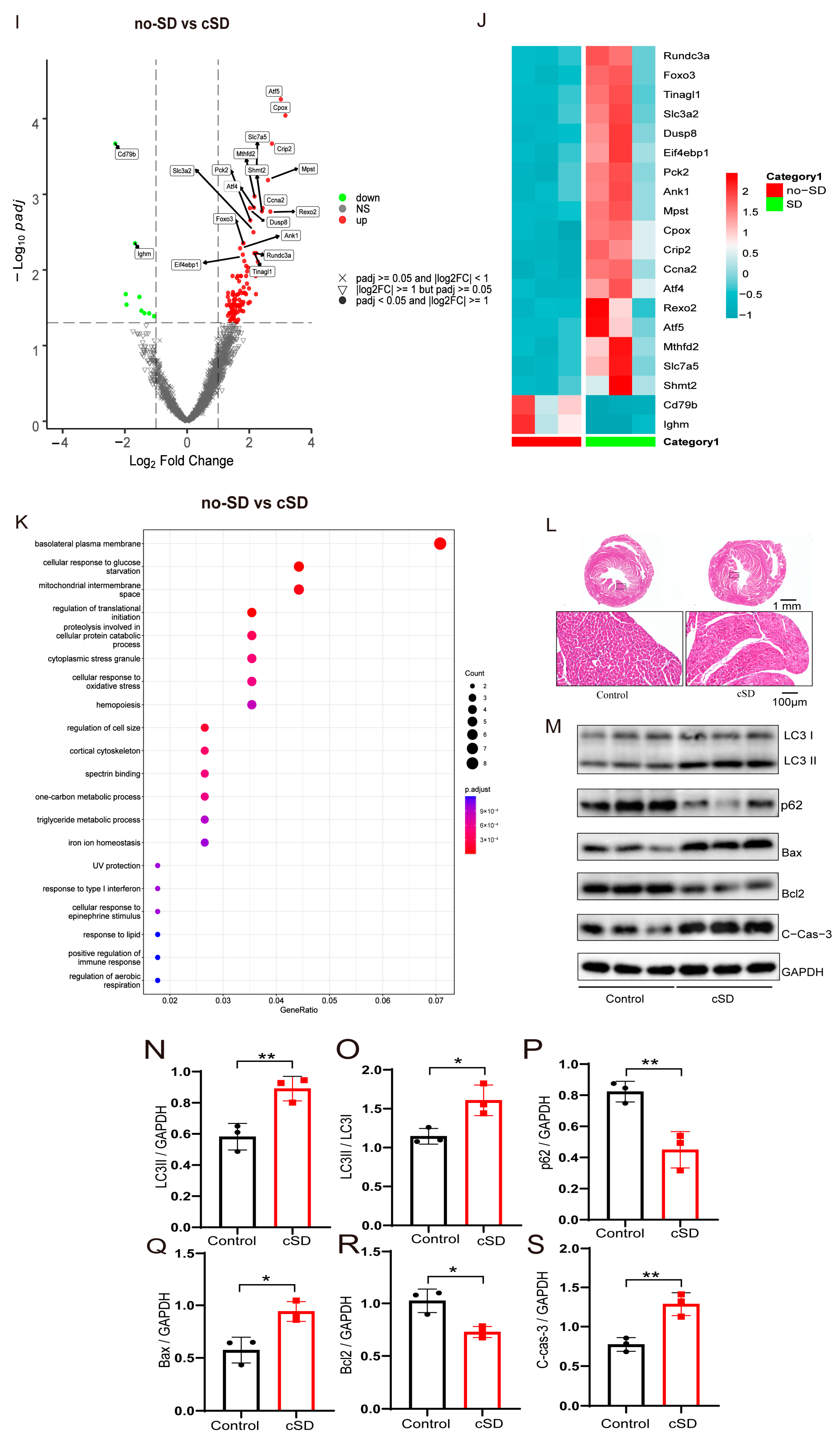
3.1. Chronic Sleep Deprivation (cSD) Decreased the Heart Rate, Increased Blood Pressure, Changed the Blood Transcriptome, and Enhanced the Autophagy and Apoptosis of Cardiac Tissue
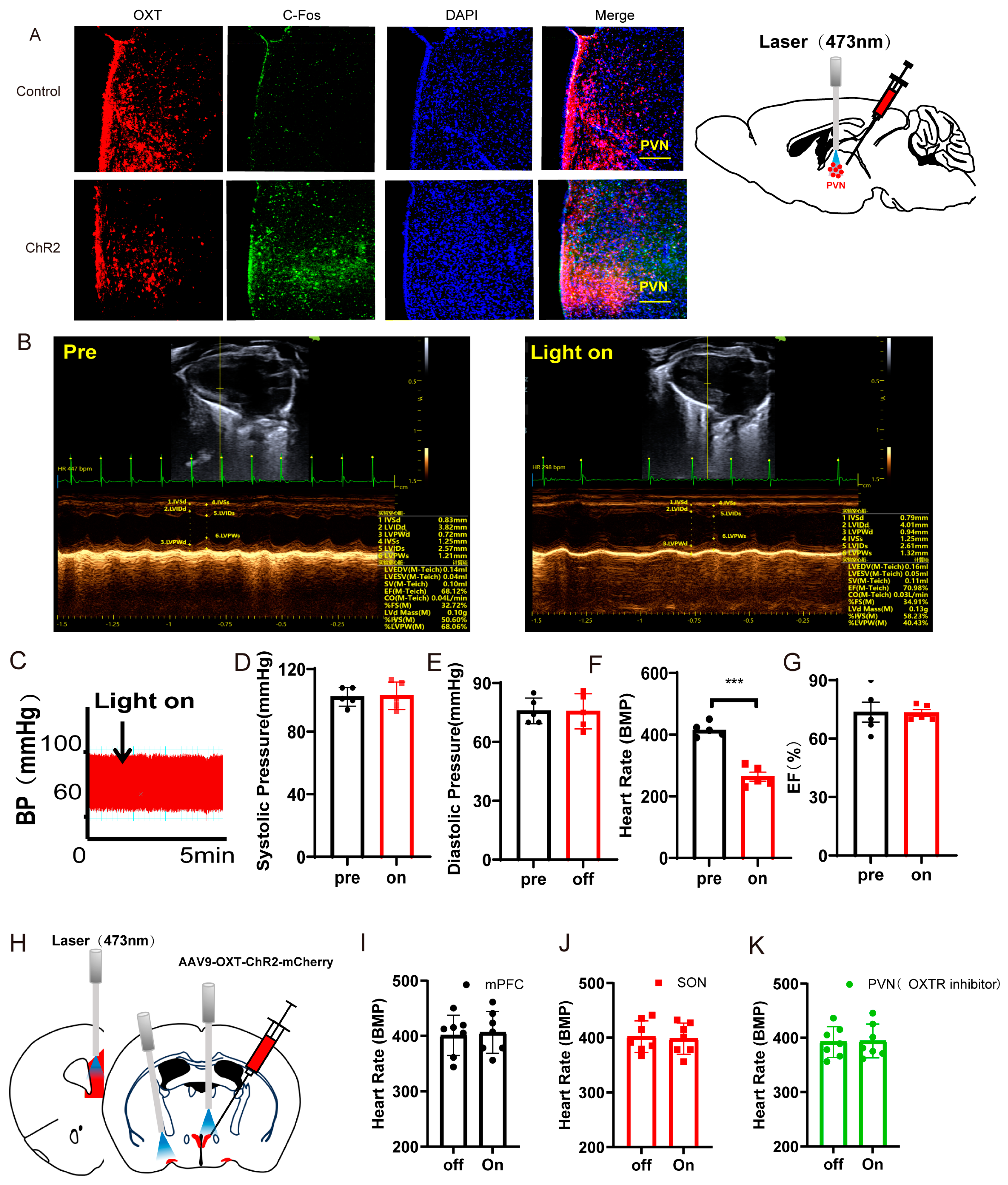
3.2. Optogenetic Activation of PVNOXT Neurons of Normal Mice Decreased Heart Rates Without Affecting the Left Ventricular Function and Blood Pressure
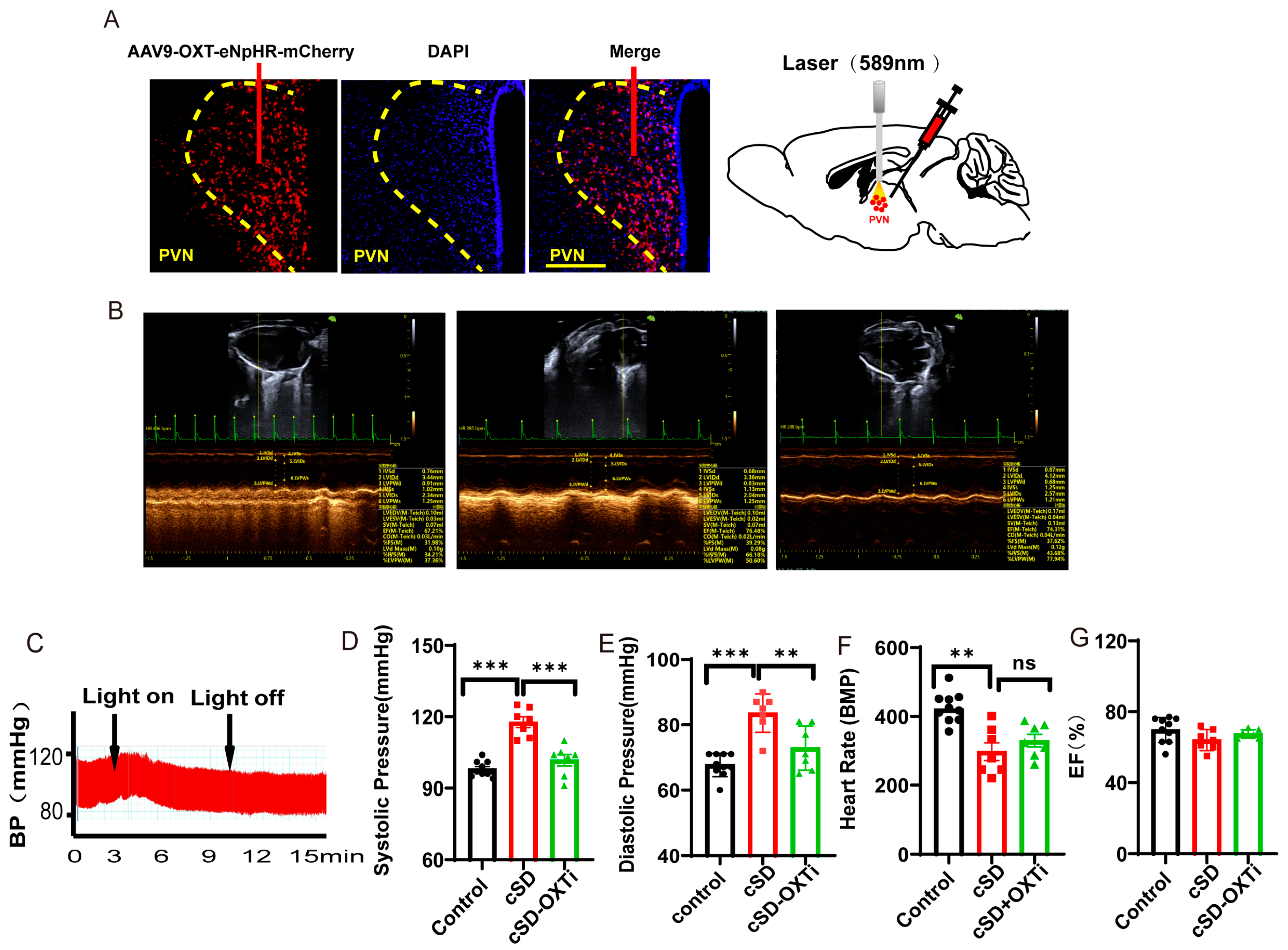
3.3. Instant Optogenetic Inhibition of PVNOXT Neurons Could Reverse the Change of Blood Pressure Induced by the cSD but Not the Change of Heart Rates
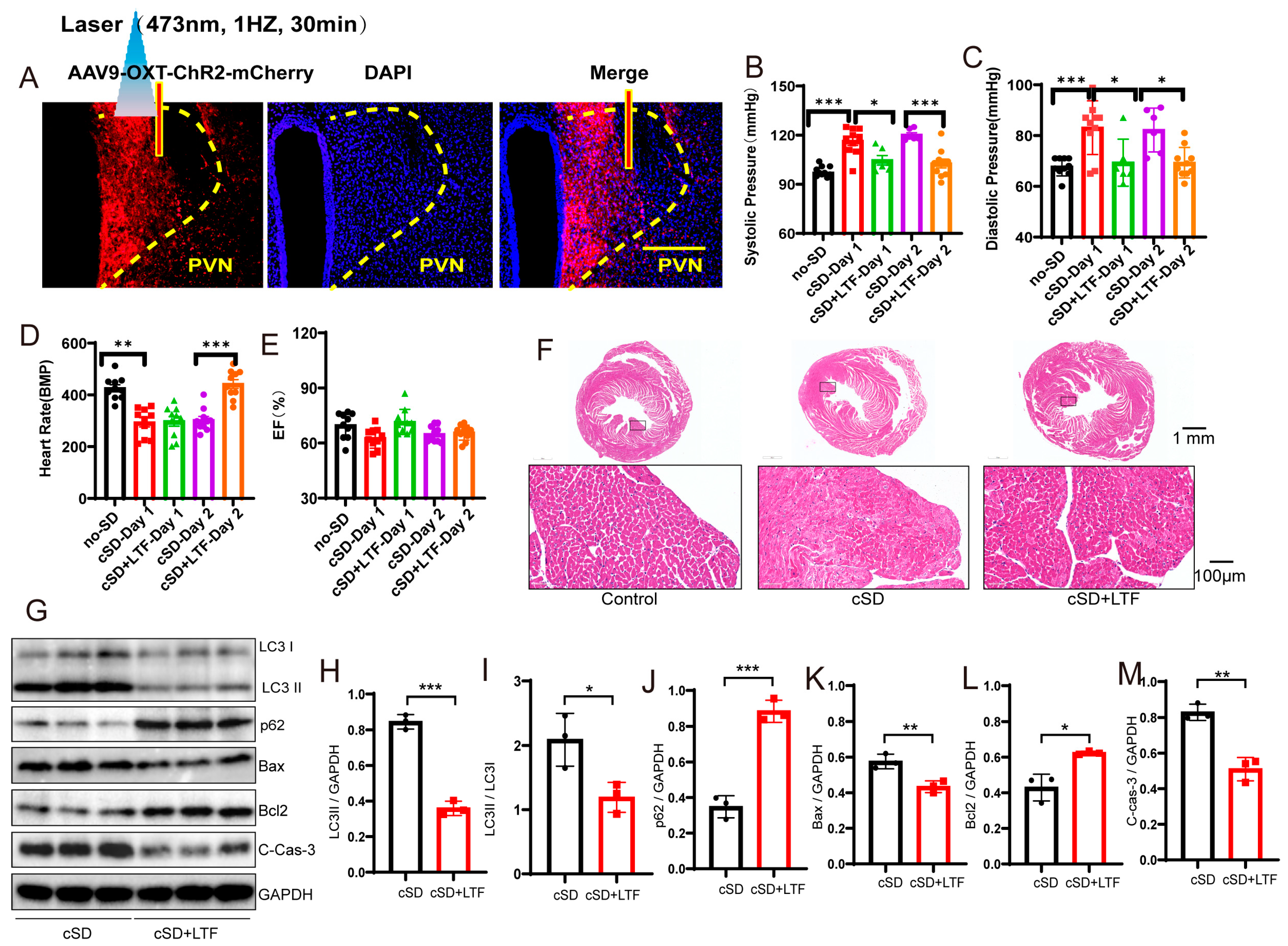
3.4. Long-Term Low-Frequencies (LTF) Stimulation of PVNOXT Neurons Could Reverse the Change in Blood Pressures, Heart Rates, the Transcriptome of the Blood, the Morphological Structure of Myocardial Cells, and the Autophagy and Apoptosis of Cardiac Tissue Induced by the cSD
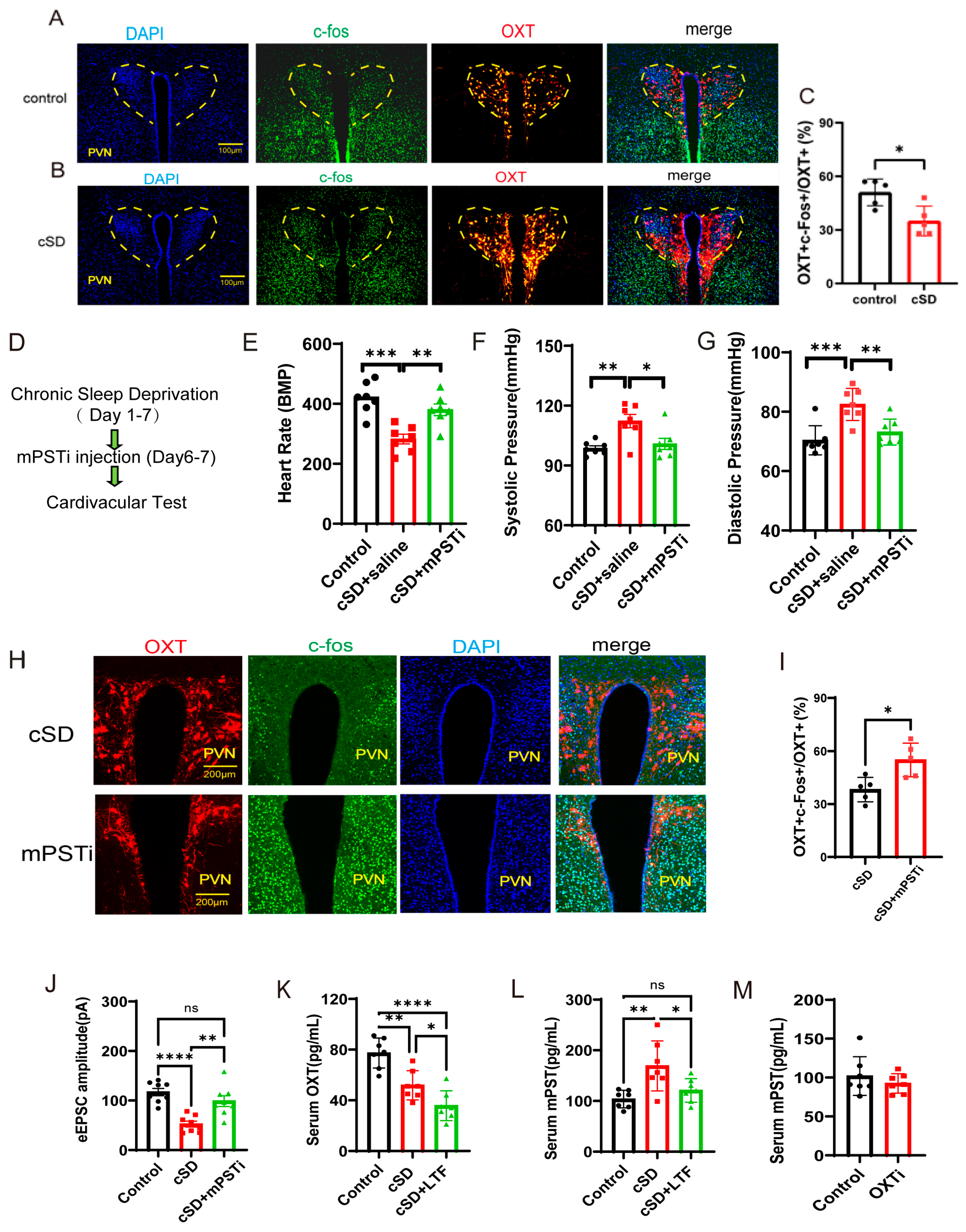
3.5. The Increased mPST Release from the Peripheral Blood Was Associated with Reduced Excitability of PVNOXT Neurons and Abnormal Cardiovascular Response Following cSD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cSD | chronic sleep deprivation |
| PVN | paraventricular nucleus |
| OXT | oxytocin |
| LTF | long-term low-frequencies |
| CIH | chronic intermittent hypoxia |
| LVIDd | left ventricular internal diameter during diastole |
| LVIDs | left ventricular internal diameter during systole |
| EF | ejection fraction |
| FS | fractional shortening |
| DEGs | differentially expressed genes |
| OXTi | inhibition of OXT neurons |
| OXTRs | OXT receptors |
References
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385,292 UK biobank participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef]
- Huynh, P.; Hoffmann, J.D.; Gerhardt, T.; Kiss, M.G.; Zuraikat, F.M.; Cohen, O.; Wolfram, C.; Yates, A.G.; Leunig, A.; Heiser, M.; et al. Myocardial infarction augments sleep to limit cardiac inflammation and damage. Nature 2024, 635, 168–177. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L.; American Heart Association Obesity, Behavior Change, Diabetes; Nutrition Committees of the Council on Lifestyle; Cardiometabolic Health; et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, K.A.; Ahles, A.; Dueck, A.; Esfandyari, D.; Pichler, P.; Weber, K.; Kotschi, S.; Bartelt, A.; Sinicina, I.; Graw, M.; et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 2023, 381, 285–290. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, C.S.; Kiss, M.G.; Rattik, S.; He, S.; Vassalli, A.; Valet, C.; Anzai, A.; Chan, C.T.; Mindur, J.E.; Kahles, F.; et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019, 566, 383–387. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef]
- Aghajani, M.; Faghihi, M.; Imani, A.; Vaez Mahdavi, M.R.; Shakoori, A.; Rastegar, T.; Parsa, H.; Mehrabi, S.; Moradi, F.; Kazemi Moghaddam, E. Post-infarct sleep disruption and its relation to cardiac remodeling in a rat model of myocardial infarction. Chronobiol. Int. 2017, 34, 587–600. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Guo, L.; Wang, L.; Chen, N.; Xiao, Y.; Wang, E. Acute sleep deprivation increases inflammation and aggravates heart failure after myocardial infarction. J. Sleep Res. 2022, 31, e13679. [Google Scholar] [CrossRef]
- Bourdillon, N.; Jeanneret, F.; Nilchian, M.; Albertoni, P.; Ha, P.; Millet, G.P. Sleep Deprivation Deteriorates Heart Rate Variability and Photoplethysmography. Front. Neurosci. 2021, 15, 642548. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension 2006, 47, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Lin, C.C.; Tzeng, N.S.; Tung, C.S.; Liu, Y.P. Effects of oxytocin on prosocial behavior and the associated profiles of oxytocinergic and corticotropin-releasing hormone receptors in a rodent model of posttraumatic stress disorder. J. Biomed. Sci. 2019, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- Perisic, M.; Woolcock, K.; Hering, A.; Mendel, H.; Muttenthaler, M. Oxytocin and vasopressin signaling in health and disease. Trends Biochem. Sci. 2024, 49, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Japundzic-Zigon, N.; Lozic, M.; Sarenac, O.; Murphy, D. Vasopressin & Oxytocin in Control of the Cardiovascular System: An Updated Review. Curr. Neuropharmacol. 2020, 18, 14–33. [Google Scholar] [CrossRef]
- Menaouar, A.; Florian, M.; Wang, D.; Danalache, B.; Jankowski, M.; Gutkowska, J. Anti-hypertrophic effects of oxytocin in rat ventricular myocytes. Int. J. Cardiol. 2014, 175, 38–49. [Google Scholar] [CrossRef]
- Althammer, F.; Roy, R.K.; Kirchner, M.K.; Lira, E.C.; Schimmer, S.; Charlet, A.; Grinevich, V.; Stern, J.E. Impaired oxytocin signalling in the central amygdala in rats with chronic heart failure. J. Physiol. 2024, 602, 6259–6280. [Google Scholar] [CrossRef]
- Wasserman, A.H.; Huang, A.R.; Lewis-Israeli, Y.R.; Dooley, M.D.; Mitchell, A.L.; Venkatesan, M.; Aguirre, A. Oxytocin promotes epicardial cell activation and heart regeneration after cardiac injury. Front. Cell Dev. Biol. 2022, 10, 985298. [Google Scholar] [CrossRef]
- Rodriguez, J.; Escobar, J.B.; Cheung, E.C.; Kowalik, G.; Russo, R.; Dyavanapalli, J.; Alber, B.R.; Harral, G.; Gill, A.; Melkie, M.; et al. Hypothalamic Oxytocin Neuron Activation Attenuates Intermittent Hypoxia-Induced Hypertension and Cardiac Dysfunction in an Animal Model of Sleep Apnea. Hypertension 2023, 80, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.; Wang, D.; Hajjar, F.; Mukaddam-Daher, S.; McCann, S.M.; Gutkowska, J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc. Natl. Acad. Sci. USA 2000, 97, 6207–6211. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Reitz, J.; Brauer, S.; Trossen, C. Oxytocin receptors: Ligand binding, signalling and cholesterol dependence. Prog. Brain Res. 2008, 170, 193–204. [Google Scholar] [CrossRef]
- Lozic, M.; Greenwood, M.; Sarenac, O.; Martin, A.; Hindmarch, C.; Tasic, T.; Paton, J.; Murphy, D.; Japundzic-Zigon, N. Overexpression of oxytocin receptors in the hypothalamic PVN increases baroreceptor reflex sensitivity and buffers BP variability in conscious rats. Br. J. Pharmacol. 2014, 171, 4385–4398. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Lundeberg, T.; Uvnas-Moberg, K. Short-term increase and long-term decrease of blood pressure in response to oxytocin-potentiating effect of female steroid hormones. J. Cardiovasc. Pharmacol. 1999, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hur, T.Y.; Hong, Y. Circadian Rhythm Disruption and Subsequent Neurological Disorders in Night-Shift Workers. J. Lifestyle Med. 2017, 7, 45–50. [Google Scholar] [CrossRef]
- Stanic, D.; Plecas-Solarovic, B.; Mirkovic, D.; Jovanovic, P.; Dronjak, S.; Markovic, B.; Dordevic, T.; Ignjatovic, S.; Pesic, V. Oxytocin in corticosterone-induced chronic stress model: Focus on adrenal gland function. Psychoneuroendocrinology 2017, 80, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Cho, J.H.; Bae, J.Y.; O’Leary, T.P.; Johnson, J.D.; Bae, Y.C.; Kim, E.K. Insulin synthesized in the paraventricular nucleus of the hypothalamus regulates pituitary growth hormone production. JCI Insight 2020, 5, e135412. [Google Scholar] [CrossRef]
- Jameson, H.; Bateman, R.; Byrne, P.; Dyavanapalli, J.; Wang, X.; Jain, V.; Mendelowitz, D. Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1549–H1557. [Google Scholar] [CrossRef]
- Domingos-Souza, G.; Martinez, D.; Sinkler, S.; Heesch, C.M.; Kline, D.D. Alpha adrenergic receptor signaling in the hypothalamic paraventricular nucleus is diminished by the chronic intermittent hypoxia model of sleep apnea. Exp. Neurol. 2021, 335, 113517. [Google Scholar] [CrossRef]
- Sharpe, A.L.; Calderon, A.S.; Andrade, M.A.; Cunningham, J.T.; Mifflin, S.W.; Toney, G.M. Chronic intermittent hypoxia increases sympathetic control of blood pressure: Role of neuronal activity in the hypothalamic paraventricular nucleus. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1772–H1780. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Gua, C.; Wu, B. Elevated Oxidative Stress and Inflammation in Hypothalamic Paraventricular Nucleus Are Associated With Sympathetic Excitation and Hypertension in Rats Exposed to Chronic Intermittent Hypoxia. Front. Physiol. 2018, 9, 840. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.M.; Hu, K.; Zhou, X.F.; Tang, S. Sensory plasticity of carotid body is correlated with oxidative stress in paraventricular nucleus during chronic intermittent hypoxia. J. Cell Physiol. 2019, 234, 13534–13543. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N. Multiple role of 3-mercaptopyruvate sulfurtransferase: Antioxidative function, H2S and polysulfide production and possible SOx production. Br. J. Pharmacol. 2018, 175, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Katsouda, A.; Markou, M.; Zampas, P.; Varela, A.; Davos, C.H.; Vellecco, V.; Cirino, G.; Bucci, M.; Papapetropoulos, A. CTH/MPST double ablation results in enhanced vasorelaxation and reduced blood pressure via upregulation of the eNOS/sGC pathway. Front. Pharmacol. 2023, 14, 1090654. [Google Scholar] [CrossRef]
- Nagahara, N.; Nagano, M.; Ito, T.; Shimamura, K.; Akimoto, T.; Suzuki, H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: A model for human mercaptolactate-cysteine disulfiduria. Sci. Rep. 2013, 3, 1986. [Google Scholar] [CrossRef]
- Nagahara, N. Regulation of mercaptopyruvate sulfurtransferase activity via intrasubunit and intersubunit redox-sensing switches. Antioxid. Redox Signal 2013, 19, 1792–1802. [Google Scholar] [CrossRef]
- Denoix, N.; Merz, T.; Unmuth, S.; Hoffmann, A.; Nespoli, E.; Scheuerle, A.; Huber-Lang, M.; Gundel, H.; Waller, C.; Radermacher, P.; et al. Cerebral Immunohistochemical Characterization of the H2S and the Oxytocin Systems in a Porcine Model of Acute Subdural Hematoma. Front. Neurol. 2020, 11, 649. [Google Scholar] [CrossRef]
- Sang, D.; Lin, K.; Yang, Y.; Ran, G.; Li, B.; Chen, C.; Li, Q.; Ma, Y.; Lu, L.; Cui, X.Y.; et al. Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals. Cell 2023, 186, 5500–5516.e5521. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Won, E.; Kim, Y.K. Neuroinflammation-Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 6546. [Google Scholar] [CrossRef]
- King, C.E.; Griffin, W.C.; Lopez, M.F.; Becker, H.C. Activation of hypothalamic oxytocin neurons reduces binge-like alcohol drinking through signaling at central oxytocin receptors. Neuropsychopharmacology 2021, 46, 1950–1957. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Liang, J.; Li, J.; Huang, K.; Li, J.; Liu, C. The neuroprotective effect of oxytocin on vincristine-induced neurotoxicity in mice. Toxicol. Lett. 2021, 340, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Simsek, Y.; Celik, O.; Karaer, A.; Gul, M.; Yilmaz, E.; Koc, O.; Colak, C.; Zengin, S.; Aydin, N.E. Therapeutic efficiency of Atosiban, an oxytocin receptor blocking agent in the treatment of experimental endometriosis. Arch. Gynecol. Obstet. 2012, 286, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jia, X.; Yang, L.; Zhang, S.; Chen, Z.; Gui, Q.; Li, T.; Pu, Z.; Qi, H.; Zhang, J. New therapeutic target NCF1-directed multi-bioactive conjugate therapies prevent preterm birth and adverse pregnancy outcomes. Sci. Bull. 2024, 69, 2604–2621. [Google Scholar] [CrossRef]
- Fan, D.; Jin, Z.; Cao, J.; Li, Y.; He, T.; Zhang, W.; Peng, L.; Liu, H.; Wu, X.; Chen, M.; et al. Leucine zipper protein 1 prevents doxorubicin-induced cardiotoxicity in mice. Redox Biol. 2023, 64, 102780. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Chen, M.; Bi, L.L. Optogenetic Long-Term Depression Induction in the PVT-CeL Circuitry Mediates Decreased Fear Memory. Mol. Neurobiol. 2019, 56, 4855–4865. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Gutkowska, J.; Jankowski, M. Oxytocin revisited: Its role in cardiovascular regulation. J. Neuroendocrinol. 2012, 24, 599–608. [Google Scholar] [CrossRef]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. Oxytocin and cardioprotection in diabetes and obesity. BMC Endocr. Disord. 2016, 16, 34. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Gaffke, L.; Zabinska, M.; Cyske, Z.; Rintz, E.; Wisniewska, K.; Podlacha, M.; Wegrzyn, G. Roles of the Oxytocin Receptor (OXTR) in Human Diseases. Int. J. Mol. Sci. 2023, 24, 3887. [Google Scholar] [CrossRef]
- Wsol, A.; Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E.; Kowalewski, S.; Puchalska, L. Oxytocin in the cardiovascular responses to stress. J. Physiol. Pharmacol. 2008, 59 (Suppl. S8), 123–127. [Google Scholar] [PubMed]
- Yang, Y.; Liu, J.; Wang, L.; Wu, W.; Wang, Q.; Zhao, Y.; Qian, X.; Wang, Z.; Fu, N.; Wang, Y.; et al. Oxytocin attenuates cardiac hypertrophy by improving cardiac glucose metabolism and regulating OXTR/JAK2/STAT3 axis. Peptides 2024, 182, 171323. [Google Scholar] [CrossRef]
- Schunke, K.J.; Rodriguez, J.; Dyavanapalli, J.; Schloen, J.; Wang, X.; Escobar, J.; Kowalik, G.; Cheung, E.C.; Ribeiro, C.; Russo, R.; et al. Outcomes of hypothalamic oxytocin neuron-driven cardioprotection after acute myocardial infarction. Basic. Res. Cardiol. 2023, 118, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qiu, L.; Xiao, W.; Ni, H.; Chen, L.; Wang, F.; Mai, W.; Wu, J.; Bao, A.; Hu, H.; et al. Reconstruction of the Hypothalamo-Neurohypophysial System and Functional Dissection of Magnocellular Oxytocin Neurons in the Brain. Neuron 2021, 109, 331–346.e337. [Google Scholar] [CrossRef] [PubMed]
- Thibord, F.; Johnson, A.D. Sources of variability in the human platelet transcriptome. Thromb. Res. 2023, 231, 255–263. [Google Scholar] [CrossRef]
- Supernat, A.; Popeda, M.; Pastuszak, K.; Best, M.G.; Gresner, P.; Veld, S.I.; Siek, B.; Bednarz-Knoll, N.; Rondina, M.T.; Stokowy, T.; et al. Transcriptomic landscape of blood platelets in healthy donors. Sci. Rep. 2021, 11, 15679. [Google Scholar] [CrossRef]
- Bhagat, A.; Shrestha, P.; Kleinerman, E.S. The Innate Immune System in Cardiovascular Diseases and Its Role in Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 14649. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Walker, C.H.; Pedersen, C.A.; Mason, G.A. Sexual activity decreases oxytocin receptor densities in the thymus. Life Sci. 1993, 52, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Noiseux, N.; Borie, M.; Desnoyers, A.; Menaouar, A.; Stevens, L.M.; Mansour, S.; Danalache, B.A.; Roy, D.C.; Jankowski, M.; Gutkowska, J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology 2012, 153, 5361–5372. [Google Scholar] [CrossRef]
- Ndiaye, K.; Poole, D.H.; Pate, J.L. Expression and regulation of functional oxytocin receptors in bovine T lymphocytes. Biol. Reprod. 2008, 78, 786–793. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Wang, S.C.; Wang, Y.F. Approaches Mediating Oxytocin Regulation of the Immune System. Front. Immunol. 2016, 7, 693. [Google Scholar] [CrossRef]
- Liccardi, G.; Bilo, M.; Mauro, C.; Salzillo, A.; Piccolo, A.; D’Amato, M.; Liccardi, A.; D’Amato, G. Oxytocin: An unexpected risk for cardiologic and broncho-obstructive effects, and allergic reactions in susceptible delivering women. Multidiscip. Respir. Med. 2013, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Saji, F.; Samejima, Y.; Kamiura, S.; Sawai, K.; Shimoya, K.; Kimura, T. Cytokine production in chorioamnionitis. J. Reprod. Immunol. 2000, 47, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.Y.; Zhang, J.; Sullivan, S.; Newman, R.; Singh, I. N-acetylcysteine prevents preterm birth by attenuating the LPS-induced expression of contractile associated proteins in an animal model. J. Matern. Fetal Neonatal Med. 2012, 25, 2395–2400. [Google Scholar] [CrossRef]
- Dyavanapalli, J.; Rodriguez, J.; Rocha Dos Santos, C.; Escobar, J.B.; Dwyer, M.K.; Schloen, J.; Lee, K.M.; Wolaver, W.; Wang, X.; Dergacheva, O.; et al. Activation of Oxytocin Neurons Improves Cardiac Function in a Pressure-Overload Model of Heart Failure. JACC Basic Transl. Sci. 2020, 5, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.; Clark, C.; Kennedy, B.; Christian Gillin, J.; Ziegler, M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav. Immun. 2003, 17, 365–372. [Google Scholar] [CrossRef]
- Irwin, M.; Thompson, J.; Miller, C.; Gillin, J.C.; Ziegler, M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: Clinical implications. J. Clin. Endocrinol. Metab. 1999, 84, 1979–1985. [Google Scholar] [CrossRef]
- Irwin, M.R.; Ziegler, M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension 2005, 45, 252–257. [Google Scholar] [CrossRef]
- Yao, S.; Chen, Y.; Zhuang, Q.; Zhang, Y.; Lan, C.; Zhu, S.; Becker, B.; Kendrick, K.M. Sniffing oxytocin: Nose to brain or nose to blood? Mol. Psychiatry 2023, 28, 3083–3091. [Google Scholar] [CrossRef]
- Niu, J.; Tong, J.; Blevins, J.E. Oxytocin as an Anti-obesity Treatment. Front. Neurosci. 2021, 15, 743546. [Google Scholar] [CrossRef]
- Kou, J.; Zhang, Y.; Zhou, F.; Gao, Z.; Yao, S.; Zhao, W.; Li, H.; Lei, Y.; Gao, S.; Kendrick, K.M.; et al. Anxiolytic Effects of Chronic Intranasal Oxytocin on Neural Responses to Threat Are Dose-Frequency Dependent. Psychother. Psychosom. 2022, 91, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, T. Of Man and Mice: Translational Research in Neurotechnology. Neuron 2020, 105, 12–15. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Xu, Z.; Kong, X.; Wang, H.; Lu, Z.; Chen, M.; Bi, L. The Role of Oxytocin Neurons in the Paraventricular Nucleus in Chronic-Sleep-Deprivation-Mediated Abnormal Cardiovascular Responses. Curr. Issues Mol. Biol. 2025, 47, 220. https://doi.org/10.3390/cimb47040220
Zhang Y, Wang Y, Xu Z, Kong X, Wang H, Lu Z, Chen M, Bi L. The Role of Oxytocin Neurons in the Paraventricular Nucleus in Chronic-Sleep-Deprivation-Mediated Abnormal Cardiovascular Responses. Current Issues in Molecular Biology. 2025; 47(4):220. https://doi.org/10.3390/cimb47040220
Chicago/Turabian StyleZhang, Yifei, Yuxin Wang, Zhendong Xu, Xiangjie Kong, Hairong Wang, Zhibing Lu, Ming Chen, and Linlin Bi. 2025. "The Role of Oxytocin Neurons in the Paraventricular Nucleus in Chronic-Sleep-Deprivation-Mediated Abnormal Cardiovascular Responses" Current Issues in Molecular Biology 47, no. 4: 220. https://doi.org/10.3390/cimb47040220
APA StyleZhang, Y., Wang, Y., Xu, Z., Kong, X., Wang, H., Lu, Z., Chen, M., & Bi, L. (2025). The Role of Oxytocin Neurons in the Paraventricular Nucleus in Chronic-Sleep-Deprivation-Mediated Abnormal Cardiovascular Responses. Current Issues in Molecular Biology, 47(4), 220. https://doi.org/10.3390/cimb47040220






