Dark Sweet Cherry (Prunus avium L.) Juice Phenolics Rich in Anthocyanins Exhibit Potential to Inhibit Drug Resistance Mechanisms in 4T1 Breast Cancer Cells via the Drug Metabolism Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Antibodies, and Reagents
2.2. Extraction of Phenolics Enriched in Anthocyanins (ACN)
2.3. Cell Line
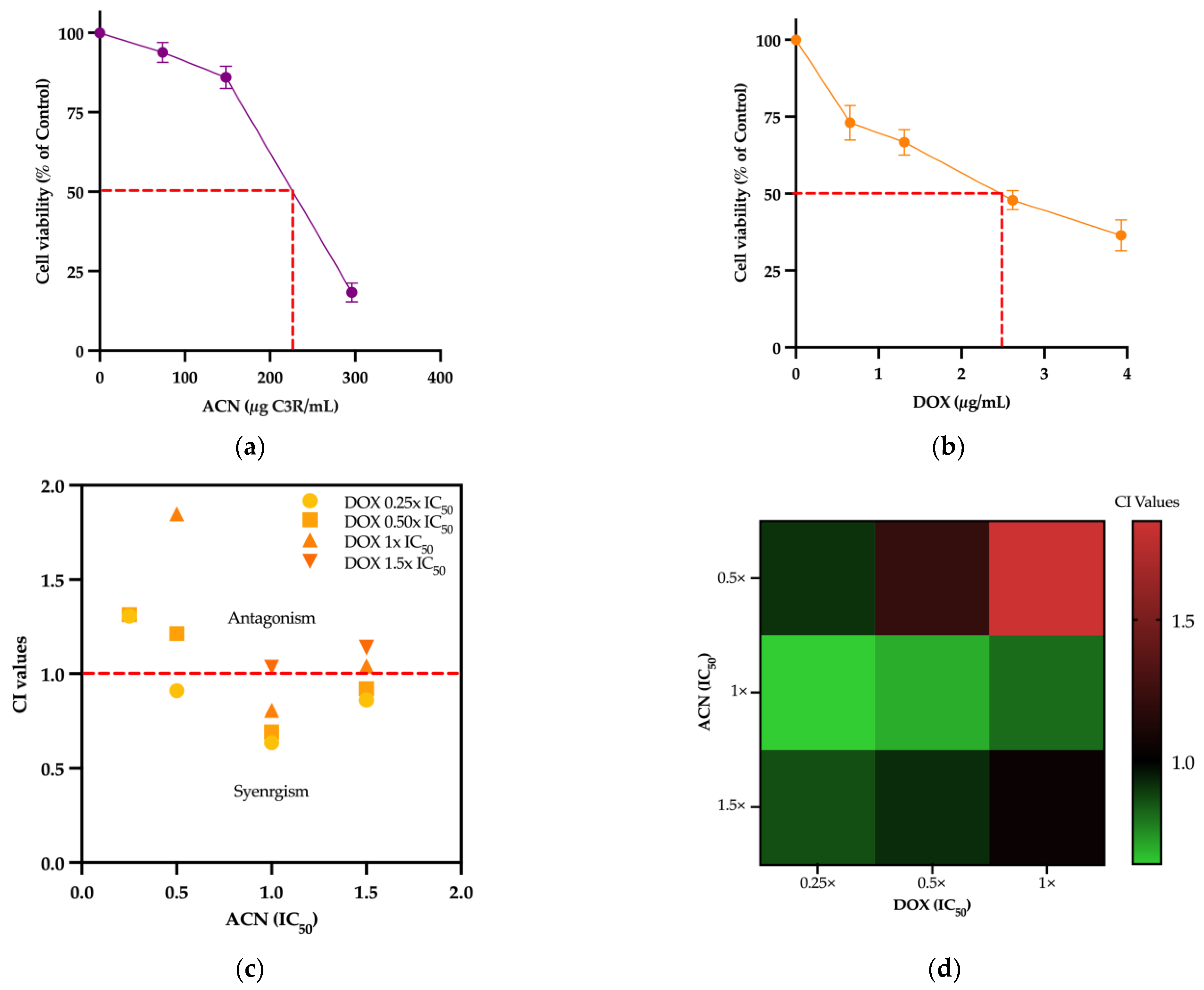
2.4. Cell Viability
2.5. Synergistic Effect of ACN and DOX Combination
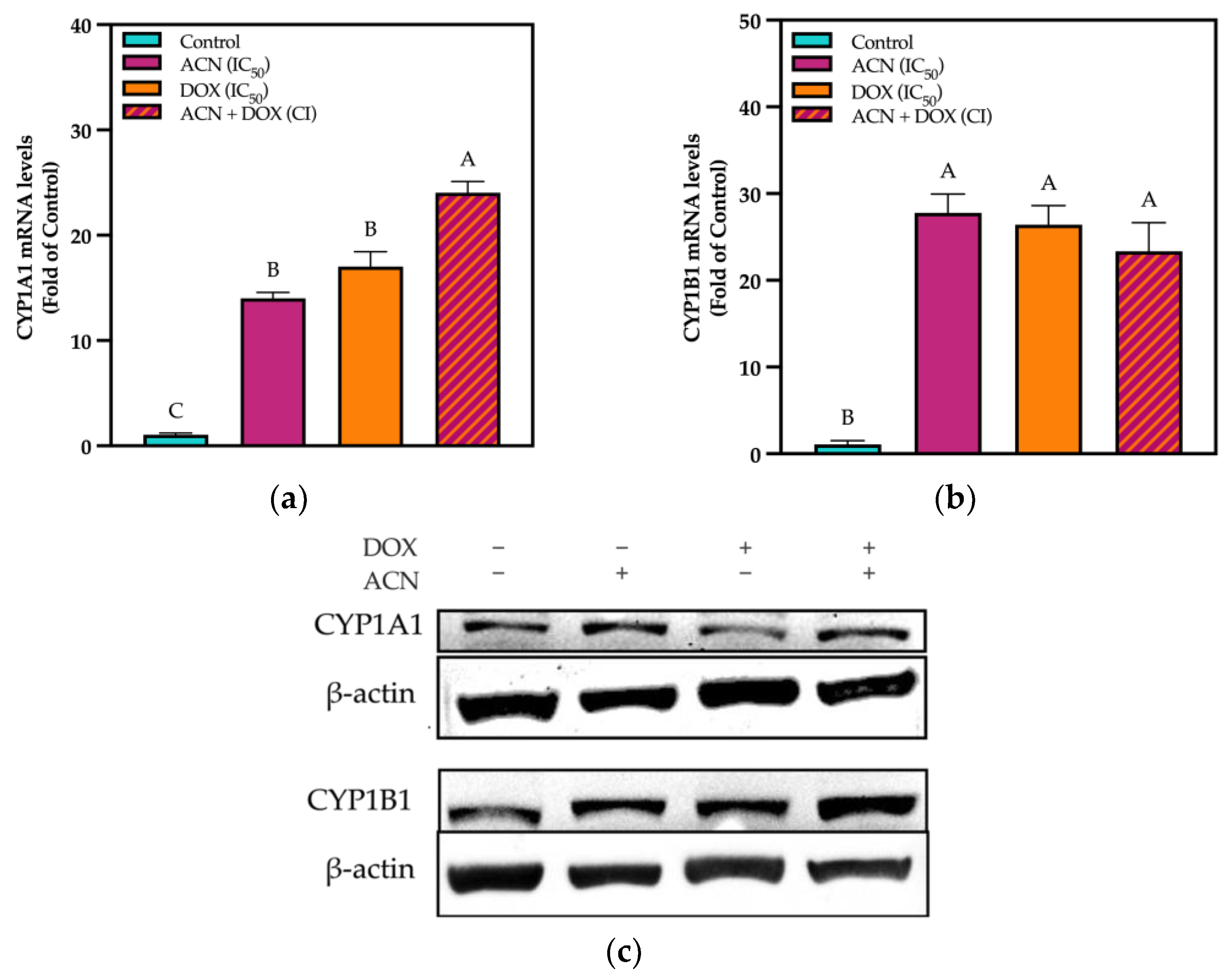
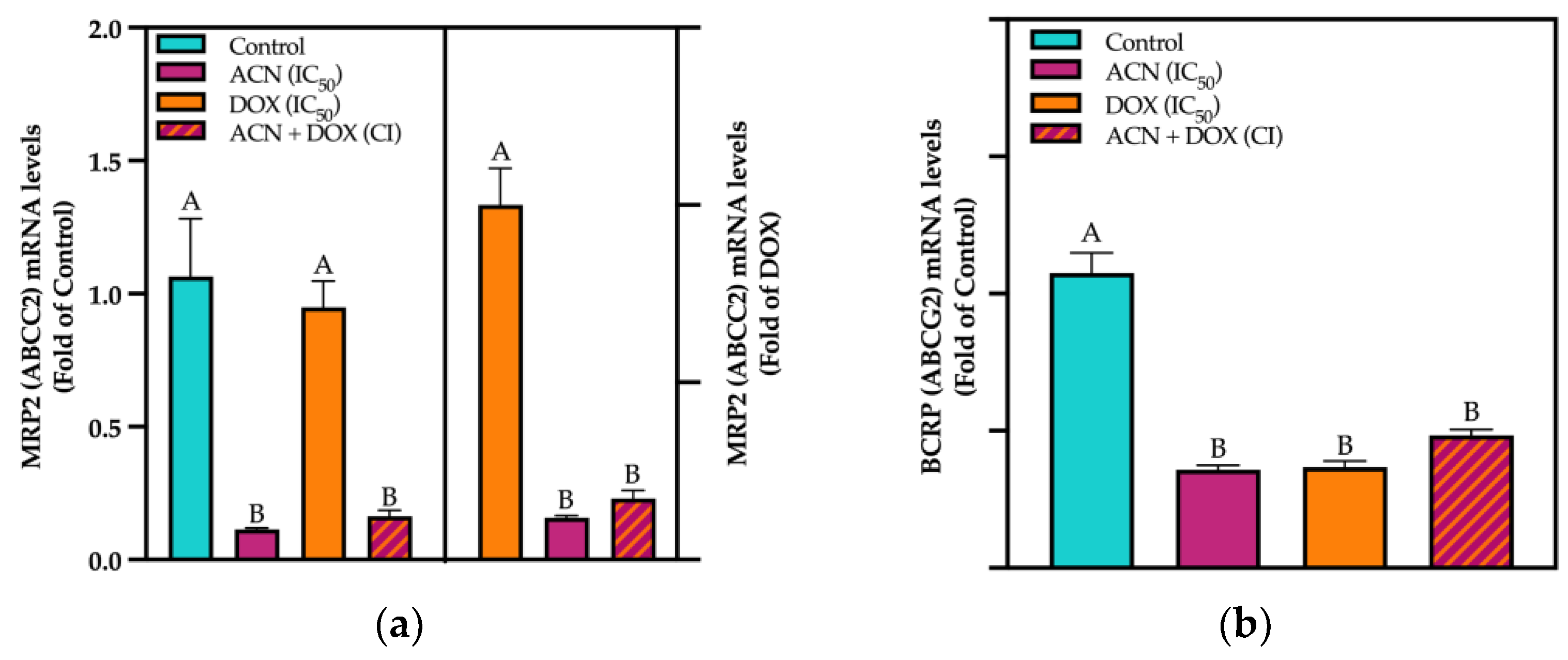
2.6. Gene Expression
2.7. Protein Expression
2.8. CYP1 Enzymatic Activity
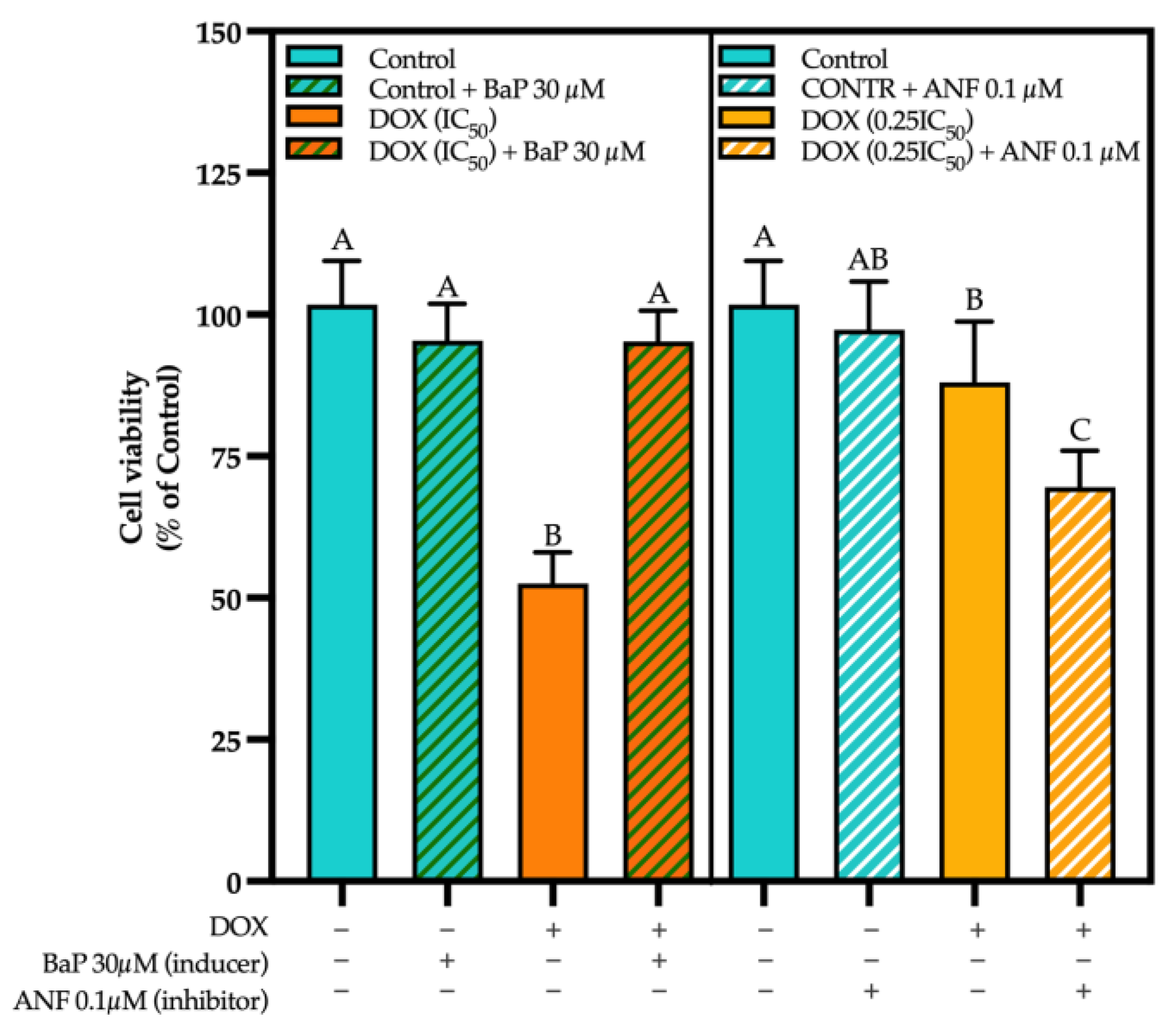
2.9. Effect of CYP Modulation on Cell Viability
2.10. Rhodamine123 Efflux Assay
2.11. Effect of P-gp Modulation on Cell Viability
2.12. Statistical Analysis
3. Results and Discussion
3.1. ACN and DOX Synergized to Inhibit 4T1 Cell Viability
3.2. Modulation of Phase I Drug Metabolizing in TNBC 4T1 Cells by ACN
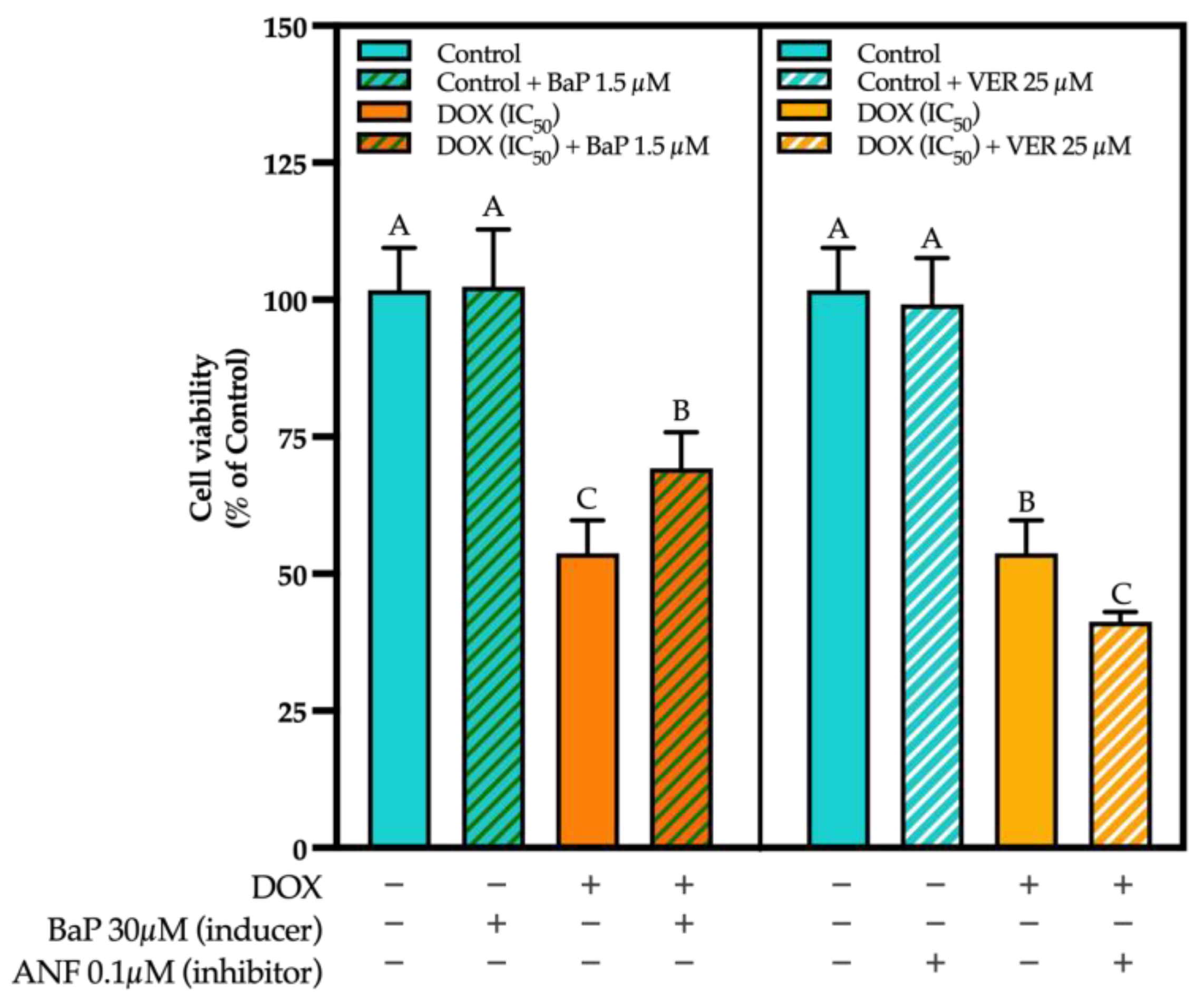
3.3. CYP Modulation by ACN Enhanced Cell Viability Inhibition by DOX
3.4. ACN Target Phase III Drug Transporters
3.5. ACN-Induced Drug Efflux Inhibition Contributed to the ACN-DOX Synergism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | dark sweet cherries |
| ACNs | anthocyanins |
| BC | breast cancer |
| TNBC | triple negative breast cancer |
| DR | drug resistance |
| DOX | Doxorubicin |
| CYP | Cytochrome P450 |
| P-gp | P-glycoprotein |
| HER2 | human epidermal growth factor receptor type 2 |
| ER | estrogen receptor |
| PR | progesterone receptor |
| AhR | aryl hydrocarbon receptor |
| ABC | ATP-Binding Cassette |
| MDR-1 | multidrug resistance protein 1 |
| ABCB1 | ATP-Binding Cassette Subfamily Member 1 |
| BCRP | BC resistance protein |
| ABCG2 | ATP-Binding Cassette Subfamily G Member 2 |
| MRP2 | multi-drug resistance protein 2 |
| ABCC2 | ATP-Binding Cassette Subfamily C 2 |
| CI | combination index |
| ANF | alpha-naphthoflavone |
| BaP | Benzo[a]Pyrene |
| Rho | Rhodamine-123 |
| VER | Verapamil |
References
- Foulkes, W.D.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Boušová, I.; Skálová, L. Possibilities to Increase the Effectiveness of Doxorubicin in Cancer Cells Killing. Drug Metab. Rev. 2011, 43, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Sritharan, S.; Sivalingam, N. A Comprehensive Review on Time-Tested Anticancer Drug Doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Kurmi, B.D. A Comprehensive Review on Doxorubicin: Mechanisms, Toxicity, Clinical Trials, Combination Therapies and Nanoformulations in Breast Cancer. Drug Deliv. Transl. Res. 2025, 15, 102–133. [Google Scholar] [CrossRef]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark Sweet Cherry (Prunus avium) Phenolics Enriched in Anthocyanins Exhibit Enhanced Activity against the Most Aggressive Breast Cancer Subtypes without Toxicity to Normal Breast Cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Jodoin, J.; Demeule, M.; Béliveau, R. Inhibition of the Multidrug Resistance P-Glycoprotein Activity by Green Tea Polyphenols. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Cooray, H.C.; Janvilisri, T.; Van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the Breast Cancer Resistance Protein with Plant Polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef]
- Hussain, S.A.; Sulaiman, A.A.; Balch, C.; Chauhan, H.; Alhadidi, Q.M.; Tiwari, A.K. Natural Polyphenols in Cancer Chemoresistance. Nutr. Cancer 2016, 68, 879–891. [Google Scholar] [CrossRef]
- Costea, T.; Vlad, O.C.; Miclea, L.-C.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Alleviation of Multidrug Resistance by Flavonoid and Non-Flavonoid Compounds in Breast, Lung, Colorectal and Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 401. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Go, R.-E.; Hwang, K.-A.; Choi, K.-C. Cytochrome P450 1 Family and Cancers. J. Steroid Biochem. Mol. Biol. 2015, 147, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hutter, M.; Goh, W.W.B.; Bureik, M. CYP4Z1—A Human Cytochrome P450 Enzyme That Might Hold the Key to Curing Breast Cancer. Curr. Pharm. Des. 2017, 23, 2060–2064. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Yu, X.; Chen, B.; Hao, L.; Chen, P.; Chen, X.; Tian, Y.; Zeng, J.; Hua, H.; Dai, Y.; et al. Tetrahydrocurcumin Regulates the Tumor Immune Microenvironment to Inhibit Breast Cancer Proliferation and Metastasis via the CYP1A1/NF-κB Signaling Pathway. Cancer Cell Int. 2023, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Scatena, C.; Ghilli, M.; Bargagna, I.; Lorenzini, G.; Nicolini, A. Molecular Mechanisms, Biomarkers and Emerging Therapies for Chemotherapy Resistant TNBC. Int. J. Mol. Sci. 2022, 23, 1665. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural Products as Multidrug Resistance Modulators in Cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- Silveira Rabelo, A.C.; Mertens-Talcott, S.U.; Chew, B.P.; Noratto, G. Dark Sweet Cherry (Prunus avium) Anthocyanins Suppressed ERK1/2-Akt/mTOR Cell Signaling and Oxidative Stress: Implications for TNBC Growth and Invasion. Molecules 2022, 27, 7245. [Google Scholar] [CrossRef]
- Du, G.; Lin, H.; Wang, M.; Zhang, S.; Wu, X.; Lu, L.; Ji, L.; Yu, L. Quercetin Greatly Improved Therapeutic Index of Doxorubicin against 4T1 Breast Cancer by Its Opposing Effects on HIF-1α in Tumor and Normal Cells. Cancer Chemother. Pharmacol. 2010, 65, 277–287. [Google Scholar] [CrossRef]
- Gheybi, F.; Alavizadeh, S.H.; Rezayat, S.M.; Zendedel, E.; Jaafari, M. Chemotherapeutic Activity of Silymarin Combined with Doxorubicin Liposomes in 4T1 Breast Cancer Cells. Nanomed. Res. J. 2019, 4, 29–34. [Google Scholar] [CrossRef]
- Singh, R.D.; Avadhesh, A.; Sharma, G.; Dholariya, S.; Shah, R.B.; Goyal, B.; Gupta, S.C. Potential of Cytochrome P450, a Family of Xenobiotic Metabolizing Enzymes, in Cancer Therapy. Antioxid. Redox Signal. 2023, 38, 853–876. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Wang, C.C.N.; Liao, W.-C.; Lan, Y.-H.; Hung, C.-C. Caffeic Acid Attenuates Multi-Drug Resistance in Cancer Cells by Inhibiting Efflux Function of Human P-Glycoprotein. Molecules 2020, 25, 247. [Google Scholar] [CrossRef]
- Hou, X.-L.; Takahashi, K.; Tanaka, K.; Tougou, K.; Qiu, F.; Komatsu, K.; Takahashi, K.; Azuma, J. Curcuma Drugs and Curcumin Regulate the Expression and Function of P-Gp in Caco-2 Cells in Completely Opposite Ways. Int. J. Pharm. 2008, 358, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Jakobušić Brala, C.; Karković Marković, A.; Kugić, A.; Torić, J.; Barbarić, M. Combination Chemotherapy with Selected Polyphenols in Preclinical and Clinical Studies—An Update Overview. Molecules 2023, 28, 3746. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Awajan, D.; Hamed, R.A.; Azzam, A.O.; Mahmod, A.I.; AL-Yasari, I.H. Combination Anticancer Therapies Using Selected Phytochemicals. Molecules 2022, 27, 5452. [Google Scholar] [CrossRef] [PubMed]
- Wakharde, A.; Awad, A.; Bhagat, A.; Karuppayil, S. Synergistic Activation of Doxorubicin against Cancer: A Review. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1009. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Sinal, C.J.; Bend, J.R. Aryl Hydrocarbon Receptor-Dependent Induction of Cyp1a1 by Bilirubin in Mouse Hepatoma Hepa 1c1c7 Cells. Mol. Pharmacol. 1997, 52, 590–599. [Google Scholar] [CrossRef]
- Noratto, G.; Layosa, M.A.; Lage, N.N.; Atienza, L.; Ivanov, I.; Mertens-Talcott, S.U.; Chew, B.P. Antitumor Potential of Dark Sweet Cherry Sweet (Prunus avium) Phenolics in Suppressing Xenograft Tumor Growth of MDA-MB-453 Breast Cancer Cells. J. Nutr. Biochem. 2020, 84, 108437. [Google Scholar] [CrossRef]
- Layosa, M.A.A.; Lage, N.N.; Chew, B.P.; Atienza, L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark Sweet Cherry (Prunus avium) Phenolics Enriched in Anthocyanins Induced Apoptosis in MDA-MB-453 Breast Cancer Cells through MAPK-Dependent Signaling and Reduced Invasion via Akt and PLCγ-1 Downregulation. Nutr. Cancer 2021, 73, 1985–1997. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic Interactions Between Anticancer Chemotherapeutics and Phenolic Compounds and Anticancer Synergy Between Polyphenols. Postep. Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- D Georgiev, K.; J Slavov, I.; A Iliev, I. Synergistic Growth Inhibitory Effects of Lycium Barbarum (Goji berry) Extract with Doxorubicin against Human Breast Cancer Cells. J. Pharm. Pharmacol. Res. 2019, 3, 51–58. [Google Scholar] [CrossRef]
- Liu, W.; Xu, J.; Liu, Y.; Yu, X.; Tang, X.; Wang, Z.; Li, X. Anthocyanins Potentiate the Activity of Trastuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer Cells In Vitro and In Vivo. Mol. Med. Rep. 2014, 10, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, J.; Wang, W.; Lyu, L.; Wu, W.; Li, W. The Extraction and High Antiproliferative Effect of Anthocyanin from Gardenblue Blueberry. Molecules 2023, 28, 2850. [Google Scholar] [CrossRef]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the Oral Bioavailability of Beneficial Polyphenols through Designed Synergies. Genes. Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Szaefer, H. Modulation of Carcinogen-Metabolizing Cytochromes P450 by Phytochemicals in Humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 927–941. [Google Scholar] [CrossRef]
- Korobkova, E.A. Effect of Natural Polyphenols on CYP Metabolism: Implications for Diseases. Chem. Res. Toxicol. 2015, 28, 1359–1390. [Google Scholar] [CrossRef]
- McFadyen, M.C.E.; Melvin, W.T.; Murray, G.I. Cytochrome P. 450 Enzymes: Novel Options for Cancer Therapeutics. Mol. Cancer Ther. 2004, 3, 363–371. [Google Scholar] [CrossRef]
- Satomi, Y.; Nishino, H. Inhibition of the Enzyme Activity of Cytochrome P450 1A1, 1A2 and 3A4 by Fucoxanthin, a Marine Carotenoid. Oncol. Lett. 2013, 6, 860–864. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider Roles in Cancer Progression and Prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef]
- Barata, I.S.; Gomes, B.C.; Rodrigues, A.S.; Rueff, J.; Kranendonk, M.; Esteves, F. The Complex Dynamic of Phase I Drug Metabolism in the Early Stages of Doxorubicin Resistance in Breast Cancer Cells. Genes 2022, 13, 1977. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Choi, J.-S. Effect of Genistein on the Pharmacokinetics of Paclitaxel Administered Orally or Intravenously in Rats. Int. J. Pharm. 2007, 337, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Choi, J.; Li, X. Enhanced Bioavailability of Tamoxifen after Oral Administration of Tamoxifen with Quercetin in Rats. Int. J. Pharm. 2006, 313, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yu, L.; Lu, P.; Wei, Y.; Wang, X.; Chen, L. Evaluate the Inhibition of Cytochrome P450 1A1 for Enhancing Breast Cancer Chemotherapy with a Turn-on Fluorescent Probe. Sens. Actuators B Chem. 2021, 344, 130233. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Daschner, P.J.; Wang, T.T.Y.; Yeh, G.C. Effect of Curcumin on the Aryl Hydrocarbon Receptor and Cytochrome P450 1A1 in MCF-7 Human Breast Carcinoma Cells. Biochem. Pharmacol. 1998, 56, 197–206. [Google Scholar] [CrossRef]
- Chan, H.Y.; Chen, Z.; Tsang, D.S.C.; Leung, L.K. Baicalein Inhibits DMBA–DNA Adduct Formation by Modulating CYP1A1 and CYP1B1 Activities. Biomed. Pharmacother. 2002, 56, 269–275. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Yeh, G.C. Inhibition of Aryl Hydrocarbon-Induced Cytochrome P-450 1A1 Enzyme Activity and CYP1A1 Expression by Resveratrol. Mol. Pharmacol. 1999, 56, 760–767. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Crawford, R.R.; Potukuchi, P.K.; Schuetz, E.G.; Schuetz, J.D. Beyond Competitive Inhibition: Regulation of ABC Transporters by Kinases and Protein-Protein Interactions as Potential Mechanisms of Drug-Drug Interactions. Drug Metab. Dispos. 2018, 46, 567–580. [Google Scholar] [CrossRef]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wu, H. Ubiquitination-Proteasome System (UPS) and Autophagy Two Main Protein Degradation Machineries in Response to Cell Stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Couraud, P.-O.; Suzuki, T.; Terasaki, T. Oxidative Stress-Induced Activation of Abl and Src Kinases Rapidly Induces P-Glycoprotein Internalization via Phosphorylation of Caveolin-1 on Tyrosine-14, Decreasing Cortisol Efflux at the Blood–Brain Barrier. J. Cereb. Blood Flow. Metab. 2020, 40, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Shin, S.-C.; Choi, J.-S. Effects of Myricetin on the Bioavailability of Doxorubicin for Oral Drug Delivery in Rats: Possible Role of CYP3A4 and P-Glycoprotein Inhibition by Myricetin. Arch. Pharm. Res. 2011, 34, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rouibah, H.; Kebsa, W.; Lahouel, M.; Zihlif, M.; Ahram, M.; Aburmaileh, B.; Al Shhab, M.A.M.; Al-Ameer, H.J.; Mustafa, E. Algerian Propolis: Between Protection of Normal Cells and Potentialisation of the Anticancer Effects of Doxorubicin against Breast Cancer Cells via p-Glycoprotein Inhibition and Cell Cycle Arrest in the S Phase. J. Physiol. Pharmacol. 2021, 72, 239–248. [Google Scholar] [CrossRef]
- Yumrutaş, Ö.; Yumrutaş, P. Phenolic Compounds That Modulate Multi Drug Resistance through Inhibiting of P-Glycoprotein Encoded by Gene ABCB1. Eurasian J. Biol. Chem. Sci. 2022, 5, 162–165. [Google Scholar] [CrossRef]


| ACN (IC50) | DOX (IC50) | |||
| 0.25× | 0.5× | 1× | 1.5× | |
| 0.25× | 0.25:0.25 | 0.25:0.5 | 0.25:1 | 0.25:1.5 |
| 0.5× | 0.5:0.25 | 0.5:0.5 | 0.5:1 | 0.5:1.5 |
| 1× | 1:0.25 | 1:0.5 | 1:1 | 1:1.5 |
| 1.5× | 1.5:0.25 | 1.5:0.5 | 1.5:1 | 1.5:1.5 |
| Primer | Sequence |
|---|---|
| CYP1A1 | Forward 5′-GGAACTAGACACAGTGATTG-3′ |
| Reverse 3′-TTGGGGATATAGAAGCCATTC-5′ | |
| CYP1B1 | Forward 5′-ACTATTACGGACATCTTCGG-3′ |
| Reverse 3′-ATCTGGTAAAGAGGATGAGC-5′ | |
| ABCB1 | Forward 5′-CCCATCATTGCAATAGCAGG-3′ |
| Reverse 3′-GTTCAAACTTCTGCTCCTGA-5′ | |
| ABCC1 | Forward 5′-GAGTCAAAGCCGGTGGAAAAT-3′ |
| Reverse 3′-TTAGCTCCAGCCTTCTGCAGTT-5′ | |
| ABCG2 | Forward 5′-AGCTCCGATGGATTGCCAG-3′ |
| Reverse 3′-GAGGGTTCCCGAGCAAGT-TT-5′ | |
| RPL-19 | Forward 5′-GAAGGTCAAAGGGAATGTGTTC-3′ |
| Reverse 3′-CCTTGTCTGCCTTCAGCTTGT-5′ |
| ACN (µg C3R/mL) | Cell Viability (% of Control) (Mean ± SD) |
|---|---|
| 0 | 100 |
| 74.12 | 93.81 ± 7.04 |
| 148.24 | 84.21 ± 7.88 |
| 296.49 | 18.30 ± 5.07 |
| DOX (µg/mL) | Cell Viability (% of Control) (Mean ± SD) |
| 0 | 100 |
| 0.655 | 73.10 ± 8.02 |
| 1.25 | 66.75 ± 10.28 |
| 2.6 | 47.89 ± 7.65 |
| 3.93 | 36.49 ± 11.19 |
| ACN (IC50) | DOX (IC50) | Combination Index (CI) |
|---|---|---|
| 0.25× | 0.25× | 1.3064 |
| 0.25× | 0.50× | 1.3145 |
| 0.25× | 1× | 2.9162 |
| 0.25× | 1.5× | 4.0968 |
| 0.50× | 0.25× | 0.9102 |
| 0.50× | 0.50× | 1.2126 |
| 0.50× | 1× | 1.8487 |
| 0.50× | 1.5× | 2.3838 |
| 1× | 0.25× | 0.6353 |
| 1× | 0.50× | 0.6908 |
| 1× | 1× | 0.8071 |
| 1× | 1.5× | 1.0344 |
| 1.5× | 0.25× | 0.8602 |
| 1.5× | 0.50× | 0.9208 |
| 1.5× | 1× | 1.0409 |
| 1.5× | 1.5× | 1.1391 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Ochoa, A.; Mertens-Talcott, S.U.; Talcott, S.T.; Noratto, G.D. Dark Sweet Cherry (Prunus avium L.) Juice Phenolics Rich in Anthocyanins Exhibit Potential to Inhibit Drug Resistance Mechanisms in 4T1 Breast Cancer Cells via the Drug Metabolism Pathway. Curr. Issues Mol. Biol. 2025, 47, 213. https://doi.org/10.3390/cimb47030213
Nava-Ochoa A, Mertens-Talcott SU, Talcott ST, Noratto GD. Dark Sweet Cherry (Prunus avium L.) Juice Phenolics Rich in Anthocyanins Exhibit Potential to Inhibit Drug Resistance Mechanisms in 4T1 Breast Cancer Cells via the Drug Metabolism Pathway. Current Issues in Molecular Biology. 2025; 47(3):213. https://doi.org/10.3390/cimb47030213
Chicago/Turabian StyleNava-Ochoa, Ana, Susanne U. Mertens-Talcott, Stephen T. Talcott, and Giuliana D. Noratto. 2025. "Dark Sweet Cherry (Prunus avium L.) Juice Phenolics Rich in Anthocyanins Exhibit Potential to Inhibit Drug Resistance Mechanisms in 4T1 Breast Cancer Cells via the Drug Metabolism Pathway" Current Issues in Molecular Biology 47, no. 3: 213. https://doi.org/10.3390/cimb47030213
APA StyleNava-Ochoa, A., Mertens-Talcott, S. U., Talcott, S. T., & Noratto, G. D. (2025). Dark Sweet Cherry (Prunus avium L.) Juice Phenolics Rich in Anthocyanins Exhibit Potential to Inhibit Drug Resistance Mechanisms in 4T1 Breast Cancer Cells via the Drug Metabolism Pathway. Current Issues in Molecular Biology, 47(3), 213. https://doi.org/10.3390/cimb47030213






