USP5 Suppresses Ferroptosis in Bladder Cancer Through Stabilization of GPX4
Abstract
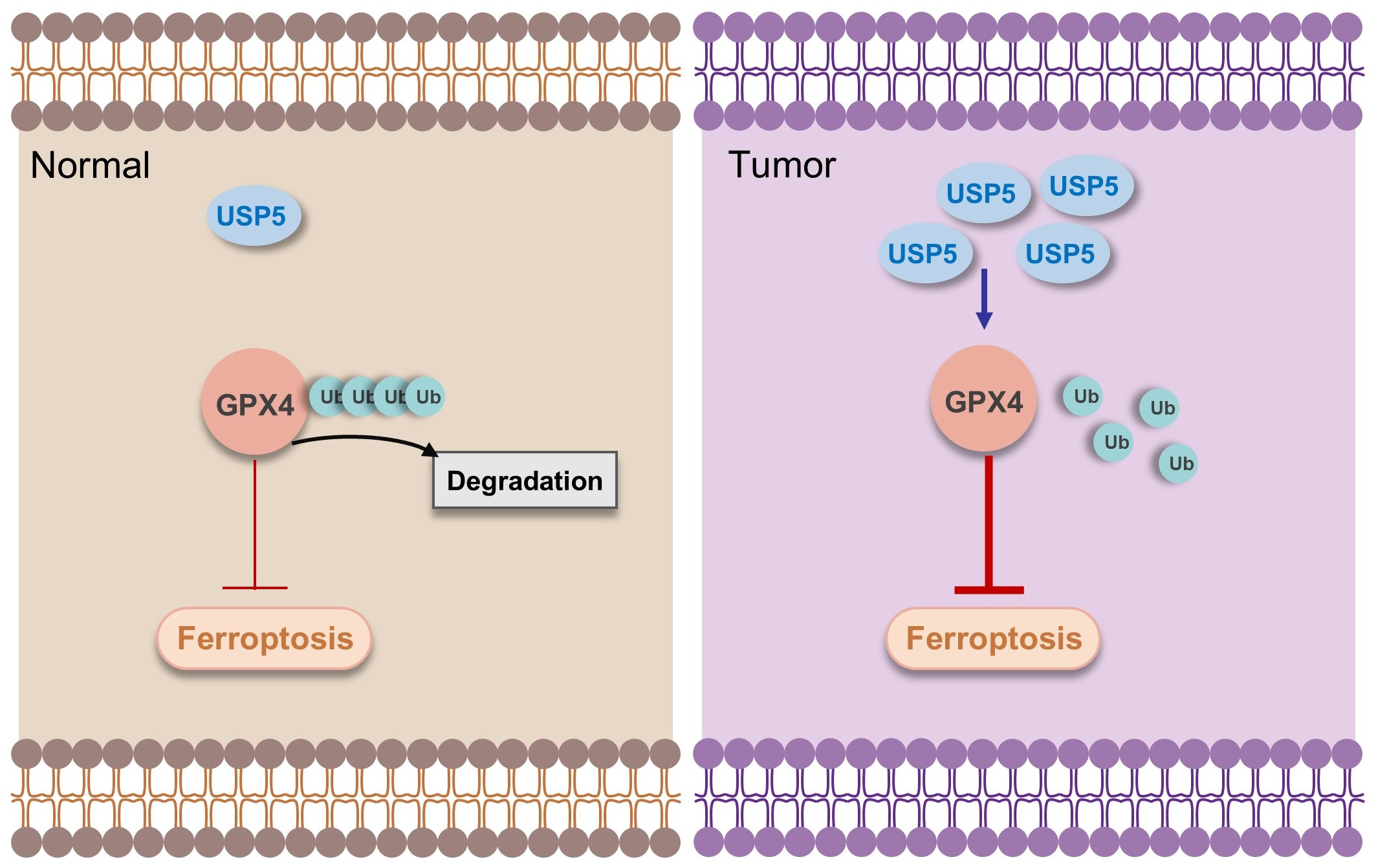
1. Introduction
2. Materials and Methods
2.1. Antibodies and Plasmids
2.2. Cell Culture and Cell Lines
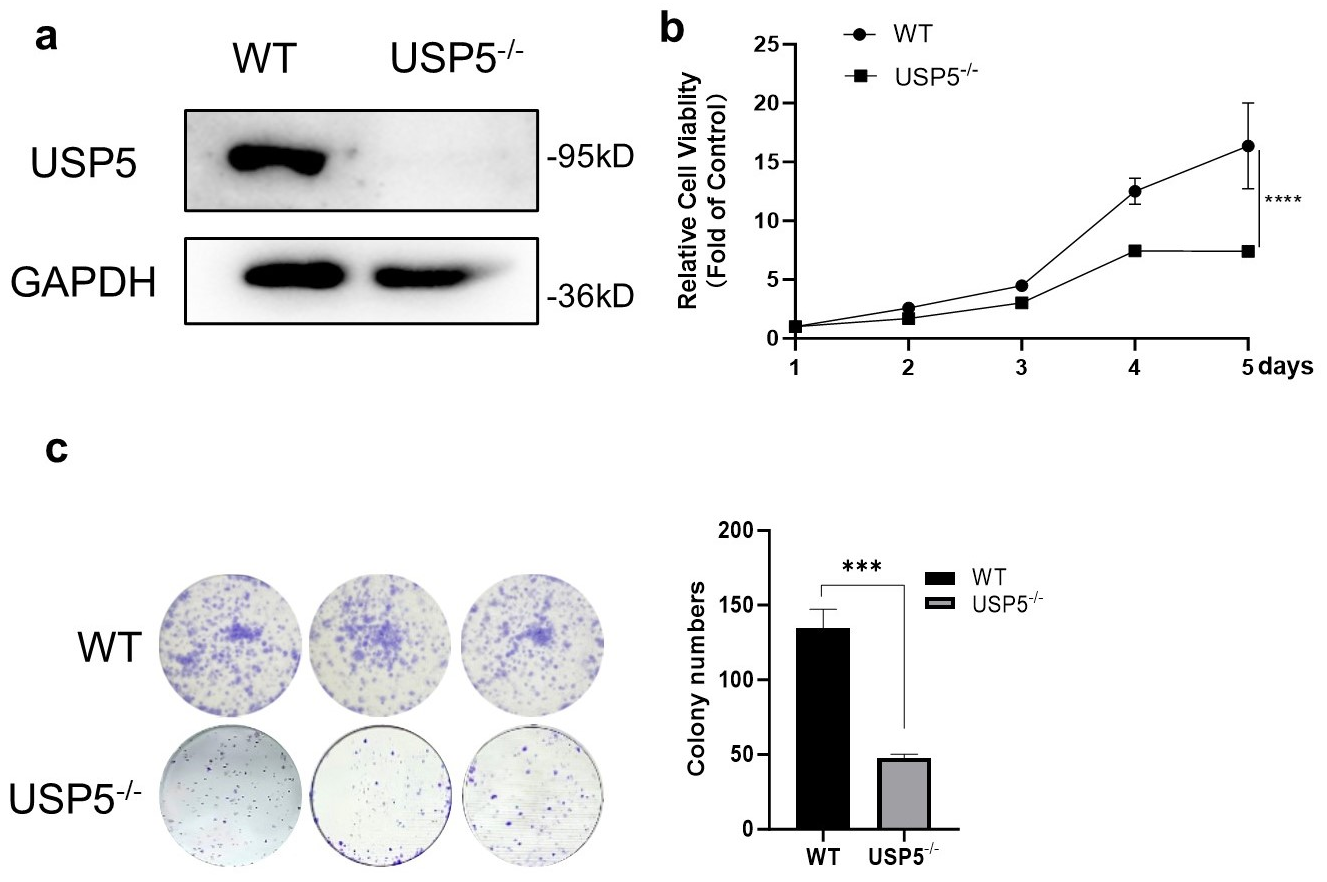
2.3. Cell Proliferation and Colony Formation
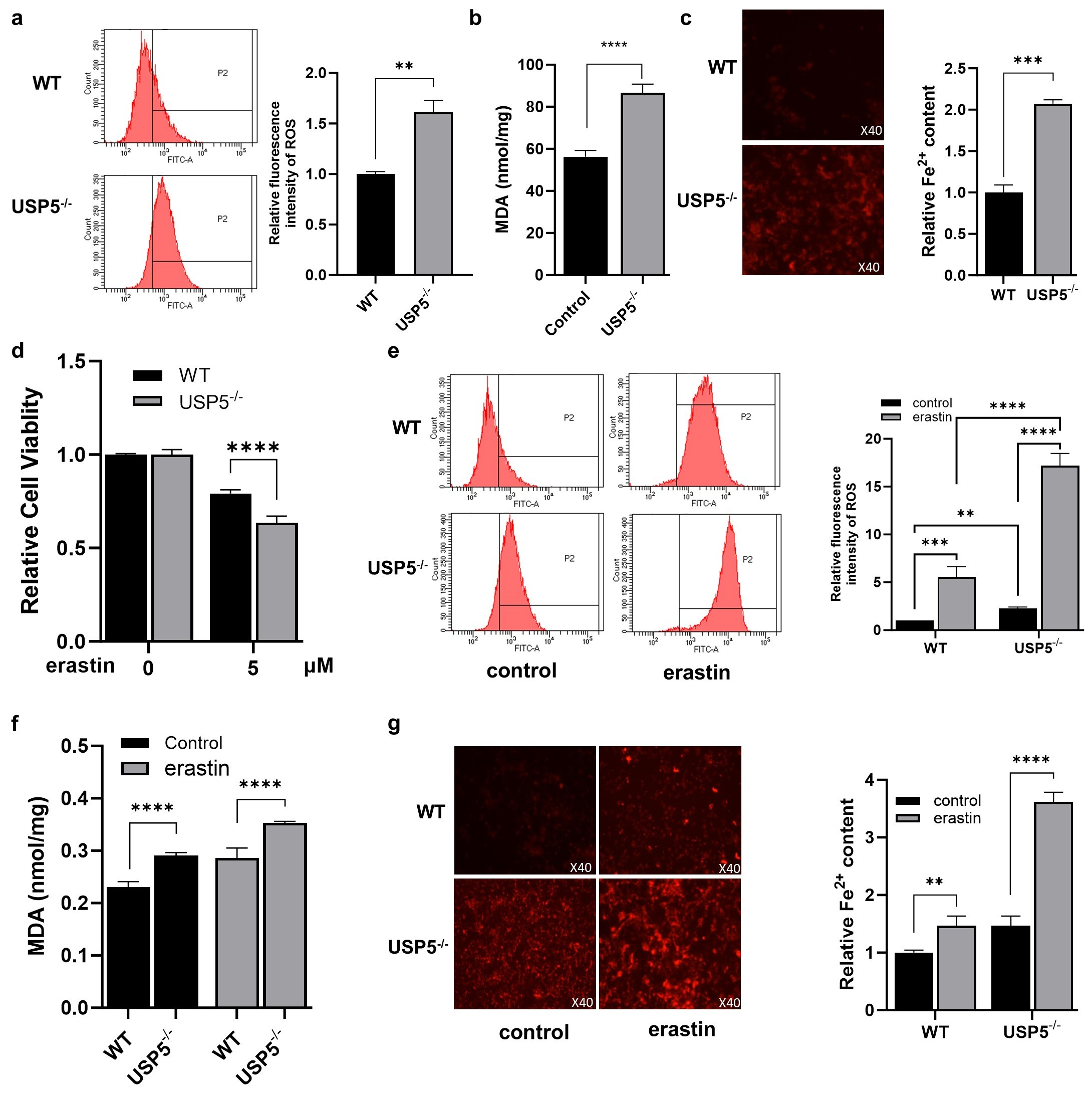
2.4. Determination of Intracellular ROS
2.5. Measurement of the Intracellular Fe2+ and MDA Contents
2.6. Western Blot Analysis
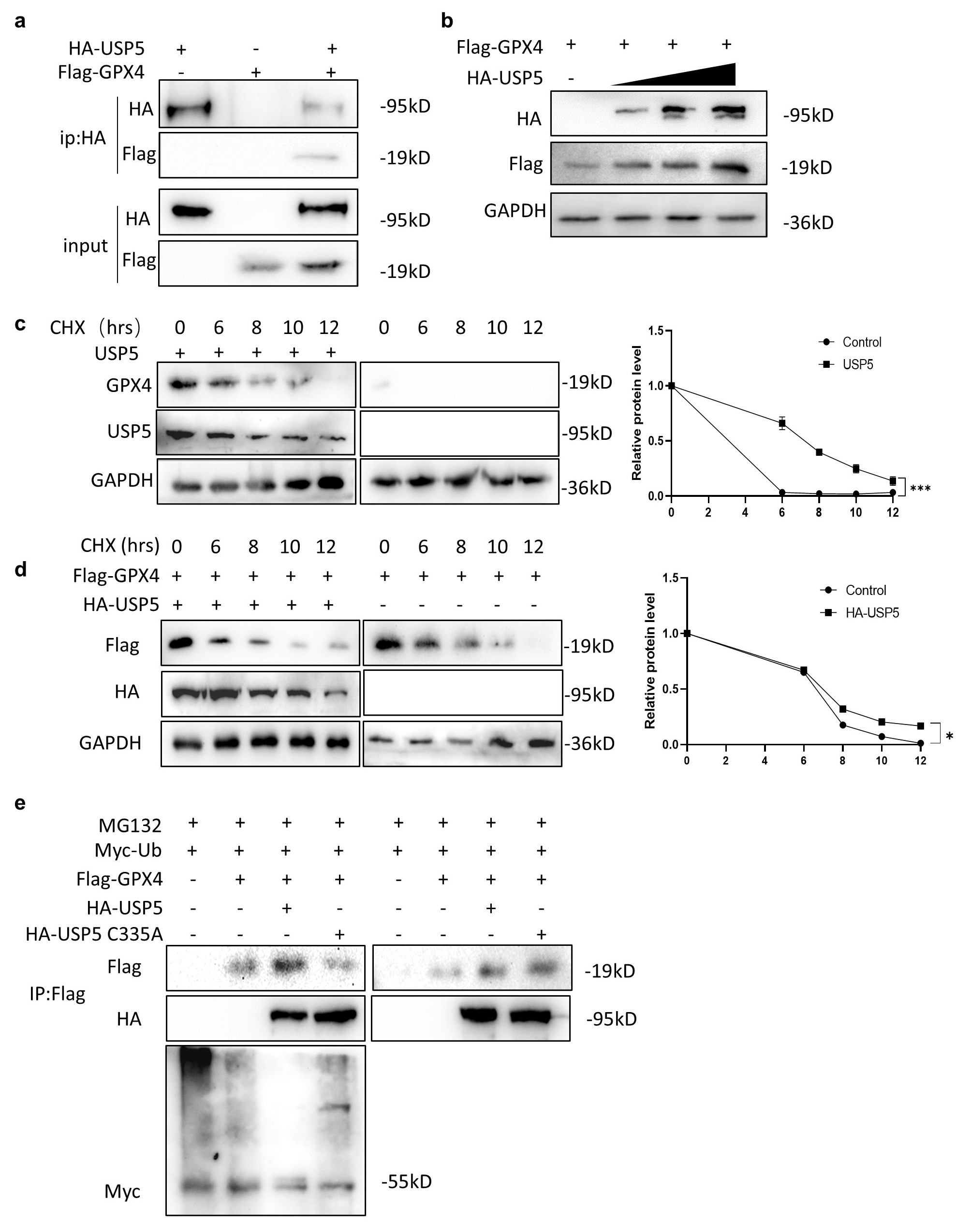
2.7. Coimmunoprecipitation
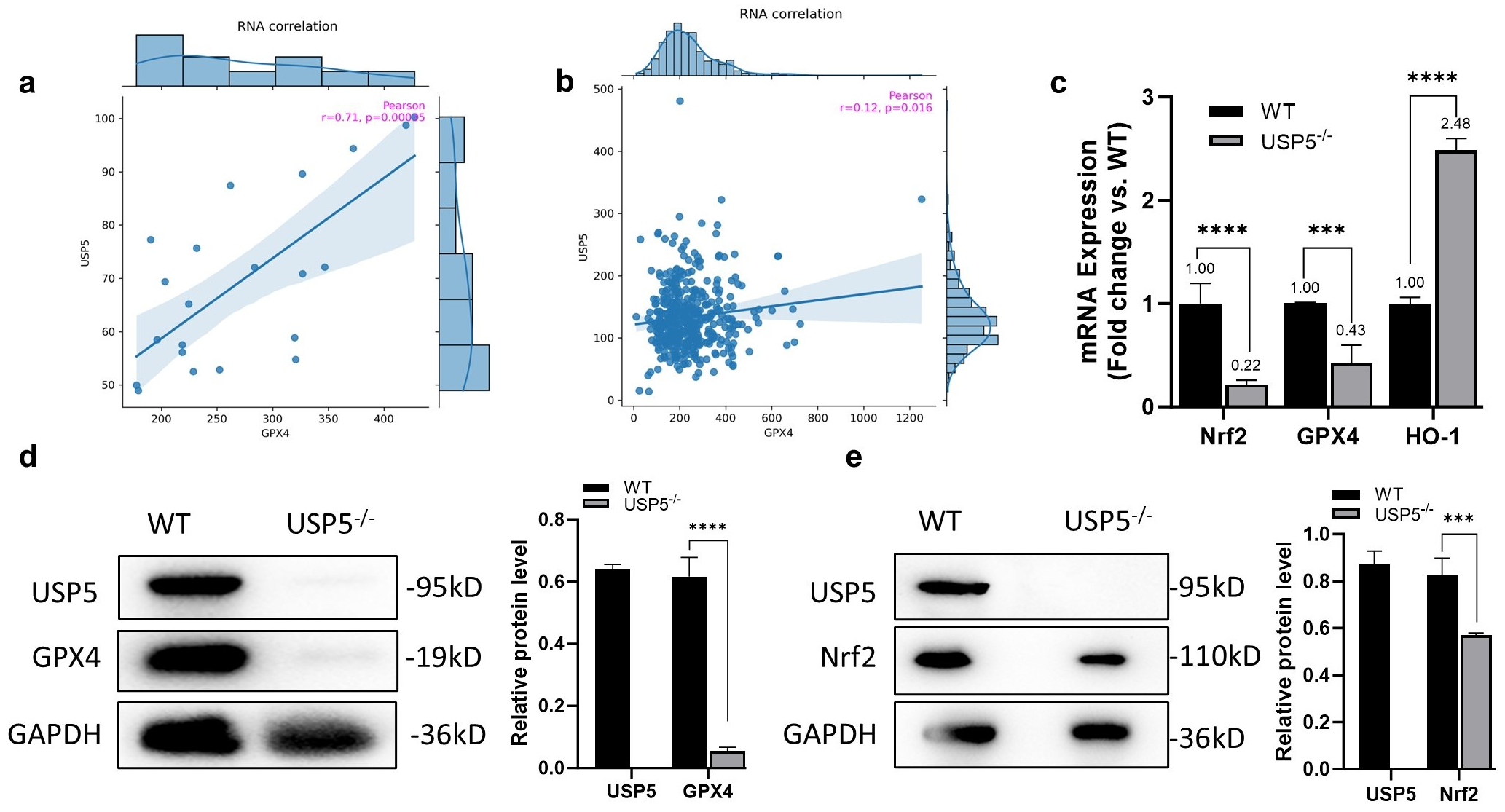
2.8. Real-Time Quantitative PCR
2.9. Statistical Analysis
3. Results
3.1. USP5 Deficiency Inhibits Cell Viability and Proliferation
3.2. USP5 Deficiency Promotes Ferroptosis in T24 Cells
3.3. USP5 Modulates Ferroptosis by Targeting GPX4
3.4. USP5 Interacts with and Stabilizes GPX4
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina 2021, 57, 749. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Lobo, N.; Afferi, L.; Moschini, M.; Mostafid, H.; Porten, S.; Psutka, S.P.; Gupta, S.; Smith, A.B.; Williams, S.B.; Lotan, Y. Epidemiology, Screening, and Prevention of Bladder Cancer. Eur. Urol. Oncol. 2022, 5, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Liu, S.H.; Lin, W.C.; Chow, P.M.; Chang, Y.W.; Yang, S.P.; Shi, C.S.; Hsu, C.H.; Liao, S.M.; Chang, H.C.; et al. The Deubiquitinating Enzyme Inhibitor PR-619 Enhances the Cytotoxicity of Cisplatin via the Suppression of Anti-Apoptotic Bcl-2 Protein: In Vitro and In Vivo Study. Cells 2019, 8, 1268. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Xu, J.; Wang, X.; Zhang, G.; Tang, T.; Shen, X.; Liang, T.; Bai, X. Deubiquitinating Enzyme: A Potential Secondary Checkpoint of Cancer Immunity. Front. Oncol. 2020, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Graham, S.H. Role of UCHL1 in the pathogenesis of neurodegenerative diseases and brain injury. Ageing Res. Rev. 2023, 86, 101856. [Google Scholar] [CrossRef]
- Ren, J.; Yu, P.; Liu, S.; Li, R.; Niu, X.; Chen, Y.; Zhang, Z.; Zhou, F.; Zhang, L. Deubiquitylating Enzymes in Cancer and Immunity. Adv. Sci. 2023, 10, e2303807. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Z.; Guo, Y.; He, Q.; Pan, C. Recent advances in small molecule inhibitors of deubiquitinating enzymes. Eur. J. Med. Chem. 2025, 287, 117324. [Google Scholar] [CrossRef]
- Avvakumov, G.V.; Walker, J.R.; Xue, S.; Allali-Hassani, A.; Asinas, A.; Nair, U.B.; Fang, X.; Zuo, X.; Wang, Y.X.; Wilkinson, K.D.; et al. Two ZnF-UBP domains in isopeptidase T (USP5). Biochemistry 2012, 51, 1188–1198. [Google Scholar] [CrossRef]
- Xu, X.; Huang, A.; Cui, X.; Han, K.; Hou, X.; Wang, Q.; Cui, L.; Yang, Y. Ubiquitin specific peptidase 5 regulates colorectal cancer cell growth by stabilizing Tu translation elongation factor. Theranostics 2019, 9, 4208–4220. [Google Scholar] [CrossRef]
- Du, Y.; Lin, J.; Zhang, R.; Yang, W.; Quan, H.; Zang, L.; Han, Y.; Li, B.; Sun, H.; Wu, J. Ubiquitin specific peptidase 5 promotes ovarian cancer cell proliferation through deubiquitinating HDAC2. Aging 2019, 11, 9778–9793. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, X.; Zhang, Y.; Deng, M.; Li, G.; Chen, G.; Yu, L.; Jin, L.; Liu, T.; Wang, Y.; et al. USP5 promotes breast cancer cell proliferation and metastasis by stabilizing HIF2α. J. Cell Physiol. 2022, 237, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Qiao, Y.; Chen, C.; Zang, H.; Zhang, X.; Qi, F.; Chang, C.; Yang, F.; Sun, M.; Lin, S.; et al. USP5 facilitates non-small cell lung cancer progression through stabilization of PD-L1. Cell Death Dis. 2021, 12, 1051. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, H.; Lu, H.; Xu, K.; Jiang, X.; Jiang, Y.; Gongye, X.; Chen, Z.; Liu, J.; Chen, X.; et al. METTL5 stabilizes c-Myc by facilitating USP5 translation to reprogram glucose metabolism and promote hepatocellular carcinoma progression. Cancer Commun. 2023, 43, 338–364. [Google Scholar] [CrossRef]
- Dayal, S.; Sparks, A.; Jacob, J.; Allende-Vega, N.; Lane, D.P.; Saville, M.K. Suppression of the deubiquitinating enzyme USP5 causes the accumulation of unanchored polyubiquitin and the activation of p53. J. Biol. Chem. 2009, 284, 5030–5041. [Google Scholar] [CrossRef]
- Li, X.Y.; Wu, H.Y.; Mao, X.F.; Jiang, L.X.; Wang, Y.X. USP5 promotes tumorigenesis and progression of pancreatic cancer by stabilizing FoxM1 protein. Biochem. Biophys. Res. Commun. 2017, 492, 48–54. [Google Scholar] [CrossRef]
- Tung, C.H.; Wu, J.E.; Huang, M.F.; Wang, W.L.; Wu, Y.Y.; Tsai, Y.T.; Hsu, X.R.; Lin, S.H.; Chen, Y.L.; Hong, T.M. Ubiquitin-specific peptidase 5 facilitates cancer stem cell-like properties in lung cancer by deubiquitinating β-catenin. Cancer Cell Int. 2023, 23, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Zhang, A.Q.; Peng, P.; Huang, L.; Liu, C.Y.; Nie, X.R.; Hou, D.F.; Zhang, X.; Li, S.Z. USP5 facilitates bladder cancer progression by stabilizing the c-Jun protein. Cancer Cell Int. 2024, 24, 32. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. The roles of ferroptosis in cancer: Tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell 2024, 42, 513–534. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Y.; Wang, Y.; Zhu, R.; Li, H.; Liu, Y.; Shen, N. The E3 ligase TRIM26 suppresses ferroptosis through catalyzing K63-linked ubiquitination of GPX4 in glioma. Cell Death Dis. 2023, 14, 695. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Yao, Y.; Ke, S.; Zhang, N.; Xiong, W.; Shi, J.; He, C.; Xiao, X.; Yu, H.; et al. USP8-governed GPX4 homeostasis orchestrates ferroptosis and cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2024, 121, e2315541121. [Google Scholar] [CrossRef]
- Shan, Z.; Tang, W.; Shi, Z.; Shan, T. Ferroptosis: An Emerging Target for Bladder Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 8201–8214. [Google Scholar] [CrossRef]
- Zeng, F.; Lan, Y.; Wang, N.; Huang, X.; Zhou, Q.; Wang, Y. Ferroptosis: A new therapeutic target for bladder cancer. Front. Pharmacol. 2022, 13, 1043283. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Yagoda, N.; von Rechenberg, M.; Zaganjor, E.; Bauer, A.J.; Yang, W.S.; Fridman, D.J.; Wolpaw, A.J.; Smukste, I.; Peltier, J.M.; Boniface, J.J.; et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007, 447, 864–868. [Google Scholar] [CrossRef]
- Yang, R.; Gao, W.; Wang, Z.; Jian, H.; Peng, L.; Yu, X.; Xue, P.; Peng, W.; Li, K.; Zeng, P. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine 2024, 122, 155135. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Li, B.; Zheng, C.; Liu, G.; Liu, Z.; Zhang, L.; Xu, P. Discovery of ML210-Based glutathione peroxidase 4 (GPX4) degrader inducing ferroptosis of human cancer cells. Eur. J. Med. Chem. 2023, 254, 115343. [Google Scholar] [CrossRef] [PubMed]
- Moosmayer, D.; Hilpmann, A.; Hoffmann, J.; Schnirch, L.; Zimmermann, K.; Badock, V.; Furst, L.; Eaton, J.K.; Viswanathan, V.S.; Schreiber, S.L.; et al. Crystal structures of the selenoprotein glutathione peroxidase 4 in its apo form and in complex with the covalently bound inhibitor ML162. Acta Crystallogr. D Struct. Biol. 2021, 77, 237–248. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Sun, Y.; Berleth, N.; Wu, W.; Schlütermann, D.; Deitersen, J.; Stuhldreier, F.; Berning, L.; Friedrich, A.; Akgün, S.; Mendiburo, M.J.; et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell Death Dis. 2021, 12, 1028. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liao, Y.; Zhu, C.; Zou, Z. GPX4, ferroptosis, and diseases. Biomed. Pharmacother. 2024, 174, 116512. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Dong, C.; Chen, T.; Dong, A.; Ren, J.; Li, W.; Shu, G.; Yang, J.; Shen, W.; et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene 2023, 42, 83–98. [Google Scholar] [CrossRef]
| Name | Sequence |
|---|---|
| GAPDH | Forward GACAAGCTTCCCGTTCTCAGReverse GAGTCAACGGATTTGGTCGT |
| GPX4 | Forward GAGGCAAGACCGAAGTAAACTACReverse CCGAACTGGTTACACGGGAA |
| NRF2 | Forward CTTTTGGCGCAGACATTCCCReverse GACTGGGCTCTCGATGTGAC |
| HO-1 | Forward CAGTGCCACCAAGTTCAAGCReverse GTTGAGCAGGAACGCAGTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Deng, Y.; Huang, L.; Nie, X.; Jiang, Y.; Zhang, X.; Zhang, H. USP5 Suppresses Ferroptosis in Bladder Cancer Through Stabilization of GPX4. Curr. Issues Mol. Biol. 2025, 47, 211. https://doi.org/10.3390/cimb47030211
Liu C, Deng Y, Huang L, Nie X, Jiang Y, Zhang X, Zhang H. USP5 Suppresses Ferroptosis in Bladder Cancer Through Stabilization of GPX4. Current Issues in Molecular Biology. 2025; 47(3):211. https://doi.org/10.3390/cimb47030211
Chicago/Turabian StyleLiu, Caiying, Yanong Deng, Liang Huang, Xinrui Nie, Yuxuan Jiang, Xia Zhang, and Huihui Zhang. 2025. "USP5 Suppresses Ferroptosis in Bladder Cancer Through Stabilization of GPX4" Current Issues in Molecular Biology 47, no. 3: 211. https://doi.org/10.3390/cimb47030211
APA StyleLiu, C., Deng, Y., Huang, L., Nie, X., Jiang, Y., Zhang, X., & Zhang, H. (2025). USP5 Suppresses Ferroptosis in Bladder Cancer Through Stabilization of GPX4. Current Issues in Molecular Biology, 47(3), 211. https://doi.org/10.3390/cimb47030211






