Adaptations of the Genus Bradyrhizobium to Selected Elements, Heavy Metals and Pesticides Present in the Soil Environment
Abstract
1. Introduction
2. The Influence of Selected Elements Present in Soil on Bradyrhizobia and Their Symbiotic Processes
2.1. Iron
2.1.1. Iron Deficiency
2.1.2. Excess Iron
2.2. Phosphorus
2.2.1. Phosphorus Deficiency
2.2.2. Excess Phosphorus
2.3. Sulfur
2.3.1. Sulfur Deficiency
2.3.2. Excess Sulfur
2.4. Calcium
2.4.1. Calcium Deficiency
2.4.2. Excess Calcium
2.5. Manganese
2.5.1. Manganese Deficiency
2.5.2. Excess Manganese
3. Adaptation of Bradyrhizobia to Environments Rich in Heavy Metals and Radionuclides
3.1. Lead
3.2. Mercury
3.3. Arsenic
3.4. Radionuclides
4. Bradyhizobia and Pesticides Used in Agriculture
4.1. Herbicides and Their Effect on Bradyrhizobia
4.1.1. Glyphosate
4.1.2. Sulfentrazone
4.1.3. Chlorophenoxy Herbicides
4.1.4. Flumioxazin and Imidazolinone
4.1.5. Atrazine
4.2. Effects of Insecticides and Fungicides on Bradyrhizobium
4.3. Bradyrhizobia as Biopesticides
5. Conclusions
Funding
Conflicts of Interest
References
- Ledermann, R.; Schulte, C.C.M.; Poole, P.S. How Rhizobia Adapt to the Nodule Environment. J. Bacteriol. 2021, 203, e0053920. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.; Pereira, A.; Menezes, L.D.; Sawaikar, R. Rhizobium as a Biofertilizer for Non-Leguminous Plants. Discov. Food 2024, 4, 85. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Mohammad, R.H.; Alobaidy, B.S.J. The Effect of Rhizobia Inoculum and Mineral Fertilizers on the Number of Root Nodes, Growth, and Yield of Groundnut. IOP Conf. Ser. Earth Environ. Sci. 2023, 1252, 012027. [Google Scholar] [CrossRef]
- Avontuur, J.R.; Palmer, M.; Beukes, C.W.; Chan, W.Y.; Tasiya, T.; van Zyl, E.; Coetzee, M.P.A.; Stepkowski, T.; Venter, S.N.; Steenkamp, E.T. Bradyrhizobium altum sp. nov., Bradyrhizobium oropedii sp. nov. and Bradyrhizobium acaciae sp. nov. from South Africa Show Locally Restricted and Pantropical nodA Phylogeographic Patterns. Mol. Phylogenet. Evol. 2022, 167, 107338. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Babalola, O.O. The Multifaceted Plant-Beneficial Rhizobacteria toward Agricultural Sustainability. Plant Protect. Sci. 2021, 57, 95–111. [Google Scholar] [CrossRef]
- Bitire, T.D.; Abberton, M.; Oyatomi, O.; Babalola, O.O. Effect of Bradyrhizobium japonicum Strains and Inorganic Nitrogen Fertilizer on the Growth and Yield of Bambara Groundnut (Vigna subterranea (L.) Verdc) Accessions. Front. Sustain. Food Syst. 2022, 6, 913239. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, C.-Z.; Zhuo, Y.; Li, T.; Jin, F.-J.; Lee, H.-G.; Jin, L. Genetic Diversity into a Novel Free-Living Species of Bradyrhizobium from Contaminated Freshwater Sediment. Front. Microbiol. 2023, 14, 1295854. [Google Scholar] [CrossRef]
- Avontuur, J.R.; Wilken, P.M.; Palmer, M.; Coetzee, M.P.A.; Stępkowski, T.; Venter, S.N.; Steenkamp, E.T. Complex Evolutionary History of Photosynthesis in Bradyrhizobium. Microb. Genom. 2023, 9, 001105. [Google Scholar] [CrossRef]
- Gao, Y.; Øverlie Arntzen, M.; Kjos, M.; Bakken, L.R.; Frostegård, Å. Denitrification by Bradyrhizobia under Feast and Famine and the Role of the bc1 Complex in Securing Electrons for N2O Reduction. Appl. Environ. Microbiol. 2023, 89, e0174522. [Google Scholar] [CrossRef]
- Qu, Y.; Spain, J. Molecular and Biochemical Characterization of the 5-Nitroanthranilic Acid Degradation Pathway in Bradyrhizobium sp. Strain JS329. J. Bacteriol. 2011, 193, 3057–3063. [Google Scholar] [CrossRef]
- Eppinger, E.; Stolz, A.; Ferraroni, M. Crystal Structure of the Monocupin Ring-Cleaving Dioxygenase 5-Nitrosalicylate 1,2-Dioxygenase from Bradyrhizobium sp. Acta Crystallogr. Sect. D 2023, 79, 632–640. [Google Scholar] [CrossRef]
- Kalyoncu, S.; Heaner, D.P.; Kurt, Z.; Bethel, C.M.; Ukachukwu, C.U.; Chakravarthy, S.; Spain, J.C.; Lieberman, R.L. Enzymatic hydrolysis by transition-metal-dependent nucleophilic aromatic substitution. Nat. Chem. Biol. 2016, 12, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, R.; Cao, L.; Yusha, L.; Liu, J.; Feng, J.; Fu, W.-J.; Li, X.; Li, B. High-Efficiency Biodegradation of Chloramphenicol by Enriched Bacterial Consortia: Kinetics Study and Bacterial Community Characterization. J. Hazard. Mater. 2019, 384, 121344. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N.; Galloway, J. An Earth-System Perspective of the Global Nitrogen Cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Zhang, F.; O’Brian, M.R. The Divalent Metal Ion Exporter IhpABC Is Required to Maintain Iron Homeostasis under Low to Moderate Environmental Iron Conditions in the Bacterium Bradyrhizobium japonicum. Mol. Microbiol. 2024, 121, 85–97. [Google Scholar] [CrossRef]
- Braun, V.; Killmann, H. Bacterial Solutions to the Iron-Supply Problem. Trends Biochem. Sci. 1999, 24, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ouati, D. Iron and Oxidative Stress in Bacteria. Arch. Biochem. Biophys. 2000, 373, 1–6. [Google Scholar] [CrossRef]
- Frawley, E.R.; Fang, F.C. The Ins and Outs of Bacterial Iron Metabolism. Mol. Microbiol. 2014, 93, 609–616. [Google Scholar] [CrossRef]
- Dutta, K.; Shityakov, S.; Maruyama, F. DSF Inactivator RpfB Homologous FadD Upregulated in Bradyrhizobium japonicum under Iron Limiting Conditions. Sci. Rep. 2023, 13, 8701. [Google Scholar] [CrossRef]
- Niehus, R.; Picot, A.; Oliveira, N.M.; Mitri, S.; Foster, K.R. The Evolution of Siderophore Production as a Competitive Trait. Evolution 2017, 71, 1443–1455. [Google Scholar] [CrossRef]
- Ong, A.; O’Brian, M.R. The Bradyrhizobium japonicum fsrB Gene Is Essential for Utilization of Structurally Diverse Ferric Siderophores to Fulfill Its Nutritional Iron Requirement. Mol. Microbiol. 2023, 119, 340–349. [Google Scholar] [CrossRef]
- Pi, H.; Helmann, J.D. Ferrous Iron Efflux Systems in Bacteria. Metallomics 2017, 9, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.G.; Ong, C.Y.; Djoko, K.Y.; West, N.P.; Davies, M.R.; McEwan, A.G.; Walker, M.J. The PerR-Regulated P1B-4-Type ATPase (PmtA) Acts as a Ferrous Iron Efflux Pump in Streptococcus pyogenes. Infect. Immun. 2017, 85, e00140-17. [Google Scholar] [CrossRef] [PubMed]
- Meade, J.C. P-Type Transport ATPases in Leishmania and Trypanosoma. Parasite 2019, 26, 69. [Google Scholar] [CrossRef] [PubMed]
- Frawley, E.R.; Crouch, M.L.; Bingham-Ramos, L.K.; Robbins, H.F.; Wang, W.; Wright, G.D.; Fang, F.C. Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 2013, 110, 12054–12059. [Google Scholar] [CrossRef]
- Bennett, B.D.; Brutinel, E.D.; Gralnick, J.A. A ferrous iron exporter mediates iron resistance in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 2015, 81, 7938–7944. [Google Scholar] [CrossRef]
- Alves de Souza, A.; Michel, D.; Aragão, O.O.; Castro, J.; Ferreira, M.; Moreira, F. Symbiotic efficiency with Enterolobium contortisiliquum, adaptability, and functional genes of Bradyrhizobium strains isolated from soils with high Fe and Mn content. Authorea, 2024; preprint. [Google Scholar] [CrossRef]
- Sankari, S.; O’Brian, M.R. Synthetic lethality of the bfr and mbfA genes reveals a functional relationship between iron storage and iron export in managing stress responses in Bradyrhizobium japonicum. PLoS ONE 2016, 11, e0157250. [Google Scholar] [CrossRef]
- Rangel, W.M.; Thijs, S.; Janssen, J.; Oliveira Longatti, S.M.; Bonaldi, D.S.; Ribeiro, P.R.; Jambon, I.; Eevers, N.; Weyens, N.; Vangronsveld, J.; et al. Native rhizobia from Zn mining soil promote the growth of Leucaena leucocephala on contaminated soil. Int. J. Phytoremediat. 2017, 19, 142–156. [Google Scholar] [CrossRef]
- Jinal, H.N.; Gopi, K.; Prittesh, P.; Kartik, V.P.; Amaresan, N. Phytoextraction of Iron from Contaminated Soils by Inoculation of Iron-Tolerant Plant Growth-Promoting Bacteria in Brassica juncea L. Czern. Environ. Sci. Pollut. Res. Int. 2019, 26, 32815–32823. [Google Scholar] [CrossRef] [PubMed]
- Chandwani, S.; Chavan, S.M.; Paul, D.; Amaresan, N. Bacterial Inoculations Mitigate Different Forms of Iron (Fe) Stress and Enhance Nutrient Uptake in Rice Seedlings (Oryza sativa L.). Environ. Technol. Innov. 2022, 26, 102326. [Google Scholar] [CrossRef]
- Upadhyay, R.; Saini, R.; Shukla, P.K.; Tiwari, K.N. Role of Secondary Metabolites in Plant Defense Mechanisms: A Molecular and Biotechnological Insight. Phytochem. Rev. 2024, 24, 953–983. [Google Scholar] [CrossRef]
- Buoso, S.; Zamboni, A.; Franco, A.; Commisso, M.; Guzzo, F.; Varanini, Z.; Pinton, R.; Tomasi, N.; Zanin, L. Nodulating white lupins take advantage of the reciprocal interplay between N and P nutritional responses. Physiol. Plant. 2022, 174, e13607. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Alexander, P.; Cordell, D.; Maslin, M.; Metson, G.S.; Sutton, M.A.; Spears, B.M. National phosphorus planning for food and environmental security. Curr. Opin. Biotechnol. 2024, 90, 103226. [Google Scholar] [CrossRef]
- Tiessen, H. Phosphorus in the Global Environment. In Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer: New York, NY, USA, 2008; pp. 1–8. [Google Scholar]
- Valentine, A.J.; Kleinert, A.; Benedito, V.A. Adaptive strategies for nitrogen metabolism in phosphate deficient legume nodules. Plant Sci. 2017, 256, 46–52. [Google Scholar] [CrossRef]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Hufnagel, B.; Marques, A.; Soriano, A.; Marquès, L.; Divol, F.; Doumas, P.; Sallet, E.; Mancinotti, D.; Carrere, S.; Marande, W.; et al. High-quality genome sequence of white lupin provides insight into soil exploration and seed quality. Nat. Commun. 2020, 11, 492. [Google Scholar] [CrossRef]
- Gardner, W.K.; Parbery, D.G.; Barber, D.A. The acquisition of phosphorus by Lupinus albus L. I. Some characteristics of the soil/root interface. Plant Soil 1982, 67, 19–32. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Quiñones, M.A.; Coba de la Peña, T.; Fedorova, E.E.; Lucas, M.M. Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Front. Plant Sci. 2021, 12, 644218. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Knight, J.D.; Vance, C.P.; Allan, D.L. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant Cell Environ. 2002, 22, 801–810. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, G.; Pinton, R.; Varanini, Z.; Nannipieri, P.; Torrent, J.; Martinoia, E.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Lamont, B.B. Structure, ecology and physiology of root clusters—A review. Plant Soil 2003, 248, 1–19. [Google Scholar] [CrossRef]
- Su, M.; Meng, L.; Zhao, L.; Tang, Y.; Qiu, J.; Tian, D.; Li, Z. Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 2021, 404, 115311. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Schulze, J.; Temple, G.; Temple, S.J.; Beschow, H.; Vance, C.P. Nitrogen fixation by white lupin under phosphorus deficiency. Ann. Bot. 2006, 98, 731–740. [Google Scholar] [CrossRef]
- Raven, J.A. The evolution of autotrophy in relation to phosphorus requirement. J. Exp. Bot. 2013, 64, 4023–4046. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Zaheer, R.; Finan, T.M. Regulation and properties of PstSCAB, a high-affinity, high-velocity phosphate transport system of Sinorhizobium meliloti. J. Bacteriol. 2006, 188, 1089–1102. [Google Scholar] [CrossRef]
- iTsvetkova, G.; Georgiev, G. Effect of phosphorus nutrition on the nodulation, nitrogen fixation and nutrient-use efficiency of Bradyrhizobiumjaponicun soybean (Glycine max L. Merr.) symbiosis. Bulg. J. Plant Physiol. 2003, 3, 315–335. [Google Scholar]
- Autry, A.R.; Fitzgerald, J.W. Sulfonate S: A major form of forest soil organic sulfur. Biol. Fertil. Soils 1990, 10, 50–56. [Google Scholar] [CrossRef]
- Saleem, S.; Mushtaq, N.U.; Rasool, A.; Shah, W.H.; Tahir, I.; Rehman, R.U. Chapter Two—Plant Nutrition and Soil Fertility: Physiological and Molecular Avenues for Crop Improvement. In Sustainable Plant Nutrition; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 23–49. [Google Scholar] [CrossRef]
- Berhe, A.; Barnes, R.; Six, J.; Marin-Spiotta, E. Role of Soil Erosion in Biogeochemical Cycling of Essential Elements: Carbon, Nitrogen, and Phosphorus. Annu. Rev. Earth Planet. Sci. 2018, 46, 521–548. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Q.; Fan, S.; Zhang, Y.; Zhang, M.; Zhang, J. Distinction Between Cr and Other Heavy-Metal-Resistant Bacteria Involved in C/N Cycling in Contaminated Soils of Copper Producing Sites. J. Hazard. Mater. 2020, 402, 123454. [Google Scholar] [CrossRef]
- Kuykendall, F.M.; Hashem, W.J.; Hunter, W.J. Enhanced Competitiveness of a Bradyrhizobium japonicum Mutant Strain Improved for Nodulation and Nitrogen Fixation. Plant Soil 1996, 186, 121–125. [Google Scholar] [CrossRef]
- Rao, N.S.S.; Tilak, K.V.B.R.; Singh, C.S. Dual Inoculation with Rhizobium sp. and Glomus fasciculatum Enhances Nodulation, Yield, and Nitrogen Fixation in Chickpea (Cicer arietinum Linn.). Plant Soil 1986, 95, 351–359. [Google Scholar] [CrossRef]
- Deak, E.A.; Martin, T.N.; Stecca, J.D.L.; Conceição, G.M.; Ferreira, M.M.; Rumpel, V.S.; Grolli Carvalho, A.F.; Baena, F.J.L. Sulfur Fertilization and Inoculation of Soybean with Azospirillum brasilense and Bradyrhizobium spp. Can Improve Grain Yield and Quality. Braz. J. Microbiol. 2024, 56, 573–588. [Google Scholar] [CrossRef]
- Habetamu, G.; Gebreslassie, H.; Hirut, B. Effect of Sulfur and Bradyrhizobium Inoculation on Nodulation and Yield of Soybean (Glycine max L.) on Nitisols of Southwestern Ethiopia. Agric. For. Fish. 2021, 10, 280–287. [Google Scholar] [CrossRef]
- Becana, M.; Wienkoop, S.; Matamoros, M.A. Sulfur Transport and Metabolism in Legume Root Nodules. Front. Plant Sci. 2018, 9, 1434. [Google Scholar] [CrossRef]
- Sugawara, M.; Shah, G.R.; Sadowsky, M.J.; Paliy, O.; Speck, J.; Vail, A.W.; Gyaneshwar, P. Expression and Functional Roles of Bradyrhizobium japonicum Genes Involved in the Utilization of Inorganic and Organic Sulfur Compounds in Free-Living and Symbiotic Conditions. Mol. Plant-Microbe Interact. 2011, 24, 451–457. [Google Scholar] [CrossRef]
- Speck, J.J.; James, E.K.; Sugawara, M.; Sadowsky, M.J.; Gyaneshwar, P. An Alkane Sulfonate Monooxygenase Is Required for Symbiotic Nitrogen Fixation by Bradyrhizobium diazoefficiens (syn. Bradyrhizobium japonicum) USDA110T. Appl. Environ. Microbiol. 2019, 85, e01552-19. [Google Scholar] [CrossRef]
- Karimizarchi, M.; Aminuddin, H.; Khanif, M.; Radziah, O. Elemental Sulphur Application Effects on Nutrient Availability and Sweet Maize (Zea mays L.) Response in a High pH Soil of Malaysia. Malays. J. Soil Sci. 2014, 18, 75–86. [Google Scholar]
- Kobayashi, H.; Broughton, W.J. Fine-Tuning of Symbiotic Genes in Rhizobia: Flavonoid Signal Transduction Cascade. Nitrogen Fixat. Orig. Appl. Res. Prog. 2008, 7, 117–152. [Google Scholar] [CrossRef]
- Yuan, P.; Luo, F.; Gleason, C.; Poovaiah, B.W. Calcium/Calmodulin-Mediated Microbial Symbiotic Interactions in Plants. Front. Plant Sci. 2022, 13, 984909. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Wang, C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef]
- Kadreva, I.; Ignatov, G. Role of Ca2+ in Bradyrhizobium japonicum Strain 273 Attachment Ability and Accumulation on Soybean Root Surface. J. Plant Physiol. 1995, 145, 577–579. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Liu, Y.; Zou, D.; Wang, D.; Xie, Z. Effects of Calcium and Signal Sensing Systems on Azorhizobium caulinodans Biofilm Formation and Host Colonization. Front. Microbiol. 2020, 11, 563367. [Google Scholar] [CrossRef]
- Vitiello, G.; Oliva, R.; Petraccone, L.; Vecchio, P.D.; Heenan, R.K.; Molinaro, A.; Silipo, A.; D’Errico, G.; Paduano, L. Covalently Bonded Hopanoid-Lipid A from Bradyrhizobium: The Role of Unusual Molecular Structure and Calcium Ions in Regulating the Lipid Bilayers Organization. J. Colloid Interface Sci. 2021, 594, 891–901. [Google Scholar] [CrossRef]
- Tookmanian, E.; Junghans, L.; Kulkarni, G.; Ledermann, R.; Saenz, J.; Newman, D.K. Hopanoids Confer Robustness to Physicochemical Variability in the Niche of the Plant Symbiont Bradyrhizobium diazoefficiens. J. Bacteriol. 2022, 204, e0044221. [Google Scholar] [CrossRef]
- Andreeva, I.N.; Kozharinova, G.M.; Izmailov, S.F. Senescence of legume nodules. Rus. J. Plant Physiol. 1998, 45, 101–112. [Google Scholar]
- Zhou, S.; Zhang, C.; Huang, Y.; Chen, H.; Yuan, S.; Zhou, X. Characteristics and Research Progress of Legume Nodule Senescence. Plants 2021, 10, 1103. [Google Scholar] [CrossRef]
- Yokel, R.A. Manganese flux across the blood-brain barrier. Neuromol. Med. 2009, 11, 297–310. [Google Scholar] [CrossRef]
- Dey, S.; Tripathy, B.; Kumar, M.S.; Das, A.P. Ecotoxicological consequences of manganese mining pollutants and their biological remediation. Environ. Chem. Ecotoxicol. 2023, 5, 55–61. [Google Scholar] [CrossRef]
- Sobota, J.M.; Imlay, J.A. Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc. Natl. Acad. Sci. USA 2011, 108, 5402–5407. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Yuan, X.Z.; Jia, Y.; Feng, L.J.; Zhu, F.P.; Dong, S.S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef]
- Hohle, T.H.; O’Brian, M.R. Manganese is required for oxidative metabolism in unstressed Bradyrhizobium japonicum cells. Mol. Microbiol. 2012, 84, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Hohle, T.H.; O’Brian, M.R. The mntH gene encodes the major Mn(2+) transporter in Bradyrhizobium japonicum and is regulated by manganese via the Fur protein. Mol. Microbiol. 2009, 72, 399–409. [Google Scholar] [CrossRef]
- Pisarek, I.; Grata, K. The Influence of Sewage Sludge and Fly Ash Fertilization on the Total Number of Bacteria (TNB) and Bradyrhizobium Species in Soybean Agroecosystem. Agriculture 2023, 13, 201. [Google Scholar] [CrossRef]
- Nogueira, M.; Magalhaes, G.; Cardoso, E. Manganese toxicity in mycorrhizal and phosphorus-fertilized soybean plants. J. Plant Nutr. 2007, 27, 141–156. [Google Scholar] [CrossRef]
- El-Jaoual, T.; Cox, D.A. Manganese toxicity in plants. J. Plant Nutr. 2008, 21, 353–386. [Google Scholar] [CrossRef]
- Swędrzyńska, D.; Sawicka, A. The effect of copper on bacteria of the genus Azospirillum in the rhizosphere of maize and wheat seedlings. Water-Environ. Rural. Areas 2010, 10, 167–178. [Google Scholar]
- Brzezińska, A.; Mrozek-Niećko, A. The effect of seeds treatment with a micronutrient fertilizer on chlorophyll content in leaves and yield of soya. Fragm. Agron. 2019, 36, 7–15. [Google Scholar] [CrossRef]
- Brzezińska, A.; Mrozek-Niećko, A. Effect of selected micronutrient seed fertilizers on the viability of Bradyrhizobium japonicum. Prog. Plant Prot. 2021, 61, 17–23. [Google Scholar] [CrossRef]
- Lamin, H.; Alami, S.; Lamrabet, M.; Bouhnik, O.; Bennis, M.; Abdelmoumen, H.; Bedmar, E.J.; Missbah-El Idrissi, M. Bradyrhizobium sp. sv. retamae nodulates Retama monosperma grown in a lead and zinc mine tailings in Eastern Morocco. Braz. J. Microbiol. 2021, 52, 639–649. [Google Scholar] [CrossRef]
- Wang, Y.; Wiatrowski, H.A.; John, R.; Lin, C.C.; Young, L.Y.; Kerkhof, L.J.; Yee, N.; Barkay, T. Impact of mercury on denitrification and denitrifying microbial communities in nitrate enrichments of subsurface sediments. Biodegradation 2013, 24, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Talano, M.A.; Cejas, R.B.; González, P.S.; Agostini, E. Arsenic effect on the model crop symbiosis Bradyrhizobium-soybean. Plant Physiol. Biochem. 2013, 63, 8–14. [Google Scholar] [CrossRef]
- Thomas, J.C.; Oladeinde, A.; Kieran, T.J.; Finger, J.W., Jr.; Bayona-Vásquez, N.J.; Cartee, J.C.; Beasley, J.C.; Seaman, J.C.; McArthur, J.V.; Rhodes, O.E., Jr.; et al. Co-occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microb. Biotechnol. 2020, 13, 1179–1200. [Google Scholar] [CrossRef] [PubMed]
- Arregui, G.; Hipólito, P.; Pallol, B.; Lara-Dampier, V.; García-Rodríguez, D.; Varela, H.P.; Tavakoli Zaniani, P.; Balomenos, D.; Paape, T.; Coba de la Peña, T.; et al. Mercury-Tolerant Ensifer medicae Strains Display High Mercuric Reductase Activity and a Protective Effect on Nitrogen Fixation in Medicago truncatula Nodules Under Mercury Stress. Front. Plant Sci. 2021, 11, 560768. [Google Scholar] [CrossRef]
- Agashe, R.; George, J.; Pathak, A.; Fasakin, O.; Seaman, J.; Chauhan, A. Shotgun Metagenomics Analysis Indicates Bradyrhizobium spp. as the Predominant Genera for Heavy Metal Resistance and Bioremediation in a Long-Term Heavy Metal-Contaminated Ecosystem. Microbiol. Resour. Announc. 2024, 13, e0024524. [Google Scholar] [CrossRef]
- Salmi, A.; Boulila, F. Heavy Metals Multi-Tolerant Bradyrhizobium Isolated from Mercury Mining Region in Algeria. J. Environ. Manag. 2021, 289, 112547. [Google Scholar] [CrossRef]
- Singh, S.B.; Srivastava, P.K. Bioavailability of Arsenic in Agricultural Soils Under the Influence of Different Soil Properties. SN Appl. Sci. 2020, 2, 153. [Google Scholar] [CrossRef]
- Seraj, M.F.; Rahman, T.; Lawrie, A.C.; Reichman, S.M. Assessing the Plant Growth Promoting and Arsenic Tolerance Potential of Bradyrhizobium japonicum CB1809. Environ. Manag. 2020, 66, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Vezza, M.E.; Pramparo, R.D.P.; Wevar Oller, A.L.; Agostini, E.; Talano, M.A. Promising Co-Inoculation Strategies to Reduce Arsenic Toxicity in Soybean. Environ. Sci. Pollut. Res. Int. 2022, 29, 88066–88077. [Google Scholar] [CrossRef] [PubMed]
- Peralta, J.M.; Travaglia, C.N.; Romero-Puertas, M.C.; Furlan, A.; Castro, S.; Bianucci, E. Unraveling the Impact of Arsenic on the Redox Response of Peanut Plants Inoculated with Two Different Bradyrhizobium sp. Strains. Chemosphere 2020, 259, 127410. [Google Scholar] [CrossRef]
- Seiler, C.; Berendonk, T.U. Heavy Metal Driven Co-selection of Antibiotic Resistance in Soil and Water Bodies Impacted by Agriculture and Aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of Antibiotic and Metal Resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Salomao, H.M.; Trezzi, M.M.; Viecelli, M.; Pagnoncelli Junior, F.D.B.; Patel, F.; Damo, L.; Frizzon, G. Weed Management with Pre-emergent Herbicides in Soybean Crops. Commun. Plant Sci. 2021, 11, 60–66. [Google Scholar] [CrossRef]
- Rodrigues, T.F.; Bender, F.R.; Sanzovo, A.W.S.; Ferreira, E.; Nogueira, M.A.; Hungria, M. Impact of Pesticides in Properties of Bradyrhizobium spp. and in the Symbiotic Performance with Soybean. World J. Microbiol. Biotechnol. 2020, 36, 172. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chang, W.; Sui, X.; Liu, Y.; Song, G.; Song, F.; Feng, F. Changes in Rhizobacterial Community Mediating Atrazine Dissipation by Arbuscular Mycorrhiza. Chemosphere 2020, 256, 127046. [Google Scholar] [CrossRef]
- Feng, L.; Xu, N.; Qu, Q.; Zhang, Z.; Ke, M.; Lu, T.; Qian, H. Synergetic Toxicity of Silver Nanoparticle and Glyphosate on Wheat (Triticum aestivum L.). Sci. Total Environ. 2021, 797, 149200. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Reddy, K.N. Impact of Glyphosate on the Bradyrhizobium japonicum Symbiosis with Glyphosate-Resistant Transgenic Soybean: A Minireview. J. Environ. Qual. 2004, 33, 825–831. [Google Scholar] [CrossRef]
- Verma, J.; Singh, D.; Kumar, A. Herbicide-Induced Oxidative Stress in Bradyrhizobium and Its Effects on Symbiotic Nitrogen Fixation. J. Hazard. Mater. 2017, 336, 84–91. [Google Scholar] [CrossRef]
- Saxena, S.; Kumar, V.; Chandra, R. Impact of Herbicides on Soil Microbial Communities and Resistance of Bradyrhizobium to Herbicides. Agric. Microbiol. 2020, 72, 323–333. [Google Scholar]
- Zhang, C.; Hu, X.; Luo, J.; Wu, Z.; Wang, L.; Li, B.; Wang, Y.; Sun, G. Degradation Dynamics of Glyphosate in Different Types of Citrus Orchard Soils in China. Molecules 2015, 20, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Wang, L.; Xu, Q.; An, J.; Mei, Y.; Liu, G. Influence of glyphosate and its metabolite aminomethylphosphonic acid on aquatic plants in different ecological niches. Ecotoxicol. Environ. Saf. 2022, 246, 114155. [Google Scholar] [CrossRef] [PubMed]
- Hernández Guijarro, K.; De Gerónimo, E.; Erijman, L. Glyphosate Biodegradation Potential in Soil Based on Glycine Oxidase Gene (thiO) from Bradyrhizobium. Curr. Microbiol. 2021, 78, 1991–2000. [Google Scholar] [CrossRef]
- Shaner, D.L. Field Dissipation of Sulfentrazone and Pendimethalin in Colorado. Weed Technol. 2012, 26, 633–637. [Google Scholar] [CrossRef]
- Martinez, C.O.; Silva, C.M.; Fay, E.F.; Abakerli, R.B.; Maia, A.H.; Durrant, L.R. Microbial degradation of sulfentrazone in a Brazilian rhodic hapludox soil. Braz. J. Microbiol. 2010, 41, 209–217. [Google Scholar] [CrossRef]
- Mielke, K.C.; Bertuani, R.R.; Pires, F.R.; Bueno Cotta, A.J.; Egreja Filho, F.B.; Madalão, J.C. Does Canavalia ensiformis inoculation with Bradyrhizobium sp. enhance phytoremediation of sulfentrazone-contaminated soil? Chemosphere 2020, 255, 127033. [Google Scholar] [CrossRef]
- Muhammad, J.B.; Shehu, D.; Usman, S.; Dankaka, S.M.; Gimba, M.Y.; Jagaba, A.H. Biodegradation potential of 2,4 dichlorophenoxyacetic acid by Cupriavidus campinensis isolated from rice farm cultivated soil. Case Stud. Chem. Environ. Eng. 2023, 8, 100434. [Google Scholar] [CrossRef]
- Serbent, M.P.; Rebelo, A.M.; Pinheiro, A.; Giongo, A.; Tavares, L.B.B. Biological agents for 2,4-dichlorophenoxyacetic acid herbicide degradation. Appl. Microbiol. Biotechnol. 2019, 103, 5065–5078. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Tanaka, S.; Takao, S.; Kobayashi, S.; Suyama, K.; Itoh, K. Multiple Gene Clusters and Their Role in the Degradation of Chlorophenoxyacetic Acids in Bradyrhizobium sp. RD5-C2 Isolated from Non-Contaminated Soil. Microb. Environ. 2021, 36, ME21016. [Google Scholar] [CrossRef]
- Eason, K.; Grey, T.; Cabrera, M.; Basinger, N.; Hurdle, N. Assessment of Flumioxazin Soil Behavior and Thermal Stability in Aqueous Solutions. Chemosphere 2022, 288, 132477. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, B.; Jia, Y.; Guo, X.; Lv, F. Biodegradation of Imazethapyr by Bacterial Strain IM9601 Isolated from Agricultural Soil. Curr. Microbiol. 2023, 81, 33. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Pertile, M.; Costa, R.M.; Costa, M.K.L.; de Aviz, R.O.; Mendes, L.W.; de Medeiros, E.V.; da Costa, D.P.; Melo, V.M.M.; Pereira, A.P.A. Short-term responses of plant growth-promoting bacterial community to the herbicides imazethapyr and flumioxazin. Chemosphere 2023, 328, 138581. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, S.; Rong, Y.; Mao, L.; Yang, S.; Qian, L.; Li, R.; Zheng, Y. Enhanced phytoremediation of atrazine-contaminated soil by vetiver (Chrysopogon zizanioides L.) and associated bacteria. Environ. Sci. Pollut. Res. Int. 2023, 30, 44415–44429. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Y.; Wang, J.; Lv, X.; Jiang, Z.; Cao, B.; Qu, J.; Ma, S.; Zhang, Y. Exogenous application of Bradyrhizobium japonicum AC20 enhances soybean tolerance to atrazine via regulating rhizosphere soil microbial community and amino acid, carbohydrate metabolism related genes expression. Plant Physiol. Biochem. PPB 2023, 196, 472–483. [Google Scholar] [CrossRef]
- Aguiar, L.M.; Souza, M.F.; de Laia, M.L.; de Oliveira Melo, J.; da Costa, M.R.; Gonçalves, J.F.; Silva, D.V.; Dos Santos, J.B. Metagenomic analysis reveals mechanisms of atrazine biodegradation promoted by tree species. Environ. Pollut. (Barking Essex 1987) 2020, 267, 115636. [Google Scholar] [CrossRef]
- Khalid, U.; Aftab, Z.E.; Anjum, T.; Bokhari, N.A.; Akram, W.; Anwar, W. Harnessing the Biocontrol Potential of Bradyrhizobium japonicum FCBP-SB-406 to Manage Charcoal Rot of Soybean with Increased Yield Response for the Development of Sustainable Agriculture. Microorganisms 2024, 12, 304. [Google Scholar] [CrossRef]
- Mufti, R.; Bano, A. PGPR-induced defense responses in the soybean plant against charcoal rot disease. Eur. J. Plant Pathol. 2019, 155, 983–1000. [Google Scholar] [CrossRef]
| Type of Element | Studied Bacterial Strain | Effect of Elements | References |
|---|---|---|---|
| Iron | Bradyrhizobium japonicum | Under iron deficiency, the signaling molecule DSFs from other bacteria are deactivated | Dutta K. et al. (2023) [20] |
| Bradyrhizobium japonicum | Under iron deficiency, fsrB expression is upregulated | Ong A., O’Brian M.R. (2023) [22] | |
| Phosphorus | Bradyrhizobium sp. | Under phosphorus deficiency, reduction in nitrogen assimilation | Williams A. et al. (2022) [47] |
| Sulfur | Bradyrhizobium sp. | Under sulfur deficiency, reduction in metabolic activity | Habetamu G. et al. (2021) [59] |
| Calcium | Bradyrhizobium spp. | Under calcium deficiency, inactivation of nitrogenase | Ledermann R. et al. (2021) [1] |
| Manganese | Bradyrhizobium japonicum | Manganese excess limits symbiosis | Pisarek I. (2023) [79] |
| Bradyrhizobium japonicum | Limitation of cellular processes | Brzezińska A., Mrozek-Niećko A. (2021) [84] |
| Type of Pesticide | Pesticide | Soil Persistence | Studied Bacterial Strain | Effect of Pesticide | References |
|---|---|---|---|---|---|
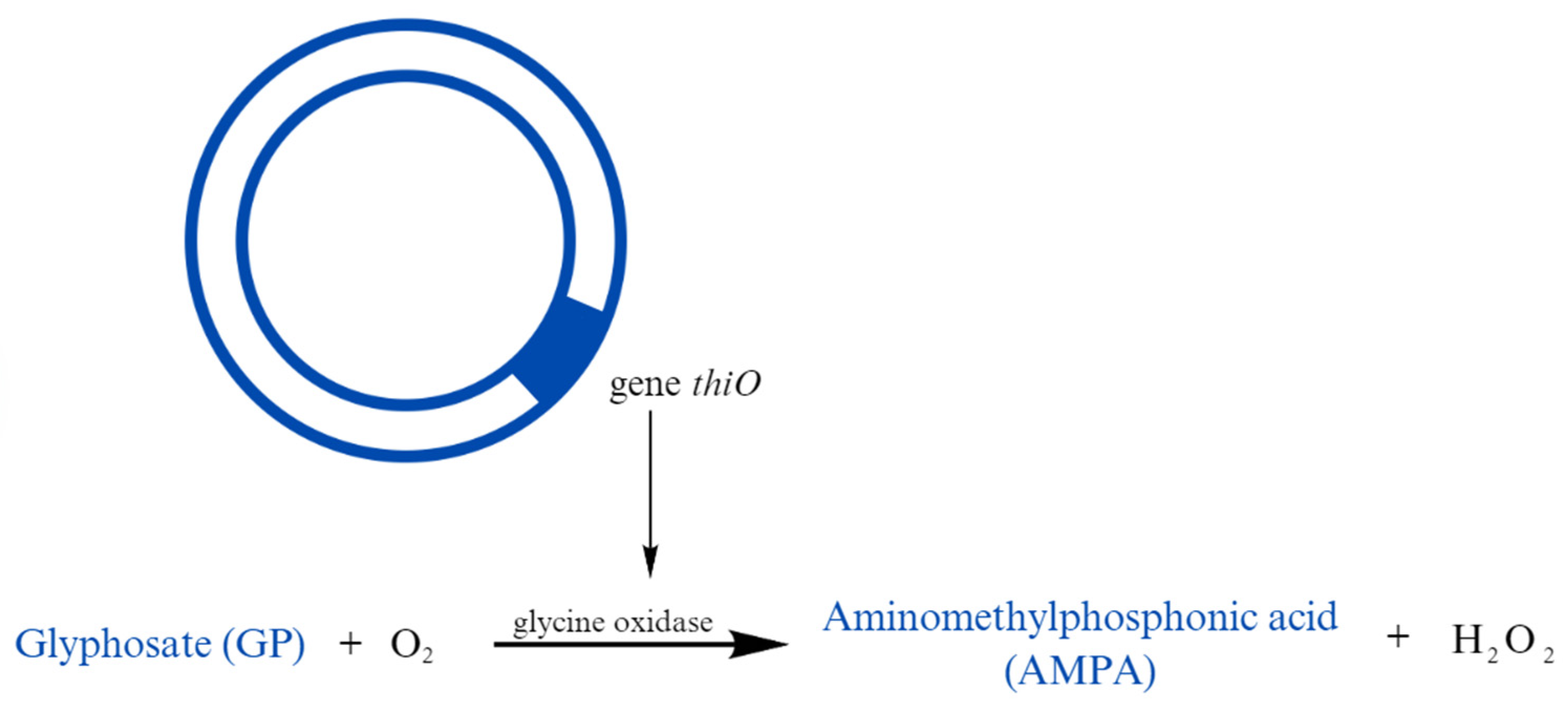
| Herbicides | Glyphosate | Low to medium persistence | Bradyrhizobium sp. | Bacteria have a thiO gene, which oxidizes GP to AMPA | Hernánde Guliaro K. et al. (2021) [109] |
| Sulfentrazone | Medium persistence | Bradyrhizobium japonicum SEMIA 5079 | Increasing herbicide tolerance in plants | Mielke K.C. et al. (2020) [112] | |
| 2,4-dichlorophenoxyacetic acid | Low to medium persistence | Bradyrhizobium sp. | The bacterium has a cad1 cluster | Hayashi S. et al. (2020) [115] | |
| Flumioxazin | Medium persistence | Bradyrhizobium sp. | Temporary positive effect on nitrogen fixation | Araújo A. S. et al. (2023) [118] | |
| Imazapyr | High persistence | ||||
| Atrazine | Medium to high persistence | Bradyrhizobium sp. | Potential impact on pesticide degradation | Zhang, F. et al. (2023) [119] | |
| Bradyrhizobium japonicum AC20 | Mitigation of pesticide effect on soil | Jiang, D et al. (2023) [120] | |||
| Bradyrhizobium sp. | Possess genes degrading atrazine (atzD, atzE, atzF) | Aguiar, L et al. (2020) [121] | |||
| Insecticides and fungicides | Pyraclostrobin | Medium persistence | Bradyrhizobium japonicum SEMIA 5079, Bradyrhizobium elkanii SEMIA 587, Bradyrhizobium diazoeficiens SEMIA 5080 | Morphological changes in strains | Rodrigues T. et al. (2020) [99] |
| Thiofanate-methyl | Medium to high persistence | ||||
| Fipronil | High persistence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasiewicz, J.; Gumowska, A.; Hołubek, A.; Orzechowski, S. Adaptations of the Genus Bradyrhizobium to Selected Elements, Heavy Metals and Pesticides Present in the Soil Environment. Curr. Issues Mol. Biol. 2025, 47, 205. https://doi.org/10.3390/cimb47030205
Banasiewicz J, Gumowska A, Hołubek A, Orzechowski S. Adaptations of the Genus Bradyrhizobium to Selected Elements, Heavy Metals and Pesticides Present in the Soil Environment. Current Issues in Molecular Biology. 2025; 47(3):205. https://doi.org/10.3390/cimb47030205
Chicago/Turabian StyleBanasiewicz, Joanna, Aleksandra Gumowska, Agata Hołubek, and Sławomir Orzechowski. 2025. "Adaptations of the Genus Bradyrhizobium to Selected Elements, Heavy Metals and Pesticides Present in the Soil Environment" Current Issues in Molecular Biology 47, no. 3: 205. https://doi.org/10.3390/cimb47030205
APA StyleBanasiewicz, J., Gumowska, A., Hołubek, A., & Orzechowski, S. (2025). Adaptations of the Genus Bradyrhizobium to Selected Elements, Heavy Metals and Pesticides Present in the Soil Environment. Current Issues in Molecular Biology, 47(3), 205. https://doi.org/10.3390/cimb47030205






