A Novel Ferroptosis-Related Gene Prognosis Signature and Identifying Atorvastatin as a Potential Therapeutic Agent for Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Data Source
2.3. Extraction of Differentially Expressed Ferroptosis-Related Genes
2.4. Identification and Validation of a Prognostic Model for HCC Associated with Ferroptosis
2.5. Functional Enrichment Analysis
2.6. Screening for Potential Therapeutic Agents in the Database
2.7. Detection of Cell Migration and Activity
2.8. Intracellular Reactive Oxygen Species Detection
2.9. Western Blotting Analysis
2.10. In Vivo Xenograft Model
2.11. Statistical Analysis
3. Results
3.1. Characteristics of Hepatocellular Carcinoma Datasets from TCGA and ICGC
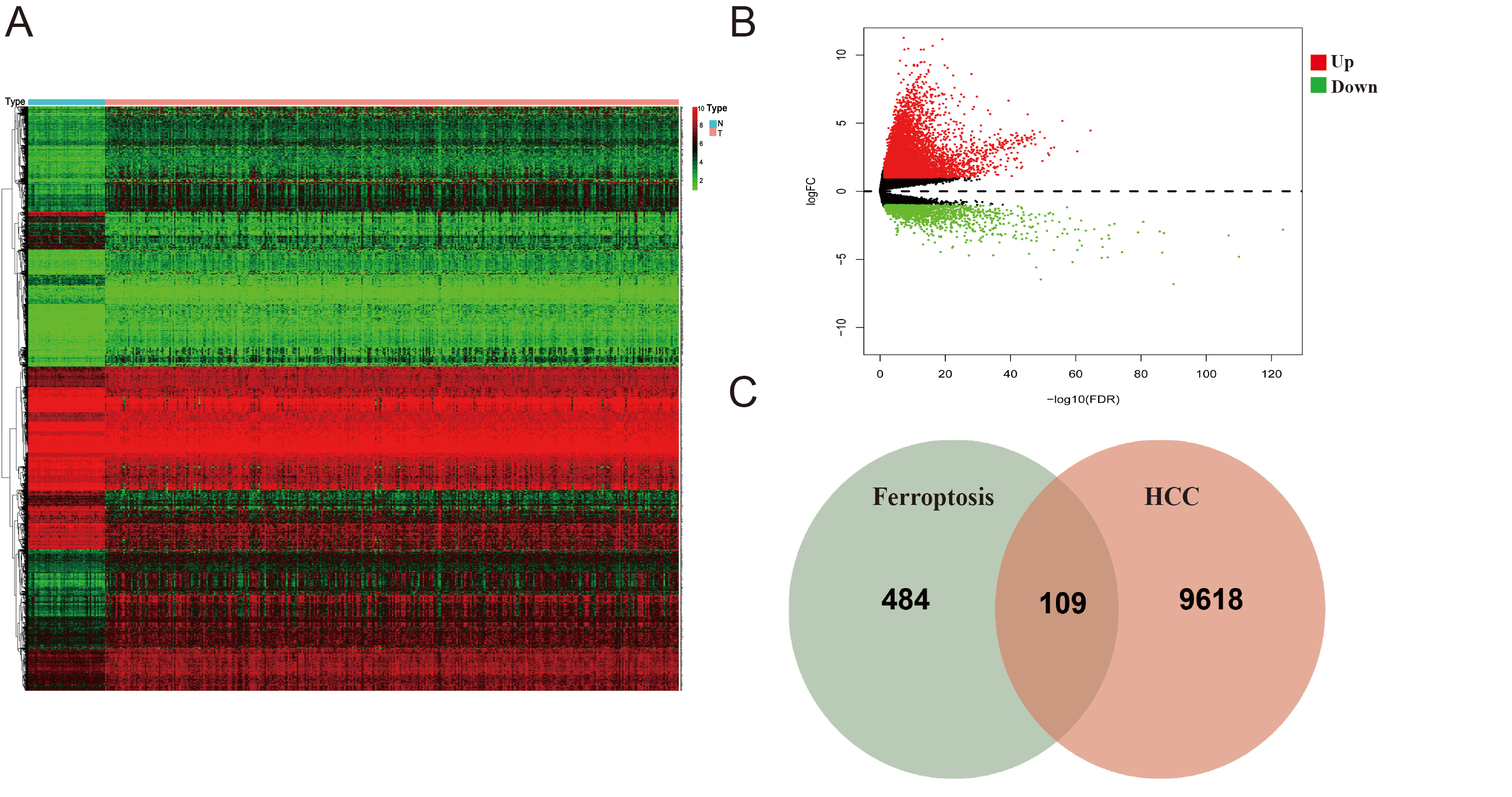
3.2. Identification of Ferroptosis-Associated Differential Genes in the TCGA Cohort
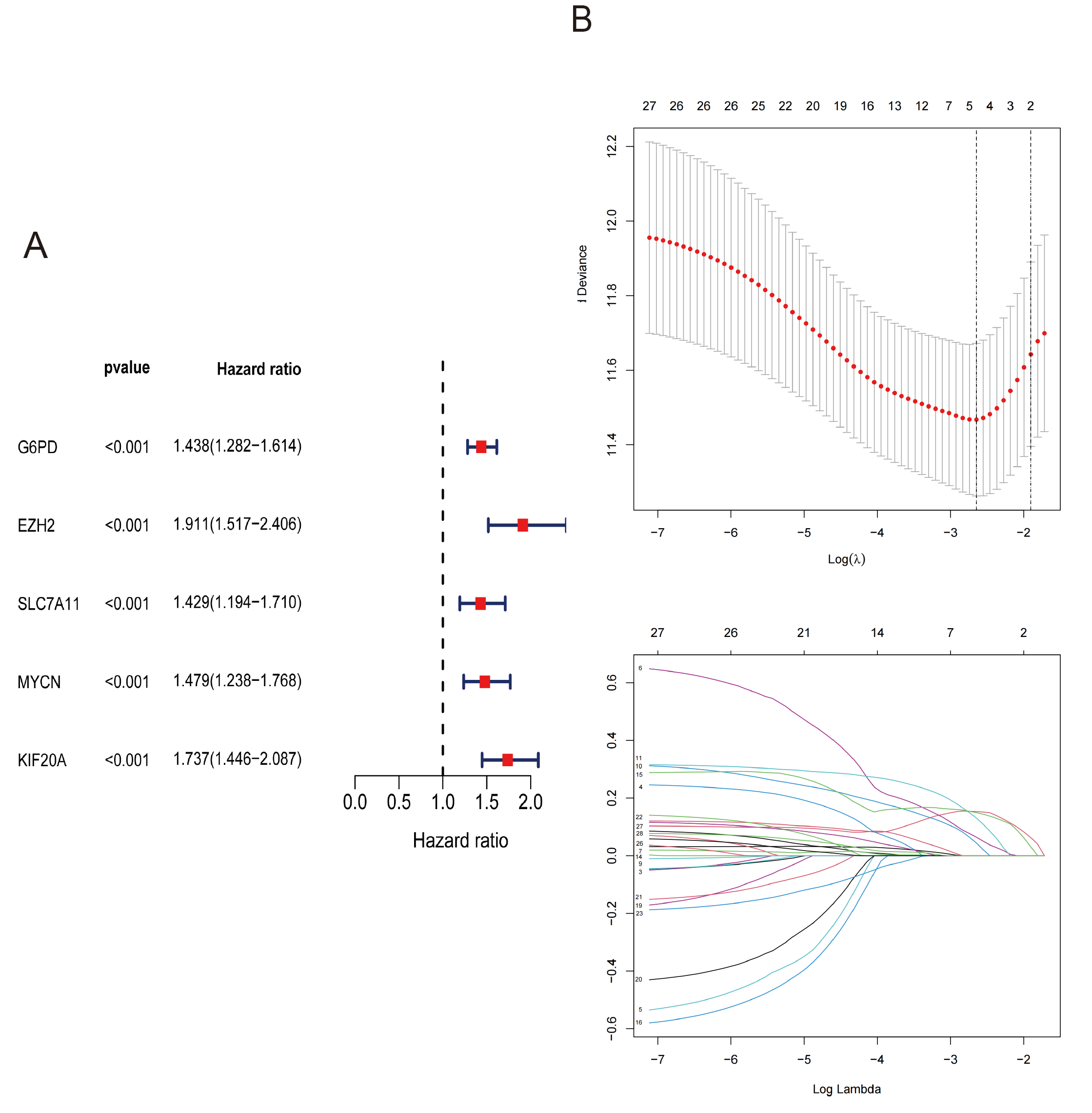
3.3. Identification of Prognostic Ferroptosis-Related Genes in the TCGA Cohort
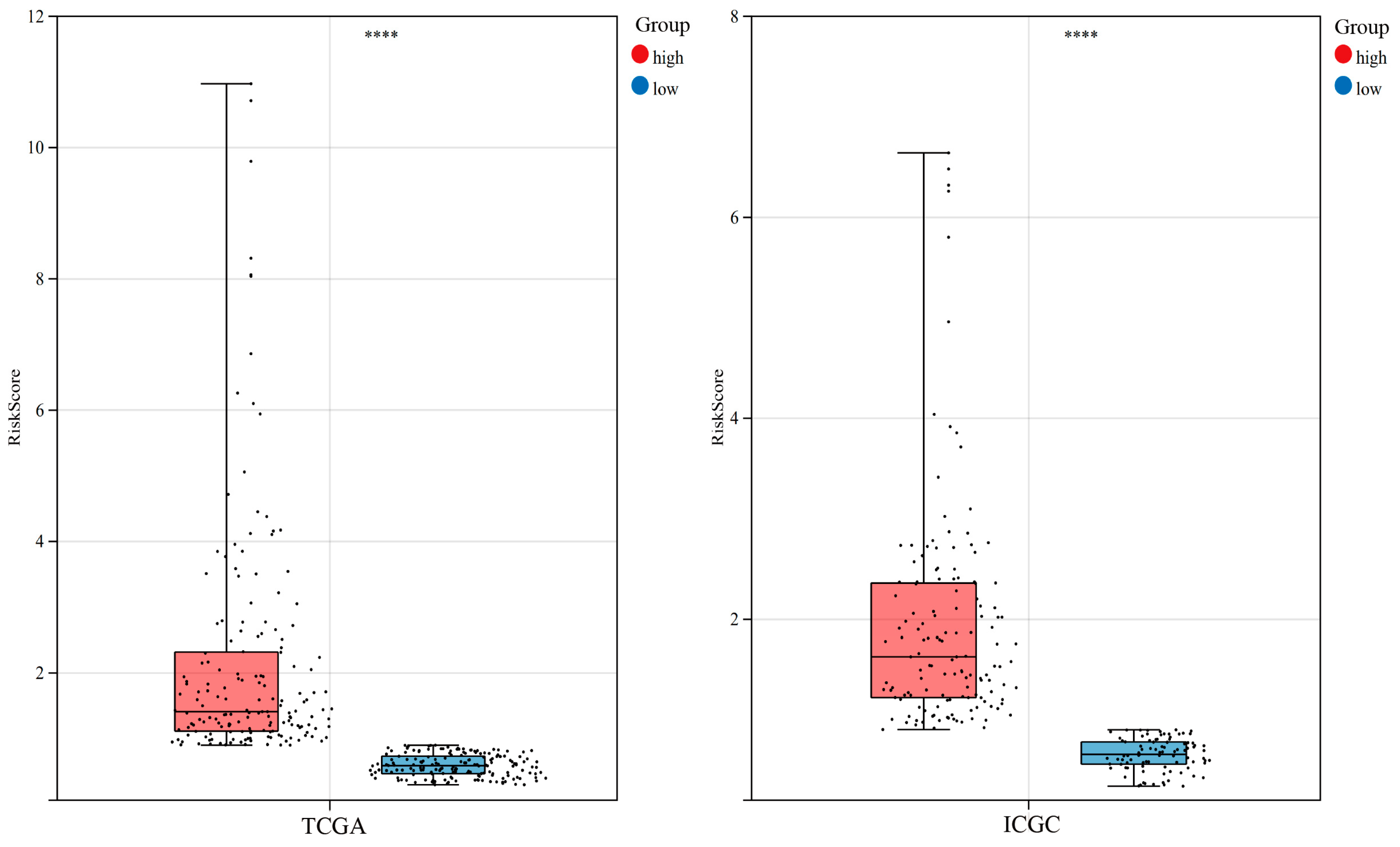
3.4. Construction of Risk Score in the TCGA Cohort
3.5. Verification of the Prognostic Model of the TCGA Cohort Based on the ICGC Cohort
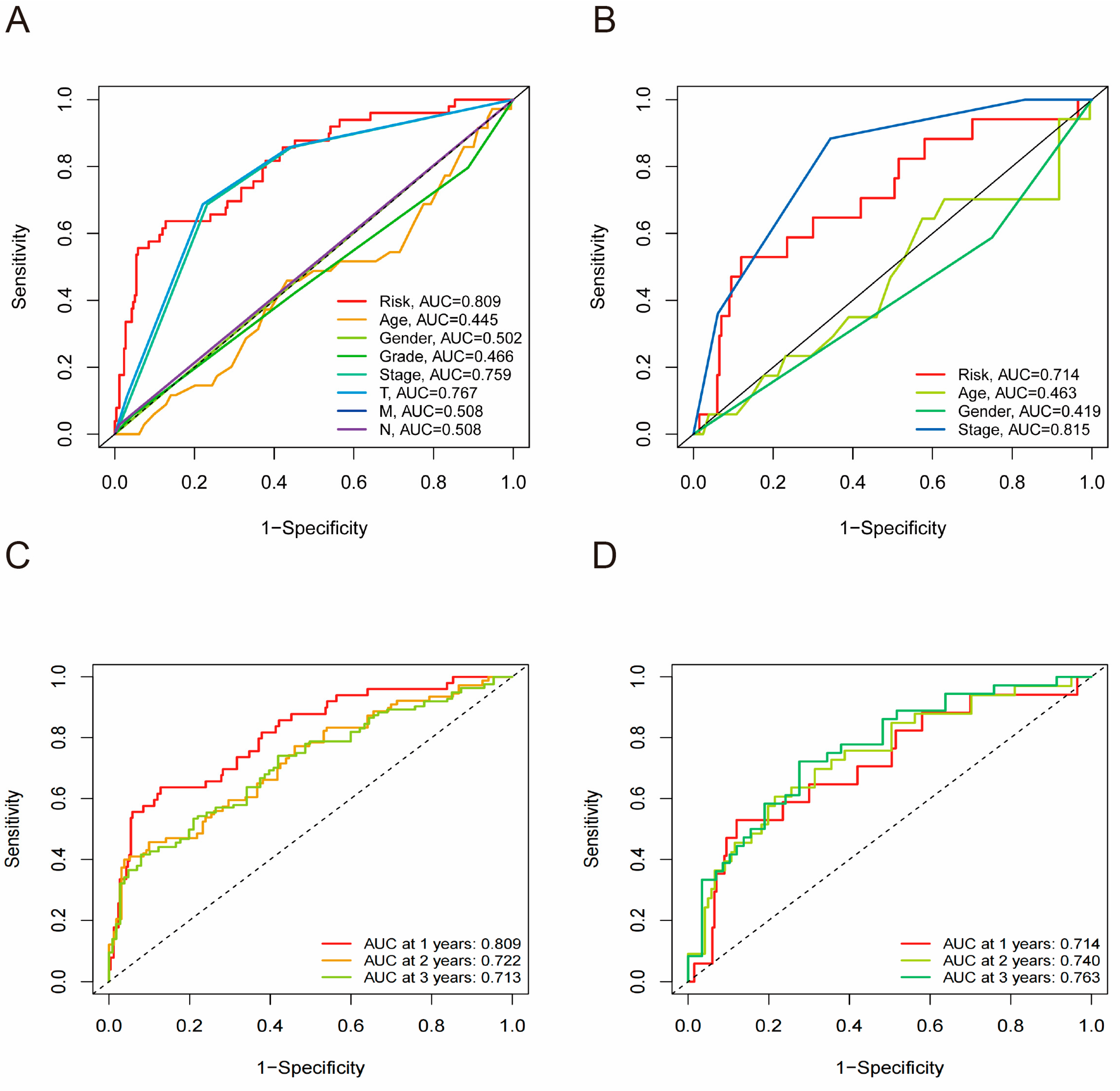
3.6. Risk Score as an Independent Predictor in HCC
3.7. Receiver Operating Characteristic Curve Analysis
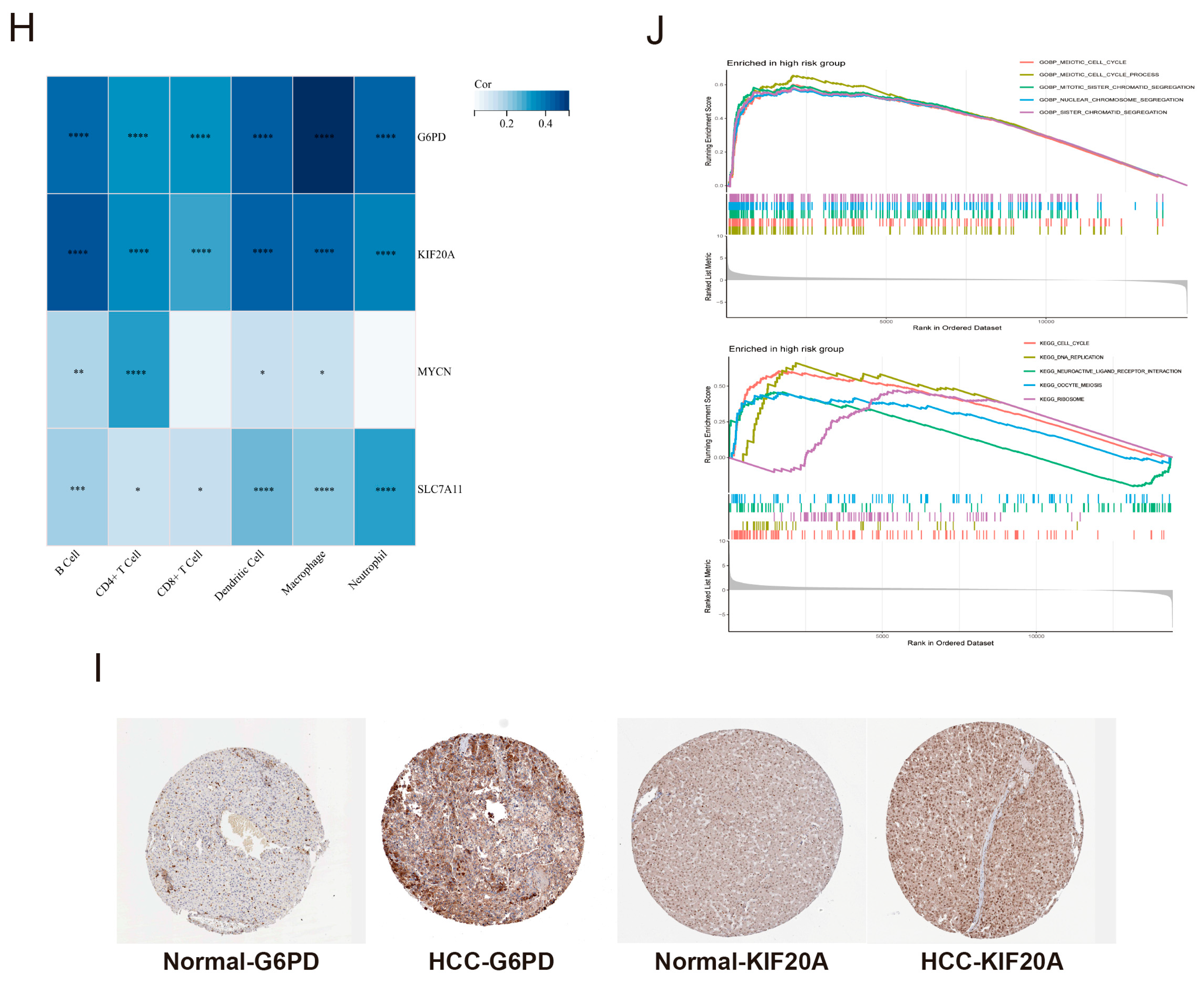
3.8. The Expression Levels of Core Genes and GSEA Enrichment Analysis of the Risk Score
3.9. Screening the Ferroptosis-Targeting Therapeutics for HCC from the CMap Database
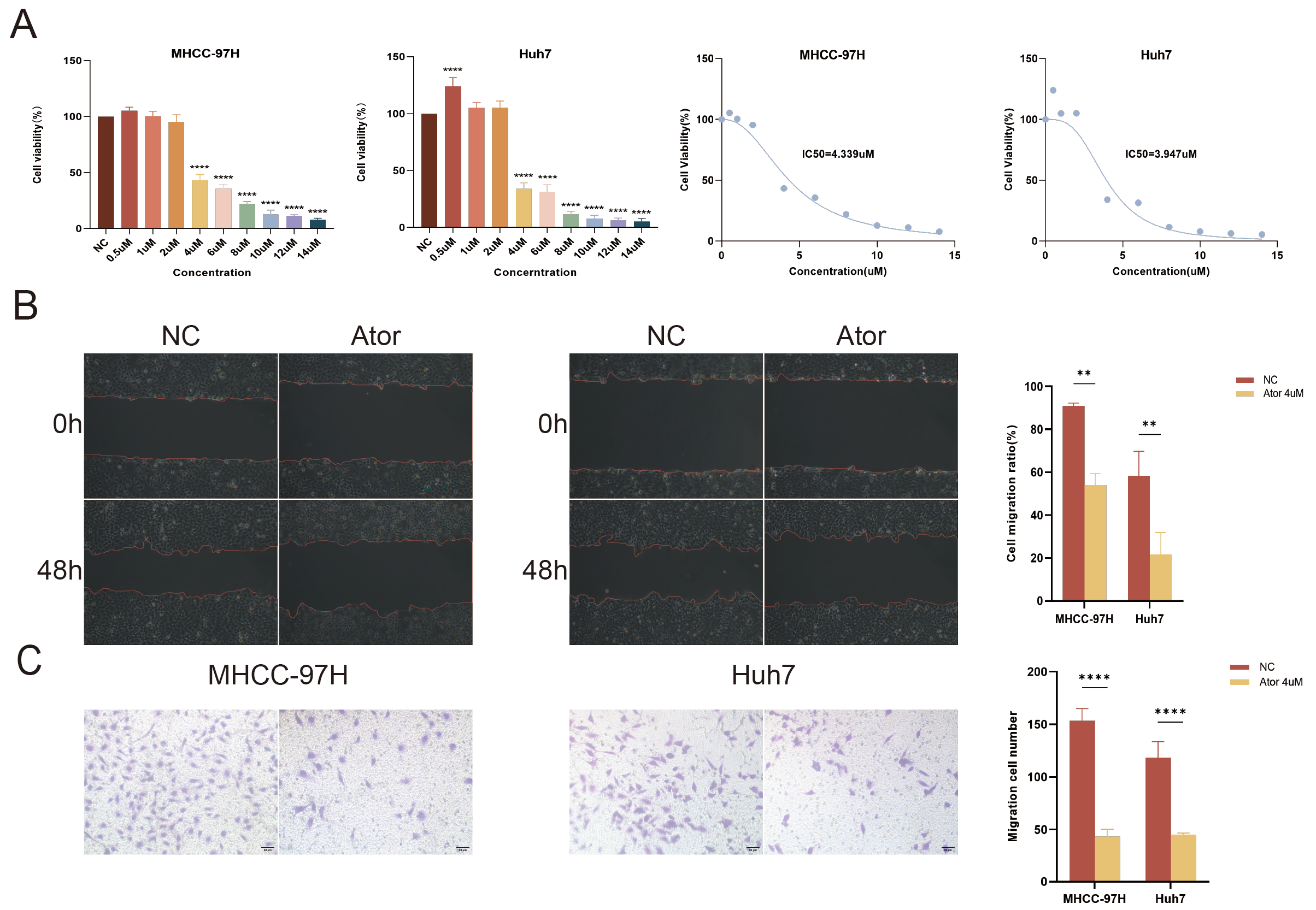
3.10. Atorvastatin Inhibits the Proliferation and Migration of HCC Cells
3.11. Atorvastatin Induces Ferroptosis in HCC Cells
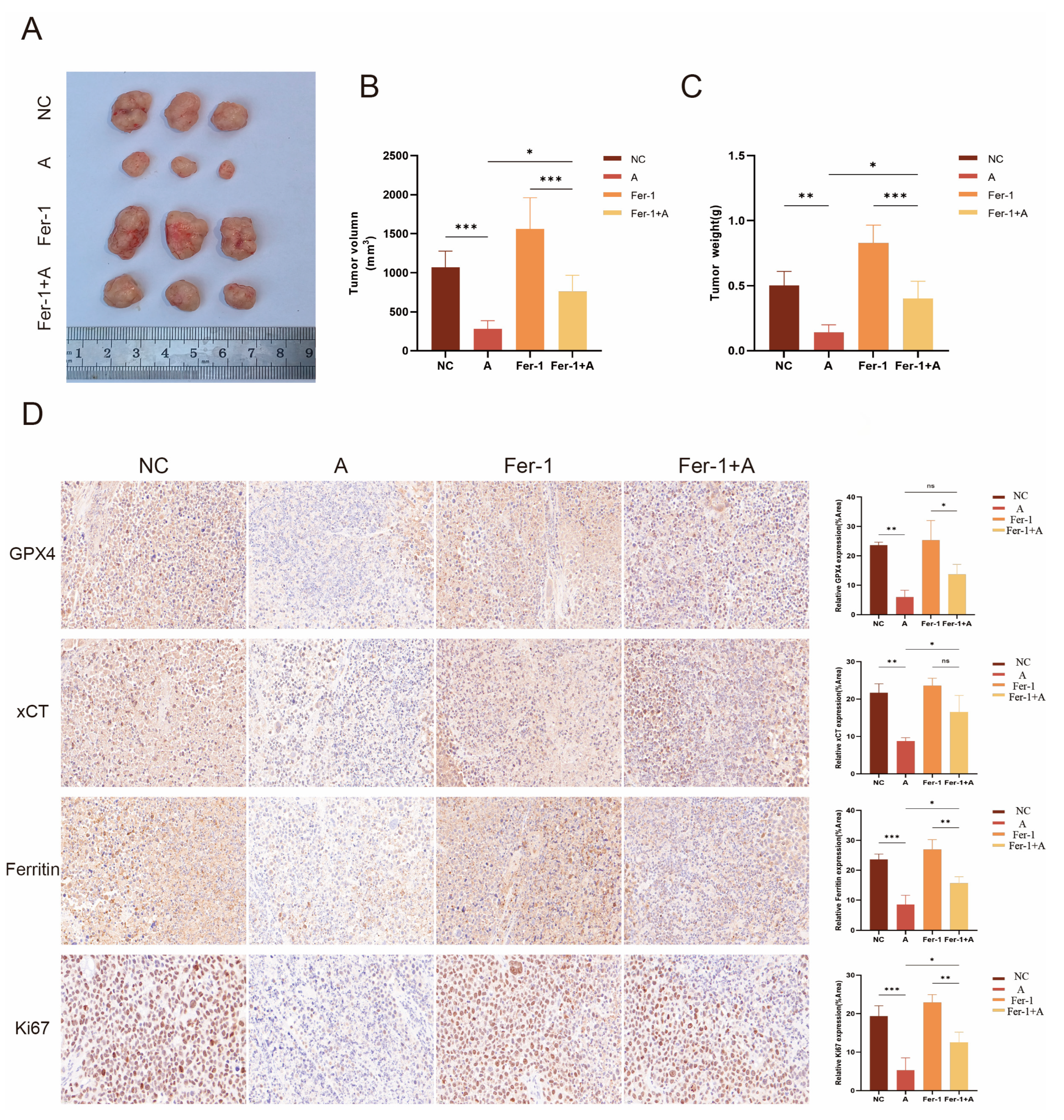
3.12. Atorvastatin Exhibits an Anti-HCC Effect In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Villanueva, A.; Hoshida, Y.; Battiston, C.; Tovar, V.; Sia, D.; Alsinet, C.; Cornella, H.; Liberzon, A.; Kobayashi, M.; Kumada, H.; et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology 2011, 140, 1501–1512.e1502. [Google Scholar] [CrossRef] [PubMed]
- Pinto Marques, H.; Gomes da Silva, S.; De Martin, E.; Agopian, V.G.; Martins, P.N. Emerging biomarkers in hcc patients: Current status. Int. J. Surg. 2020, 82, 70–76. [Google Scholar] [CrossRef]
- Wan, S.; Lei, Y.; Li, M.; Wu, B. A prognostic model for hepatocellular carcinoma patients based on signature ferroptosis-related genes. Hepatol. Int. 2022, 16, 112–124. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, P.; Wu, Y.; Zhou, H.; Wu, H.; Jin, Y.; Wu, D.; Wu, G. SLC25A19 is a novel prognostic biomarker related to immune invasion and ferroptosis in hcc. Int. Immunopharmacol. 2024, 136, 112367. [Google Scholar] [CrossRef]
- Xie, H.; Shi, M.; Liu, Y.; Cheng, C.; Song, L.; Ding, Z.; Jin, H.; Cui, X.; Wang, Y.; Yao, D.; et al. Identification of m6a- and ferroptosis-related lncrna signature for predicting immune efficacy in hepatocellular carcinoma. Front. Immunol. 2022, 13, 914977. [Google Scholar] [CrossRef]
- Chen, F.; Kang, R.; Tang, D.; Liu, J. Ferroptosis: Principles and significance in health and disease. J. Hematol. Oncol. 2024, 17, 41. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D.; Huang, J.; Tao, Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021, 29, 2185–2208. [Google Scholar] [CrossRef] [PubMed]
- Houessinon, A.; François, C.; Sauzay, C.; Louandre, C.; Mongelard, G.; Godin, C.; Bodeau, S.; Takahashi, S.; Saidak, Z.; Gutierrez, L.; et al. Metallothionein-1 as a biomarker of altered redox metabolism in hepatocellular carcinoma cells exposed to sorafenib. Mol. Cancer 2016, 15, 38. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting ferroptosis to iron out cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Lai, W.; Ping, Z.; Chen, Y.; Wang, J.; Liu, Y.; Zou, S.; Wang, J.; Zhang, T.; Ren, W.; Wang, W. Multi-omics analysis reveals BZW1’s regulation of EMT via the Wnt pathway in lung adenocarcinoma. J. Cell Mol. Med. 2024, 28, e70163. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.; Saki, M.; Cheng, F.; He, L.; Zhang, L.; Ioannidis, A.; Nathanson, D.; Tsang, J.; Bensinger, S.J.; Nghiemphu, P.L.; et al. Dopamine receptor antagonists, radiation, and cholesterol biosynthesis in mouse models of glioblastoma. J. Natl. Cancer Inst. 2021, 113, 1094–1104. [Google Scholar] [CrossRef]
- Henslee, A.B.; Steele, T.A. Combination statin and chemotherapy inhibits proliferation and cytotoxicity of an aggressive natural killer cell leukemia. Biomark. Res. 2018, 6, 26. [Google Scholar] [CrossRef]
- Yang, C.; Li, L.; Ye, Z.; Zhang, A.; Bao, Y.; Wu, X.; Ren, G.; Jiang, C.; Wang, O.; Wang, Z. Mechanisms underlying neutrophils adhesion to triple-negative breast cancer cells via cd11b-icam1 in promoting breast cancer progression. Cell Commun. Signal 2024, 22, 340. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chrysavgis, L.; Giannakodimos, I.; Diamantopoulou, P.; Cholongitas, E. Non-alcoholic fatty liver disease and hepatocellular carcinoma: Clinical challenges of an intriguing link. World J. Gastroenterol. 2022, 28, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Brar, G.; Greten, T.F.; Graubard, B.I.; McNeel, T.S.; Petrick, J.L.; McGlynn, K.A.; Altekruse, S.F. Hepatocellular carcinoma survival by etiology: A seer-medicare database analysis. Hepatol. Commun. 2020, 4, 1541–1551. [Google Scholar] [CrossRef]
- Ding, J.; Wen, Z. Survival improvement and prognosis for hepatocellular carcinoma: Analysis of the seer database. BMC Cancer 2021, 21, 1157. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Riester, M.; Xu, Q.; Moreira, A.; Zheng, J.; Michor, F.; Downey, R.J. The warburg effect: Persistence of stem-cell metabolism in cancers as a failure of differentiation. Ann. Oncol. 2018, 29, 264–270. [Google Scholar] [CrossRef]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The multiple roles of glucose-6-phosphate dehydrogenase in tumorigenesis and cancer chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Li, Y.; Xie, Y.; Huang, C.; Zhao, H.; Miyagishi, M.; Kasim, V. Transcription factor yy1 promotes cell proliferation by directly activating the pentose phosphate pathway. Cancer Res. 2018, 78, 4549–4562. [Google Scholar] [CrossRef]
- Luo, X.; Wei, M.; Li, W.; Zhao, H.; Kasim, V.; Wu, S. PBX3 promotes pentose phosphate pathway and colorectal cancer progression by enhancing g6pd expression. Int. J. Biol. Sci. 2023, 19, 4525–4538. [Google Scholar] [CrossRef]
- Cao, F.; Luo, A.; Yang, C. G6pd inhibits ferroptosis in hepatocellular carcinoma by targeting cytochrome p450 oxidoreductase. Cell Signal 2021, 87, 110098. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, J.; Liu, X.; Feng, L.; Gong, Z.; Koppula, P.; Sirohi, K.; Li, X.; Wei, Y.; Lee, H.; et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 2018, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, L.; Tavana, O.; Gu, W. The deubiquitylase otub1 mediates ferroptosis via stabilization of slc7a11. Cancer Res. 2019, 79, 1913–1924. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of n-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Garson, J.A.; McIntyre, P.G.; Kemshead, J.T. N-myc amplification in malignant astrocytoma. Lancet 1985, 2, 718–719. [Google Scholar] [CrossRef]
- Williamson, D.; Lu, Y.J.; Gordon, T.; Sciot, R.; Kelsey, A.; Fisher, C.; Poremba, C.; Anderson, J.; Pritchard-Jones, K.; Shipley, J. Relationship between mycn copy number and expression in rhabdomyosarcomas and correlation with adverse prognosis in the alveolar subtype. J. Clin. Oncol. 2005, 23, 880–888. [Google Scholar] [CrossRef]
- Wong, A.J.; Ruppert, J.M.; Eggleston, J.; Hamilton, S.R.; Baylin, S.B.; Vogelstein, B. Gene amplification of c-myc and n-myc in small cell carcinoma of the lung. Science 1986, 233, 461–464. [Google Scholar] [CrossRef]
- Lin, D.; Hu, B.; Zhu, S.; Wu, Y. Exploring a ferroptosis and oxidative stress-based prognostic model for clear cell renal cell carcinoma. Front. Oncol. 2023, 13, 1131473. [Google Scholar] [CrossRef]
- Qiu, L.; Zhou, R.; Luo, Z.; Wu, J.; Jiang, H. Cdc27-odc1 axis promotes metastasis, accelerates ferroptosis and predicts poor prognosis in neuroblastoma. Front. Oncol. 2022, 12, 774458. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K.; Pogoda, K.; Nandi, S.; Mathieu, S.; Kasri, A.; Klein, E.; Radvanyi, F.; Goud, B.; Janmey, P.A.; Manneville, J.B. Role of a kinesin motor in cancer cell mechanics. Nano Lett. 2019, 19, 7691–7702. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.R.; Chapeaublanc, E.; Kirkwood, L.; Nicolle, R.; Benhamou, S.; Lebret, T.; Allory, Y.; Southgate, J.; Radvanyi, F.; Goud, B. Deregulation of rab and rab effector genes in bladder cancer. PLoS ONE 2012, 7, e39469. [Google Scholar] [CrossRef]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; Mackellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3d matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, Y.M. The role of kinesin family proteins in tumorigenesis and progression: Potential biomarkers and molecular targets for cancer therapy. Cancer 2010, 116, 5150–5160. [Google Scholar] [CrossRef]
- Liu, S.L.; Lin, H.X.; Qiu, F.; Zhang, W.J.; Niu, C.H.; Wen, W.; Sun, X.Q.; Ye, L.P.; Wu, X.Q.; Lin, C.Y.; et al. Overexpression of kinesin family member 20a correlates with disease progression and poor prognosis in human nasopharyngeal cancer: A retrospective analysis of 105 patients. PLoS ONE 2017, 12, e0169280. [Google Scholar] [CrossRef]
- Imai, K.; Hirata, S.; Irie, A.; Senju, S.; Ikuta, Y.; Yokomine, K.; Harao, M.; Inoue, M.; Tomita, Y.; Tsunoda, T.; et al. Identification of hla-a2-restricted ctl epitopes of a novel tumour-associated antigen, kif20a, overexpressed in pancreatic cancer. Br. J. Cancer 2011, 104, 300–307. [Google Scholar] [CrossRef]
- Gasnereau, I.; Boissan, M.; Margall-Ducos, G.; Couchy, G.; Wendum, D.; Bourgain-Guglielmetti, F.; Desdouets, C.; Lacombe, M.L.; Zucman-Rossi, J.; Sobczak-Thépot, J. Kif20a mrna and its product mklp2 are increased during hepatocyte proliferation and hepatocarcinogenesis. Am. J. Pathol. 2012, 180, 131–140. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Lin, S.; Liu, Y.; Li, W. Suppressing the kif20a/nuak1/nrf2/gpx4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging 2021, 13, 13515–13534. [Google Scholar] [CrossRef]
- Ekins, S.; Puhl, A.C.; Zorn, K.M.; Lane, T.R.; Russo, D.P.; Klein, J.J.; Hickey, A.J.; Clark, A.M. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 2019, 18, 435–441. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Bai, J.; Lu, E.; Yang, C.; Liu, J.; Wen, Z.; Liu, X.; Jin, Z.; Xu, M.; Jiang, L.; et al. Identification of novel drug candidate for epithelial ovarian cancer via in silico investigation and in vitro validation. Front. Oncol. 2021, 11, 745590. [Google Scholar] [CrossRef]
- Pu, F.; Chen, F.; Zhang, Z.; Shi, D.; Zhong, B.; Lv, X.; Tucker, A.B.; Fan, J.; Li, A.J.; Qin, K.; et al. Ferroptosis as a novel form of regulated cell death: Implications in the pathogenesis, oncometabolism and treatment of human cancer. Genes. Dis. 2022, 9, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Foroutan, Z.; Katsiki, N.; Jamialahmadi, T.; Mantzoros, C.S. Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: Is there a role for statin therapy? Metabolism 2023, 146, 155659. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Yao, X.; Xie, R.; Cao, Y.; Tang, J.; Men, Y.; Peng, H.; Yang, W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnol. 2021, 19, 311. [Google Scholar] [CrossRef]
- Elakkad, Y.E.; Mohamed, S.N.S.; Abuelezz, N.Z. Potentiating the cytotoxic activity of a novel simvastatin-loaded cubosome against breast cancer cells: Insights on dual cell death via ferroptosis and apoptosis. Breast Cancer (Dove Med. Press) 2021, 13, 675–689. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, H.; Chen, Y.; Luo, X.; Chen, C.; Xiao, B.; Ding, X.; Zhao, P.; Lu, Y.; Chen, A.F.; et al. atorvastatin induces mitochondria-dependent ferroptosis via the modulation of nrf2-xct/gpx4 axis. Front. Cell Dev. Biol. 2022, 10, 806081. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liao, B.; Zhou, Y.; Zeng, W.; Zeng, Z.Y. Atorvastatin inhibits ferroptosis of h9c2 cells by regulatingsmad7/hepcidin expression to improve ischemia-reperfusion injury. Cardiol. Res. Pract. 2022, 2022, 3972829. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wu, J.; Zhang, T.; Ma, X.; Du, Z.; Xu, J.; You, J.; Wang, L.; Chen, N.; Luo, M.; et al. Statin-induced geranylgeranyl pyrophosphate depletion promotes ferroptosis-related senescence in adipose tissue. Nutrients 2022, 14, 4365. [Google Scholar] [CrossRef]
- Kitagawa, K.; Moriya, K.; Kaji, K.; Saikawa, S.; Sato, S.; Nishimura, N.; Namisaki, T.; Akahane, T.; Mitoro, A.; Yoshiji, H. Atorvastatin augments gemcitabine-mediated anti-cancer effects by inhibiting yes-associated protein in human cholangiocarcinoma cells. Int. J. Mol. Sci. 2020, 21, 7588. [Google Scholar] [CrossRef]
- Wang, S.T.; Huang, S.W.; Liu, K.T.; Lee, T.Y.; Shieh, J.J.; Wu, C.Y. Atorvastatin-induced senescence of hepatocellular carcinoma is mediated by downregulation of htert through the suppression of the il-6/stat3 pathway. Cell Death Discov. 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.M.; Li, P.C.; Nguyen, M.T.; Lin, Y.T.; Huang, Y.T.; Cheng, T.S.; Nguyen, T.H.; Tran, T.H.; Huang, T.Y.; Hoang, T.H.; et al. Repurposing pitavastatin and atorvastatin to overcome chemoresistance of metastatic colorectal cancer under high glucose conditions. Cancer Cell Int. 2025, 25, 79. [Google Scholar] [CrossRef]
- Noh, J.K.; Lee, M.K.; Lee, Y.; Bae, M.; Min, S.; Kong, M.; Lee, J.W.; Kim, S.I.; Lee, Y.C.; Ko, S.G.; et al. Targeting ferroptosis for improved radiotherapy outcomes in hpv-negative head and neck squamous cell carcinoma. Mol. Oncol. 2024, 19, 540–557. [Google Scholar] [CrossRef]






| Variables | TCGA (n = 326) | ICGC (n = 258) |
|---|---|---|
| Age, n (%) | ||
| <65 | 201 (61.66) | 91 (35.27) |
| ≥65 | 125 (38.34) | 167 (64.73) |
| Gender, n (%) | ||
| Female | 101 (30.98) | 214 (82.95) |
| Male | 225 (69.02) | 44 (17.05) |
| OS days | 879 | 803 |
| Survival status, n (%) | ||
| Alive | 215 (65.95) | 68 (26.36) |
| Dead | 111 (34.05) | 190 (73.64) |
| Stage, n (%) | ||
| I | 164 (50.30) | 40 (15.51) |
| II | 78 (23.93) | 117 (45.35) |
| III | 81 (24.85) | 78 (30.23) |
| IV | 3 (0.92) | 23 (8.91) |
| T, n (%) | ||
| T1 | 165 (50.61) | NA |
| T2 | 79 (24.23) | |
| T3 | 72 (22.09) | |
| T4 | 10 (3.07) | |
| M, n (%) | ||
| Mx | 74 (22.70) | NA |
| M0 | 249 (76.38) | |
| M1 | 3 (0.92) | |
| N, n (%) | ||
| Nx | 79 (24.23) | NA |
| N0 | 244 (74.85) | |
| N1 | 3 (0.92) |
| Number | Name | Score | FDR |
|---|---|---|---|
| 1 | PHF2 | −1.88 | 15.65 |
| 2 | BRSK2 | −1.87 | 15.65 |
| 3 | tiabendazole | −1.86 | 15.65 |
| 4 | reversine | −1.84 | 15.65 |
| 5 | etoposide | −1.83 | 15.65 |
| 6 | PON3 | −1.83 | 15.65 |
| 7 | HEBP1 | −1.82 | 15.65 |
| 8 | TWS-119 | −1.80 | 15.65 |
| 9 | ZNF385B | −1.80 | 15.65 |
| 10 | GPR64 | −1.80 | 15.65 |
| Number | Name | Score | FDR |
|---|---|---|---|
| 1 | azacitidine | −1.99 | 15.65 |
| 2 | anisomycin | −1.99 | 15.65 |
| 3 | heliomycin | −1.92 | 15.65 |
| 4 | BRAF | −1.92 | 15.65 |
| 5 | clofarabine | −1.90 | 15.65 |
| 6 | etoposide | −1.89 | 15.65 |
| 7 | pralatrexate | −1.88 | 15.65 |
| 8 | CRH | −1.87 | 15.35 |
| 9 | GPR146 | −1.87 | 15.35 |
| 10 | eltrombopag | −1.86 | 15.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; He, X.; Shen, Y.; Chen, J.; Chen, Y.; Zhou, Z.; Xu, X. A Novel Ferroptosis-Related Gene Prognosis Signature and Identifying Atorvastatin as a Potential Therapeutic Agent for Hepatocellular Carcinoma. Curr. Issues Mol. Biol. 2025, 47, 201. https://doi.org/10.3390/cimb47030201
Wang L, He X, Shen Y, Chen J, Chen Y, Zhou Z, Xu X. A Novel Ferroptosis-Related Gene Prognosis Signature and Identifying Atorvastatin as a Potential Therapeutic Agent for Hepatocellular Carcinoma. Current Issues in Molecular Biology. 2025; 47(3):201. https://doi.org/10.3390/cimb47030201
Chicago/Turabian StyleWang, Ling, Xiaoqin He, Yang Shen, Jiayu Chen, Yukai Chen, Zhuolin Zhou, and Ximing Xu. 2025. "A Novel Ferroptosis-Related Gene Prognosis Signature and Identifying Atorvastatin as a Potential Therapeutic Agent for Hepatocellular Carcinoma" Current Issues in Molecular Biology 47, no. 3: 201. https://doi.org/10.3390/cimb47030201
APA StyleWang, L., He, X., Shen, Y., Chen, J., Chen, Y., Zhou, Z., & Xu, X. (2025). A Novel Ferroptosis-Related Gene Prognosis Signature and Identifying Atorvastatin as a Potential Therapeutic Agent for Hepatocellular Carcinoma. Current Issues in Molecular Biology, 47(3), 201. https://doi.org/10.3390/cimb47030201





