Exploring Oxylipins in Host–Microbe Interactions and Their Impact on Infection and Immunity
Abstract
1. Introduction
2. Oxylipin Immunomodulation
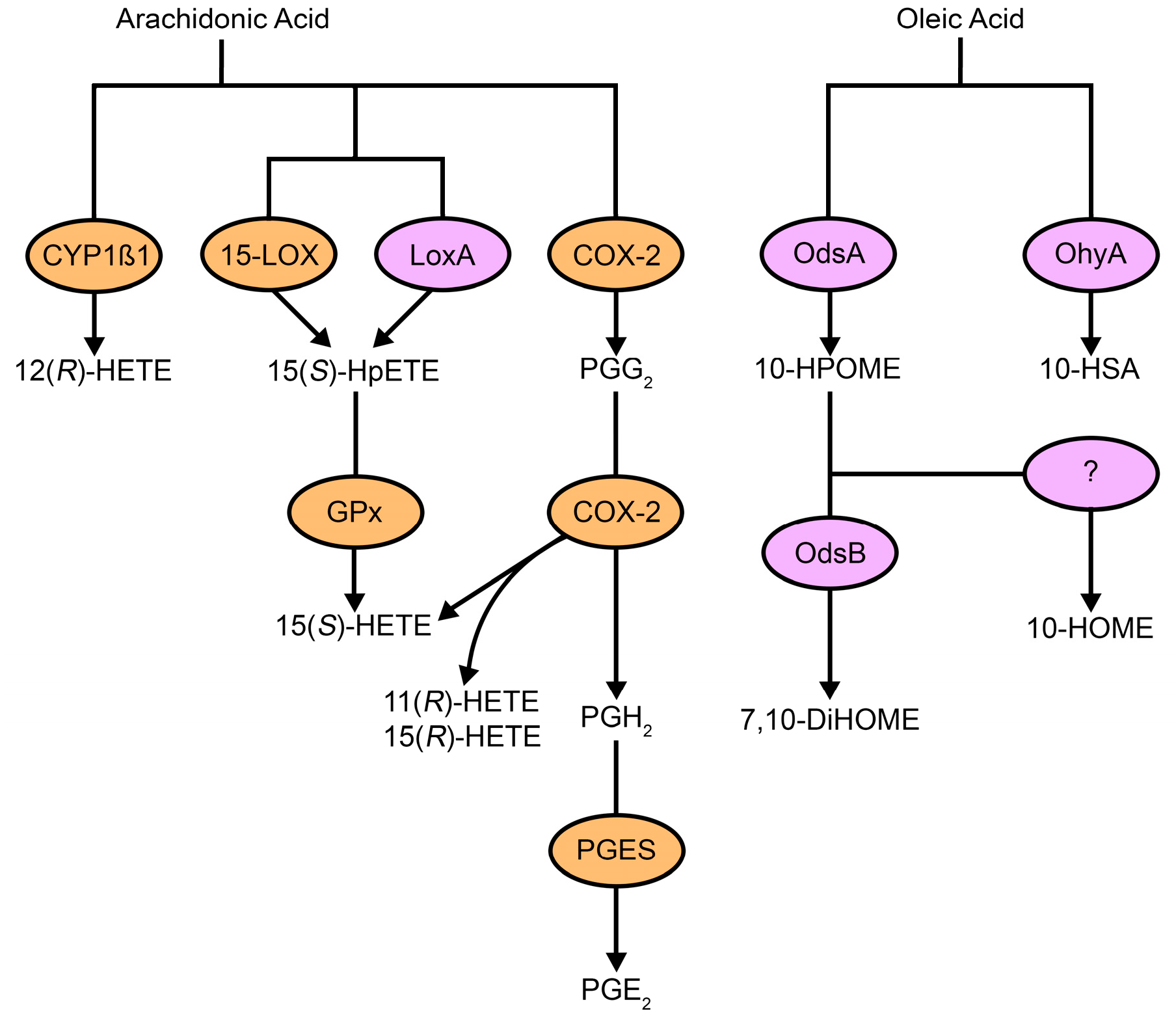
3. Fungal Oxylipins
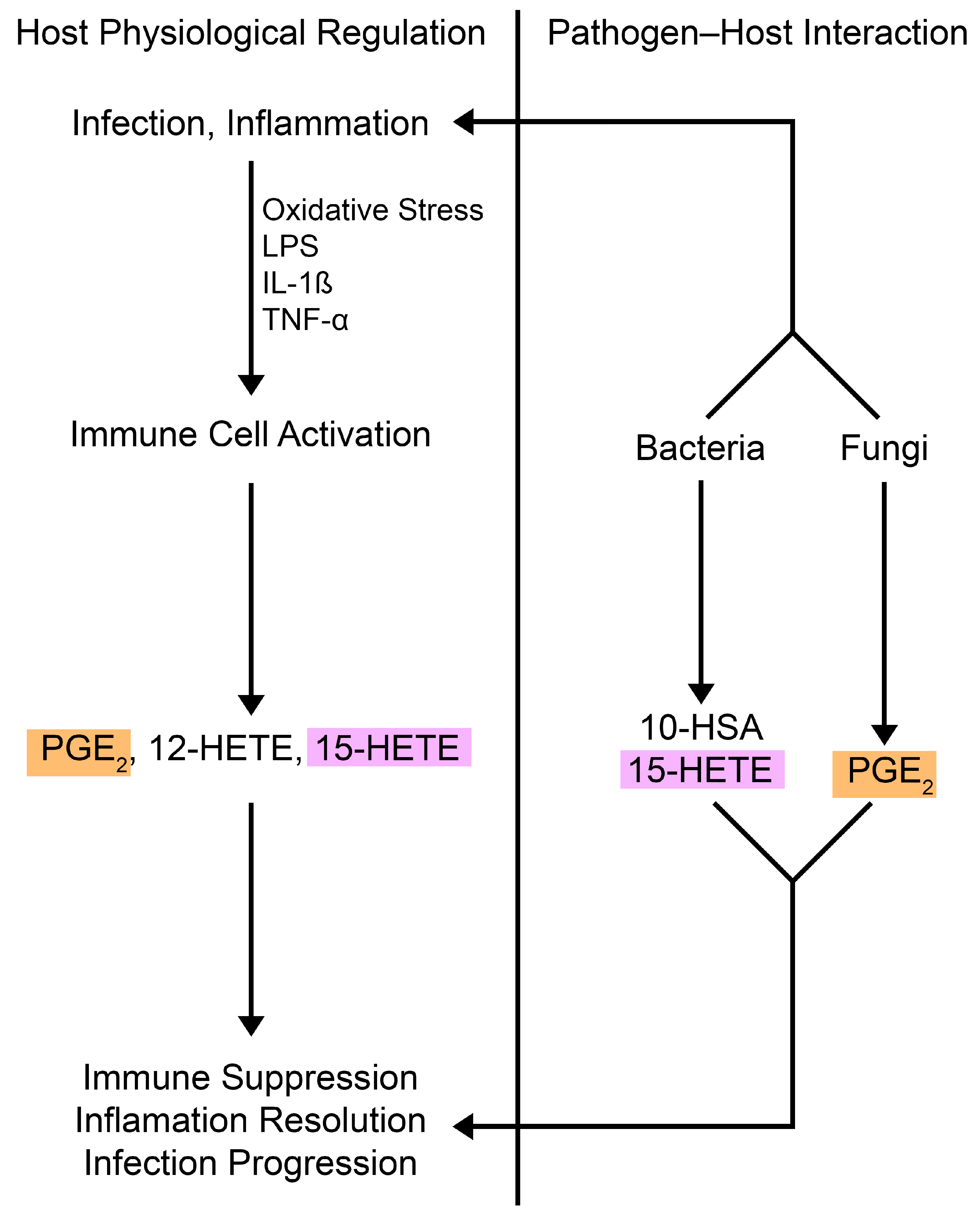
4. Bacterial Oxylipins
5. Targeting Oxylipins in Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van der Vusse, G.J. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef]
- Quehenberger, O.; Dennis, E.A. The human plasma lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef]
- Leaf, A. Plasma nonesterified fatty acid concentration as a risk factor for sudden cardiac death: The Paris Prospective Study. Circulation 2001, 104, 744–745. [Google Scholar] [CrossRef] [PubMed]
- McLennan, P.L. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am. J. Clin. Nutr. 1993, 57, 207–212. [Google Scholar] [CrossRef]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [Google Scholar] [CrossRef]
- Buczynski, M.W.; Dumlao, D.S.; Dennis, E.A. Thematic review series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009, 50, 1015–1038. [Google Scholar] [CrossRef]
- Du, Y.; Taylor, C.G.; Aukema, H.M.; Zahradka, P. Role of oxylipins generated from dietary PUFAs in the modulation of endothelial cell function. Prostaglandins Leukot. Essent. Fatty Acids 2020, 160, 102160. [Google Scholar] [CrossRef]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Tyson, G.W.; Vivanco, J.M.; Schenk, P.M. Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS ONE 2013, 8, e56457. [Google Scholar] [CrossRef]
- Konya, V.; Mjosberg, J. Lipid mediators as regulators of human ILC2 function in allergic diseases. Immunol. Lett. 2016, 179, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Beermann, C.; Neumann, S.; Fussbroich, D.; Zielen, S.; Schubert, R. Combinations of distinct long-chain polyunsaturated fatty acid species for improved dietary treatment against allergic bronchial asthma. Nutrition 2016, 32, 1165–1170. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef]
- Durand, T.; Bultel-Ponce, V.; Guy, A.; Berger, S.; Mueller, M.J.; Galano, J.M. New bioactive oxylipins formed by non-enzymatic free-radical-catalyzed pathways: The phytoprostanes. Lipids 2009, 44, 875–888. [Google Scholar] [CrossRef]
- Samuchiwal, S.K.; Boyce, J.A. Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology. J. Allergy Clin. Immunol. 2018, 141, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Reddy, S.T.; Herschman, H.R. Ligand-induced prostaglandin synthesis requires expression of the TIS10/PGS-2 prostaglandin synthase gene in murine fibroblasts and macrophages. J. Biol. Chem. 1994, 269, 15473–15480. [Google Scholar] [CrossRef]
- Tang, Y.; Di Pietro, L.; Feng, Y.; Wang, X. Increased TNF-a and PGI2, but not NO release from macrophages in 18-month-old rats. Mech. Ageing Dev. 2000, 114, 79–88. [Google Scholar] [CrossRef]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.W.; Burke, P.A.; Drotar, M.E.; Chavali, S.R.; Forse, R.A. Effects of prostaglandin E2, cholera toxin and 8-bromo-cyclic AMP on lipopolysaccharide-induced gene expression of cytokines in human macrophages. Immunology 1995, 84, 446–452. [Google Scholar] [PubMed]
- Ikegami, R.; Sugimoto, Y.; Segi, E.; Katsuyama, M.; Karahashi, H.; Amano, F.; Maruyama, T.; Yamane, H.; Tsuchiya, S.; Ichikawa, A. The expression of prostaglandin E receptors EP2 and EP4 and their different regulation by lipopolysaccharide in C3H/HeN peritoneal macrophages. J. Immunol. 2001, 166, 4689–4696. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and cancer: Insight into tumor progression and immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Lacher, S.B.; Dorr, J.; de Almeida, G.P.; Honninger, J.; Bayerl, F.; Hirschberger, A.; Pedde, A.M.; Meiser, P.; Ramsauer, L.; Rudolph, T.J.; et al. PGE2 limits effector expansion of tumour-infiltrating stem-like CD8(+) T cells. Nature 2024, 629, 417–425. [Google Scholar] [CrossRef]
- Burkett, J.B.; Doran, A.C.; Gannon, M. Harnessing prostaglandin E2 signaling to ameliorate autoimmunity. Trends Immunol. 2023, 44, 162–171. [Google Scholar] [CrossRef]
- Kofler, D.M.; Marson, A.; Dominguez-Villar, M.; Xiao, S.; Kuchroo, V.K.; Hafler, D.A. Decreased RORC-dependent silencing of prostaglandin receptor EP2 induces autoimmune Th17 cells. J. Clin. Investig. 2014, 124, 2513–2522. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Pellegrini, J.M.; Martin, C.; Morelli, M.P.; Schander, J.A.; Tateosian, N.L.; Amiano, N.O.; Rolandelli, A.; Palmero, D.J.; Levi, A.; Ciallella, L.; et al. PGE2 displays immunosuppressive effects during human active tuberculosis. Sci. Rep. 2021, 11, 13559. [Google Scholar] [CrossRef]
- Agard, M.; Asakrah, S.; Morici, L.A. PGE2 suppression of innate immunity during mucosal bacterial infection. Front. Cell. Infect. Microbiol. 2013, 3, 45. [Google Scholar] [CrossRef]
- Martin-Vazquez, E.; Cobo-Vuilleumier, N.; Lopez-Noriega, L.; Lorenzo, P.I.; Gauthier, B.R. The PTGS2/COX2-PGE2 signaling cascade in inflammation: Pro or anti? A case study with type 1 diabetes mellitus. Int. J. Biol. Sci. 2023, 19, 4157–4165. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Foudi, N.; Longrois, D.; Norel, X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 55–63. [Google Scholar] [CrossRef]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta 2015, 1851, 414–421. [Google Scholar] [CrossRef]
- Crittenden, S.; Goepp, M.; Pollock, J.; Robb, C.T.; Smyth, D.J.; Zhou, Y.; Andrews, R.; Tyrrell, V.; Gkikas, K.; Adima, A.; et al. Prostaglandin E2 promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci. Adv. 2021, 7, eabd7954. [Google Scholar] [CrossRef]
- Oyesola, O.O.; Tait Wojno, E.D. Prostaglandin regulation of type 2 inflammation: From basic biology to therapeutic interventions. Eur. J. Immunol. 2021, 51, 2399–2416. [Google Scholar] [CrossRef] [PubMed]
- Birrell, M.A.; Maher, S.A.; Dekkak, B.; Jones, V.; Wong, S.; Brook, P.; Belvisi, M.G. Anti-inflammatory effects of PGE2 in the lung: Role of the EP4 receptor subtype. Thorax 2015, 70, 740–747. [Google Scholar] [CrossRef]
- Kabashima, K.; Sakata, D.; Nagamachi, M.; Miyachi, Y.; Inaba, K.; Narumiya, S. Prostaglandin E2-EP4 signaling initiates skin immune responses by promoting migration and maturation of Langerhans cells. Nat. Med. 2003, 9, 744–749. [Google Scholar] [CrossRef]
- Angeli, V.; Faveeuw, C.; Roye, O.; Fontaine, J.; Teissier, E.; Capron, A.; Wolowczuk, I.; Capron, M.; Trottein, F. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 2001, 193, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Printz, M.P. Arachidonic acid metabolism in skin: Experimental contact dermatitis in guinea pigs. Int. Arch. Allergy Appl. Immunol. 1982, 69, 347–352. [Google Scholar] [CrossRef]
- Eberhard, J.; Jepsen, S.; Pohl, L.; Albers, H.K.; Acil, Y. Bacterial challenge stimulates formation of arachidonic acid metabolites by human keratinocytes and neutrophils in vitro. Clin. Diagn. Lab. Immunol. 2002, 9, 132–137. [Google Scholar] [CrossRef]
- Jira, W.; Spiteller, G.; Richter, A. Increased levels of lipid oxidation products in low density lipoproteins of patients suffering from rheumatoid arthritis. Chem. Phys. Lipids 1997, 87, 81–89. [Google Scholar] [CrossRef]
- Ku, G.; Thomas, C.E.; Akeson, A.L.; Jackson, R.L. Induction of interleukin 1 beta expression from human peripheral blood monocyte-derived macrophages by 9-hydroxyoctadecadienoic acid. J. Biol. Chem. 1992, 267, 14183–14188. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Klug, M.J. Characterization and differentiation of filamentous fungi based on fatty acid composition. Appl. Environ. Microbiol. 1996, 62, 4136–4146. [Google Scholar] [CrossRef]
- Ells, R.; Kemp, G.; Albertyn, J.; Kock, J.L.; Pohl, C.H. Phenothiazine is a potent inhibitor of prostaglandin E2 production by Candida albicans biofilms. FEMS Yeast Res. 2013, 13, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.J.; Keller, N.P. Production of cross-kingdom oxylipins by pathogenic fungi: An update on their role in development and pathogenicity. J. Microbiol. 2016, 54, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.I.; Sa-Nunes, A.; Soares, E.G.; Peres, C.M.; Silva, C.L.; Faccioli, L.H. Blockade of endogenous leukotrienes exacerbates pulmonary histoplasmosis. Infect. Immun. 2004, 72, 1637–1644. [Google Scholar] [CrossRef]
- Secatto, A.; Rodrigues, L.C.; Serezani, C.H.; Ramos, S.G.; Dias-Baruffi, M.; Faccioli, L.H.; Medeiros, A.I. 5-Lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS ONE 2012, 7, e31701. [Google Scholar] [CrossRef]
- Nicolete, R.; Secatto, A.; Pereira, P.A.; Soares, E.G.; Faccioli, L.H. Leukotriene B4-loaded microspheres as a new approach to enhance antimicrobial responses in Histoplasma capsulatum-infected mice. Int. J. Antimicrob. Agents 2009, 34, 365–369. [Google Scholar] [CrossRef]
- Odds, F.C. Candida infections: An overview. Crit. Rev. Microbiol. 1987, 15, 1–5. [Google Scholar] [CrossRef]
- Shiraki, Y.; Ishibashi, Y.; Hiruma, M.; Nishikawa, A.; Ikeda, S. Candida albicans abrogates the expression of interferon-g-inducible protein-10 in human keratinocytes. FEMS Immunol. Med. Microbiol. 2008, 54, 122–128. [Google Scholar] [CrossRef]
- Sheppe, A.E.F.; Edelmann, M.J. Roles of eicosanoids in regulating inflammation and neutrophil migration as an innate host response to bacterial infections. Infect. Immun. 2021, 89, e0009521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, K.; Wang, W.S. Identification of a feed-forward loop between 15(S)-HETE and PGE2 in human amnion at parturition. J. Lipid Res. 2022, 63, 100294. [Google Scholar] [CrossRef]
- Salari, H.; Chan-Yeung, M. Release of 15-hydroxyeicosatetraenoic acid (15-HETE) and prostaglandin E2 (PGE2) by cultured human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1989, 1, 245–250. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Betz, M.; Fox, B.S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 1991, 146, 108–113. [Google Scholar] [CrossRef]
- Romani, L.; Kaufmann, S.H. Immunity to fungi: Editorial overview. Res. Immunol. 1998, 149, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Toews, G.B.; Huffnagle, G.B. Production of prostaglandins and leukotrienes by pathogenic fungi. Infect. Immun. 2002, 70, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Smeekens, S.P.; van de Veerdonk, F.L.; van der Meer, J.W.; Kullberg, B.J.; Joosten, L.A.; Netea, M.G. The Candida Th17 response is dependent on mannan- and b-glucan-induced prostaglandin E2. Int. Immunol. 2010, 22, 889–895. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Y. Prostaglandin E2 blockade enhances the pulmonary anti-Cryptococcus neoformans immune reaction via the induction of TLR-4. Int. Immunopharmacol. 2015, 28, 376–381. [Google Scholar] [CrossRef]
- Erb-Downward, J.R.; Noverr, M.C. Characterization of prostaglandin E2 production by Candida albicans. Infect. Immun. 2007, 75, 3498–3505. [Google Scholar] [CrossRef]
- Tan, T.G.; Lim, Y.S.; Tan, A.; Leong, R.; Pavelka, N. Fungal symbionts produce prostaglandin E2 to promote their intestinal colonization. Front. Cell. Infect. Microbiol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Thuer, E.; Heijink, M.; Toth, R.; Bodai, L.; Vagvolgyi, C.; Giera, M.; Gabaldon, T.; Gacser, A. Eicosanoid biosynthesis influences the virulence of Candida parapsilosis. Virulence 2018, 9, 1019–1035. [Google Scholar] [CrossRef]
- Kabashima, K.; Saji, T.; Murata, T.; Nagamachi, M.; Matsuoka, T.; Segi, E.; Tsuboi, K.; Sugimoto, Y.; Kobayashi, T.; Miyachi, Y.; et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Investig. 2002, 109, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Haas-Stapleton, E.J.; Lu, Y.; Hong, S.; Arita, M.; Favoreto, S.; Nigam, S.; Serhan, C.N.; Agabian, N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS ONE 2007, 2, e1316. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef]
- Ishida, T.; Yoshida, M.; Arita, M.; Nishitani, Y.; Nishiumi, S.; Masuda, A.; Mizuno, S.; Takagawa, T.; Morita, Y.; Kutsumi, H.; et al. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 2010, 16, 87–95. [Google Scholar] [CrossRef]
- Oner, F.; Alvarez, C.; Yaghmoor, W.; Stephens, D.; Hasturk, H.; Firatli, E.; Kantarci, A. Resolvin E1 regulates Th17 function and T cell activation. Front. Immunol. 2021, 12, 637983. [Google Scholar] [CrossRef]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010, 184, 836–843. [Google Scholar] [CrossRef]
- Evans, R.J.; Pline, K.; Loynes, C.A.; Needs, S.; Aldrovandi, M.; Tiefenbach, J.; Bielska, E.; Rubino, R.E.; Nicol, C.J.; May, R.C.; et al. 15-keto-prostaglandin E2 activates host peroxisome proliferator-activated receptor gamma (PPAR-g) to promote Cryptococcus neoformans growth during infection. PLoS Pathog. 2019, 15, e1007597. [Google Scholar] [CrossRef]
- Noverr, M.C.; Phare, S.M.; Toews, G.B.; Coffey, M.J.; Huffnagle, G.B. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 2001, 69, 2957–2963. [Google Scholar] [CrossRef]
- Schimanski, J.; Gresnigt, M.S.; Brunner, E.; Werz, O.; Hube, B.; Garscha, U. Hyphal-associated protein expression is crucial for Candida albicans-induced eicosanoid biosynthesis in immune cells. Eur. J. Immunol. 2024, 54, e2350743. [Google Scholar] [CrossRef] [PubMed]
- Mochochoko, B.M.; Pohl, C.H.; O’Neill, H.G. Candida albicans-enteric viral interactions-the prostaglandin E2 connection and host immune responses. iScience 2023, 26, 105870. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Fradin, C.; De Groot, P.; MacCallum, D.; Schaller, M.; Klis, F.; Odds, F.C.; Hube, B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 2005, 56, 397–415. [Google Scholar] [CrossRef]
- Grubb, S.E.; Murdoch, C.; Sudbery, P.E.; Saville, S.P.; Lopez-Ribot, J.L.; Thornhill, M.H. Adhesion of Candida albicans to endothelial cells under physiological conditions of flow. Infect. Immun. 2009, 77, 3872–3878. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Gow, N.A. Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Alem, M.A.; Douglas, L.J. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 41–47. [Google Scholar] [CrossRef]
- Nemecek, J.C.; Wuthrich, M.; Klein, B.S. Global control of dimorphism and virulence in fungi. Science 2006, 312, 583–588. [Google Scholar] [CrossRef]
- Brown, A.J.; Gow, N.A. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999, 7, 333–338. [Google Scholar] [CrossRef]
- Lo, H.J.; Kohler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Tsitsigiannis, D.I.; Bok, J.W.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Keller, N.P. Aspergillus cyclooxygenase-like enzymes are associated with prostaglandin production and virulence. Infect. Immun. 2005, 73, 4548–4559. [Google Scholar] [CrossRef]
- Brakhage, A.A.; Langfelder, K.; Wanner, G.; Schmidt, A.; Jahn, B. Pigment biosynthesis and virulence. Contrib. Microbiol. 1999, 2, 205–215. [Google Scholar] [CrossRef]
- Tsai, H.F.; Chang, Y.C.; Washburn, R.G.; Wheeler, M.H.; Kwon-Chung, K.J. The developmentally regulated alb1 gene of Aspergillus fumigatus: Its role in modulation of conidial morphology and virulence. J. Bacteriol. 1998, 180, 3031–3038. [Google Scholar] [CrossRef]
- Nieminen, S.M.; Maki-Paakkanen, J.; Hirvonen, M.R.; Roponen, M.; von Wright, A. Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat. Res. 2002, 520, 161–170. [Google Scholar] [CrossRef]
- Kaleli, I.; Cevahir, N.; Demir, M.; Yildirim, U.; Sahin, R. Anticandidal activity of Pseudomonas aeruginosa strains isolated from clinical specimens. Mycoses 2007, 50, 74–78. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Hayes, D., Jr.; Wozniak, D.J. Cystic fibrosis and Pseudomonas aeruginosa: The host-microbe interface. Clin. Microbiol. Rev. 2019, 32, e00138-00118. [Google Scholar] [CrossRef] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.E.; Hong, S.; Gronert, K.; Serhan, C.N.; Mekalanos, J.J. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 2135–2139. [Google Scholar] [CrossRef]
- Banthiya, S.; Kalms, J.; Galemou Yoga, E.; Ivanov, I.; Carpena, X.; Hamberg, M.; Kuhn, H.; Scheerer, P. Structural and functional basis of phospholipid oxygenase activity of bacterial lipoxygenase from Pseudomonas aeruginosa. Biochim. Biophys. Acta 2016, 1861, 1681–1692. [Google Scholar] [CrossRef]
- Deschamps, J.D.; Ogunsola, A.F.; Jameson, J.B., 2nd; Yasgar, A.; Flitter, B.A.; Freedman, C.J.; Melvin, J.A.; Nguyen, J.V.; Maloney, D.J.; Jadhav, A.; et al. Biochemical and cellular characterization and inhibitor discovery of Pseudomonas aeruginosa 15-lipoxygenase. Biochemistry 2016, 55, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: An update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002, 68–69, 433–455. [Google Scholar] [CrossRef]
- Serhan, C.N. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): A jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins 1997, 53, 107–137. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; McCormick, B.; Neish, A.S.; Petasis, N.A.; Gronert, K.; Serhan, C.N.; Madara, J.L. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J. Clin. Investig. 1998, 101, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Fiore, S.; Maddox, J.F.; Brady, H.R.; Petasis, N.A.; Serhan, C.N. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 1997, 185, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Lamacka, M.; Sajbidor, J. The occurrence of prostaglandins and related compounds in lower organisms. Prostaglandins Leukot. Essent. Fatty Acids 1995, 52, 357–364. [Google Scholar] [CrossRef]
- Turinsky, J.; Loegering, D.J. Prostaglandin E2 and muscle protein turnover in Pseudomonas aeruginosa sepsis. Biochim. Biophys. Acta 1985, 840, 137–140. [Google Scholar] [CrossRef]
- Martinez, E.; Cosnahan, R.K.; Wu, M.; Gadila, S.K.; Quick, E.B.; Mobley, J.A.; Campos-Gomez, J. Oxylipins mediate cell-to-cell communication in Pseudomonas aeruginosa. Commun. Biol. 2019, 2, 66. [Google Scholar] [CrossRef]
- Martinez, E.; Campos-Gomez, J. Oxylipins produced by Pseudomonas aeruginosa promote biofilm formation and virulence. Nat. Commun. 2016, 7, 13823. [Google Scholar] [CrossRef]
- Beccaccioli, M.; Pucci, N.; Salustri, M.; Scortichini, M.; Zaccaria, M.; Momeni, B.; Loreti, S.; Reverberi, M.; Scala, V. Fungal and bacterial oxylipins are signals for intra- and inter-cellular communication within plant disease. Front. Plant Sci. 2022, 13, 823233. [Google Scholar] [CrossRef]
- Kurakin, G.F. Bacterial oxylipins: A key to multicellularity and to combating antimicrobial resistance? Priroda 2022, 26–32. [Google Scholar] [CrossRef]
- Estupinan, M.; Alvarez-Garcia, D.; Barril, X.; Diaz, P.; Manresa, A. In silico/in vivo insights into the functional and evolutionary pathway of Pseudomonas aeruginosa oleate-diol synthase. discovery of a new bacterial di-heme cytochrome c peroxidase subfamily. PLoS ONE 2015, 10, e0131462. [Google Scholar] [CrossRef]
- Martinez, E.; Orihuela, C.J.; Campos-Gomez, J. Pseudomonas aeruginosa secretes the oxylipin autoinducer synthases OdsA and OdsB via the Xcp type 2 secretion system. J. Bacteriol. 2022, 204, e0011422. [Google Scholar] [CrossRef]
- Burrows, L.L. Pseudomonas aeruginosa twitching motility: Type IV pili in action. Annu. Rev. Microbiol. 2012, 66, 493–520. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L. Oxidized fatty acids as inter-kingdom signaling molecules. Molecules 2014, 19, 1273–1285. [Google Scholar] [CrossRef]
- Ciofu, O.; Hansen, C.R.; Hoiby, N. Respiratory bacterial infections in cystic fibrosis. Curr. Opin. Pulm. Med. 2013, 19, 251–258. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Cunha, D.A.; Ladriere, L.; Igoillo-Esteve, M.; Bugliani, M.; Marchetti, P.; Cnop, M. In vitro use of free fatty acids bound to albumin: A comparison of protocols. Biotechniques 2015, 58, 228–233. [Google Scholar] [CrossRef]
- Valenza, G.; Tappe, D.; Turnwald, D.; Frosch, M.; Konig, C.; Hebestreit, H.; Abele-Horn, M. Prevalence and antimicrobial susceptibility of microorganisms isolated from sputa of patients with cystic fibrosis. J. Cyst. Fibros. 2008, 7, 123–127. [Google Scholar] [CrossRef]
- Bauernfeind, A.; Bertele, R.M.; Harms, K.; Horl, G.; Jungwirth, R.; Petermuller, C.; Przyklenk, B.; Weisslein-Pfister, C. Qualitative and quantitative microbiological analysis of sputa of 102 patients with cystic fibrosis. Infection 1987, 15, 270–277. [Google Scholar] [CrossRef]
- Subramanian, C.; Frank, M.W.; Batte, J.L.; Whaley, S.G.; Rock, C.O. Oleate hydratase from Staphylococcus aureus protects against palmitoleic acid, the major antimicrobial fatty acid produced by mammalian skin. J. Biol. Chem. 2019, 294, 9285–9294. [Google Scholar] [CrossRef]
- Campbell, I.M.; Crozier, D.N.; Caton, R.B. Abnormal fatty acid composition and impaired oxygen supply in cystic fibrosis patients. Pediatrics 1976, 57, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Radka, C.D.; Frank, M.W.; Simmons, T.S.; Johnson, C.N.; Rosch, J.W.; Rock, C.O. Staphylococcus aureus oleate hydratase produces ligands that activate host PPARa. Front. Cell. Infect. Microbiol. 2024, 14, 1352810. [Google Scholar] [CrossRef]
- Campbell, I.M.; Crozier, D.N.; Trim, A.; Sigrist, J. Cystic fibrosis and bacterial conversion of oleic acid to a cathartic, 10-hydroxystearic acid. Lancet 1987, 2, 107. [Google Scholar] [CrossRef]
- Wallen, L.L.; Benedict, R.G.; Jackson, R.W. The microbiological production of 10-hydroxystearic acid from oleic acid. Arch. Biochem. Biophys. 1962, 99, 249–253. [Google Scholar] [CrossRef]
- Radka, C.D.; Grace, C.R.; Hasdemir, H.S.; Li, Y.; Rodriguez, C.C.; Rodrigues, P.; Oldham, M.L.; Qayyum, M.Z.; Pitre, A.; MacCain, W.J.; et al. The carboxy terminus causes interfacial assembly of oleate hydratase on a membrane bilayer. J. Biol. Chem. 2024, 300, 105627. [Google Scholar] [CrossRef]
- Oldham, M.L.; Zuhaib Qayyum, M.; Kalathur, R.C.; Rock, C.O.; Radka, C.D. Cryo-EM reconstruction of oleate hydratase bound to a phospholipid membrane bilayer. J. Struct. Biol. 2024, 216, 108116. [Google Scholar] [CrossRef]
- Lathram, W.A.; Neff, R.J.; Zalla, A.N.; Brien, J.D.; Subramanian, V.; Radka, C.D. Dissecting the biophysical mechanisms of oleate hydratase association with membranes. Front. Mol. Biosci. 2024, 11, 1504373. [Google Scholar] [CrossRef]
- Radka, C.D.; Batte, J.L.; Frank, M.W.; Young, B.M.; Rock, C.O. Structure and mechanism of Staphylococcus aureus oleate hydratase (OhyA). J. Biol. Chem. 2021, 296, 100252. [Google Scholar] [CrossRef]
- Radka, C.D. Interfacial enzymes enable Gram-positive microbes to eat fatty acids. Membranes 2023, 13, 423. [Google Scholar] [CrossRef]
- Radka, C.D.; Batte, J.L.; Frank, M.W.; Rosch, J.W.; Rock, C.O. Oleate hydratase (OhyA) is a virulence determinant in Staphylococcus aureus. Microbiol. Spectr. 2021, 9, e0154621. [Google Scholar] [CrossRef]
- Volkov, A.; Liavonchanka, A.; Kamneva, O.; Fiedler, T.; Goebel, C.; Kreikemeyer, B.; Feussner, I. Myosin cross-reactive antigen of Streptococcus pyogenes M49 encodes a fatty acid double bond hydratase that plays a role in oleic acid detoxification and bacterial virulence. J. Biol. Chem. 2010, 285, 10353–10361. [Google Scholar] [CrossRef]
- Neff, R.J.; Lages, P.C.; Donworth, S.K.; Brien, J.D.; Radka, C.D. Independent evolution of oleate hydratase clades in Bacillales reflects molecular convergence. Front. Mol. Biosci. 2024, 11, 1485485. [Google Scholar] [CrossRef]
- Volkov, A.; Khoshnevis, S.; Neumann, P.; Herrfurth, C.; Wohlwend, D.; Ficner, R.; Feussner, I. Crystal structure analysis of a fatty acid double-bond hydratase from Lactobacillus acidophilus. Acta Crystallogr. D. Biol. Crystallogr. 2013, 69, 648–657. [Google Scholar] [CrossRef]
- Rosberg-Cody, E.; Liavonchanka, A.; Gobel, C.; Ross, R.P.; O’Sullivan, O.; Fitzgerald, G.F.; Feussner, I.; Stanton, C. Myosin-cross-reactive antigen (MCRA) protein from Bifidobacterium breve is a FAD-dependent fatty acid hydratase which has a function in stress protection. BMC Biochem. 2011, 12, 9. [Google Scholar] [CrossRef]
- Zhao, G.; Kempen, P.J.; Shetty, R.; Gu, L.; Zhao, S.; Ruhdal Jensen, P.; Solem, C. Harnessing cross-resistance-sustainable nisin production from low-value food side streams using a Lactococcus lactis mutant with higher nisin-resistance obtained after prolonged chlorhexidine exposure. Bioresour. Technol. 2022, 348, 126776. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, S.; Back, Y.W.; Lee, K.I.; Choi, H.G.; Kim, H.J. Mycobacterium tuberculosis RpfE-induced prostaglandin E2 in dendritic cells induces Th1/Th17 cell differentiation. Int. J. Mol. Sci. 2021, 22, 7535. [Google Scholar] [CrossRef]
- Pavan Kumar, N.; Moideen, K.; Nancy, A.; Viswanathan, V.; Shruthi, B.S.; Shanmugam, S.; Hissar, S.; Kornfeld, H.; Babu, S. Plasma eicosanoid levels in tuberculosis and tuberculosis-diabetes co-morbidity are associated with lung pathology and bacterial burden. Front. Cell. Infect. Microbiol. 2019, 9, 335. [Google Scholar] [CrossRef]
- Nore, K.G.; Jorgensen, M.J.; Dyrhol-Riise, A.M.; Jenum, S.; Tonby, K. Elevated levels of anti-inflammatory eicosanoids and monocyte heterogeneity in Mycobacterium tuberculosis infection and disease. Front. Immunol. 2020, 11, 579849. [Google Scholar] [CrossRef]
- Kaushal, D. Eicosanoids, prostaglandins, and the progression of tuberculosis. J. Infect. Dis. 2012, 206, 1803–1805. [Google Scholar] [CrossRef]
- Sorgi, C.A.; Soares, E.M.; Rosada, R.S.; Bitencourt, C.S.; Zoccal, K.F.; Pereira, P.A.T.; Fontanari, C.; Brandao, I.; Masson, A.P.; Ramos, S.G.; et al. Eicosanoid pathway on host resistance and inflammation during Mycobacterium tuberculosis infection is comprised by LTB4 reduction but not PGE2 increment. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165574. [Google Scholar] [CrossRef]
- Gretschel, J.; El Hage, R.; Wang, R.; Chen, Y.; Pietzner, A.; Loew, A.; Leineweber, C.G.; Wordemann, J.; Rohwer, N.; Weylandt, K.H.; et al. Harnessing oxylipins and inflammation modulation for prevention and treatment of colorectal cancer. Int. J. Mol. Sci. 2024, 25, 5408. [Google Scholar] [CrossRef]
- Misheva, M.; Johnson, J.; McCullagh, J. Role of oxylipins in the inflammatory-related diseases NAFLD, obesity, and type 2 diabetes. Metabolites 2022, 12, 1238. [Google Scholar] [CrossRef]
- Savchenko, T.; Degtyaryov, E.; Radzyukevich, Y.; Buryak, V. Therapeutic potential of plant oxylipins. Int. J. Mol. Sci. 2022, 23, 14627. [Google Scholar] [CrossRef]
- Peran, J.E.; Salvador-Reyes, L.A. Modified oxylipins as inhibitors of biofilm formation in Staphylococcus epidermidis. Front. Pharmacol. 2024, 15, 1379643. [Google Scholar] [CrossRef]
- Radka, C.D.; Rock, C.O. Mining fatty acid biosynthesis for new antimicrobials. Annu. Rev. Microbiol. 2022, 76, 281–304. [Google Scholar] [CrossRef]
- Niu, M.; Keller, N.P. Co-opting oxylipin signals in microbial disease. Cell. Microbiol. 2019, 21, e13025. [Google Scholar] [CrossRef]
- Alotaibi, A. Oxylipins in health and disease: Overcoming analytical, temporal, and functional obstacles. J. Clin. Res. Bioeth. 2024, 15, 506. [Google Scholar] [CrossRef]
- Gladine, C.; Fedorova, M. The clinical translation of eicosanoids and other oxylipins, although challenging, should be actively pursued. J. Mass Spectrom. Adv. Clin. Lab 2021, 21, 27–30. [Google Scholar] [CrossRef]
- Biagini, D.; Franzini, M.; Oliveri, P.; Lomonaco, T.; Ghimenti, S.; Bonini, A.; Vivaldi, F.; Macera, L.; Balas, L.; Durand, T.; et al. MS-based targeted profiling of oxylipins in COVID-19: A new insight into inflammation regulation. Free Radic. Biol. Med. 2022, 180, 236–243. [Google Scholar] [CrossRef]
- Stanger, L.; Yamaguchi, A.; Yalavarthi, P.; Lambert, S.; Gilmore, D.; Rickenberg, A.; Luke, C.; Kumar, K.; Obi, A.T.; White, A.; et al. The oxylipin analog CS585 prevents platelet activation and thrombosis through activation of the prostacyclin receptor. Blood 2023, 142, 1556–1569. [Google Scholar] [CrossRef]
- Hateley, C.; Olona, A.; Halliday, L.; Edin, M.L.; Ko, J.H.; Forlano, R.; Terra, X.; Lih, F.B.; Beltran-Debon, R.; Manousou, P.; et al. Multi-tissue profiling of oxylipins reveal a conserved up-regulation of epoxide:diol ratio that associates with white adipose tissue inflammation and liver steatosis in obesity. eBioMedicine 2024, 103, 105127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neff, R.J.; Radka, C.D. Exploring Oxylipins in Host–Microbe Interactions and Their Impact on Infection and Immunity. Curr. Issues Mol. Biol. 2025, 47, 190. https://doi.org/10.3390/cimb47030190
Neff RJ, Radka CD. Exploring Oxylipins in Host–Microbe Interactions and Their Impact on Infection and Immunity. Current Issues in Molecular Biology. 2025; 47(3):190. https://doi.org/10.3390/cimb47030190
Chicago/Turabian StyleNeff, Robert J., and Christopher D. Radka. 2025. "Exploring Oxylipins in Host–Microbe Interactions and Their Impact on Infection and Immunity" Current Issues in Molecular Biology 47, no. 3: 190. https://doi.org/10.3390/cimb47030190
APA StyleNeff, R. J., & Radka, C. D. (2025). Exploring Oxylipins in Host–Microbe Interactions and Their Impact on Infection and Immunity. Current Issues in Molecular Biology, 47(3), 190. https://doi.org/10.3390/cimb47030190





