Exploring the Therapeutic Potential of the DOT1L Inhibitor EPZ004777 Using Bioinformatics and Molecular Docking Approaches in Acute Myeloid Leukemia
Abstract
1. Background
2. Methods
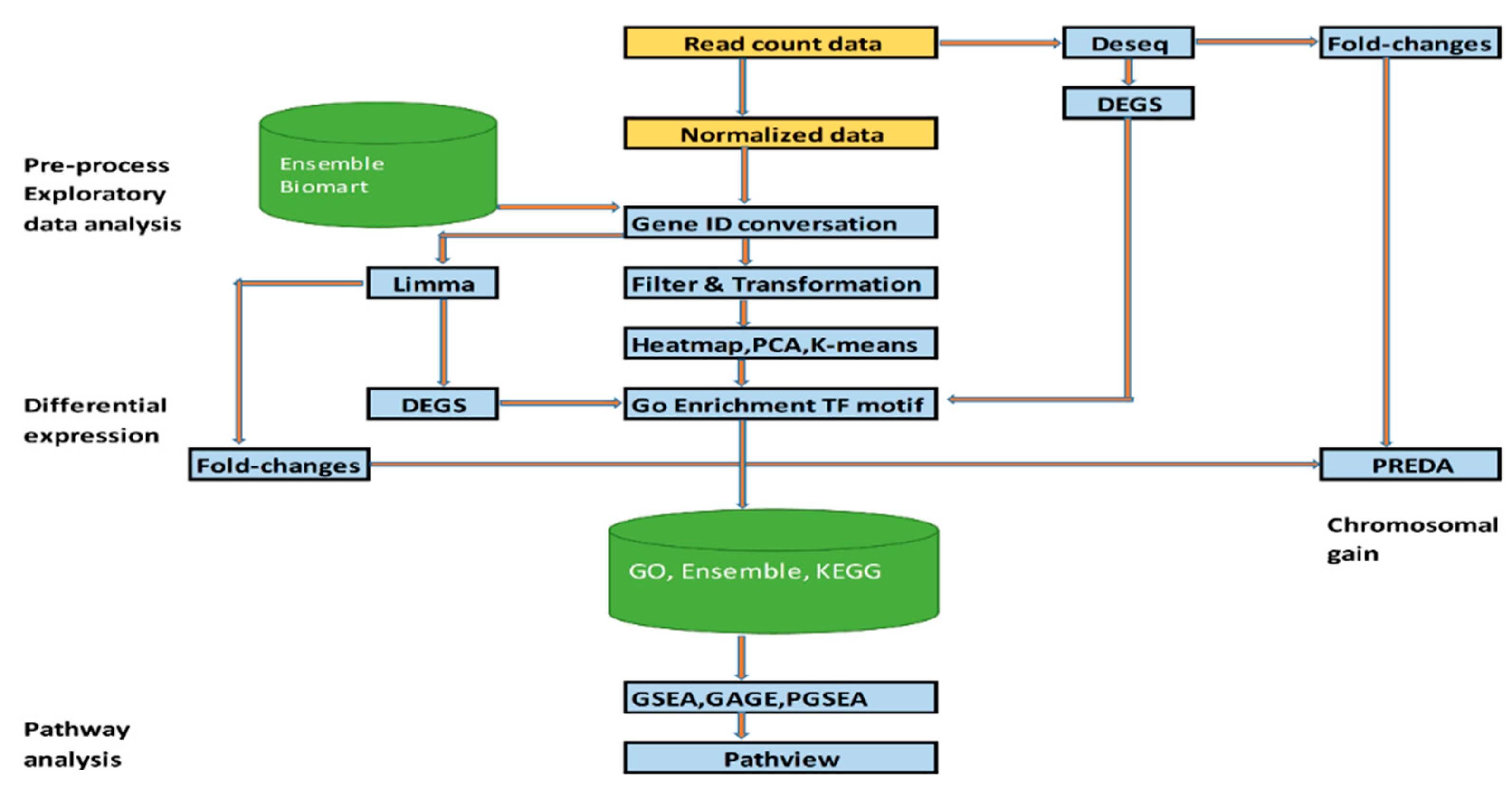
2.1. Dataset and RNA-Seq Data Analysis
2.2. Data Preprocessing
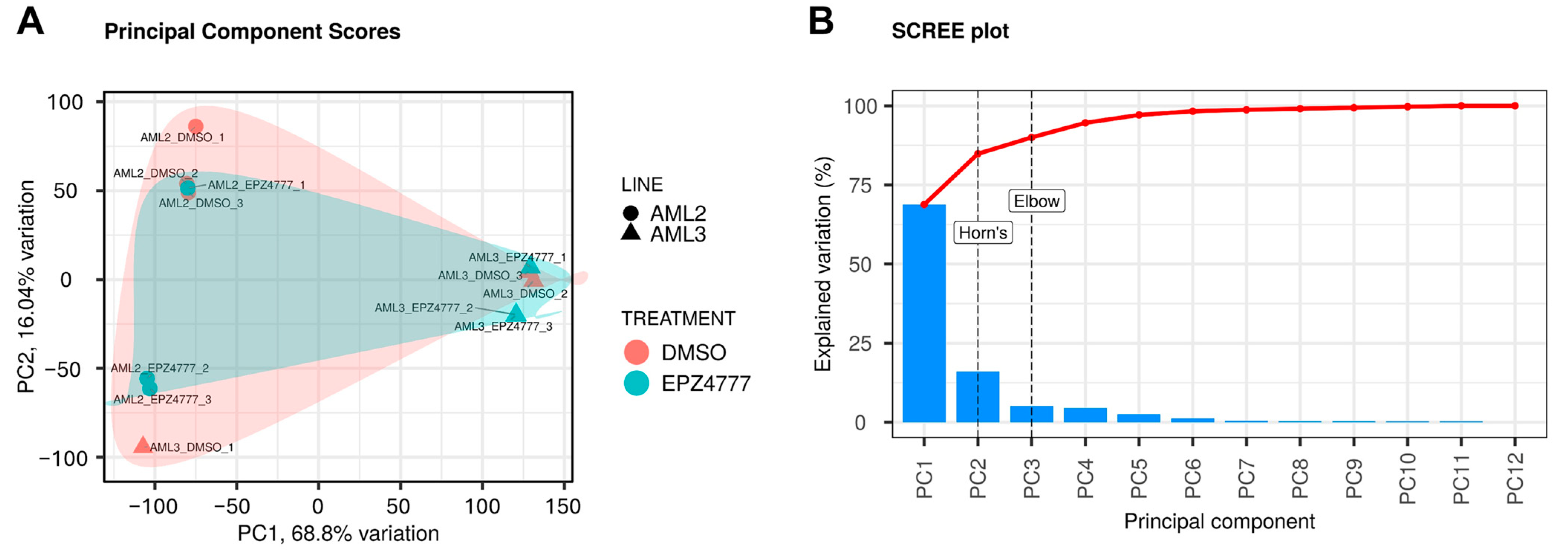
2.3. PCA
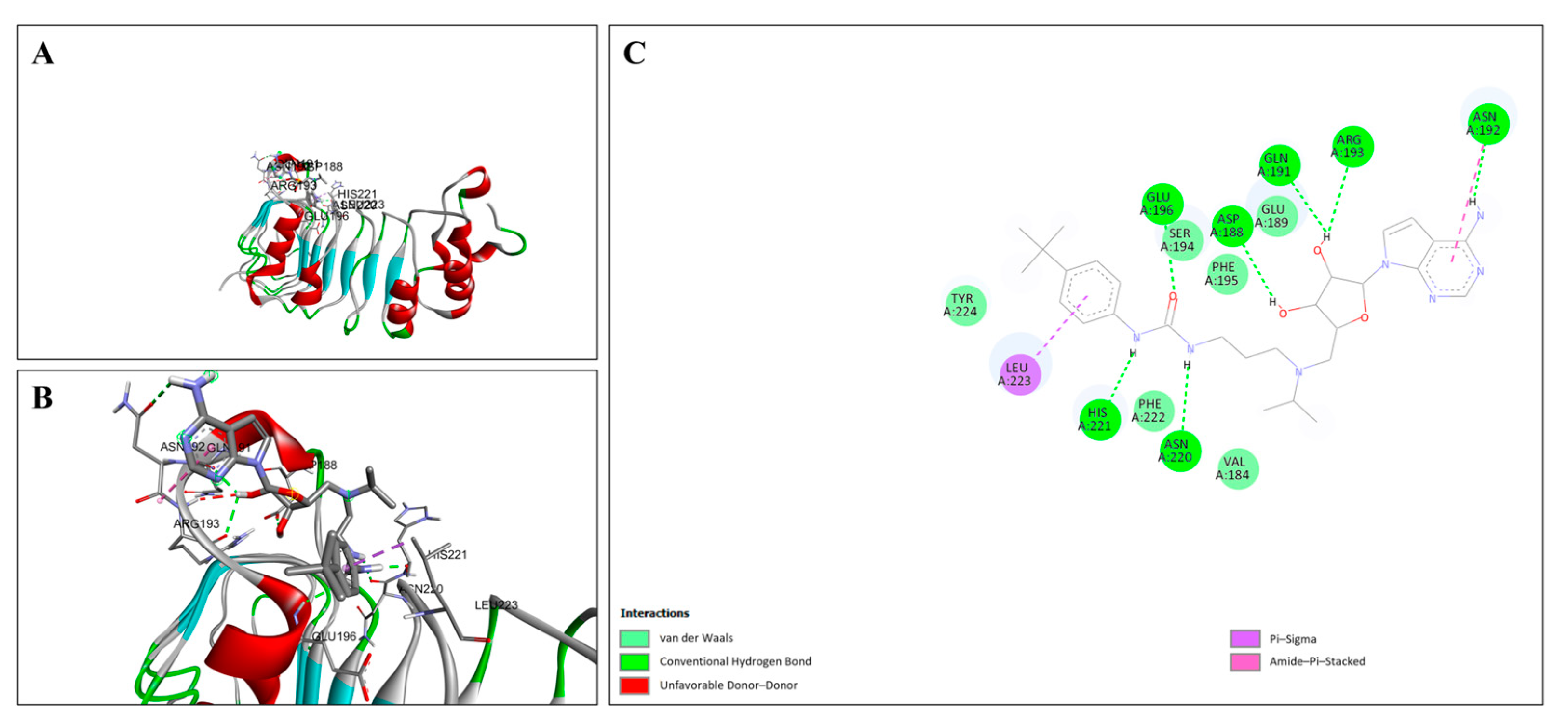
2.4. Molecular Docking Analysis
3. Results
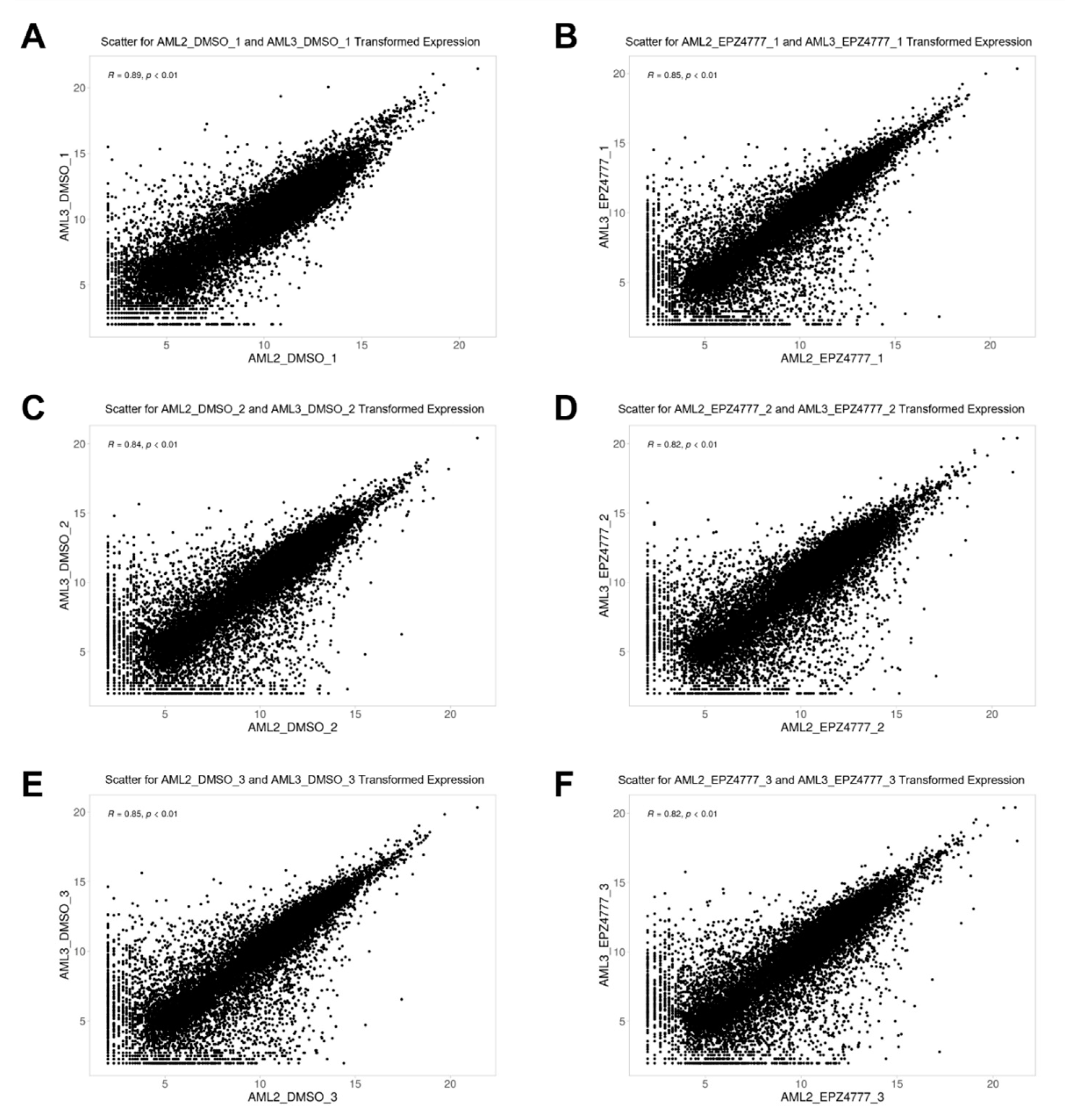
3.1. Correlation Matrix Between Cell Lines
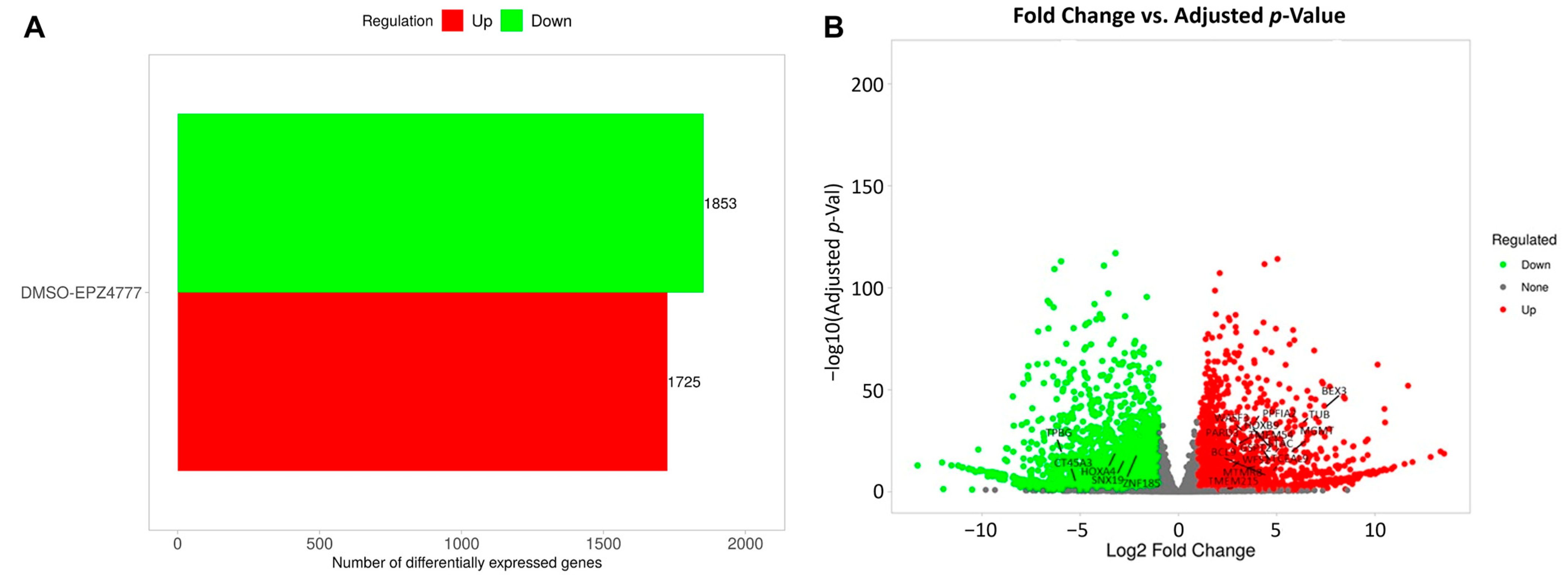
3.2. DEG2 (Differentially Expressed Genes2) Analysis
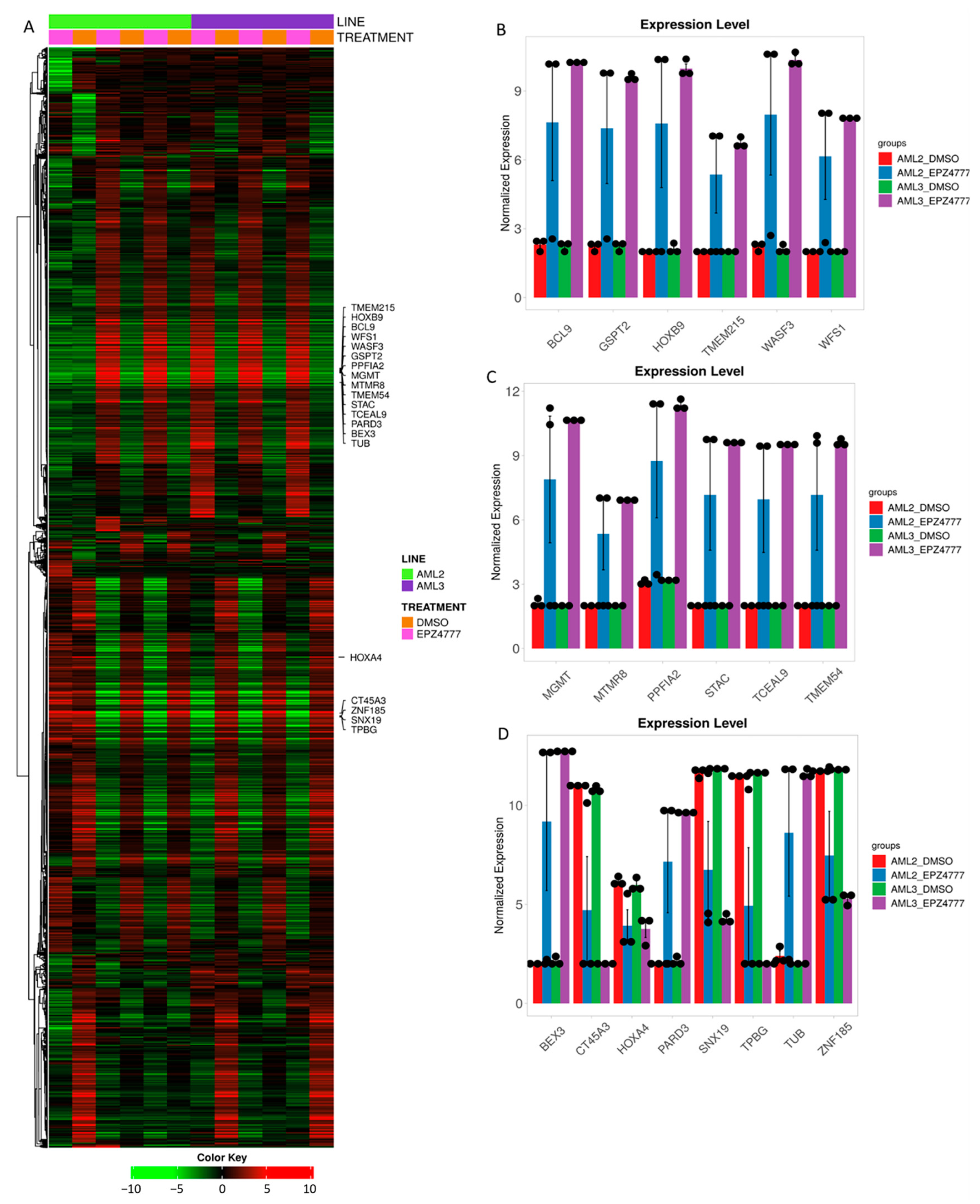
3.3. DEG2 Profiles in AML2 and AML3 Cell Lines Following EPZ004777 Treatment
3.4. Pathway Enrichment Analysis Reveals Differential Regulation of Signaling and Immune Pathways in AML Cell Lines Following EPZ004777 Treatment
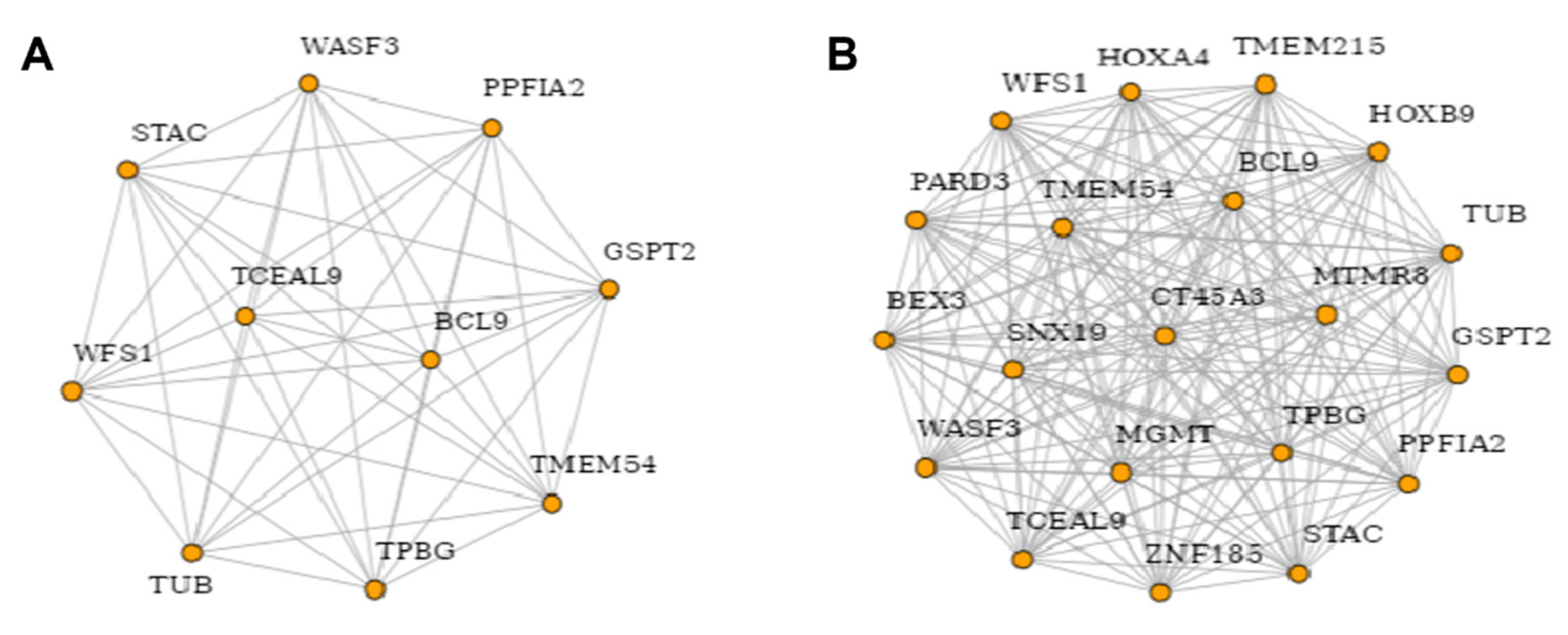
3.5. Network Analysis
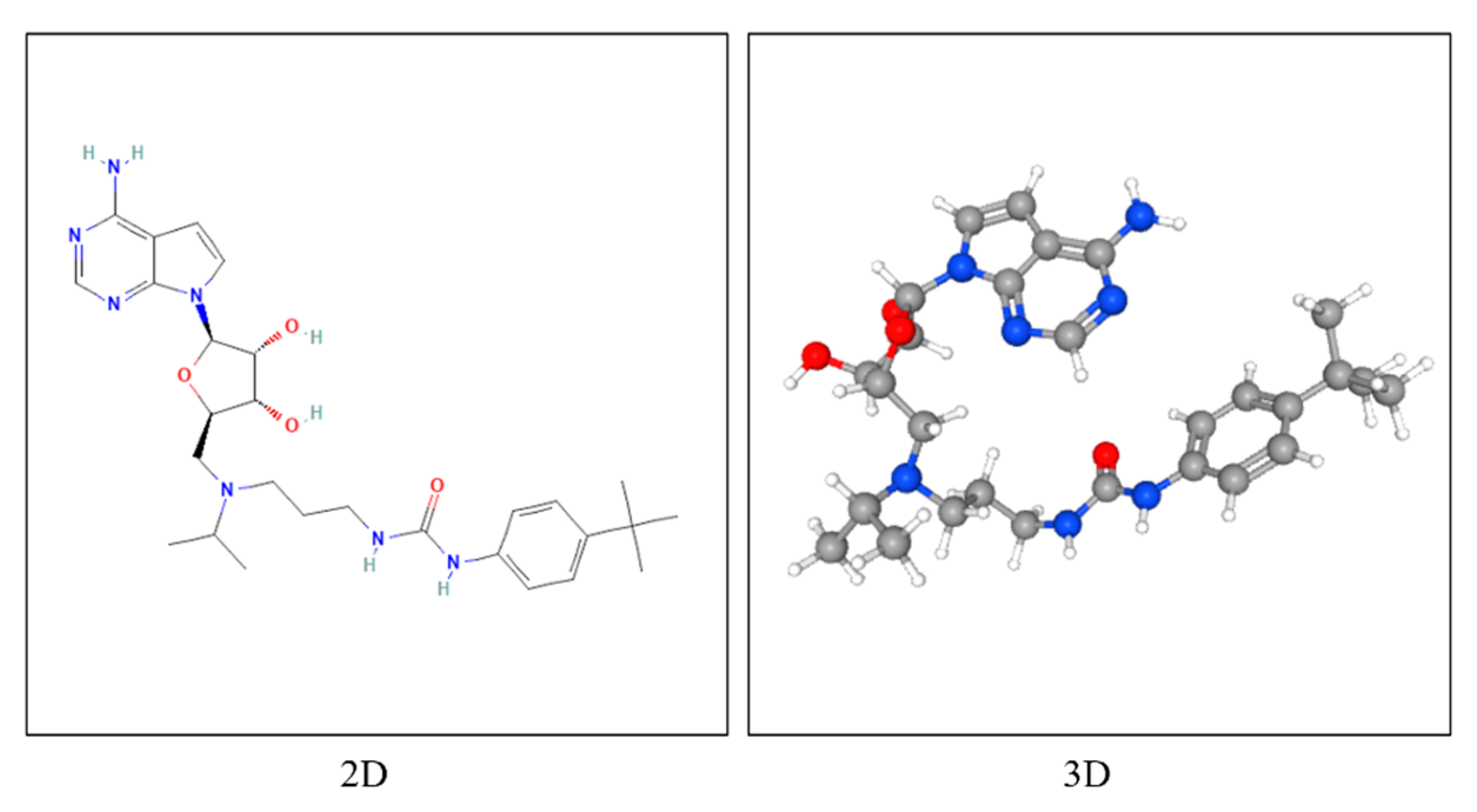
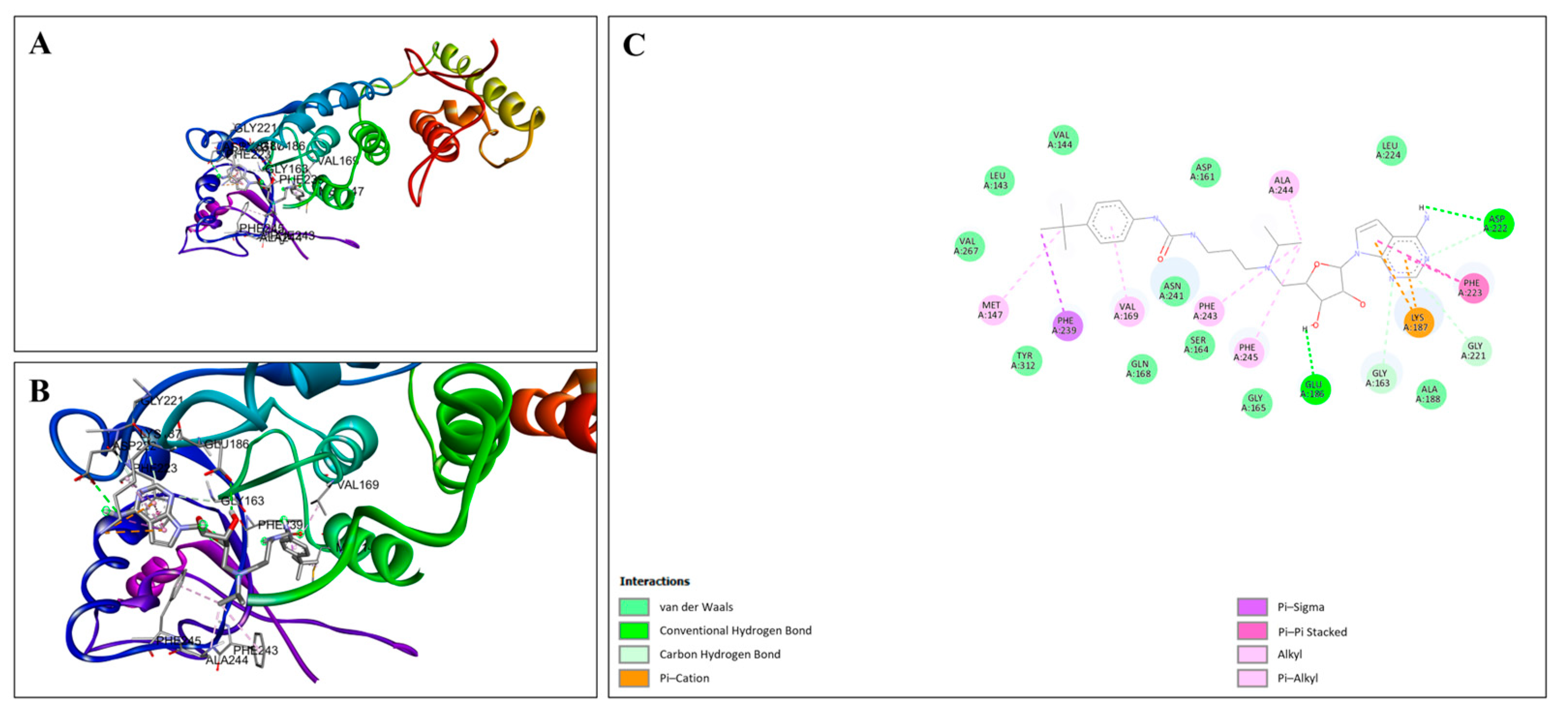
3.6. Molecular Docking of EPZ004777 with Target Proteins
4. Discussion
4.1. Effects of EPZ004777 on Gene Expression
4.2. Pathway Enrichment Implications
4.3. Insights from Molecular Docking Analysis
4.4. Clinical Relevance and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef]
- Borer, R.; Lehner, C.; Eppenberger, H.; Nigg, E. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989, 56, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Grisendi, S.; Mecucci, C.; Falini, B.; Pandolfi, P.P. Nucleophosmin and cancer. Nat. Rev. Cancer 2006, 6, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.M.; Chan, S.M.; Minden, M.D.; Murphy, T.; Shlush, L.I.; Schimmer, A.D. Biological and clinical consequences of NPM1 mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef]
- Brunetti, L.; Gundry, M.C.; Sorcini, D.; Guzman, A.G.; Huang, Y.H.; Ramabadran, R.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Nabet, B.; et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell 2018, 34, 499–512.e9. [Google Scholar] [CrossRef]
- Alcalay, M.; Tiacci, E.; Bergomas, R.; Bigerna, B.; Venturini, E.; Minardi, S.P.; Meani, N.; Diverio, D.; Bernard, L.; Tizzoni, L.; et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc + AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood 2005, 106, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Krivtsov, A.V.; Armstrong, S.A. MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer 2007, 7, 823–833. [Google Scholar] [CrossRef]
- Martelli, M.P.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Pierangeli, S.; Mulas, F.; Pacini, R.; Tabarrini, A.; Pettirossi, V.; Rossi, R.; et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 2015, 125, 3455–3465. [Google Scholar] [CrossRef]
- Nabbouh, A.I.; Hleihel, R.S.; Saliba, J.L.; Karam, M.M.; Hamie, M.H.; Wu, H.-C.J.M.; Berthier, C.P.; Tawil, N.M.; Bonnet, P.-A.A.; Deleuze-Masquefa, C.; et al. Imidazoquinoxaline derivative EAPB0503: A promising drug targeting mutant nucleophosmin 1 in acute myeloid leukemia. Cancer 2017, 123, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Balusu, R.; Fiskus, W.; Rao, R.; Chong, D.G.; Nalluri, S.; Mudunuru, U.; Ma, H.; Chen, L.; Venkannagari, S.; Ha, K.; et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood 2011, 118, 3096–3106. [Google Scholar] [CrossRef] [PubMed]
- Kühn, M.W.M.; Song, E.; Feng, Z.; Sinha, A.; Chen, C.-W.; Deshpande, A.J.; Cusan, M.; Farnoud, N.; Mupo, A.; Grove, C.; et al. Targeting Chromatin Regulators Inhibits Leukemogenic Gene Expression in NPM1 Mutant Leukemia. Cancer Discov. 2016, 6, 1166–1181. [Google Scholar] [CrossRef]
- Daigle, S.R.; Olhava, E.J.; Therkelsen, C.A.; Majer, C.R.; Sneeringer, C.J.; Song, J.; Johnston, L.D.; Scott, M.P.; Smith, J.J.; Xiao, Y.; et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell 2011, 20, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I. Principal Component Analysis. In Encyclopedia of Statistics in Behavioral Science; Everitt, B.S., Howell, D.C., Eds.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Bixby, D.; Perl, A.; Bhatt, V.R.; Altman, J.K.; Appelbaum, F.R.; de Lima, M.; Fathi, A.T.; Foran, J.M.; Gojo, I. NCCN guidelines insights: Acute myeloid leukemia, version 2.2021: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 16–27. [Google Scholar] [CrossRef]
- Colombo, E.; Alcalay, M.; Pelicci, P.G. Nucleophosmin and its complex network: A possible therapeutic target in hematological diseases. Oncogene 2011, 30, 2595–2609. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J. Nucleophosmin1 (NPM1) abnormality in hematologic malignancies, and therapeutic targeting of mutant NPM1 in acute myeloid leukemia. Ther. Adv. Hematol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Cho, M.H.; Park, J.H.; Choi, H.J.; Park, M.K.; Won, H.Y.; Park, Y.J.; Lee, C.H.; Oh, S.H.; Song, Y.S.; Kim, H.S.; et al. DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nat. Commun. 2015, 6, 7821. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, Q.; Li, L.; Yang, J.; Dong, Z.; Cui, H. Silencing or inhibition of H3K79 methyltransferase DOT1L induces cell cycle arrest by epigenetically modulating c-Myc expression in colorectal cancer. Clin. Epigenet. 2019, 11, 199. [Google Scholar] [CrossRef]
- Vatapalli, R.; Sagar, V.; Rodriguez, Y.; Zhao, J.C.; Unno, K.; Pamarthy, S.; Lysy, B.; Anker, J.; Han, H.; Yoo, Y.A.; et al. Histone methyltransferase DOT1L coordinates AR and MYC stability in prostate cancer. Nat. Commun. 2020, 11, 4153. [Google Scholar] [CrossRef]
- Mukai, J.; Suvant, P.; Sato, T.-A. Nerve growth factor-dependent regulation of NADE-induced apoptosis. Vitam. Horm. 2003, 66, 386–404. [Google Scholar]
- Zhang, W.; Barger, C.J.; Link, P.A.; Mhawech-Fauceglia, P.; Miller, A.; Akers, S.N.; Odunsi, K.; Karpf, A.R. DNA hypomethylation-mediated activation of Cancer/Testis Antigen 45 (CT45) genes is associated with disease progression and reduced survival in epithelial ovarian cancer. Epigenetics 2015, 10, 736–748. [Google Scholar] [CrossRef]
- Urasawa, T.; Koizumi, T.; Kimura, K.; Ohta, Y.; Kawasaki, N. Quantitative Proteomics for the Development and Manufacturing of Human-Induced Pluripotent Stem Cell-Derived Neural Stem Cells Using Data-Independent Acquisition Mass Spectrometry. J. Proteome Res. 2023, 22, 1843–1854. [Google Scholar] [CrossRef]
- Spencer, D.H.; Young, M.A.; Lamprecht, T.L.; Helton, N.M.; Fulton, R.; O’Laughlin, M.; Fronick, C.; Magrini, V.; Demeter, R.T.; Miller, C.A. Epigenomic analysis of the HOX gene loci reveals mechanisms that may control canonical expression patterns in AML and normal hematopoietic cells. Leukemia 2015, 29, 1279–1289. [Google Scholar] [CrossRef]
- Furukawa, D.; Chijiwa, T.; Matsuyama, M.; Mukai, M.; Matsuo, E.-I.; Nishimura, O.; Kawai, K.; Suemizu, H.; Hiraoka, N.; Nakagohri, T. Zinc finger protein 185 is a liver metastasis-associated factor in colon cancer patients. Mol. Clin. Oncol. 2014, 2, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.H.; Volkov, P.; Bacos, K.; Dayeh, T.; Hall, E.; Nilsson, E.A.; Ladenvall, C.; Rönn, T.; Ling, C. Genome-wide associations between genetic and epigenetic variation influence mRNA expression and insulin secretion in human pancreatic islets. PLoS Genet. 2014, 10, e1004735. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Kabir, N.N.; Rönnstrand, L. Brain-Expressed X-linked (BEX) proteins in human cancers. Biochim. Biophys. Acta 2015, 1856, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y. 5T4 oncotrophoblast glycoprotein: Janus molecule in life and a novel potential target against tumors. Cell. Mol. Immunol. 2007, 4, 99–104. [Google Scholar]
- Scurr, M.; Bloom, A.; Pembroke, T.; Srinivasan, R.; Brown, C.; Smart, K.; Bridgeman, H.; Davies, M.; Hargest, R.; Phillips, S. Escalating regulation of 5T4-specific IFN-γ+ CD4+ T cells distinguishes colorectal cancer patients from healthy controls and provides a target for in vivo therapy. Cancer Immunol. Res. 2013, 1, 416–425. [Google Scholar] [CrossRef]
- Hole, N.; Stern, P.L. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br. J. Cancer 1988, 57, 239–246. [Google Scholar] [CrossRef]
- Southall, P.J.; Boxer, G.M.; Bagshawe, K.D.; Hole, N.; Bromley, M.; Stern, P.L. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br. J. Cancer 1990, 61, 89–95. [Google Scholar] [CrossRef]
- Starzynska, T.; Marsh, P.J.; Schofield, P.F.; Roberts, S.A.; Myers, K.A.; Stern, P.L. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br. J. Cancer 1994, 69, 899–902. [Google Scholar] [CrossRef]
- Wrigley, E.; McGown, A.T.; Rennison, J.; Swindell, R.; Crowther, D.; Starzynska, T.; Stern, P.L. 5T4 oncofetal antigen expression in ovarian carcinoma. Int. J. Gynecol. Cancer 1995, 5, 269–274. [Google Scholar] [CrossRef]
- Naganuma, H.; Kono, K.; Mori, Y.; Takayoshi, S.; Stern, P.L.; Tasaka, K.; Matsumoto, Y. Oncofetal antigen 5T4 expression as a prognostic factor in patients with gastric cancer. Anticancer. Res. 2002, 22, 1033–1038. [Google Scholar]
- Cullen, P.J. Endosomal sorting and signalling: An emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 2008, 9, 574–582. [Google Scholar] [CrossRef]
- Teasdale, R.D.; Collins, B.M. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem. J. 2012, 441, 39–59. [Google Scholar] [CrossRef]
- Jacques, C.; Baris, O.; Prunier-Mirebeau, D.; Savagner, F.D.R.; Rodien, P.; Rohmer, V.; Franc, B.; Guyetant, S.; Malthiery, Y.; Reynier, P. Two-Step Differential Expression Analysis Reveals a New Set of Genes Involved in Thyroid Oncocytic Tumors. J. Clin. Endocrinol. Metab. 2005, 90, 2314–2320. [Google Scholar] [CrossRef]
- Tyybäkinoja, A.; Saarinen-Pihkala, U.; Elonen, E.; Knuutila, S. Amplified, lost, and fused genes in 11q23-25 amplicon in acute myeloid leukemia, an array-CGH study. Genes Chromosomes Cancer 2006, 45, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gong, A.; Young, C.Y. ZNF185, an actin-cytoskeleton-associated growth inhibitory LIM protein in prostate cancer. Oncogene 2007, 26, 111–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, Y.; Han, X.; Li, J.; Kuang, T.; Lou, W. HEATR1 Deficiency Promotes Chemoresistance via Upregulating ZNF185 and Downregulating SMAD4 in Pancreatic Cancer. J. Oncol. 2020, 2020, 3181596. [Google Scholar] [CrossRef]
- Vanaja, D.K.; Cheville, J.C.; Iturria, S.J.; Young, C.Y. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003, 63, 3877–3882. [Google Scholar] [PubMed]
- Gonzalez, H.E.; Gujrati, M.; Frederick, M.; Henderson, Y.; Arumugam, J.; Spring, P.W.; Mitsudo, K.; Kim, H.W.; Clayman, G.L. Identification of 9 genes differentially expressed in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 754–759. [Google Scholar] [CrossRef]
- Medina, P.P.; Carretero, J.; Ballestar, E.; Angulo, B.; Lopez-Rios, F.; Esteller, M.; Sanchez-Cespedes, M. Transcriptional targets of the chromatin-remodelling factor SMARCA4/BRG1 in lung cancer cells. Hum. Mol. Genet. 2005, 14, 973–982. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, Z.; Lian, X.; Cheng, X.; Liu, B.; Zhang, B.; Wang, H.; Wang, J.; Li, A.; Ren, Z.; et al. High expression of HOXA4 in patients with glioma indicates unfavorable clinical outcomes. Cell Cycle 2022, 21, 2387–2402. [Google Scholar] [CrossRef]
- Bhatlekar, S.; Viswanathan, V.; Fields, J.Z.; Boman, B.M. Overexpression of HOXA4 and HOXA9 genes promotes self-renewal and contributes to colon cancer stem cell overpopulation. J. Cell. Physiol. 2018, 233, 727–735. [Google Scholar] [CrossRef] [PubMed]




| Experimental Design | Cell Line | Treatment |
|---|---|---|
| AML2_EPZ004777_1 | AML2 | EPZ004777 |
| AML2_DMSO_1 | AML2 | DMSO |
| AML2_EPZ004777_2 | AML2 | EPZ004777 |
| AML2_DMSO_2 | AML2 | DMSO |
| AML2_EPZ004777_3 | AML2 | EPZ004777 |
| AML2_DMSO_3 | AML2 | DMSO |
| AML3_EPZ004777_1 | AML3 | EPZ004777 |
| AML3_DMSO_1 | AML3 | DMSO |
| AML3_EPZ004777_2 | AML3 | EPZ004777 |
| AML3_DMSO_2 | AML3 | DMSO |
| AML3_EPZ004777_3 | AML3 | EPZ004777 |
| AML3_DMSO_3 | AML3 | DMSO |
| Protein | Center at (X, Y, Z) | Dimension (Å) |
|---|---|---|
| BEX3 | X: 23.7, Y: 2.969, Z: −16.044 | 100 Å × 100 Å × 100 Å |
| CT45A3 | X: 9.031, Y: 9.16, Z: 5.757 | 100 Å × 100 Å × 100 Å |
| HOXA4 | X: −15.665, Y: 5.339, Z: −0.047 | 60 Å × 60 Å × 60 Å |
| SNX19 | X: 1.419, Y: −1.15, Z: −3.045 | 60 Å × 60 Å × 60 Å |
| TPBG | X: 22.829, Y: −1.218, Z: −12.068 | 40 Å × 40 Å × 40 Å |
| ZNF185 | X: −7.809, Y: 7.241, Z: −1.59 | 60 Å × 60 Å × 60 Å |
| DOT1L | X: 29.253, Y: 57.69, Z: 2.739 | 40 Å × 40 Å × 40 Å |
| Group | Ensembl Id | Symbol | Entrezgene Id | Log2FC Values | Description |
|---|---|---|---|---|---|
| Upregulated | ENSG00000132970 | WASF3 | 10810 | 4.48 | WASP family member 3 |
| Upregulated | ENSG00000139220 | PPFIA2 | 8499 | 4.6 | PTPRF interacting protein alpha 2 |
| Upregulated | ENSG00000189369 | GSPT2 | 23708 | 4.19 | G1 to S phase transition 2 |
| Upregulated | ENSG00000121900 | TMEM54 | 113452 | 4.27 | Transmembrane protein 54 |
| Upregulated | ENSG00000166402 | TUB | 7275 | 5.25 | TUB bipartite transcription factor |
| Upregulated | ENSG00000109501 | WFS1 | 7466 | 3.24 | Wolframin ER transmembrane glycoprotein |
| Upregulated | ENSG00000144681 | STAC | 6769 | 4.32 | SH3 and cysteine rich domain |
| Upregulated | ENSG00000185222 | TCEAL9 | 51186 | 4.4 | Transcription elongation factor A like 9 |
| Upregulated | ENSG00000116128 | BCL9 | 607 | 4.7 | BCL9 transcription coactivator |
| Upregulated | ENSG00000188133 | TMEM215 | 401498 | 2.5 | Transmembrane protein 215 |
| Upregulated | ENSG00000170689 | HOXB9 | 3219 | 4.31 | Homeobox B9 |
| Upregulated | ENSG00000166681 | BEX3 | 27018 | 6.25 | Brain expressed X-linked 3 |
| Upregulated | ENSG00000148498 | PARD3 | 56288 | 4.44 | Par-3 family cell polarity regulator |
| Upregulated | ENSG00000170430 | MGMT | 4255 | 5.01 | O-6-methylguanine-DNA methyltransferase |
| Upregulated | ENSG00000102043 | MTMR8 | 55613 | 2.99 | Myotubularin related protein 8 |
| Downregulated | ENSG00000197576 | HOXA4 | 3201 | −4.3 | Homeobox A4 |
| Downregulated | ENSG00000147394 | ZNF185 | 7739 | −3.66 | Zinc finger protein 185 |
| Downregulated | ENSG00000146242 | TPBG | 7162 | −5.53 | Trophoblast Glycoprotein |
| Downregulated | ENSG00000120451 | SNX19 | 399979 | −4.1 | Sorting nexin 19 |
| Downregulated | ENSG00000269096 | CT45A3 | 441519 | −5.12 | Cancer/testis antigen family 45 member A3 |
| Direction | DEG2 Analysis: AML2 vs. AML3 Pathways | Fold Enriched | nGenes | −log10 (FDR) |
|---|---|---|---|---|
| Down | Rap1 signaling pathway | 2.279 | 43 | 4.8 |
| Cell adhesion molecules | 2.112 | 27 | 1.97 | |
| Neuroactive ligand–receptor interaction | 2.245 | 35 | 3.61 | |
| Melanogenesis | 2.336 | 19 | 2.0 | |
| Proteoglycans in cancer | 2.038 | 38 | 3.18 | |
| Up | Autoimmune thyroid disease | 5.631 | 14 | 12.8 |
| Graft-versus-host disease | 5.456 | 13 | 12.6 | |
| Allograft rejection | 5.406 | 14 | 11.9 | |
| Type I diabetes mellitus | 5.005 | 14 | 10.8 | |
| Proteoglycans in cancer | 5.019 | 28 | 17.5 |
| Protein | Minimum Binding Energy (Kcal/mol) | Key Structural Amino Acid Residues | Hydrogen Bond Distance (Å) | Number of Hydrogen Bonds |
|---|---|---|---|---|
| DOT1L | −9.7 | ASP222 | 2.74 | 5 |
| 3.58 | ||||
| GLY163 | 3.62 | |||
| GLU186 | 2.28 | |||
| GLY221 | 3.63 | |||
| SNX19 | −8.2 | PRO891 | 2.77 | 3 |
| ASN245 | 2.41 | |||
| 2.81 | ||||
| TPBG | −7.7 | GLU196 | 2.11 | 7 |
| ASN192 | 2.60 | |||
| GLN191 | 2.02 | |||
| ARG193 | 2.45 | |||
| ASP188 | 2.35 | |||
| ASN220 | 2.34 | |||
| HIS221 | 1.86 | |||
| ZNF185 | −7.2 | HIS646 | 2.35 | 6 |
| TYR581 | 2.10 | |||
| ILE674 | 2.18 | |||
| 3.53 | ||||
| 3.50 | ||||
| TYR685 | 3.48 | |||
| CT45A3 | −6.7 | HIS180 | 2.48 | 2 |
| 3.36 | ||||
| HOXA4 | −6.5 | LYS199 | 2.49 | 2 |
| LYS200 | 2.98 | |||
| BEX3 | −5.2 | ASN46 | 2.01 | 4 |
| PHE47 | 2.63 | |||
| 2.77 | ||||
| ALA44 | 3.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kivrak, M.; Nalkiran, I.; Sevim Nalkiran, H. Exploring the Therapeutic Potential of the DOT1L Inhibitor EPZ004777 Using Bioinformatics and Molecular Docking Approaches in Acute Myeloid Leukemia. Curr. Issues Mol. Biol. 2025, 47, 173. https://doi.org/10.3390/cimb47030173
Kivrak M, Nalkiran I, Sevim Nalkiran H. Exploring the Therapeutic Potential of the DOT1L Inhibitor EPZ004777 Using Bioinformatics and Molecular Docking Approaches in Acute Myeloid Leukemia. Current Issues in Molecular Biology. 2025; 47(3):173. https://doi.org/10.3390/cimb47030173
Chicago/Turabian StyleKivrak, Mehmet, Ihsan Nalkiran, and Hatice Sevim Nalkiran. 2025. "Exploring the Therapeutic Potential of the DOT1L Inhibitor EPZ004777 Using Bioinformatics and Molecular Docking Approaches in Acute Myeloid Leukemia" Current Issues in Molecular Biology 47, no. 3: 173. https://doi.org/10.3390/cimb47030173
APA StyleKivrak, M., Nalkiran, I., & Sevim Nalkiran, H. (2025). Exploring the Therapeutic Potential of the DOT1L Inhibitor EPZ004777 Using Bioinformatics and Molecular Docking Approaches in Acute Myeloid Leukemia. Current Issues in Molecular Biology, 47(3), 173. https://doi.org/10.3390/cimb47030173






