Multi-Pathway Study for Oxaliplatin Resistance Reduction
Abstract
1. Introduction
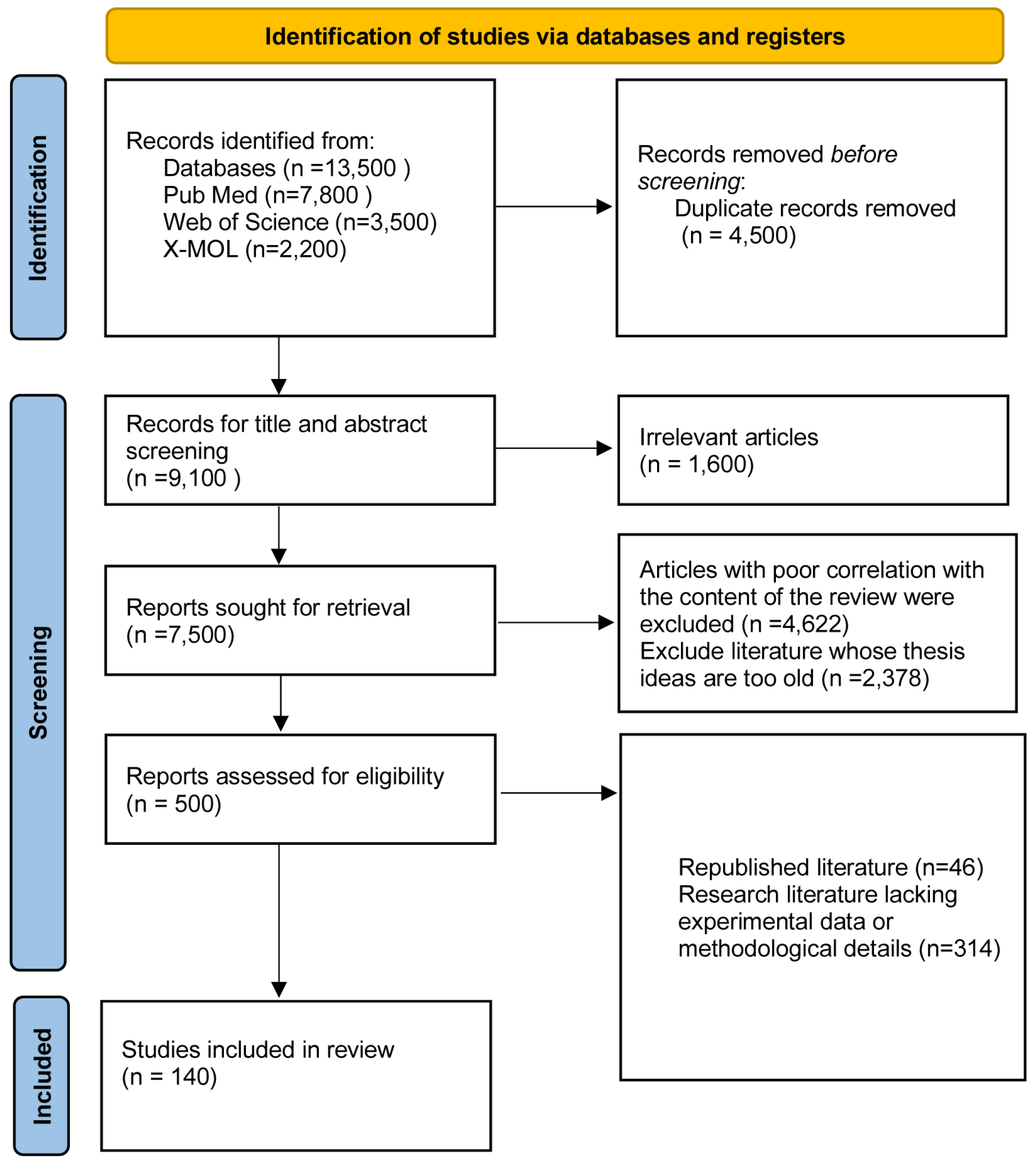
2. Information and Methodology
2.1. Sources of Information
2.1.1. Searchers and Time of the Search
2.1.2. Timeframe for Searching the Literature
2.1.3. Search Databases
2.1.4. Type of Literature Searched
2.1.5. Search Terms
2.1.6. Search Strategy
2.2. Criteria for Inclusion and Exclusion
2.2.1. Criteria for Inclusion
2.2.2. Criteria for Exclusion
2.2.3. Evaluation of the Literature Quality and Data Extraction
3. Development of First-Line Pt Drugs
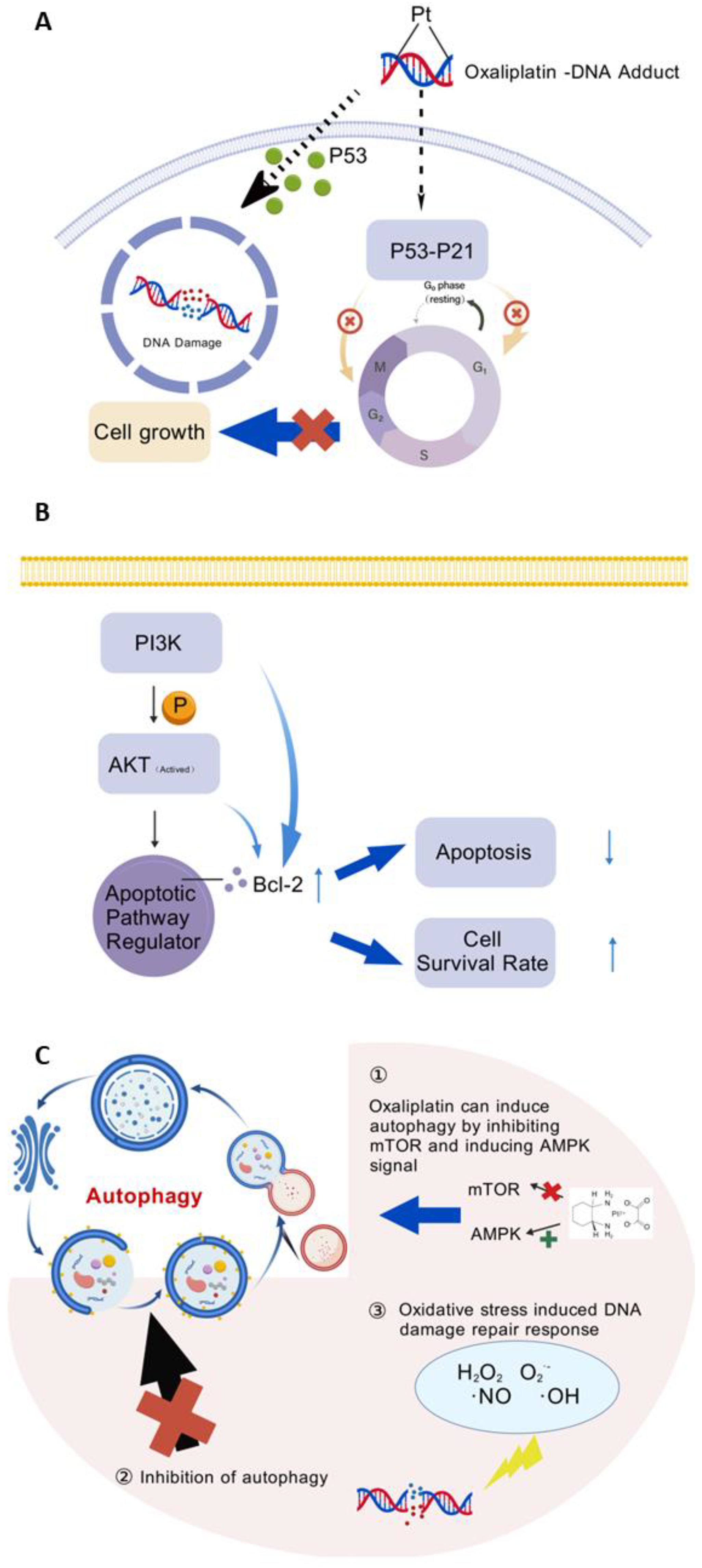
4. Molecular Mechanism of Oxaliplatin

5. Strategies to Enhance Platinum-Based Anticancer Efficiency and Reduce Systemic Resistance
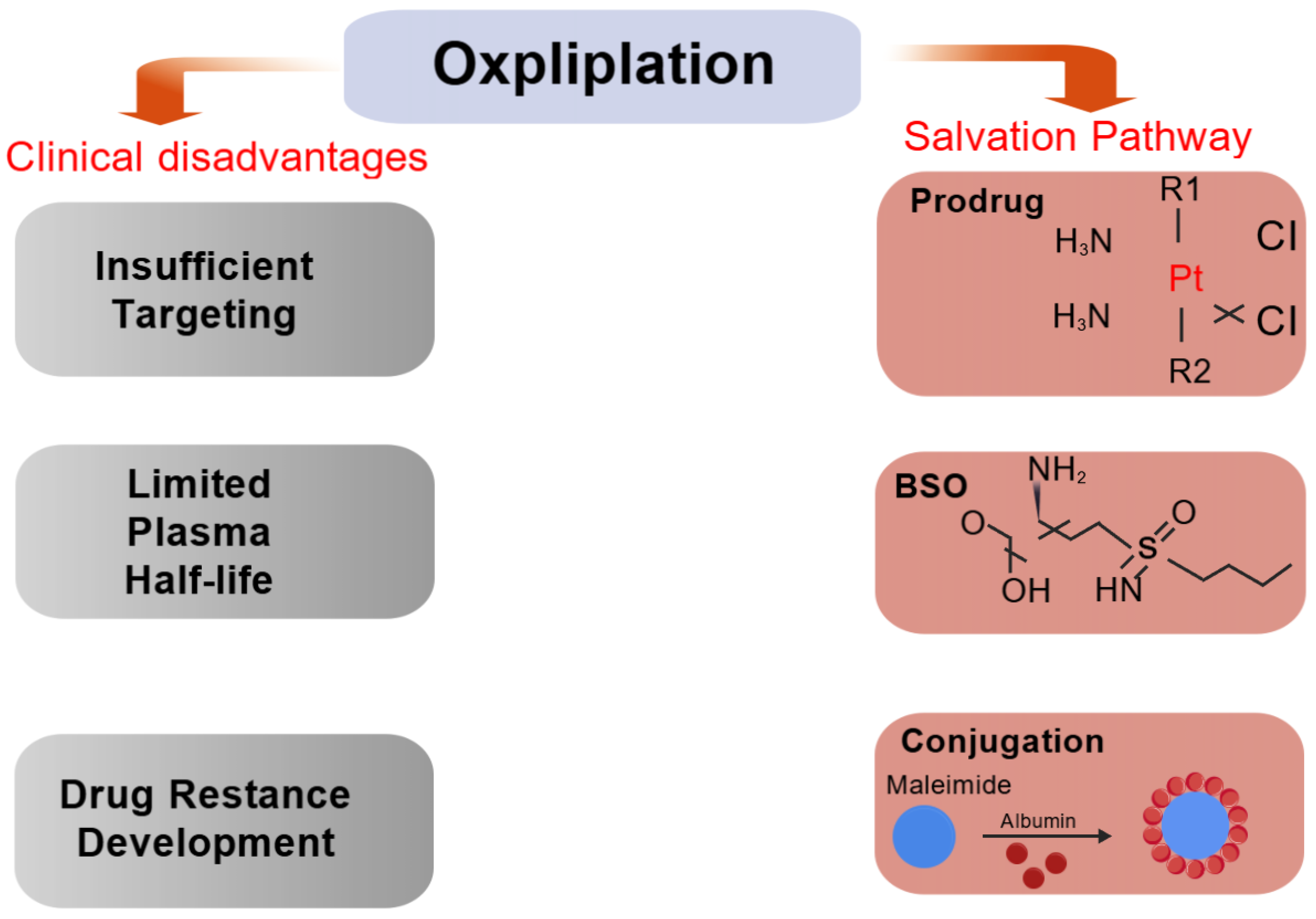
5.1. Glutathione-Based Strategies to Reduce Oxaliplatin Resistance
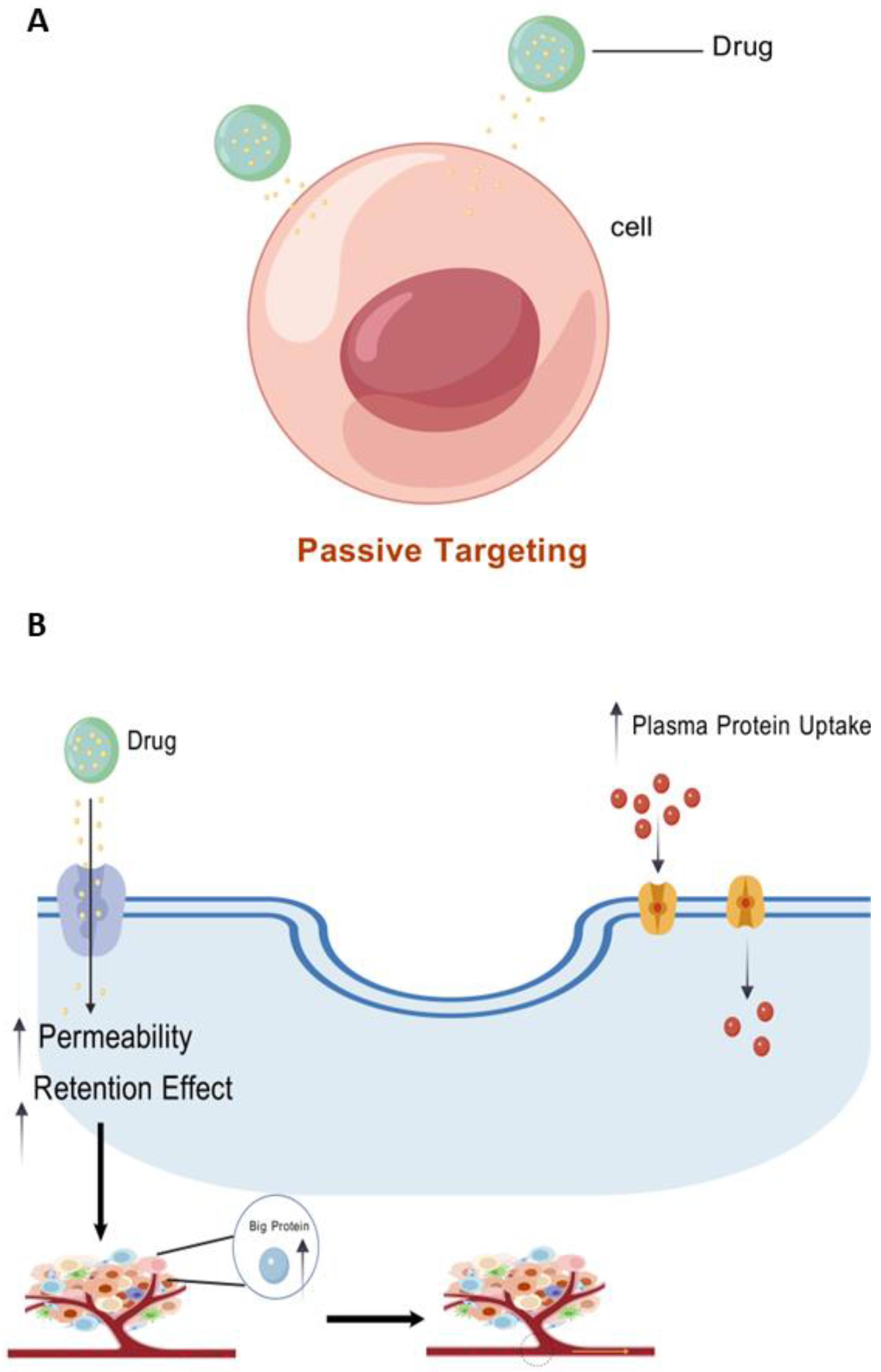
5.1.1. Improving Plasma Half-Life and Tumor Selection
- (1)
- (2)
- Open-window vascularization and decreased lymphatic drainage are caused by enhanced permeability and retention (EPR) effects, which enrich macromolecules in malignant tissues as a result of disarray [51].
5.1.2. Inhibition of Glutathione Synthesis in Malignant Tissues
5.1.3. Reduced Drug Intake or Increased Drug Exportation
5.1.4. Support for GSH Conversion Enhancements
5.2. In Vivo Anticancer Activity
5.3. Discovery of Drug Resistance Genes and Prediction of Drug Resistance
5.3.1. Discovery of Drug Resistance Genes
Platinum Prodrug Design and Its Compounds
Application of CRISPR Screening Technology in Drug Resistance Gene Discovery
5.3.2. Prediction of Drug Resistance
Application Scope of Drug Resistance Prediction Technology Based on Artificial Intelligence Technology
- (1)
- Deep research into genomic data: AI can forecast pathogen resistance to drugs using whole genome sequencing (WGS) and machine learning models. As an example, one study predicted antibiotic resistance in multidrug-resistant Acinetobacter baumannii-utilizing deep neural network (DNN) models in conjunction with WGS and gene expression data, with a prediction accuracy of 98.64% [68].
- (2)
- Analysis of mass spectrometry data: AI techniques can also be used to study mass spectrometry data to predict antimicrobial resistance. For instance, by employing machine learning algorithms to evaluate clinical strains’ mass spectrometry data and combining them with data on drug resistance, helpful classifiers like gradient-enhanced decision trees (LightGBMs) and deep neural network classifiers (MLPs) have been developed, which have greatly boosted the accuracy of drug resistance prediction [69].
- (3)
- Integration of multi-omics data: AI models could more accurately forecast drug resistance by integrating data from genomes, transcriptomics, and metabolomics. In this regard, AI models may predict resistance phenotypes and minimum inhibitory concentrations (MICs) by investigating the relationship between bacterial DNA sequences and antimicrobial resistance phenotypes [69].
Specific Applications of Artificial Intelligence in Drug Resistance Prediction
5.4. Utilizing Biomarkers and Therapeutic Targets to Diminish Oxaliplatin Resistance
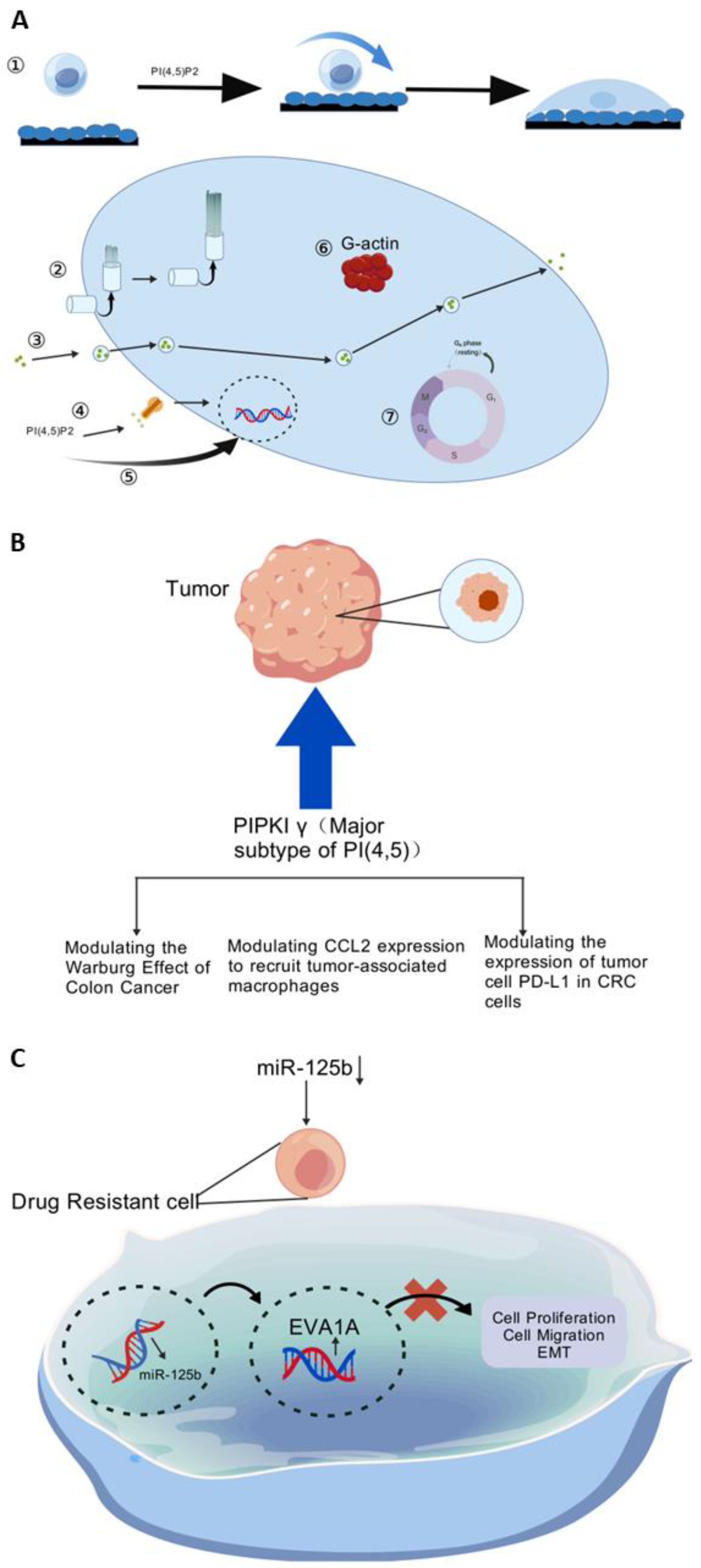
5.4.1. Targeting Kinases to Conquer Oxaliplatin Resistance
5.4.2. Targeting MiRNA to Minimize Oxaliplatin Resistance
5.5. Decreasing Oxaliplatin Resistance by Inducing Anti-Apoptotic Pathway
5.5.1. Inhibition of PLK4
5.5.2. Inhibition of Signaling Pathways
5.6. Autophagy
5.6.1. Oxidative Stress-Induced DNA Damage Repair Response
5.6.2. Autophagy Inhibition
5.7. Impact of DNA Damage Pathways on Increasing Anticancer Efficiency and Reducing Systemic Drug Resistance
5.7.1. DNA Damage Repair
5.7.2. Blocking Homologous Recombination Repair
5.7.3. Nucleus Destruction
6. Combination Therapy
6.1. XL413, a Novel CDC7 Inhibitor
6.2. Oxaliplatin and XL413 Combination Therapy in Cancer
7. Relationship Between Strategies for Enhancing Platinum Medicines’ Anticancer Activity
7.1. Synergies Between the Various Pathways
7.2. Pathways in Antagonistic Relationship
8. Conclusions and Prospects
- (1)
- Glutathione-based
- (2)
- Using biomarkers and therapeutic targets
- (3)
- DNA damage pathway
- (4)
- Combination therapy
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Monneret, C. Platinum anticancer drugs. From serendipity to rational design. Ann. Pharm. Fr. 2011, 69, 286–295. [Google Scholar] [CrossRef]
- Lázaro, A.; Balcells, C.; Quirante, J.; Badia, J.; Baldomà, L.; Ward, J.S.; Rissanen, K.; Font-Bardia, M.; Rodríguez, L.; Crespo, M.; et al. Luminescent PtII and PtIV Platinacycles with Anticancer Activity Against Multiplatinum-Resistant Metastatic CRC and CRPC Cell Models. Chem. A Eur. J. 2020, 26, 1947–1952. [Google Scholar] [CrossRef]
- Chi, R.; Yang, X.; Yang, Z.; Xia, H.; Li, H.; Pan, L.; Hao, J.; Yan, D.; Si, X.; Shi, C.; et al. Tumor microenvironment-responsive hyaluronic acid-oxaliplatin nanoparticles enhanced tumor cell elimination. Polym. Adv. Technol. 2024, 35. [Google Scholar] [CrossRef]
- Sasi, N.K.; Tiwari, K.; Soon, F.-F.; Bonte, D.; Wang, T.; Melcher, K.; Xu, H.E.; Weinreich, M. The Potent Cdc7-Dbf4 (DDK) Kinase Inhibitor XL413 Has Limited Activity in Many Cancer Cell Lines and Discovery of Potential New DDK Inhibitor Scaffolds. PLoS ONE 2014, 9, e113300. [Google Scholar] [CrossRef]
- Chava, S.; Bugide, S.; Malvi, P.; Gupta, R. Co-targeting of specific epigenetic regulators in combination with CDC7 potently inhibit melanoma growth. iScience 2022, 25, 104752. [Google Scholar] [CrossRef]
- Bian, M.; Fan, R.; Zhao, S.; Liu, W. Targeting the Thioredoxin System as a Strategy for Cancer Therapy. J. Med. Chem. 2019, 62, 7309–7321. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2020, 21, 37–50. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Anticancer Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef]
- Margalit, O.; Mamtani, R.; Kopetz, S.; Yang, Y.-X.; Lawrence, Y.R.; Abu-Gazala, S.; Reiss, K.A.; Golan, T.; Halpern, N.; Aderka, D.; et al. Refining the Use of Adjuvant Oxaliplatin in Clinical Stage II or III Rectal Adenocarcinoma. Oncol. 2019, 24, e671–e676. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef]
- Jin, Z.; Zhao-Xia, L.; Fan-Ke, P.; Wen-Juan, Z.; Min-Li, W.; Han-Yi, Z. Progress in the study of reproductive toxicity of platinum-based antitumor drugs and their means of prevention. Front. Pharmacol. 2024, 15, 1327502. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Y.; Lin, X.; Ma, W.; Chen, G.; Li, W.; Wang, X.; Yu, Z. Platinum(iv) prodrugs with long lipid chains for drug delivery and overcoming cisplatin resistance. Chem. Commun. 2018, 54, 5369–5372. [Google Scholar] [CrossRef]
- Turiel-Fernández, D.; Gutiérrez-Romero, L.; Corte-Rodriguez, M.; Bettmer, J.; Montes-Bayón, M. Ultrasmall iron oxide nanoparticles cisplatin (IV) prodrug nanoconjugate: ICP-MS based strategies to evaluate the formation and drug delivery capabilities in single cells. Anal. Chim. Acta 2021, 1159, 338356. [Google Scholar] [CrossRef]
- Gandioso, A.; Shaili, E.; Massaguer, A.; Artigas, G.; González-Cantó, A.; Woods, J.A.; Sadler, P.J.; Marchán, V. An integrin-targeted photoactivatable Pt(iv) complex as a selective anticancer pro-drug: Synthesis and photoactivation studies. Chem. Commun. 2015, 51, 9169–9172. [Google Scholar] [CrossRef]
- Hu, X.; Li, F.; Noor, N.; Ling, D. Platinum drugs: From Pt(II) compounds, Pt(IV) prodrugs, to Pt nanocrystals/nanoclusters. Sci. Bull. 2017, 62, 589–596. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- de la Rosa, S.Y.G.; Diaz, R.M.; Gutiérrez, P.T.V.; Patakfalvi, R.; Coronado, Ó.G. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Chaney, S.G.; Taamma, A.; Cvitkovic, E. Oxaliplatin: A review of preclinical and clinical studies. Ann. Oncol. 1998, 9, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Abu Ammar, A.; Raveendran, R.; Gibson, D.; Nassar, T.; Benita, S. A Lipophilic Pt(IV) Oxaliplatin Derivative Enhances Antitumor Activity. J. Med. Chem. 2016, 59, 9035–9046. [Google Scholar] [CrossRef] [PubMed]
- Chaney, S.G.; Vaisman, A. Specificity of platinum–DNA adduct repair. J. Inorg. Biochem. 1999, 77, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Schoch, S.; Gajewski, S.; Rothfuß, J.; Hartwig, A.; Köberle, B. Comparative Study of the Mode of Action of Clinically Approved Platinum-Based Chemotherapeutics. Int. J. Mol. Sci. 2020, 21, 6928. [Google Scholar] [CrossRef]
- Boulet, M.H.C.; Bolland, H.R.; Hammond, E.M.; Sedgwick, A.C. Oxali(IV)Fluors: Fluorescence Responsive Oxaliplatin(IV) Complexes Identify a Hypoxia-Dependent Reduction in Cancer Cells. J. Am. Chem. Soc. 2023, 145, 12998–13002. [Google Scholar] [CrossRef]
- Reardon, J.T.; Vaisman, A.; Chaney, S.G.; Sancar, A. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and Bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res. 1999, 59, 3968–3971. [Google Scholar]
- Fink, D.; Aebi, S.; Howell, S.B. The role of DNA mismatch repair in drug resistance. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1998, 4, 1–6. [Google Scholar]
- Ahmad, S. Platinum–DNA Interactions and Subsequent Cellular Processes Controlling Sensitivity to Anticancer Platinum Complexes. Chem. Biodivers. 2010, 7, 543–566. [Google Scholar] [CrossRef]
- Graham, M.A.; Lockwood, G.F.; Greenslade, D.; Brienza, S.; Bayssas, M.; Gamelin, E. Clinical pharmacokinetics of oxaliplatin: A critical review. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 1205–1218. [Google Scholar]
- Slyskova, J.; Muniesa-Vargas, A.; da Silva, I.T.; Drummond, R.; Park, J.; Häckes, D.; Poetsch, I.; Ribeiro-Silva, C.; Moretton, A.; Heffeter, P.; et al. Detection of oxaliplatin- and cisplatin-DNA lesions requires different global genome repair mechanisms that affect their clinical efficacy. NAR Cancer 2023, 5, zcad057. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gan, Y.; Liu, J.; Li, J.; Zhou, Z.; Tian, R.; Sun, R.; Liu, J.; Xiao, Q.; Li, Y.; et al. Downregulation of MEIS1 mediated by ELFN1-AS1/EZH2/DNMT3a axis promotes tumorigenesis and oxaliplatin resistance in colorectal cancer. Signal Transduct. Target. Ther. 2022, 7, 87. [Google Scholar] [CrossRef]
- O’Dowd, P.D.; Sutcliffe, D.F.; Griffith, D.M. Oxaliplatin and its derivatives—An overview. Coord. Chem. Rev. 2023, 497. [Google Scholar] [CrossRef]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; de Porras, V.R.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 53, D1670–D1676. [Google Scholar] [CrossRef]
- El-Bendary, M.M.; Saleh, T.S.; Alomari, M.M.; Ali, E.M.M.; Davaasuren, B.; Jaremko, M.; Babgi, B.A. Potential Anticancer Activities and Catalytic Oxidation Efficiency of Platinum(IV) Complex. Molecules 2022, 27, 4406. [Google Scholar] [CrossRef]
- Fabijańska, M.; Kasprzak, M.M.; Ochocki, J. Ruthenium(II) and Platinum(II) Complexes with Biologically Active Aminoflavone Ligands Exhibit In Vitro Anticancer Activity. Int. J. Mol. Sci. 2021, 22, 7568. [Google Scholar] [CrossRef]
- Hu, W.; Fang, L.; Hua, W.; Gou, S. Biotin-Pt (IV)-indomethacin hybrid: A targeting anticancer prodrug providing enhanced cancer cellular uptake and reversing cisplatin resistance. J. Inorg. Biochem. 2017, 175, 47–57. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Q.; Huang, Z.; Yang, X.; Nie, Q.; Hao, W.; Wang, P.G.; Wang, X. Glycosylated Platinum(IV) Complexes as Substrates for Glucose Transporters (GLUTs) and Organic Cation Transporters (OCTs) Exhibited Cancer Targeting and Human Serum Albumin Binding Properties for Drug Delivery. J. Med. Chem. 2017, 60, 5736–5748. [Google Scholar] [CrossRef]
- El-Bendary, M.M.; Saleh, T.S.; Al-Bogami, A.S. Synthesis and structural characterization of a palladium complex as an anticancer agent, and a highly efficient and reusable catalyst for the Heck coupling reaction under ultrasound irradiation: A convenient sustainable green protocol. Polyhedron 2021, 194. [Google Scholar] [CrossRef]
- Seo, H.; Jamison, T.F. Catalytic Generation and Use of Ketyl Radical from Unactivated Aliphatic Carbonyl Compounds. Org. Lett. 2019, 21, 10159–10163. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Ma, X.; McDonald, T.J.; Huang, C.-H.; Sharma, V.K. Overlooked Role of Chromium(V) and Chromium(IV) in Chromium Redox Reactions of Environmental Importance. ACS ES&T Water 2022, 2, 932–942. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Khanaposhtani, M.M.; Larijani, B.; Mahdavi, M. Dimethyl Sulfoxide: Yesterday’s Solvent, Today’s Reagent. Adv. Synth. Catal. 2019, 362, 65–86. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, J.; Chen, D.; Young, D.J.; Li, H.-X. Transition metal-catalyzed olefination of alkyl heteroarenes with alcohols. Tetrahedron 2024, 168, 134306. [Google Scholar] [CrossRef]
- Nikitas, N.F.; Tzaras, D.I.; Triandafillidi, I.; Kokotos, C.G. Photochemical oxidation of benzylic primary and secondary alcohols utilizing air as the oxidant. Green Chem. 2019, 22, 471–477. [Google Scholar] [CrossRef]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin Nanoparticle-Based Drug Delivery Systems. Int. J. Nanomed. 2024, ume 19, 6945–6980. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Fronik, P.; Gutmann, M.; Vician, P.; Stojanovic, M.; Kastner, A.; Heffeter, P.; Pirker, C.; Keppler, B.K.; Berger, W.; Kowol, C.R. A platinum(IV) prodrug strategy to overcome glutathione-based oxaliplatin resistance. Commun. Chem. 2022, 5, 46. [Google Scholar] [CrossRef]
- Davidson, S.M.; Jonas, O.; Keibler, M.A.; Hou, H.W.; Luengo, A.; Mayers, J.R.; Wyckoff, J.; Del Rosario, A.M.; Whitman, M.; Chin, C.R.; et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 2016, 23, 235–241. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ben-Josef, E.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Williams, T.M. Caveolae-Mediated Endocytosis Is Critical for Albumin Cellular Uptake and Response to Albumin-Bound Chemotherapy. Cancer Res. 2017, 77, 5925–5937. [Google Scholar] [CrossRef]
- Sleep, D. Albumin and its application in drug delivery. Expert Opin. Drug Deliv. 2014, 12, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Petruzzella, E.; Braude, J.P.; Aldrich-Wright, J.R.; Gandin, V.; Gibson, D. A Quadruple-Action Platinum(IV) Prodrug with Anticancer Activity Against KRAS Mutated Cancer Cell Lines. Angew. Chem. Int. Ed. Engl. 2017, 56, 11539–11544. [Google Scholar] [CrossRef] [PubMed]
- Yempala, T.; Babu, T.; Karmakar, S.; Nemirovski, A.; Ishan, M.; Gandin, V.; Gibson, D. Expanding the Arsenal of PtIV Anticancer Agents: Multi-action PtIV Anticancer Agents with Bioactive Ligands Possessing a Hydroxy Functional Group. Angew. Chem. Int. Ed. Engl. 2019, 58, 18218–18223. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Man, S.; Xie, L.; Ma, L.; Gao, W. Pathogenesis and therapeutic strategy in platinum resistance lung cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2021, 1876, 188577. [Google Scholar] [CrossRef]
- Wijdeven, R.H.; Pang, B.; Assaraf, Y.G.; Neefjes, J. Old drugs, novel ways out: Drug resistance toward cytotoxic chemotherapeutics. Drug Resist. Updat. 2016, 28, 65–81. [Google Scholar] [CrossRef]
- Aizawa, F.; Kajimoto, H.; Okabayashi, A.; Moriyama, D.; Yagi, K.; Takahashi, S.; Sonoda, Y.; Shibata, T.; Goda, M.; Niimura, T.; et al. Statins ameliorate oxaliplatin- and paclitaxel-induced peripheral neuropathy via glutathione S-transferase. Neurochem. Int. 2024, 180, 105863. [Google Scholar] [CrossRef]
- Lee, K.G.Z.; Babak, M.V.; Weiss, A.; Dyson, P.J.; Nowak-Sliwinska, P.; Montagner, D.; Ang, W.H. Development of an Efficient Dual-Action GST-Inhibiting Anticancer Platinum(IV) Prodrug. ChemMedChem 2018, 13, 1210–1217. [Google Scholar] [CrossRef]
- Zhang, L.H.; Tang, M.; Tao, X.; Shao, Q.; Thomas, V.; Shimizu, S.; Kasano, M.; Ishikawa, Y.; Inukai, T.; Nomura, D.K. Covalent Targeting of Glutamate Cysteine Ligase to Inhibit Glutathione Synthesis**. ChemBioChem 2023, 24, e202300371. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Gouveia-Fernandes, S.; Gonçalves, L.G.; Nunes, C.; Faustino, I.; Silva, F.; Félix, A.; Pereira, S.A.; Serpa, J. HNF1β drives glutathione (GSH) synthesis underlying intrinsic carboplatin resistance of ovarian clear cell carcinoma (OCCC). Tumor Biol. 2015, 37, 4813–4829. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Hamilton, T.C.; LaCreta, F.P.; Gallo, J.M.; Kilpatrick, D.; Halbherr, T.; Brennan, J.; Bookman, M.A.; Hoffman, J.; Young, R.C.; et al. Phase I trial of buthionine sulfoximine in combination with melphalan in patients with cancer. J. Clin. Oncol. 1996, 14, 249–256. [Google Scholar] [CrossRef]
- Oliveira, C.d.R.; Pereira, J.C.; Ibiapina, A.B.; Martins, I.R.R.; Sousa, J.M.d.C.e.; Ferreira, P.M.P.; da Silva, F.C.C. Buthionine sulfoximine and chemoresistance in cancer treatments: A systematic review with meta-analysis of preclinical studies. J. Toxicol. Environ. Health Part B 2023, 26, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Galvez, L.; Rusz, M.; Schwaiger-Haber, M.; El Abiead, Y.; Hermann, G.; Jungwirth, U.; Berger, W.; Keppler, B.K.; Jakupec, M.A.; Koellensperger, G. Preclinical studies on metal based anticancer drugs as enabled by integrated metallomics and metabolomics. Metallomics 2019, 11, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Nasr, R.; Lorendeau, D.; Khonkarn, R.; Dury, L.; Pérès, B.; Boumendjel, A.; Cortay, J.-C.; Falson, P.; Chaptal, V.; Baubichon-Cortay, H. Molecular analysis of the massive GSH transport mechanism mediated by the human Multidrug Resistant Protein 1/ABCC1. Sci. Rep. 2020, 10, 7616. [Google Scholar] [CrossRef] [PubMed]
- Mohn, C.; Häcker, H.-G.; Hilger, R.A.; Gütschow, M.; Jaehde, U. Defining the role of MRP-mediated efflux and glutathione in detoxification of oxaliplatin. Die Pharm. 2013, 68, 622–627. [Google Scholar]
- Novohradsky, V.; Babu, T.; Kostrhunova, H.; Plaskow, M.; Markova, L.; Acharya, S.; Gibson, D.; Brabec, V. Cisplatin-eugenol Pt(IV) prodrugs target colon cancer stem cells: A novel strategy for enhanced anticancer efficacy. Biomed. Pharmacother. 2025, 183, 117854. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, P.; Wang, C.; Sun, Y.; Gao, C. Recent updates in nanoscale delivery systems of platinum(IV) antitumor prodrugs. Coord. Chem. Rev. 2024, 508, 215774. [Google Scholar] [CrossRef]
- Zhong, C.; Jiang, W.-J.; Yao, Y.; Li, Z.; Li, Y.; Wang, S.; Wang, X.; Zhu, W.; Wu, S.; Wang, J.; et al. CRISPR screens reveal convergent targeting strategies against evolutionarily distinct chemoresistance in cancer. Nat. Commun. 2024, 15, 5502. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Zhuang, Y.; Wu, Y.; Shi, S.; Sun, Q.; He, F.; Liang, S.; Wang, J.; Draz, M.S.; et al. Neural network-based predictions of antimicrobial resistance phenotypes in multidrug-resistant Acinetobacter baumannii from whole genome sequencing and gene expression. Antimicrob. Agents Chemother. 2024, 68, e0144624. [Google Scholar] [CrossRef]
- Cesaro, A.; Hoffman, S.C.; Das, P.; de la Fuente-Nunez, C. Challenges and applications of artificial intelligence in infectious diseases and antimicrobial resistance. npj Antimicrob. Resist. 2025, 3, 2. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Cai, C.; Ding, Y.; Kim, R.S.; Lipchik, C.; Gavin, P.G.; Yothers, G.; Allegra, C.J.; Petrelli, N.J.; et al. Machine Learning Predicts Oxaliplatin Benefit in Early Colon Cancer. J. Clin. Oncol. 2024, 42, 1520–1530. [Google Scholar] [CrossRef]
- Khanagar, S.B.; Al-Ehaideb, A.; Vishwanathaiah, S.; Maganur, P.C.; Patil, S.; Naik, S.; Baeshen, H.A.; Sarode, S.S. Scope and performance of artificial intelligence technology in orthodontic diagnosis, treatment planning, and clinical decision-making—A systematic review. J. Dent. Sci. 2020, 16, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Gori, V.; Bonazzina, E.; Amatu, A.; Tosi, F.; Bencardino, K.; Ruggieri, L.; Patelli, G.; Arena, S.; Bardelli, A.; et al. Oxaliplatin retreatment in metastatic colorectal cancer: Systematic review and future research opportunities. Cancer Treat. Rev. 2020, 91, 102112. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gong, T.; Wang, Z.; Wang, Z.; Lin, X.; Chen, S.; Sun, C.; Zhao, W.; Kong, Y.; Ai, H.; et al. Colorectal cancer organoid models uncover oxaliplatin-resistant mechanisms at single cell resolution. Cell. Oncol. 2022, 45, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, H.; Zhao, W.; Ge, X.; Huang, W.; Lin, F.; Tang, W.; Li, A.; Liu, S.; Li, R.-K.; et al. Targeting type Iγ phosphatidylinositol phosphate kinase overcomes oxaliplatin resistance in colorectal cancer. Theranostics 2022, 12, 4386–4398. [Google Scholar] [CrossRef]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2018, 506, 307–314. [Google Scholar] [CrossRef]
- Wu, Z.; Li, X.; Sunkara, M.; Spearman, H.; Morris, A.J.; Huang, C. PIPKIγ Regulates Focal Adhesion Dynamics and Colon Cancer Cell Invasion. PLoS ONE 2011, 6, e24775. [Google Scholar] [CrossRef]
- Thapa, N.; Tan, X.; Choi, S.; Wise, T.; Anderson, R.A. PIPKIγ and talin couple phosphoinositide and adhesion signaling to control the epithelial to mesenchymal transition. Oncogene 2016, 36, 899–911. [Google Scholar] [CrossRef]
- Ye, Y.; Xie, Y.; Pei, L.; Jiang, Z.; Wu, C.; Liu, S. Platycodin D induces neutrophil apoptosis by downregulating PD-L1 expression to inhibit breast cancer pulmonary metastasis. Int. Immunopharmacol. 2023, 115, 109733. [Google Scholar] [CrossRef]
- Shen, B.; Huang, D.; Ramsey, A.J.; Ig-Izevbekhai, K.; Zhang, K.; Lajud, S.A.; O’malley, B.W.; Li, D. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma. Br. J. Cancer 2019, 122, 640–647. [Google Scholar] [CrossRef]
- Peng, W.; Huang, W.; Ge, X.; Xue, L.; Zhao, W.; Xue, J. Type Iγ phosphatidylinositol phosphate kinase promotes tumor growth by facilitating Warburg effect in colorectal cancer. EBioMedicine 2019, 44, 375–386. [Google Scholar] [CrossRef]
- Xue, J.; Ge, X.; Zhao, W.; Xue, L.; Dai, C.; Lin, F.; Peng, W. PIPKIγ Regulates CCL2 Expression in Colorectal Cancer by Activating AKT-STAT3 Signaling. J. Immunol. Res. 2019, 2019, 3690561. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Chen, C.; Qi, M.; Huang, Y.; Wang, L.; Gao, Y.; Dong, H.; Ling, K. Type Iγ phosphatidylinositol phosphate kinase regulates PD-L1 expression by activating NF-κB. Oncotarget 2017, 8, 42414–42427. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Li, C.; Dong, S. LINC00963/miR-4458 regulates the effect of oxaliplatin in gastric cancer by mediating autophagic flux through targeting of ATG16L1. Sci. Rep. 2021, 11, 20951. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.-W.; Li, D.-D.; Chen, X.; Li, X.-L.; He, Y.-P.; Guo, L.-H.; Liu, L.-N.; Sun, L.-P.; Zhang, X.-P. Correction to: MicroRNA-125b reverses oxaliplatin resistance in hepatocellular carcinoma by negatively regulating EVA1A mediated autophagy. Cell Death Dis. 2018, 9, 843. [Google Scholar] [CrossRef]
- Marjaneh, R.M.; Khazaei, M.; Ferns, G.A.; Avan, A.; Aghaee-Bakhtiari, S.H. MicroRNAs as potential therapeutic targets to predict responses to oxaliplatin in colorectal cancer: From basic evidence to therapeutic implication. IUBMB Life 2019, 71, 1428–1441. [Google Scholar] [CrossRef]
- Ghanbarian, M.; Afgar, A.; Yadegarazari, R.; Najafi, R.; Teimoori-Toolabi, L. Through oxaliplatin resistance induction in colorectal cancer cells, increasing ABCB1 level accompanies decreasing level of miR-302c-5p, miR-3664-5p and miR-129-5p. Biomed. Pharmacother. 2018, 108, 1070–1080. [Google Scholar] [CrossRef]
- Fang, T.; Xie, X.; Lu, W.; Hong, Z.; Peng, W.; Zhou, J.; Wang, M.; Yao, B. Patient-Derived Organoids on a Microarray for Drug Resistance Study in Breast Cancer. Anal. Chem. 2024, 96, 18384–18391. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, H.; Ma, W.; Wu, K.; Peng, G.; Ou, T.; Wu, S. Down-regulation of Polo-like kinase 4 (PLK4) induces G1 arrest via activation of the p38/p53/p21 signaling pathway in bladder cancer. FEBS Open Bio 2021, 11, 2631–2646. [Google Scholar] [CrossRef]
- Nakamura, T.; Saito, H.; Takekawa, M. SAPK pathways and p53 cooperatively regulate PLK4 activity and centrosome integrity under stress. Nat. Commun. 2013, 4, 1775. [Google Scholar] [CrossRef]
- Qian, X.-L.; Zhou, F.; Xu, S.; Jiang, J.; Chen, Z.-P.; Wang, S.-K.; Zuo, Y.; Ni, C. MiR-454-3p Promotes Oxaliplatin Resistance by Targeting PTEN in Colorectal Cancer. Front. Oncol. 2021, 11, 638537. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, N.; Jiang, G.; Sun, H.; Yu, D. Nobiletin sensitizes colorectal cancer cells to oxaliplatin by PI3K Akt MTOR pathway. Front. Biosci. 2019, 24, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Nkwocha, J.; Hawkins, E.; Pei, X.; Parker, R.E.; Kmieciak, M.; Leverson, J.D.; Sampath, D.; Ferreira-Gonzalez, A.; Grant, S. Cotargeting BCL-2 and PI3K Induces BAX-Dependent Mitochondrial Apoptosis in AML Cells. Cancer Res. 2018, 78, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- García-Murria, M.J.; Duart, G.; Grau, B.; Diaz-Beneitez, E.; Rodríguez, D.; Mingarro, I.; Martínez-Gil, L. Viral Bcl2s’ transmembrane domain interact with host Bcl2 proteins to control cellular apoptosis. Nat. Commun. 2020, 11, 6056. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, S.; Han, Q.; Chu, T.; Wang, H.; Yu, L.; Zhang, W.; Liu, J.; Liang, W.; Xue, J.; et al. Liensinine sensitizes colorectal cancer cells to oxaliplatin by targeting HIF-1α to inhibit autophagy. Phytomedicine 2024, 129, 155647. [Google Scholar] [CrossRef]
- Yang, J.-H.; Yu, K.; Si, X.-K.; Li, S.; Cao, Y.-J.; Li, W.; Zhang, J.-X. Liensinine inhibited gastric cancer cell growth through ROS generation and the PI3K/AKT pathway. J. Cancer 2019, 10, 6431–6438. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Zhou, L.; Han, J.; Liu, R.; Zhang, H.; Ning, T.; Gao, Z.; Liu, B.; Chen, X.; et al. The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer. Theranostics 2020, 10, 1981–1996. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Zhang, X.; Cai, H.; Zhang, C.; Qu, H.; Liu, L.; Zhang, M.; Fu, J.; Zhang, J.; et al. Oxidative stress activates NORAD expression by H3K27ac and promotes oxaliplatin resistance in gastric cancer by enhancing autophagy flux via targeting the miR-433-3p. Cell Death Dis. 2021, 12, 90. [Google Scholar] [CrossRef]
- Riddell, I.A.; Lippard, S.J. Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions. Met. Ions Life Sci. 2018, 18, 1–42. [Google Scholar]
- Fu, X.-T.; Song, K.; Zhou, J.; Shi, Y.-H.; Liu, W.-R.; Shi, G.-M.; Gao, Q.; Wang, X.-Y.; Ding, Z.-B.; Fan, J. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019, 19, 71. [Google Scholar] [CrossRef]
- Qiu, Z.; He, S.; Lu, B.; Sun, Y.; Zhang, T.; Lv, W.; Shen, D. The E3 ubiquitin ligase RNF135 modulates chemotherapy resistance to oxaliplatin for colorectal cancer by modulating autophagy. Tissue Cell 2023, 86, 102282. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Z.; Zhu, S.; Lu, H.; Peng, D.; Soutto, M.; Naz, H.; Peek, R.; Xu, H.; Zaika, A.; et al. PRDX2 protects against oxidative stress induced by H. pylori and promotes resistance to cisplatin in gastric cancer. Redox Biol. 2019, 28, 101319. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.; Aguilar-Medina, M.; Lizárraga-Verdugo, E.; Avendaño-Félix, M.; Silva-Benítez, E.; López-Camarillo, C.; Ramos-Payán, R. LncRNAs as Regulators of Autophagy and Drug Resistance in Colorectal Cancer. Front. Oncol. 2019, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Molejon, M.I.; Swayden, M.; Fanale, D.; Bintz, J.; Gayet, O.; Soubeyran, P.; Iovanna, J. Chloroquine plays a cell-dependent role in the response to treatment of pancreatic adenocarcinoma. Oncotarget 2018, 9, 30837–30846. [Google Scholar] [CrossRef]
- Nie, Y.; Liang, X.; Liu, S.; Guo, F.; Fang, N.; Zhou, F. WASF3 Knockdown Sensitizes Gastric Cancer Cells to Oxaliplatin by Inhibiting ATG12-Mediated Autophagy. Am. J. Med. Sci. 2020, 359, 287–295. [Google Scholar] [CrossRef]
- Wible, D.J.; Chao, H.-P.; Tang, D.G.; Bratton, S.B. ATG5 cancer mutations and alternative mRNA splicing reveal a conjugation switch that regulates ATG12–ATG5-ATG16L1 complex assembly and autophagy. Cell Discov. 2019, 5, 42. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Liao, W.; Feng, M.; Zhang, M.; Wu, Q.; Zhou, K.; Wen, F.; Lei, W.; Zhang, N.; et al. The role of non-apoptotic cell death in the treatment and drug-resistance of digestive tumors. Exp. Cell Res. 2021, 405, 112678. [Google Scholar] [CrossRef]
- Hao, C.; Liu, G.; Tian, G. Autophagy inhibition of cancer stem cells promotes the efficacy of cisplatin against non-small cell lung carcinoma. Ther. Adv. Respir. Dis. 2019, 13, 1753466619866097. [Google Scholar] [CrossRef]
- Lan, H.; Liu, Y.; Liu, J.; Wang, X.; Guan, Z.; Du, J.; Jin, K. Tumor-Associated Macrophages Promote Oxaliplatin Resistance via METTL3-Mediated m6A of TRAF5 and Necroptosis in Colorectal Cancer. Mol. Pharm. 2021, 18, 1026–1037. [Google Scholar] [CrossRef]
- Nechay, M.; Wang, D.; Kleiner, R.E. Inhibition of nucleolar transcription by oxaliplatin involves ATM/ATR kinase signaling. Cell Chem. Biol. 2023, 30, 906–919.e4. [Google Scholar] [CrossRef]
- Vaughn, C.M.; Selby, C.P.; Yang, Y.; Hsu, D.S.; Sancar, A. Genome-wide single-nucleotide resolution of oxaliplatin–DNA adduct repair in drug-sensitive and -resistant colorectal cancer cell lines. J. Biol. Chem. 2020, 295, 7584–7594. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.S.; O’dowd, P.D.; Pigg, H.C.; Alley, K.R.; Griffith, D.M.; DeRose, V.J. Comparison of click-capable oxaliplatin and cisplatin derivatives to better understand Pt(ii)-induced nucleolar stress. RSC Chem. Biol. 2023, 4, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Han, Z.; Sun, Z.; Feng, H.; Zhao, L.; Yuan, Q.; Chen, C.; Yu, S.; Hu, Y.; Yu, J.; et al. PAK6 promotes homologous-recombination to enhance chemoresistance to oxaliplatin through ATR/CHK1 signaling in gastric cancer. Cell Death Dis. 2022, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, L.; Gout, J.; Roger, E.; de Almeida, F.K.; Simões, C.B.; Wiesmüller, L.; Seufferlein, T.; Kleger, A. DNA damage repair as a target in pancreatic cancer: State-of-the-art and future perspectives. Gut 2020, 70, 606–617. [Google Scholar] [CrossRef]
- Chang, Y.-W.E.; Bean, R.R.; Jakobi, R. Targeting RhoA/Rho Kinase and p21-Activated Kinase Signaling to Prevent Cancer Development and Progression. Recent Patents Anti-Cancer Drug Discov. 2009, 4, 110–124. [Google Scholar] [CrossRef]
- Schmidt, H.B.; Jaafar, Z.A.; Wulff, B.E.; Rodencal, J.J.; Hong, K.; Aziz-Zanjani, M.O.; Jackson, P.K.; Leonetti, M.D.; Dixon, S.J.; Rohatgi, R.; et al. Oxaliplatin disrupts nucleolar function through biophysical disintegration. Cell Rep. 2022, 41, 111629. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Wang, J.; Cheng, F.; Huang, X.; Cheng, Y.; Wang, K. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016, 377, 117–125. [Google Scholar] [CrossRef]
- Wang, J.-J.; Siu, M.K.-Y.; Jiang, Y.-X.; Leung, T.H.-Y.; Chan, D.W.; Wang, H.-G.; Ngan, H.Y.-S.; Chan, K.K.-L. A Combination of Glutaminase Inhibitor 968 and PD-L1 Blockade Boosts the Immune Response against Ovarian Cancer. Biomolecules 2021, 11, 1749. [Google Scholar] [CrossRef]
- Kinosada, H.; Okada-Iwasaki, R.; Kunieda, K.; Suzuki-Imaizumi, M.; Takahashi, Y.; Miyagi, H.; Suzuki, M.; Motosawa, K.; Watanabe, M.; Mie, M.; et al. The dual pocket binding novel tankyrase inhibitor K-476 enhances the efficacy of immune checkpoint inhibitor by attracting CD8(+) T cells to tumors. Am. J. Cancer Res. 2021, 11, 264–276. [Google Scholar] [CrossRef]
- Jin, S.; Ma, H.; Yang, W.; Ju, H.; Wang, L.; Zhang, Z. Cell division cycle 7 is a potential therapeutic target in oral squamous cell carcinoma and is regulated by E2F1. J. Mol. Med. 2018, 96, 513–525. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, P.; Chen, Y.; Liu, S.; Chen, J.; Yang, Z.; Chen, J.; Fan, X.; Wang, P.; Cai, Z.; et al. Inhibition of the PLK1-Coupled Cell Cycle Machinery Overcomes Resistance to Oxaliplatin in Colorectal Cancer. Adv. Sci. 2021, 8, 2100759. [Google Scholar] [CrossRef]
- Wang, J.; Cao, W.; Zhang, W.; Dou, B.; Ding, X.; Wang, M.; Ma, J.; Li, X. Tumor-Targeted Oxaliplatin(IV) Prodrug Delivery Based on ROS-Regulated Cancer-Selective Glycan Labeling. J. Med. Chem. 2024, 67, 8296–8308. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Barbero, B.; Álvarez-Fernández, M.; Zapatero-Solana, E.; El Bakkali, A.; Menéndez, M.d.C.; López-Casas, P.P.; Di Domenico, T.; Xie, T.; VanArsdale, T.; Shields, D.J.; et al. CDK4/6 Inhibitors Impair Recovery from Cytotoxic Chemotherapy in Pancreatic Adenocarcinoma. Cancer Cell 2020, 37, 340–353.e6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Z.; Deng, P.; Wu, X. Abstract 972: Pharmacological targeting CDC7 sensitizes oxaliplatin treatment in colorectal cancer. In Proceedings of the AACR Annual Meeting 2021, Philadelphia, PA, USA, 10–15 April 2021 and 17–21 May 2021; p. 972. [Google Scholar]
- Li, J.; Hu, H.; He, J.; Hu, Y.; Liu, M.; Cao, B.; Chen, D.; Ye, X.; Zhang, J.; Zhang, Z.; et al. Effective sequential combined therapy with carboplatin and a CDC7 inhibitor in ovarian cancer. Transl. Oncol. 2023, 39, 101825. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Liao, W.; Zheng, L.; Qian, S.; Mao, L. Matrine alkaloids modulating DNA damage repair in chemoresistant non-small cell lung cancer cells. BMC Cancer 2024, 24, 1283. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, L.; Guo, Q.; Zhu, J.; Deng, Y.; Wu, X. Identification of Chemoresistance-Associated Key Genes and Pathways in High-Grade Serous Ovarian Cancer by Bioinformatics Analyses. Cancer Manag. Res. 2020, ume 12, 5213–5223. [Google Scholar] [CrossRef]
- Ma, D.; Gilbert, T.; Pignanelli, C.; Tarade, D.; Noel, M.; Mansour, F.; Gupta, M.; Ma, S.; Ropat, J.; Curran, C.; et al. Exploiting mitochondrial and oxidative vulnerabilities with a synthetic analog of pancratistatin in combination with piperlongumine for cancer therapy. FASEB J. 2017, 32, 417–430. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, T.; Zhang, Z.; Liu, Z.; Qian, R.; Qi, R.; Zhou, F.; Li, M. Unraveling the immune landscape and therapeutic biomarker PMEPA1 for oxaliplatin resistance in colorectal cancer: A comprehensive approach. Biochem. Pharmacol. 2024, 222, 116117. [Google Scholar] [CrossRef]
- Modi, S.; Kir, D.; Giri, B.; Majumder, K.; Arora, N.; Dudeja, V.; Banerjee, S.; Saluja, A.K. Minnelide Overcomes Oxaliplatin Resistance by Downregulating the DNA Repair Pathway in Pancreatic Cancer. J. Gastrointest. Surg. 2016, 20, 13–24. [Google Scholar] [CrossRef]
- Al-Odat, O.S.; Guirguis, D.A.; Schmalbach, N.K.; Yao, G.; Budak-Alpdogan, T.; Jonnalagadda, S.C.; Pandey, M.K. Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2022, 24, 644. [Google Scholar] [CrossRef]
- Sekhar, K.R.; Hanna, D.N.; Cyr, S.; Baechle, J.J.; Kuravi, S.; Balusu, R.; Rathmell, K.; Baregamian, N. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroid cancer. Sci. Rep. 2022, 12, 19396. [Google Scholar] [CrossRef]

| PubMed database |
| #1 Oxaliplatin [Title/Abstract] #2 XL413 [Title/Abstract] #3 CDC7 inhibitor [Title/Abstract] #4 Drug resistance [MeSH Major Topic] #5 Cancer treatment [Title/Abstract] #6 Combination therapy [MeSH Major Topic] #7 #1 OR #2 OR #3 AND #4 #8 #1 OR #2 OR #3 AND #5 #9 #1 OR #2 OR #3 AND #6 |
| Generation | Pt Drug | Molecular Structure | Market Time | Listed Country |
|---|---|---|---|---|
| First | Cisplatin | 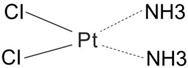 | 1978 | Japan/Italy |
| Second | Carboplatin | 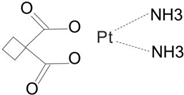 | 1986 | America |
| Third | Oxaliplatin | 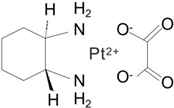 | 1996 | France |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Wu, C.; Na, J.; Liu, X.; Huang, Y. Multi-Pathway Study for Oxaliplatin Resistance Reduction. Curr. Issues Mol. Biol. 2025, 47, 172. https://doi.org/10.3390/cimb47030172
Ye T, Wu C, Na J, Liu X, Huang Y. Multi-Pathway Study for Oxaliplatin Resistance Reduction. Current Issues in Molecular Biology. 2025; 47(3):172. https://doi.org/10.3390/cimb47030172
Chicago/Turabian StyleYe, Tong, Chen Wu, Jintong Na, Xiyu Liu, and Yong Huang. 2025. "Multi-Pathway Study for Oxaliplatin Resistance Reduction" Current Issues in Molecular Biology 47, no. 3: 172. https://doi.org/10.3390/cimb47030172
APA StyleYe, T., Wu, C., Na, J., Liu, X., & Huang, Y. (2025). Multi-Pathway Study for Oxaliplatin Resistance Reduction. Current Issues in Molecular Biology, 47(3), 172. https://doi.org/10.3390/cimb47030172






