Enzymatic Activity and Nutrient Profile Assessment of Three Pleurotus Species Under Pasteurized Cenchrus fungigraminus Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Primary and Secondary Spawn
2.2. Preparation of Culture Medium and Cultivation
2.3. Determination of Enzyme Activity
2.4. Determination of Nutritional Content of Pleurotus Species
2.5. Statistical Analysis
3. Results
3.1. Yield and Biological Efficiency
3.2. Nutrient Composition and Heavy Metals in the Fruiting Bodies
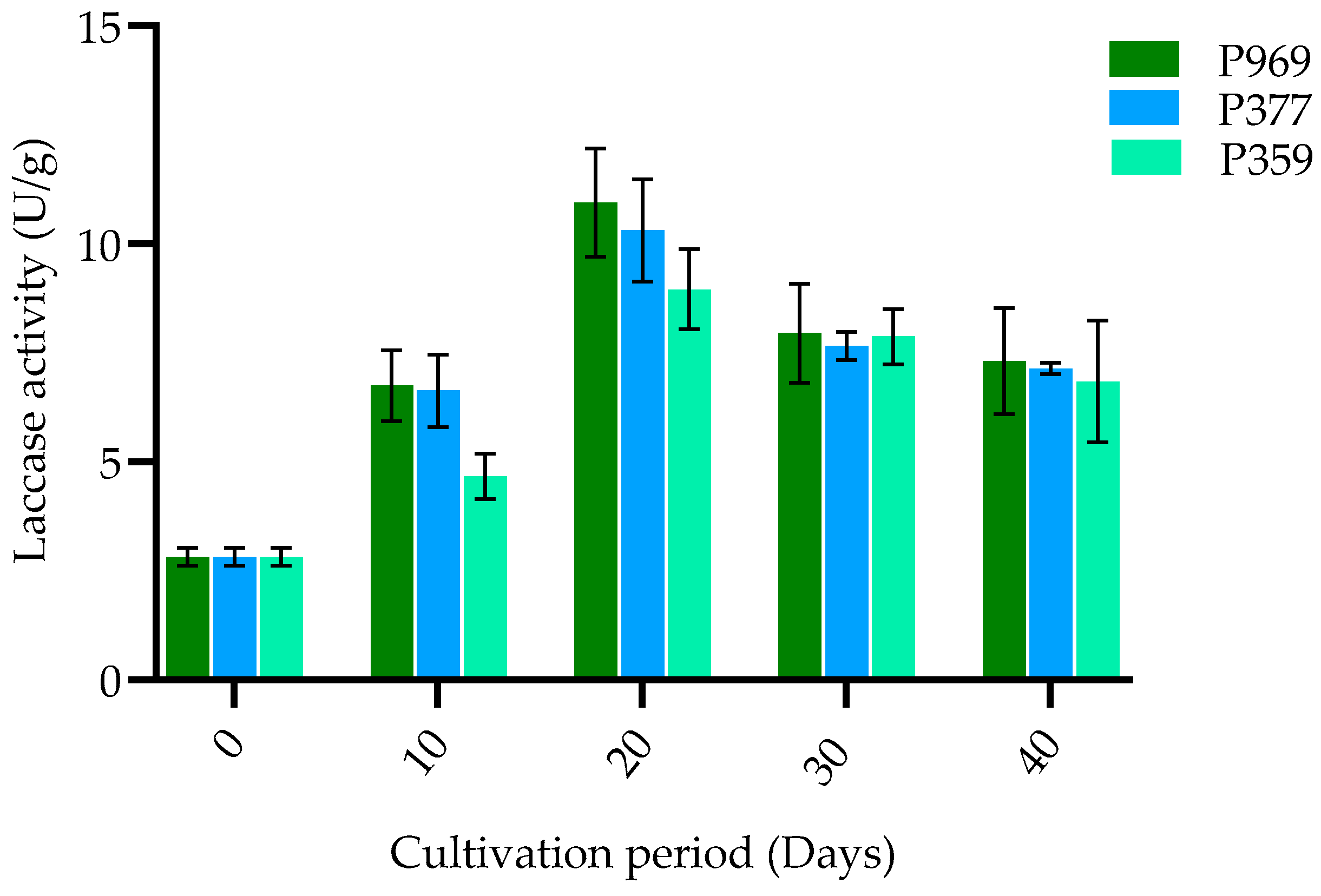
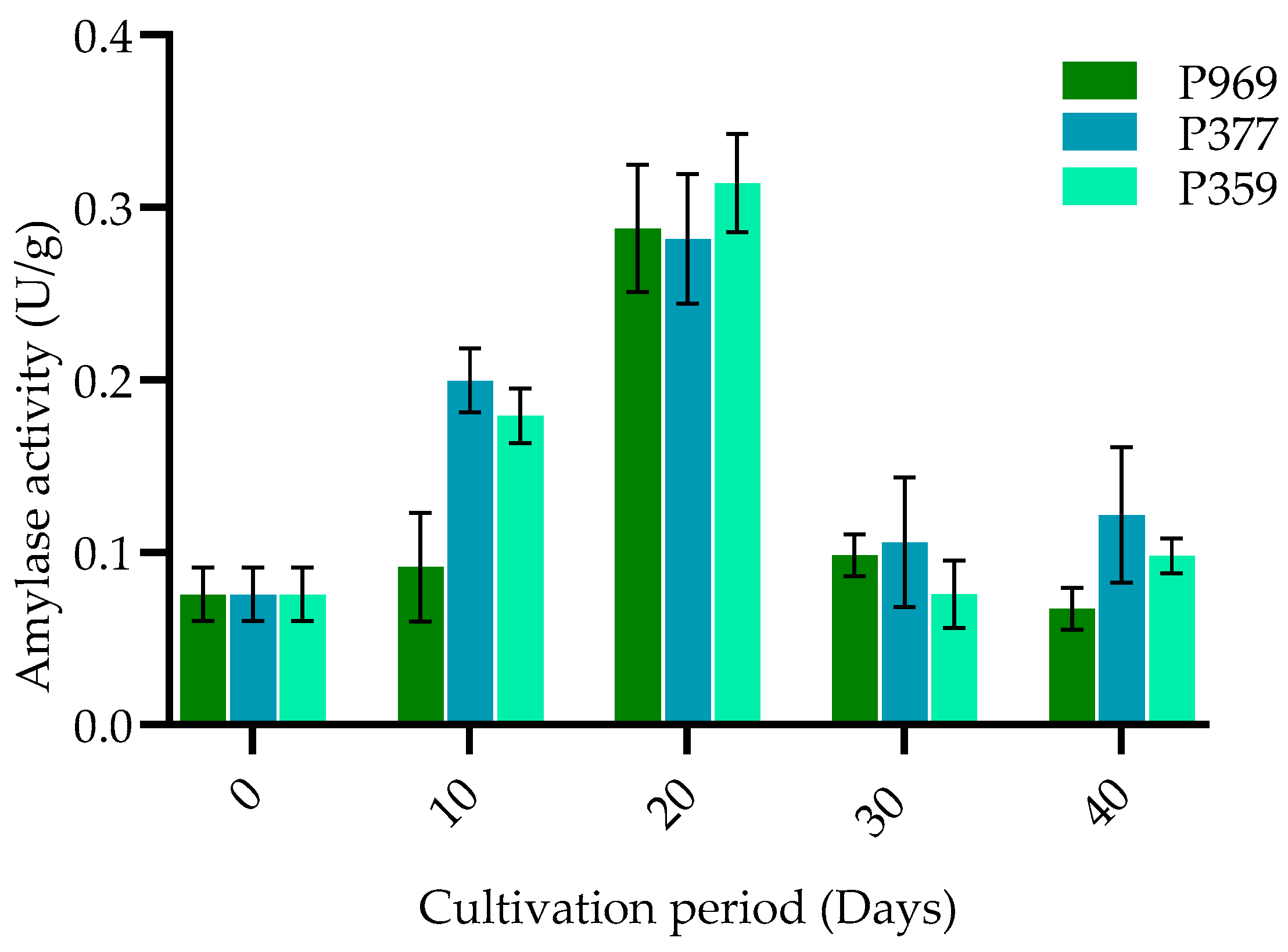
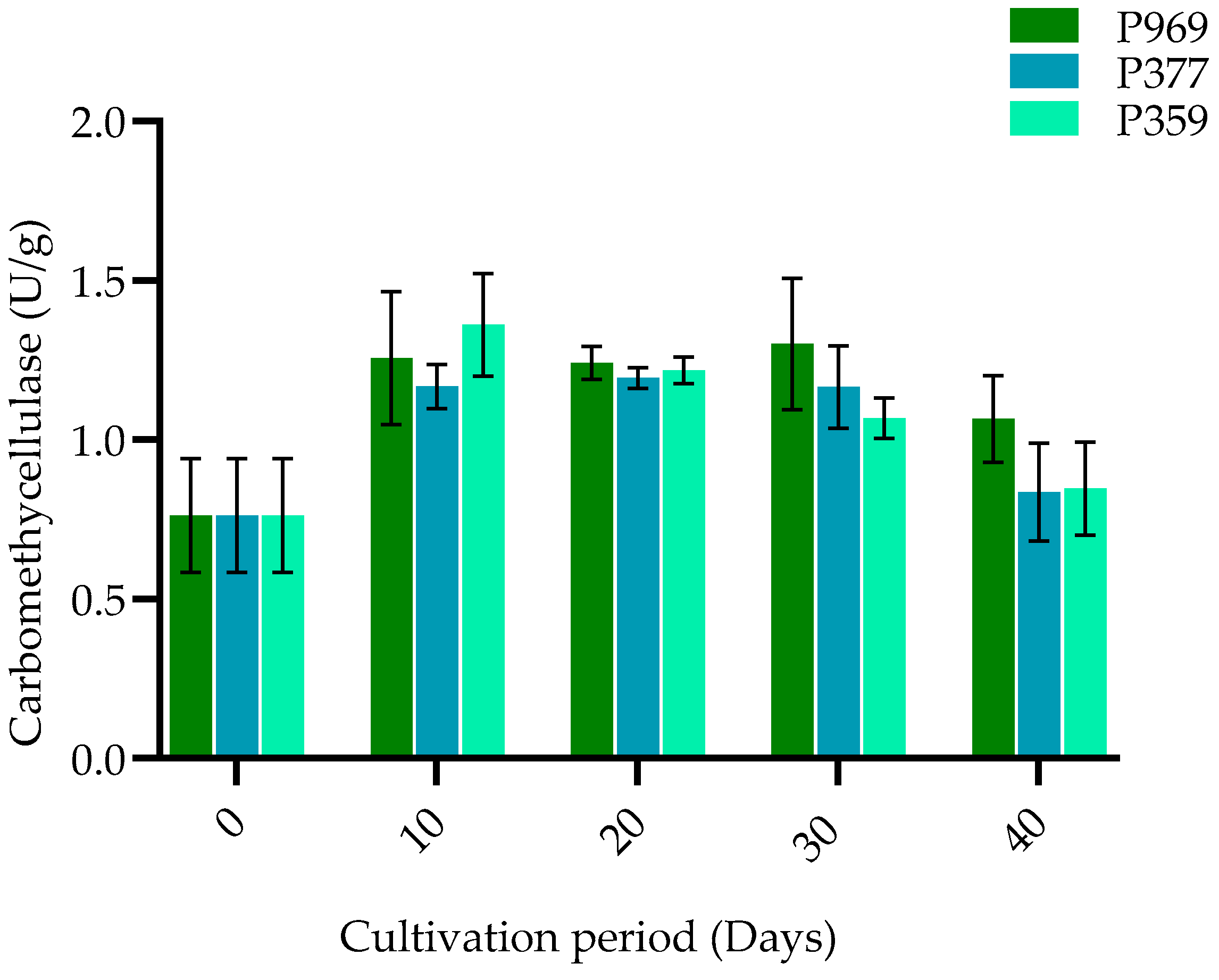
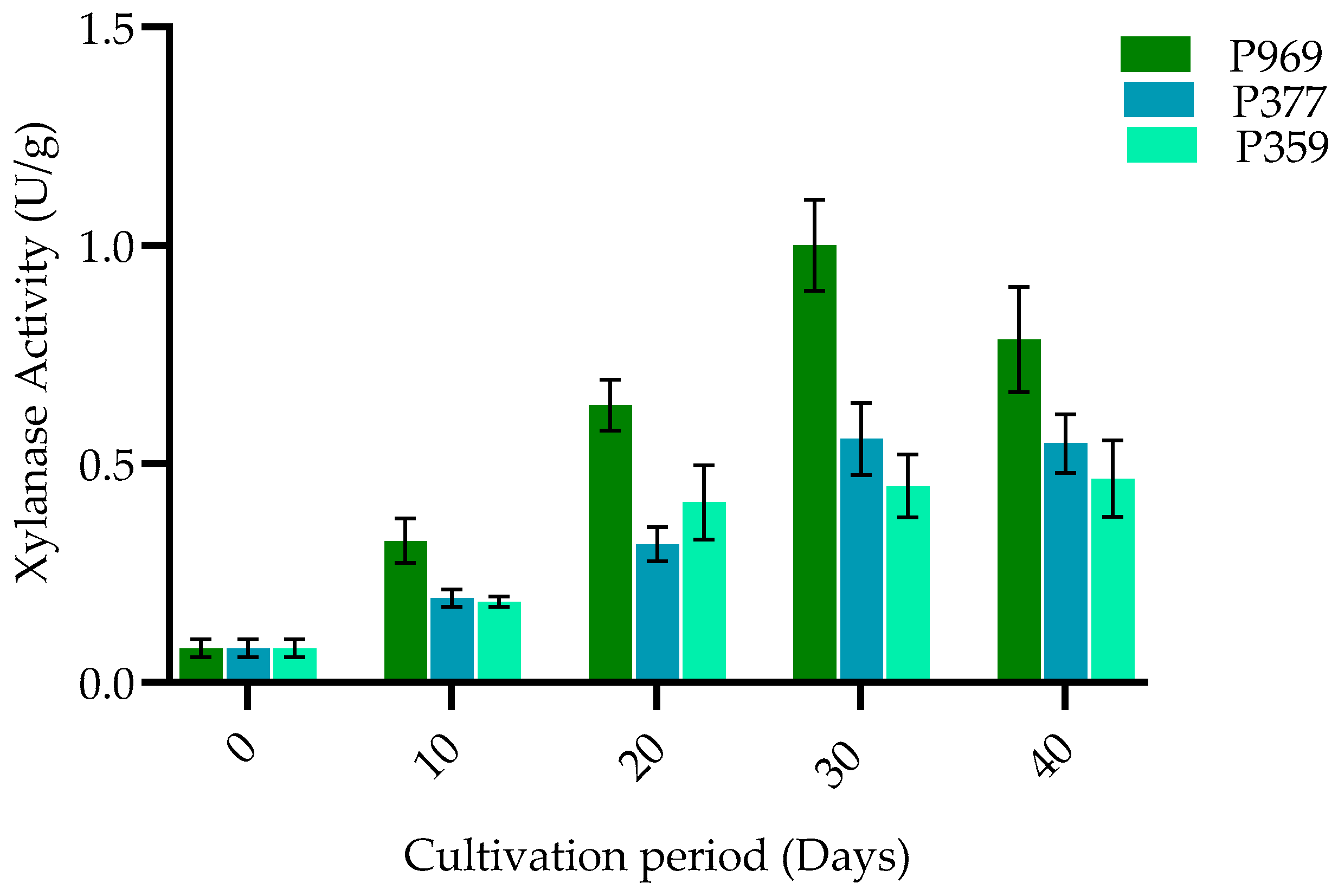
3.3. The Change in Enzymes on Mycelia Growing in the Different Growing Stages of Three Oyster Mushrooms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Chand, S.; Singh, B. Mushroom Cultivation for Increasing Income and Sustainable Development of Small and Marginal Farmers. Asian J. Agric. Res. 2022, 9, 11–16. [Google Scholar] [CrossRef]
- Nanje Gowda, N.A.; Manvi, D. Agriculture Crop Residues Disinfection Methods and Their Effects on Mushroom Growth. Proc. Indian Natl. Sci. Acad. 2020, 86, 1177–1190. [Google Scholar] [CrossRef]
- Adebayo, E.A.; Martinez-Carrera, D. Oyster Mushrooms (Pleurotus) Are Useful for Utilizing Lignocellulosic Biomass. Afr. J. Biotechnol. 2015, 14, 52–67. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.; Wen, Y.; Zhu, J.; Di, X.; Zeng, Y.; Han, X.; Que, Z.; Mediatrice, H.; Rensing, C.; et al. Short-Term Effects of Cenchrus Fungigraminus/Potato or Broad Bean Interplanting on Rhizosphere Soil Fertility, Microbial Diversity, and Greenhouse Gas Sequestration in Southeast China. Microorganisms 2024, 12, 1665. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, B.; Li, T.; Lin, H.; Lin, Z.; Lu, G.; Liu, Y.; Lin, B.; Lin, D. Isolation of Rhizobacteria from the Cenchrus Fungigraminus Rhizosphere and Characterization of Their Nitrogen-Fixing Performance and Potential Role in Plant Growth Promotion. Pland Soil 2023, 489, 405–421. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Gowda, N.A.N.; Manvi, D. Agro-Residues Disinfection Methods for Mushroom Cultivation: A Review. Agric. Rev. 2019, 40, 93–103. [Google Scholar] [CrossRef]
- Macias González, A.A.; Crespo Zafra, L.M.; Bordons, A.; Rodríguez-Porrata, B. Pasteurization Of Agricultural Substrates For Edible Mushroom Production. J. Microb. Biotech. Food Sci. 2022, 12, e5729. [Google Scholar] [CrossRef]
- Kim, S. Mushroom Ligninolytic Enzymes―Features and Application of Potential Enzymes for Conversion of Lignin into Bio-Based Chemicals and Materials. Appl. Sci. 2021, 11, 6161. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Heese, K. Characterization of Lignocellulolytic Enzymes from White-Rot Fungi. Curr. Microbiol. 2015, 70, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability 2020, 12, 7282. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhang, J.L.; Hu, K.H.; Zhang, W.G. The Relationship between Lignin Peroxidase and Manganese Peroxidase Production Capacities and Cultivation Periods of Mushrooms. Microb. Biotechnol. 2013, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ningrum, R.S.; Khairaummah, A.; Puspitasari, I.; Bazhafah, A.S.; Ratnaningtyas, N.I.; Mumpuni, A.; Ismadi, I.; Kusumah, S.S. The Properties of Particleboard Composites Made from Pleurotus Ostreatus Baglog Waste Using Citric Acid and Sucrose Adhesive. J. Bahan Alam Terbarukan 2022, 11, 115–123. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Liu, X.-B.; Yang, Z.L.; Zhao, Z.-W. Species Clarification of Oyster Mushrooms in China and Their DNA Barcoding. Mycol. Progress 2017, 16, 191–203. [Google Scholar] [CrossRef]
- Claude, I.; Aimable, N.; Mediatrice, H.; Zhou, H.; Lin, D.; Liu, P.; Lin, Z. Evaluation of the Influence of Varied Juncao Grass Substrates on Physiological and Enzymatic Reactions of Pleurotus Ostreatus. Curr. Issues Mol. Biol. 2024, 46, 9493–9502. [Google Scholar] [CrossRef]
- Deblais, L.; Helmy, Y.A.; Testen, A.; Vrisman, C.; Jimenez Madrid, A.M.; Kathayat, D.; Miller, S.A.; Rajashekara, G. Specific Environmental Temperature and Relative Humidity Conditions and Grafting Affect the Persistence and Dissemination of Salmonella Enterica subsp. Enterica Serotype Typhimurium in Tomato Plant Tissues. Appl. Environ. Microbiol. 2019, 85, e00403-19. [Google Scholar] [CrossRef] [PubMed]
- Бaндypa, I.I.; Kyлик, A.C.; Біськo, Н.А.; Xapeбa, О.В.; Цизь, О.М.; Xapeбa, В.В. Analysis of the Biological Efficiency and Quality Factors of Mushrooms of the Genus Pleurotus (Fr.) P.Kumm as a Model of Effective Cultivation of Lignicolous Fungi with High Functional Value. Plant Var. Stud. Prot. 2020, 16. [Google Scholar] [CrossRef]
- Fang, M.; Sun, X.; Yao, F.; Lu, L.; Ma, X.; Shao, K.; Kaimoyo, E. A Combination of Transcriptome and Enzyme Activity Analysis Unveils Key Genes and Patterns of Corncob Lignocellulose Degradation by Auricularia Heimuer under Cultivation Conditions. J. Fungi 2024, 10, 545. [Google Scholar] [CrossRef]
- González-Martínez, M.Á.; Puchades, R.; Maquieira, Á. Immunoanalytical Technique: Enzyme-Linked Immunosorbent Assay (ELISA). In Modern Techniques for Food Authentication; Academic Press: Cambridge, MA, USA, 2018; Volume 12, pp. 617–657. [Google Scholar] [CrossRef]
- Akata, I.; Zengin, G.; Picot, C.M.N.; Mahomoodally, M.F. Enzyme Inhibitory and Antioxidant Properties of Six Mushroom Species from the Agaricaceae Family. S. Afr. J. Bot. 2019, 120, 95–99. [Google Scholar] [CrossRef]
- Vats, A.; Kurade, A.S.; Mutnuri, S. Recovery of Lignocellulolytic Enzymes and Valorization of Spent Mushroom Substrate. Environ. Nat. Resour. J. 2022, 20, 1–9. [Google Scholar] [CrossRef]
- Chowdhary, P.; Shukla, G.; Raj, G.; Ferreira, L.F.R.; Bharagava, R.N. Microbial Manganese Peroxidase: A Ligninolytic Enzyme and Its Ample Opportunities in Research. SN Appl. Sci. 2019, 1, 45. [Google Scholar] [CrossRef]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional Composition and Biological Properties of Sixteen Edible Mushroom Species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Sawangwan, T.; Wansanit, W.; Pattani, L.; Noysang, C. Study of Prebiotic Properties from Edible Mushroom Extraction. Agric. Nat. Resour. 2018, 52, 519–524. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Lu, N.; Yu, J.; Chen, S.; Liang, Z. The Role of Camellia Shell Substrates in Modulating the Nutritional Characteristics of Pleurotus Pulmonarius. Foods 2024, 13, 2946. [Google Scholar] [CrossRef] [PubMed]
- Ab Rhaman, S.M.S.; Naher, L.; Siddiquee, S. Mushroom Quality Related with Various Substrates’ Bioaccumulation and Translocation of Heavy Metals. J. Fungi 2021, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, Y.; Li, X.; Hu, X.; Zhang, J.; Wu, X.; Fu, J. Identification, Nutrient Composition, and Evaluation of a Wild Pleurotus citrinopileatus Strain (X21156) from Tibet for Antioxidant and Cytotoxic Activities. Horticulturae 2024, 10, 377. [Google Scholar] [CrossRef]
- Ahmed, R.; Niloy, M.A.H.M.; Islam, M.S.; Reza, M.S.; Yesmin, S.; Rasul, S.B.; Khandakar, J. Optimizing Tea Waste as a Sustainable Substrate for Oyster Mushroom (Pleurotus ostreatus) Cultivation: A Comprehensive Study on Biological Efficiency and Nutritional Aspect. Front. Sustain. Food Syst. 2024, 7, 1308053. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Assessing the Nutritional Quality of Pleurotus Ostreatus (Oyster Mushroom). Front. Nutr. 2024, 10, 1279208. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, M.; Sobhi, H.R.; Esrafili, A.; FarzadKia, M.; Yeganeh, M. Heavy Metals Content in Edible Mushrooms: A Systematic Review, Meta-Analysis and Health Risk Assessment. Trends Food Sci. Technol. 2021, 109, 527–535. [Google Scholar] [CrossRef]
- FAO Explanatory Note on Heavy Metals. Available online: https://www.fao.org/fileadmin/templates/agns/pdf/jecfa/2002-09-10_Explanatory_note_Heavy_Metals.pdf (accessed on 20 May 2024).
- Margaret, I.V.; Praba, L.J.; Sathiya, G. A Comparative Study on the Growth of Oyster Mushroom in Different Substrates. Uttar Pradesh J. Zool. 2023, 44, 74–80. [Google Scholar] [CrossRef]
- Calabretti, A.; Mang, S.M.; Becce, A.; Castronuovo, D.; Cardone, L.; Candido, V.; Camele, I. Comparison of Bioactive Substances Content between Commercial and Wild-Type Isolates of Pleurotus eryngii. Sustainability 2021, 13, 3777. [Google Scholar] [CrossRef]
- Niyimbabazi, O.; Nsanzinshuti, A.; Hatungimana, M.; Lin, H.; Zhang, L.; Lin, D.; Zhanxi, L. Ability of Three Pleurotus Species for Effective Use of Giant Grass Compost. J. Hortic. Res. 2022, 30, 67–76. [Google Scholar] [CrossRef]
- Innes, C. Pleurotus spp. as Agents of Mycoremediation: A Review. Bachelor’s Thesis, Portland State University, Portland, OR, USA, 2023. University Honors Theses, Paper 1358. [Google Scholar] [CrossRef]
- Han, M.-L.; Li, X.-Q.; Zhang, C.-D.; Li, M.-X.; Zhang, M.-H.; An, M.; Dou, X.-Y.; Zhang, T.-X.; Yan, X.-Y.; Bian, L.-S.; et al. Effect of Different Lignocellulosic Biomasses on Laccase Production by Pleurotus Species. BioResources 2022, 17, 4921–4936. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]

| No | Names | Strain Code | Geographic Origin |
|---|---|---|---|
| 1 | Pleurotus florida | P377 | Yunnan |
| 2 | Pleurotus ostreatus | P969 | Fujian |
| 3 | Pleurotus pulmonarius | P359 | Fujian |
| Mushroom Species | Mycelia Growth (Days) | Stipe Length (mm) | Pileus Diameter (mm) | Fresh Fruiting Body Weight (g) | BE (%) |
|---|---|---|---|---|---|
| P. ostreatus | 25.33 ± 2.82 b | 35.94 ± 5.38 a | 70.05 ± 8.26 a | 164.50 ± 12.27 a | 78.23 ± 2.08 a |
| P. florida | 35.5 ± 2.50 a | 19.55 ± 9.10 b | 44.44 ± 11.85 b | 87.01 ± 10.50 c | 39.66 ± 5.50 c |
| P. pulmonarius | 29.33 ± 1.25 b | 36.20 ± 3.91 a | 63.73 ± 2.99 a | 117.60 ± 8.56 b | 59.88 ± 4.24 b |
| Mushroom Species | Ash Content | Polysaccharide | Protein Content | Fiber Content | Fat Content |
|---|---|---|---|---|---|
| P. ostreatus | 1.29 ± 0.10 b | 89.33 ± 2.01 b | 4.53 ± 0.01 a | 35.81 ± 0.34 a | 1.71 ± 0.09 b |
| P. pulmonarius | 1.15 ± 0.98 b | 92.29 ± 1.46 a | 2.99 ± 0.98 b | 30.42 ± 0.41 b | 2.01 ± 0.12 a |
| P. florida | 2.07 ± 0.07 a | 80.63 ± 2.06 c | 3.64 ± 0.17 b | 33.55 ± 0.02 b | 1.44 ± 0.27 b |
| Mushroom Species | Cadmium | Lead | Arsenic | Mercury |
|---|---|---|---|---|
| P. ostreatus | 0.095 ± 0.012 a | 0.628 ± 0.031 a | 0.70 ± 0.056 a | 0.054 ± 0.002 a |
| P. pulmonarius | 0.136 ± 0.090 a | 0.018 ± 0.022 b | 0.047 ± 0.022 b | 0.012 ± 0.005 a |
| P. florida | 0.117 ± 0.001 a | 0.562 ± 0.015 a | 0.091 ± 0.054 a | 0.086 ± 0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aimable, N.; Mediatrice, H.; Claude, I.; Biregeya, J.; Hu, Y.; Zhou, H.; Liu, P.; Li, J.; Lin, Z.; Lu, G.; et al. Enzymatic Activity and Nutrient Profile Assessment of Three Pleurotus Species Under Pasteurized Cenchrus fungigraminus Cultivation. Curr. Issues Mol. Biol. 2025, 47, 143. https://doi.org/10.3390/cimb47030143
Aimable N, Mediatrice H, Claude I, Biregeya J, Hu Y, Zhou H, Liu P, Li J, Lin Z, Lu G, et al. Enzymatic Activity and Nutrient Profile Assessment of Three Pleurotus Species Under Pasteurized Cenchrus fungigraminus Cultivation. Current Issues in Molecular Biology. 2025; 47(3):143. https://doi.org/10.3390/cimb47030143
Chicago/Turabian StyleAimable, Nsanzinshuti, Hatungimana Mediatrice, Irambona Claude, Jules Biregeya, Yingping Hu, Hengyu Zhou, Penghu Liu, Jing Li, Zhanxi Lin, Guodong Lu, and et al. 2025. "Enzymatic Activity and Nutrient Profile Assessment of Three Pleurotus Species Under Pasteurized Cenchrus fungigraminus Cultivation" Current Issues in Molecular Biology 47, no. 3: 143. https://doi.org/10.3390/cimb47030143
APA StyleAimable, N., Mediatrice, H., Claude, I., Biregeya, J., Hu, Y., Zhou, H., Liu, P., Li, J., Lin, Z., Lu, G., & Lin, D. (2025). Enzymatic Activity and Nutrient Profile Assessment of Three Pleurotus Species Under Pasteurized Cenchrus fungigraminus Cultivation. Current Issues in Molecular Biology, 47(3), 143. https://doi.org/10.3390/cimb47030143









