From Genes to Healing: The Protective Mechanisms of Poria cocos Polysaccharide in Endometrial Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. In Vitro Test
2.2.1. Cell Culture and Viability Assay
2.2.2. RNA Extraction and Transcriptome Sequencing
2.2.3. Transcriptome Analysis
2.2.4. Flow Cytometry Analysis
2.2.5. Fluorescent Quantitative PCR (RT-qPCR) Detection of Relevant Genes
2.2.6. Western Blot Analysis
2.3. The Efficacy Experiment of PCP in Treating Endometritis
2.3.1. Source of Polysaccharide and Ciprofloxacin Hydrochloride
2.3.2. Experimental Design and Management
2.3.3. Organ Index, Liver Antioxidant Levels, and Inflammatory Factors
2.3.4. Effect of PCP on Inflammatory Factors in Endometritis Rats
2.3.5. Pathological Changes in Uterine Tissue
2.4. Statistical Analysis
3. Results
3.1. Cell Viability Assay
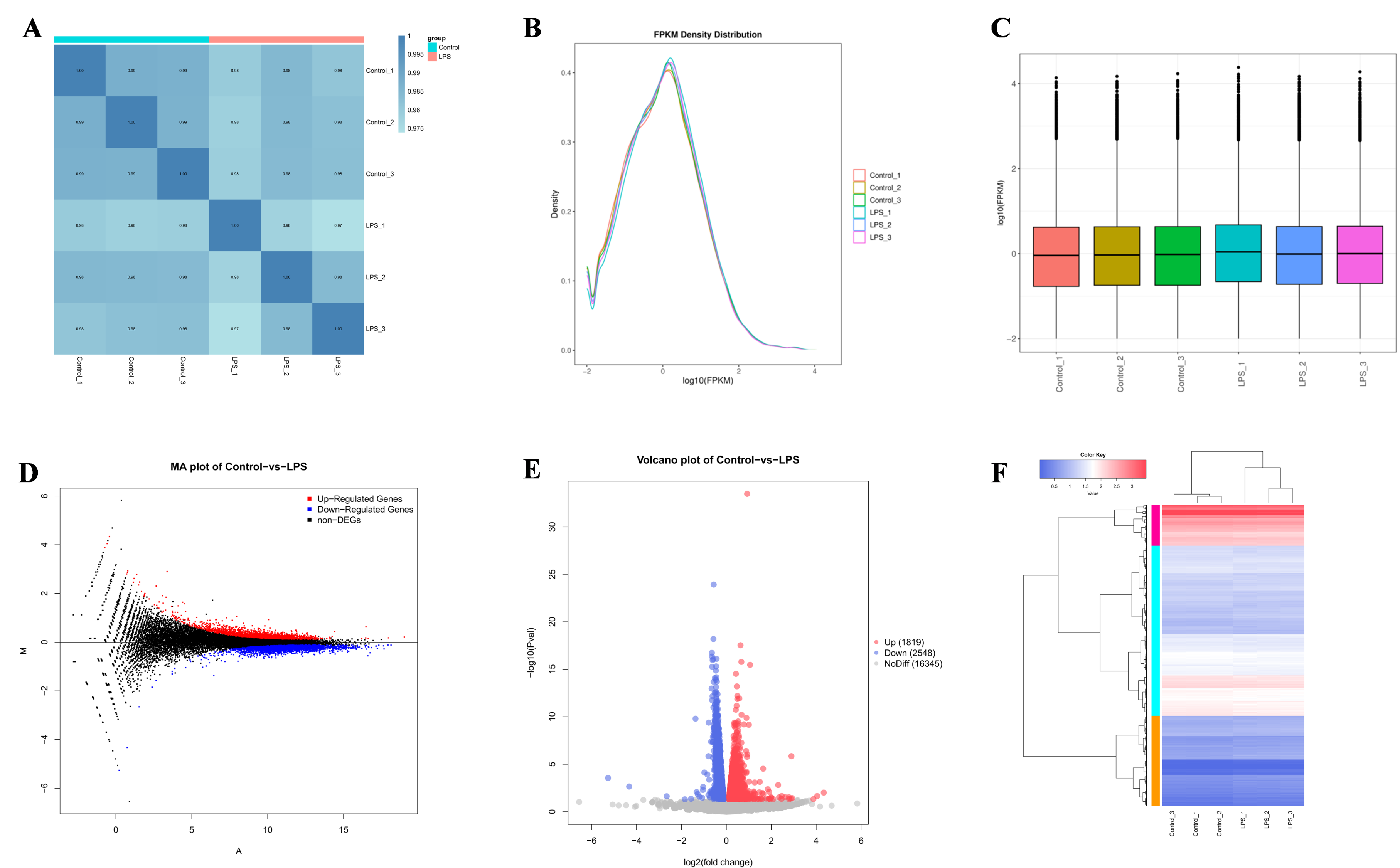
3.2. Transcriptome Data
3.3. Differences in Gene Identification
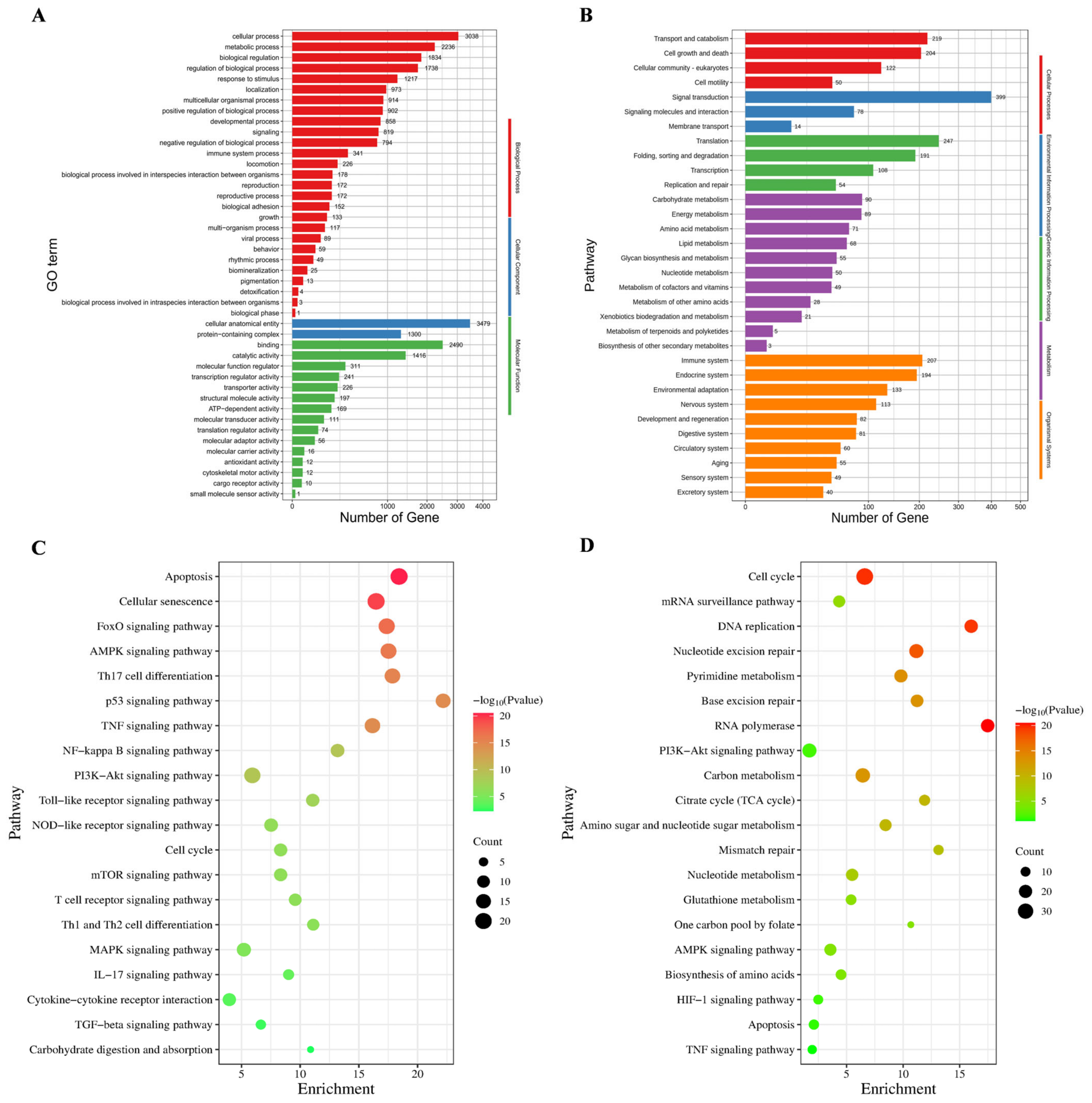
3.4. GO and KEGG Enrichment Analysis
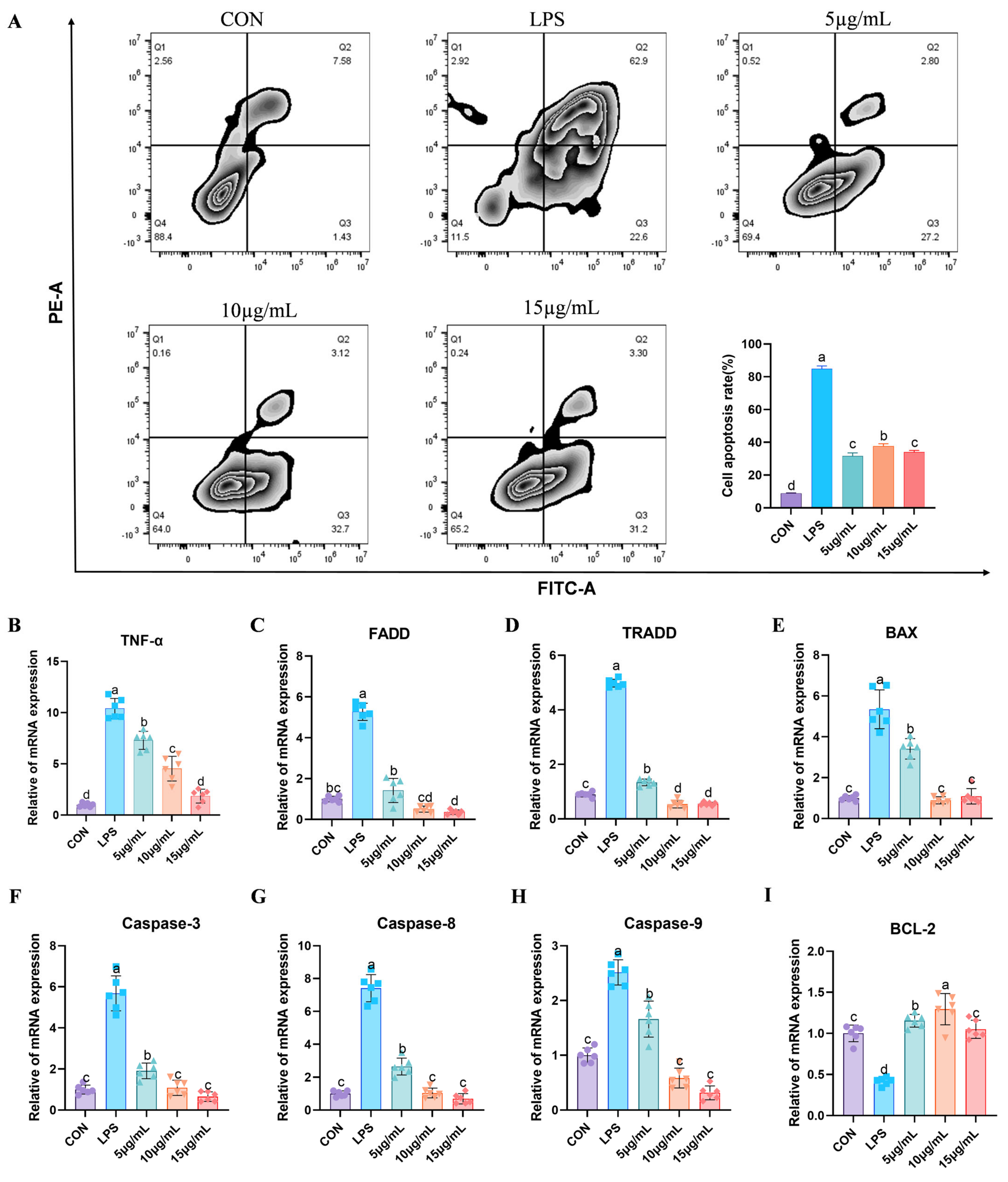
3.5. BEND Cell Apoptosis
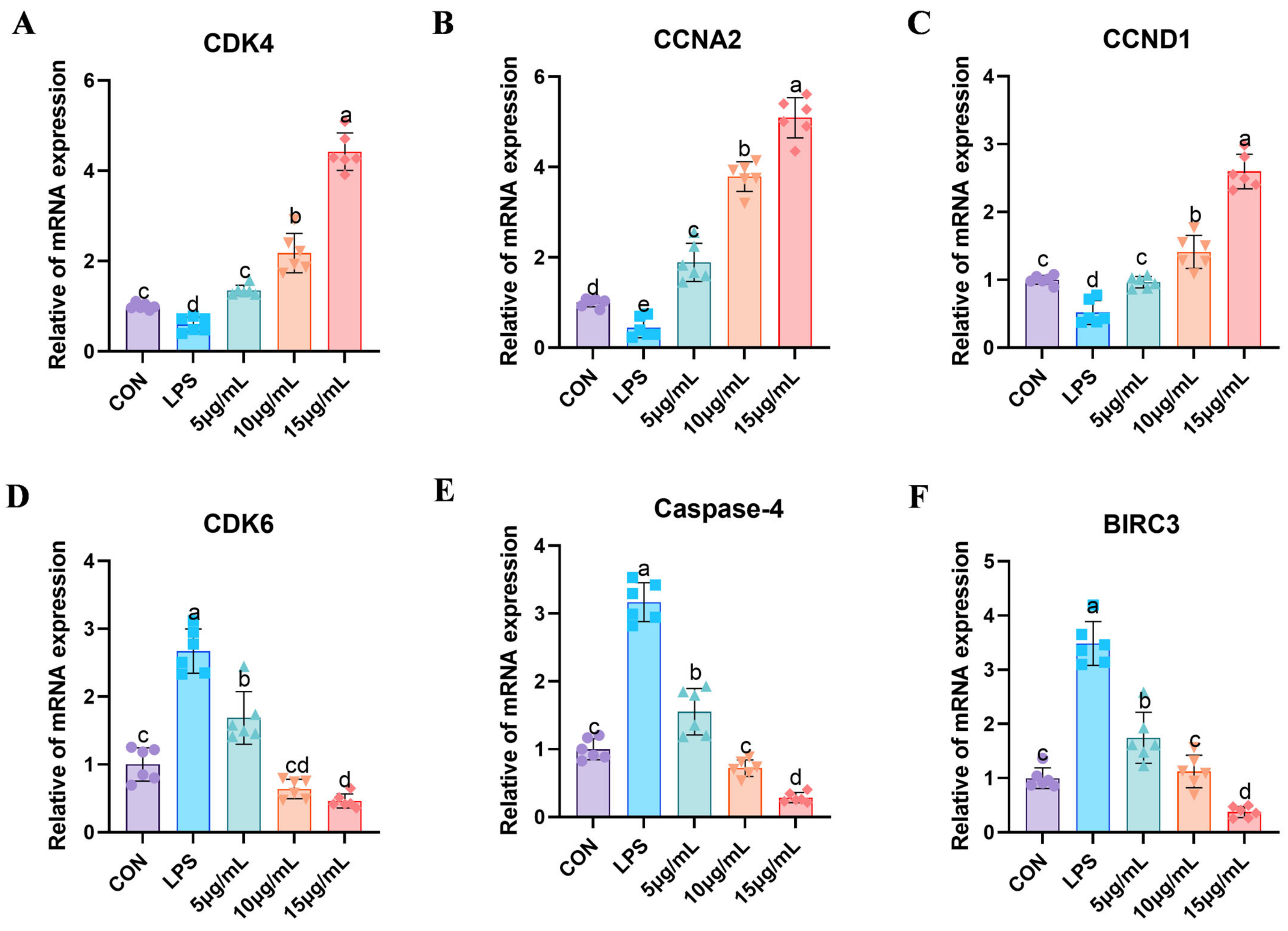
3.6. Fluorescent Quantitative PCR Detection of Relevant Genes
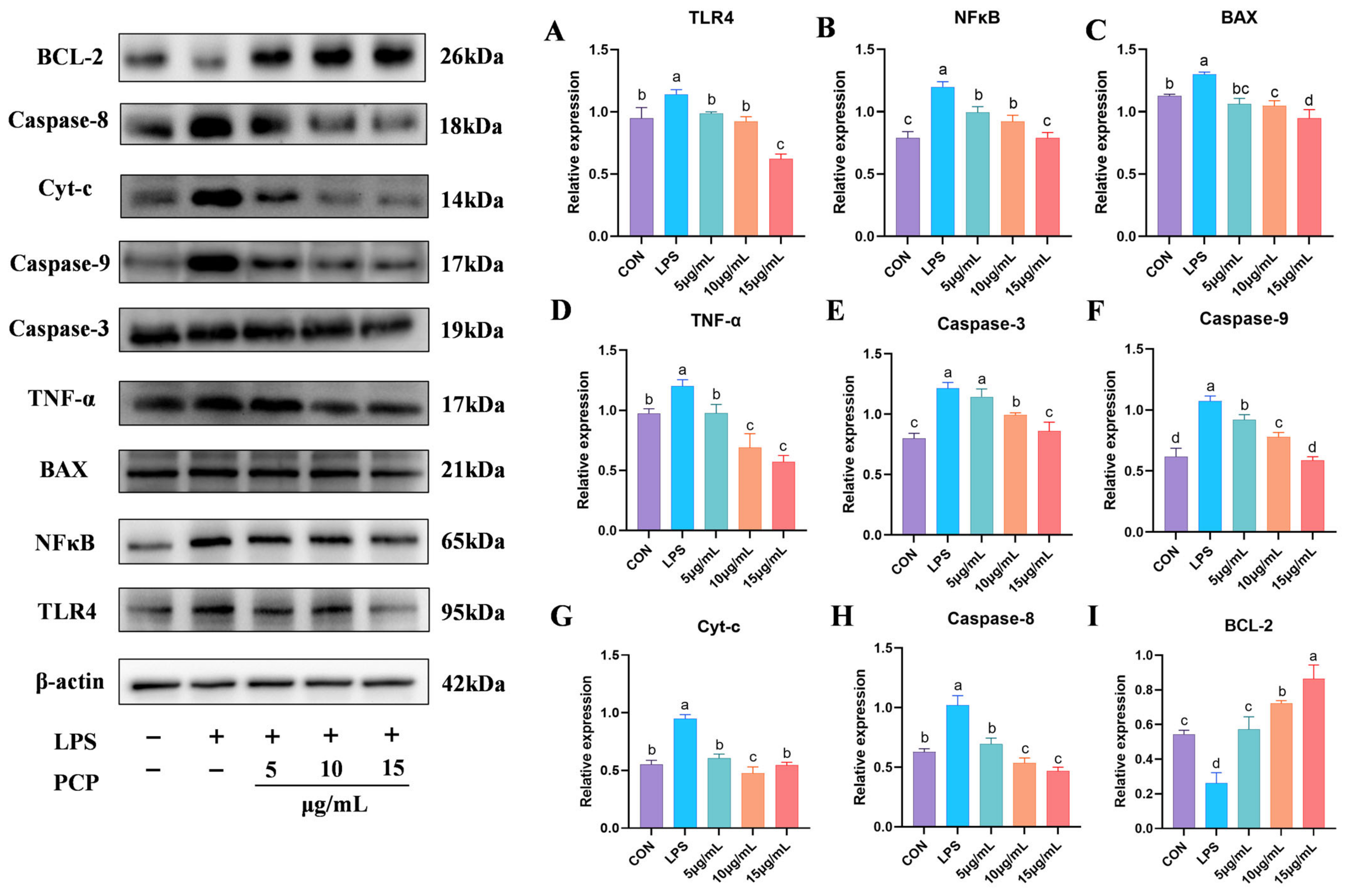
3.7. The Protein Expression Levels of NFκB and Apoptosis Signaling Pathways
3.8. Results of Organ Indexes
3.9. Results of Antioxidant Level in Liver and Inflammatory Factors
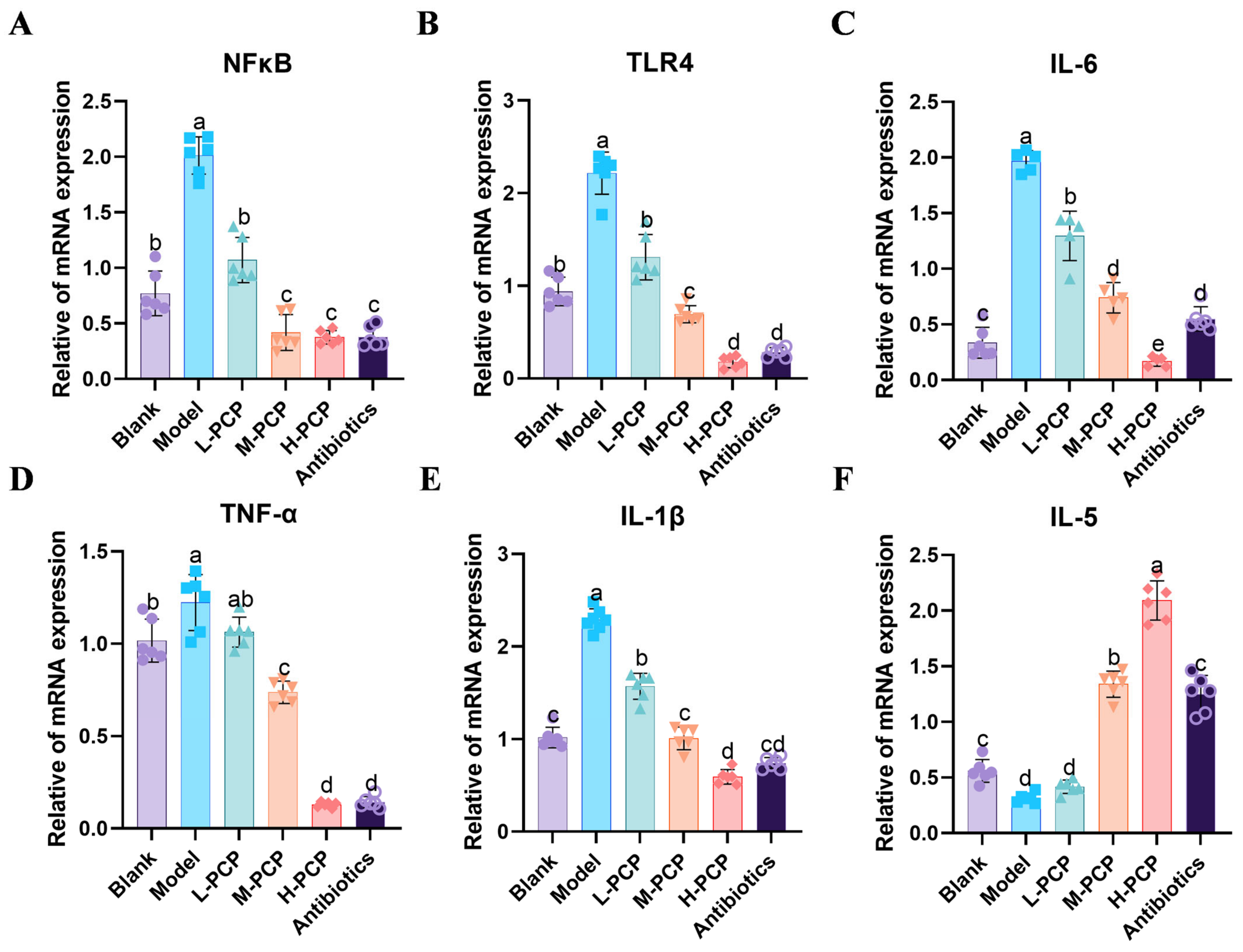
3.10. Results of mRNA Expression of Related Inflammatory Factors
3.11. Pathological Observation of Uterine Tissue in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Umar, T.; Yin, B.; Umer, S.; Ma, X.; Jiang, K.; Umar, Z.; Akhtar, M.; Shaukat, A.; Deng, G. MicroRNA: Could It Play a Role in Bovine Endometritis? Inflammation 2021, 44, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Wagener, K.; Gabler, C.; Drillich, M. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology 2017, 94, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Price, S.B.; Cronin, J.; Gilbert, R.O.; Gadsby, J.E. Mechanisms of infertility associated with clinical and subclinical endometritis in high producing dairy cattle. Reprod. Domest. Anim. 2009, 44 (Suppl. 3), 1–9. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Lavender, C.R.M.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef]
- Ghallab, R.S.; El-Karim, D.R.S.G.; Fayed, A.-H.; Rashad, A.M.A. Efficiency of conventional and nanoparticle oxytetracycline in treatment of clinical endometritis in postpartum dairy cows. Trop. Anim. Health Prod. 2023, 55, 118. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Hongzhi, D.; Xiaoying, H.; Yujie, G.; Le, C.; Yuhuan, M.; Dahui, L.; Luqi, H. Classic mechanisms and experimental models for the anti-inflammatory effect of traditional Chinese medicine. Anim. Model. Exp. Med. 2022, 5, 108–119. [Google Scholar] [CrossRef]
- Gao, J.; Yang, Z.; Zhao, C.; Tang, X.; Jiang, Q.; Yin, Y. A comprehensive review on natural phenolic compounds as alternatives to in-feed antibiotics. Sci. China Life Sci. 2023, 66, 1518–1534. [Google Scholar] [CrossRef]
- Zhao, M.; Guan, Z.; Tang, N.; Cheng, Y. The differences between the water- and alkaline-soluble Poria cocos polysaccharide: A review. Int. J. Biol. Macromol. 2023, 235, 123925. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, H.; Wang, S.; Xiang, Z.; Kong, H.; Xue, Q.; He, M.; Yu, X.; Li, Y.; Sun, D.; et al. A review on the advances in the extraction methods and structure elucidation of Poria cocos polysaccharide and its pharmacological activities and drug carrier applications. Int. J. Biol. Macromol. 2022, 217, 536–551. [Google Scholar] [CrossRef]
- Liu, J.; Hong, W.; Li, M.; Xiao, Y.; Yi, Y.; Liu, Y.; Wu, G. Transcriptome analysis reveals immune and metabolic regulation effects of Poria cocos polysaccharides on Bombyx mori larvae. Front. Immunol. 2022, 13, 1014985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, J.; Sun, M.; Duan, Y.; Wang, L.; Yu, N.; Peng, D.; Chen, W.; Wang, Y. Preparation, characterization, antioxidant and antianemia activities of Poria cocos polysaccharide iron (III) complex. Heliyon 2023, 9, e12819. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nie, F.; Liu, W.; He, W.; Guo, Y. Preparation and exploration of anti-tumor activity of Poria cocos polysaccharide gold nanorods. Int. J. Biol. Macromol. 2024, 280, 135347. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, H.; Wan, X. Protective Effect of Poria cocos Polysaccharides on Fecal Peritonitis-Induced Sepsis in Mice Through Inhibition of Oxidative Stress, Inflammation, Apoptosis, and Reduction of Treg Cells. Front. Microbiol. 2022, 13, 887949. [Google Scholar] [CrossRef]
- Yang, X.; Lu, S.; Feng, Y.; Cao, C.; Zhang, Y.; Cheng, S. Characteristics and properties of a polysaccharide isolated from Wolfiporia cocos as potential dietary supplement for IBS. Front. Nutr. 2023, 10, 1119583. [Google Scholar] [CrossRef]
- Shen, W.; Oladejo, A.O.; Ma, X.; Jiang, W.; Zheng, J.; Imam, B.H.; Wang, S.; Wu, X.; Ding, X.; Ma, B.; et al. Inhibition of Neutrophil Extracellular Traps Formation by Cl-Amidine Alleviates Lipopolysaccharide-Induced Endometritis and Uterine Tissue Damage. Animals 2022, 12, 1151. [Google Scholar] [CrossRef]
- Wu, H.; Dai, A.; Chen, X.; Yang, X.; Li, X.; Huang, C.; Jiang, K.; Deng, G. Leonurine ameliorates the inflammatory responses in lipopolysaccharide-induced endometritis. Int. Immunopharmacol. 2018, 61, 156–161. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, W.; Jin, Q.; Zhu, Y.; Chen, R.; Tian, Q.; Yan, N.; Guo, L. The effects of dietary supplementation with lotus leaf extract on the immune response and intestinal microbiota composition of broiler chickens. Poult. Sci. 2021, 100, 100925. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, P.; Jaitley, P.; Sharma, S.; Prakash, A.; Mandil, R.; Choudhury, S.; Gangwar, N.K.; Garg, S.K. Eucalyptus robusta leaves methanolic extract suppresses inflammatory mediators by specifically targeting TLR4/TLR9, MPO, COX2, iNOS and inflammatory cytokines in experimentally-induced endometritis in rats. J. Ethnopharmacol. 2018, 213, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, G.S.; Murray, R.D.; Woldehiwet, Z. Some aspects of immunology of the bovine uterus related to treatments for endometritis. Anim. Reprod. Sci. 2001, 67, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Amin, Y.A.; Abdelaziz, S.G.; Said, A.H. Treatment of postpartum endometritis induced by multidrug-resistant bacterial infection in dairy cattle by green synthesized zinc oxide nanoparticles and in vivo evaluation of its broad spectrum antimicrobial activity in cow uteri. Res. Vet. Sci. 2023, 165, 105074. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Review: Postpartum reproductive disease and fertility in dairy cows. Animal 2023, 17 (Suppl. 1), 100781. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A calculated response: Control of inflammation by the innate immune system. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Liu, T.; Jiang, J.; Zhao, X.; Fan, Y.; Zhang, W.; Ma, W.; Guo, T.; Wang, W.; Liu, Y. Salvia miltiorrhiza ameliorates endometritis in dairy cows by relieving inflammation, energy deficiency and blood stasis. Front. Pharmacol. 2024, 15, 1349139. [Google Scholar] [CrossRef]
- Menoud, V.; Holinger, M.; Graf-Schiller, S.; Mayer, P.; Gerber, L.; Walkenhorst, M.; Hirsbrunner, G. Comparison between intrauterine application of an antibiotic and an herbal product to treat clinical endometritis in dairy cattle—Randomized multicentre field study. Res. Vet. Sci. 2024, 172, 105250. [Google Scholar] [CrossRef]
- Wu, P.; Tan, H.; Zhan, J.; Wang, W.; Hu, T.; Li, S. Optimization of Bioprocess Extraction of Poria cocos Polysaccharide (PCP) with Aspergillus niger β-Glucanase and the Evaluation of PCP Antioxidant Property. Molecules 2020, 25, 5930. [Google Scholar] [CrossRef]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 112, 108709. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria cocos Polysaccharide Ameliorated Antibiotic-Associated Diarrhea in Mice via Regulating the Homeostasis of the Gut Microbiota and Intestinal Mucosal Barrier. Int. J. Mol. Sci. 2023, 24, 1423. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, X.; Huang, Z.; Zhong, X.; Liu, Y.; Cheong, K.-L.; Zhou, J.; Tang, S. The integration of single-cell sequencing, TCGA, and GEO data analysis revealed that PRRT3-AS1 is a biomarker and therapeutic target of SKCM. Front. Immunol. 2022, 13, 919145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Peng, C.; Song, C.; Zhao, Q.; Rong, J.; Wang, H.; Ding, W.; Wang, F.; Xie, Y. BICC1 as a novel prognostic biomarker in gastric cancer correlating with immune infiltrates. Int. Immunopharmacol. 2020, 87, 106828. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xu, W.-H.; Xu, F.-J.; Li, H.; Anwaier, A.; Wang, H.-K.; Wan, F.-N.; Zhu, Y.; Cao, D.-L.; Zhu, Y.-P.; et al. Identification of prognostic biomarkers in papillary renal cell carcinoma and PTTG1 may serve as a biomarker for predicting immunotherapy response. Ann. Med. 2022, 54, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Hanif, S.; Shaukat, I.; Rajput, S.A.; Shukat, R.; Huang, S.-C.; Almutairi, M.H.; Shaukat, S.; Ali, M.; Hassan, M.; et al. Up-regulation of inflammatory, oxidative stress, and apoptotic mediators via inflammatory, oxidative stress, and apoptosis-associated pathways in bovine endometritis. Microb. Pathog. 2024, 191, 106660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Cai, J.; Jiang, Q.; Loor, J.J.; Deng, G.; Li, X.; Yang, J. Interferon-tau protects bovine endometrial epithelial cells against inflammatory injury by regulating the PI3K/AKT/β-catenin/FoxO1 signaling axis. J. Dairy Sci. 2024, 107, 555–572. [Google Scholar] [CrossRef]
- Feng, R.; Qin, X.; Li, Q.; Olugbenga Adeniran, S.; Huang, F.; Li, Y.; Zhao, Q.; Zheng, P. Progesterone regulates inflammation and receptivity of cells via the NF-κB and LIF/STAT3 pathways. Theriogenology 2022, 186, 50–59. [Google Scholar] [CrossRef]
- Messer, J.S. The cellular autophagy/apoptosis checkpoint during inflammation. Cell Mol. Life Sci. 2017, 74, 1281–1296. [Google Scholar] [CrossRef]
- Thorne, A.; Bansal, A.; Necker-Brown, A.; Mostafa, M.M.; Gao, A.; Georgescu, A.; Kooi, C.; Leigh, R.; Newton, R. Differential regulation of BIRC2 and BIRC3 expression by inflammatory cytokines and glucocorticoids in pulmonary epithelial cells. PLoS ONE 2023, 18, e0286783. [Google Scholar] [CrossRef]
- Rath-Deschner, B.; Nogueira, A.V.B.; Memmert, S.; Nokhbehsaim, M.; Augusto Cirelli, J.; Eick, S.; Miosge, N.; Kirschneck, C.; Kesting, M.; Deschner, J.; et al. Regulation of Anti-Apoptotic SOD2 and BIRC3 in Periodontal Cells and Tissues. Int. J. Mol. Sci. 2021, 22, 591. [Google Scholar] [CrossRef]
- Mendoza-Rodríguez, M.; Arévalo Romero, H.; Fuentes-Pananá, E.M.; Ayala-Sumuano, J.-T.; Meza, I. IL-1β induces up-regulation of BIRC3, a gene involved in chemoresistance to doxorubicin in breast cancer cells. Cancer Lett. 2017, 390, 39–44. [Google Scholar] [CrossRef]
- Kelly, P.; Meade, K.G.; O’Farrelly, C. Non-canonical Inflammasome-Mediated IL-1β Production by Primary Endometrial Epithelial and Stromal Fibroblast Cells Is NLRP3 and Caspase-4 Dependent. Front. Immunol. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Yabas, M.; Hoyne, G.F. Immunological Phenotyping of Mice with a Point Mutation in Cdk4. Biomedicines 2023, 11, 2847. [Google Scholar] [CrossRef]
- Wang, Q.; He, G.; Hou, M.; Chen, L.; Chen, S.; Xu, A.; Fu, Y. Cell Cycle Regulation by Alternative Polyadenylation of CCND1. Sci. Rep. 2018, 8, 6824. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.-L.; Tian, F.-J.; Wu, F.; Zhang, J.; Zeng, W.-H.; Lin, Y.; Zhang, Y. Downregulation of CCNA2 disturbs trophoblast migration, proliferation, and apoptosis during the pathogenesis of recurrent miscarriage. Am. J. Reprod. Immunol. 2019, 82, e13144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dorstyn, L.; Lim, Y. The role of caspases as executioners of apoptosis. Biochem. Soc. Trans. 2022, 50, 33–45. [Google Scholar] [CrossRef]
- Fu, M.; Wang, J.; Xu, D.; Cao, N.; Li, W.; Li, F.; Liu, Z.; Li, Y.; Zhu, C.; Huang, Y.; et al. Polysaccharide of Atractylodes macrocephala Koidz alleviates LPS-induced proliferation, differentiation inhibition and excessive apoptosis in chicken embryonic myogenic cells. Vet. Med. Sci. 2024, 10, e1412. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, X.; Fu, T. Construction of a Bcl-2-shRNA expression vector and its effect on the mitochondrial apoptosis pathway in SW982 cells. Int. J. Mol. Med. 2017, 40, 1914–1920. [Google Scholar] [CrossRef][Green Version]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef]
- Taghavi, M.; Khosravi, A.; Mortaz, E.; Nikaein, D.; Athari, S.S. Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. Eur. J. Pharmacol. 2017, 808, 8–13. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Zhang, Z.; Chen, J.; Dong, G. Effects of Peptidoglycan, Lipoteichoic Acid and Lipopolysaccharide on Inflammation, Proliferation and Milk Fat Synthesis in Bovine Mammary Epithelial Cells. Toxins 2020, 12, 497. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.-F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Guo, S.; Yang, J.; Liu, J.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Matrine alleviates Staphylococcus aureus lipoteichoic acid-induced endometritis via suppression of TLR2-mediated NF-κB activation. Int. Immunopharmacol. 2019, 70, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Mahlakõiv, T.; Moeller, J.B.; Rankin, L.C.; Flamar, A.-L.; Kabata, H.; Monticelli, L.A.; Moriyama, S.; Putzel, G.G.; Rakhilin, N.; et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017, 549, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, Z.; Zhou, J.; Zhu, M.; Liu, J.; James, T.D. Recent progress in the development of fluorescent probes for imaging pathological oxidative stress. Chem. Soc. Rev. 2023, 52, 3873–3926. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Sottero, B.; Poli, G. Targeting tissue oxidative damage by means of cell signaling modulators: The antioxidant concept revisited. Pharmacol. Ther. 2010, 128, 336–374. [Google Scholar] [CrossRef]




| Sample | Clean Reads Pairs | Clean Base (bp) | Length | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| Control-1 | 45,197,184 | 13,559,155,200 | 150 | 99.14 | 96.88 | 51.40 |
| Control-2 | 42,640,925 | 12,792,277,500 | 150 | 99.07 | 96.63 | 51.34 |
| Control-3 | 41,070,775 | 12,321,232,500 | 150 | 99.07 | 96.64 | 51.02 |
| LPS-1 | 28,939,955 | 8,681,986,500 | 150 | 99.08 | 96.67 | 50.17 |
| LPS-2 | 42,217,295 | 12,665,188,500 | 150 | 99.10 | 96.77 | 51.31 |
| LPS-3 | 41,428,752 | 12,428,625,600 | 150 | 99.09 | 96.69 | 50.98 |
| Gene | Primer Sequence (5′-3′) | GenBank Accession No. |
|---|---|---|
| GAPDH | F:ATGCTGGTGCTGAGTATGTG | NC_037332.1 |
| R:CTTCTGGGTGGCAGTGAT | ||
| CDK4 | F:TTGGTGTCGGTGCCTAT | NC_037332.1 |
| R:CGAACGGTGCTGATGG | ||
| CCND1 | F:CTGGTCCTGGTGAACAAACTC | NC_037356.1 |
| R:CACAGAGGGCAACGAAGGT | ||
| CCNA2 | F:GAGTATGTCCCTGTTCCT | NC_037333.1 |
| R:TTGGTCCTGGTAAAGTAA | ||
| CDK6 | F:TGCCCACTGAAACCATAA | NC_037331.1 |
| R:GACCACTGAGGTAAGAGCC | ||
| Caspase-4 | F:GCTGCCCTTGACATCCTT | NC_037342.1 |
| R:CCCTGGCTGTGAGTTTCT | ||
| BIRC3 | F:GCCTCTTCTCAGCCTACT | NC_037342.1 |
| R:AGCATCATCCTTCGGTTC | ||
| NFκB | F:ACACGTATCGAAGGACAGCC | NC_037348.1 |
| R:GTCCTCCTTCACCTCTGTGC | ||
| TLR4 | F:TGCCTTCACTACAGGGACTT | NC_037335.1 |
| R:GGGACACCACGACAATAACC | ||
| IL-6 | F:ATCCTGAAGCAAAAGATCGCAG | NC_037331.1 |
| R:TTGCGTTCTTTACCCACTCGT | ||
| IL-1β | F:CGACGAGTTTCTGTGTGACG | NC_037338.1 |
| R:TCATGCAGAACACCACTTCTC | ||
| IL-10 | F:CACAGGCTGAGAACCACG | NC_037343.1 |
| R:CAGGGCAGAAAGCGATGA | ||
| BAX | F:CAAACTGGTGCTCAAGGC | NC_037345.1 |
| R:GCACTCCAGCCACAAAGAT | ||
| FADD | F:GGGCTTGAGGAGTGGGT | NC_037356.1 |
| R:GGTGAGCGTAGGCATCG | ||
| TRADD | F:ACTGCCCTAGCAGAGAGTGG | NC_037345.1 |
| R:CTGAAACGCAGTTGCACGAT | ||
| Caspase-3 | F:AGTGGTGCTGAGGATGAC | NC_037354.1 |
| R:ACCCGAGTAAGAATGTGC | ||
| Caspase-8 | F:TCACCCACGGAAACAAGG | NC_037329.1 |
| R:TCGGTCTCAACGGCTACA | ||
| Caspase-9 | F:CCCTTCCTTTGTTCATCTCC | NC_037343.1 |
| R:TGCTTGTCTGCTGGTCTTC | ||
| BCL-2 | F:CATGTGTGTGGAGAGCGTCA | NC_037351.1 |
| R:TACAGCTCCACAAAGGCGTC |
| Gene | Primer Sequence (5′-3′) | GenBank Accession No. |
|---|---|---|
| GAPDH | F:TTCAACGGCACAGTCAAG | NC_086022.1 |
| R:TACTCAGCACCAGCATCA | ||
| IL-6 | F:CAGAGTCATTCAGAGCAATAC | NC_086022.1 |
| R:GATGGTCTTGGTCCTTAGC | ||
| TLR4 | F:CTAGACACTTTATCCAGAGCCGTTG | NC_086023.1 |
| R:AAGGACAATGAAGATGATGCCAGAG | ||
| NFκB | F:TGTGGTGGAGGACTTGCTGAG | NC_086020.1 |
| R:GCTGCCTTGCTGTTCTTGAGTAG | ||
| IL-1β | F:GCAGCTTTCGACAGTGAGGAG | NC_086021.1 |
| R:TCTGGACAGCCCAAGTCAAG | ||
| TNF-α | F:ATGGGCTCCCTCTCATCAGT | NC_086028.1 |
| R:GCTTGGTGGTTTGCTACGAC | ||
| IL-5 | F:TGACGAGCAATGAGACGATG | NC_086038.1 |
| R:ACTTCCATTGCCCACTCTGT |
| Indicators | Group | |||||
|---|---|---|---|---|---|---|
| Blank | Model | L-PCP | M-PCP | H-PCP | Antibiotics | |
| Uterine index | 0.20 ± 0.81 e | 0.42 ± 0.71 a | 0.32 ± 0.84 ab | 0.31 ± 0.76 bc | 0.26 ± 0.78 cd | 0.24 ± 0.88 de |
| Spleen index | 0.16 ± 0.82 c | 0.22 ± 0.80 a | 0.21 ± 0.89 a | 0.19 ± 0.81 ab | 0.18 ± 0.81 bc | 0.17 ± 0.90 bc |
| Liver index | 3.21 ± 0.77 b | 3.36 ± 0.18 a | 3.27 ± 0.65 a | 3.10 ± 0.56 a | 3.13 ± 0.58 ab | 3.03 ± 0.52 ab |
| Item | Blank | Model | L-PCP | M-PCP | H-PCP | Antibiotics |
|---|---|---|---|---|---|---|
| Infiltration area of inflammatory cells (%) | 0.66 ± 0.04 d | 26.76 ± 2.56 a | 25.38 ± 1.80 a | 15.87 ± 1.76 b | 13.13 ± 1.27 c | 15.63 ± 1.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yan, P.; Zhu, J.; Gong, Y.; Liu, M.; Cheng, H.; Yi, T.; Zhang, F.; Yang, X.; Su, Y.; et al. From Genes to Healing: The Protective Mechanisms of Poria cocos Polysaccharide in Endometrial Health. Curr. Issues Mol. Biol. 2025, 47, 139. https://doi.org/10.3390/cimb47030139
Huang Y, Yan P, Zhu J, Gong Y, Liu M, Cheng H, Yi T, Zhang F, Yang X, Su Y, et al. From Genes to Healing: The Protective Mechanisms of Poria cocos Polysaccharide in Endometrial Health. Current Issues in Molecular Biology. 2025; 47(3):139. https://doi.org/10.3390/cimb47030139
Chicago/Turabian StyleHuang, Yongxi, Pupu Yan, Jun Zhu, Yinuo Gong, Man Liu, Haishan Cheng, Tilin Yi, Fuxian Zhang, Xiaolin Yang, Yingbing Su, and et al. 2025. "From Genes to Healing: The Protective Mechanisms of Poria cocos Polysaccharide in Endometrial Health" Current Issues in Molecular Biology 47, no. 3: 139. https://doi.org/10.3390/cimb47030139
APA StyleHuang, Y., Yan, P., Zhu, J., Gong, Y., Liu, M., Cheng, H., Yi, T., Zhang, F., Yang, X., Su, Y., & Guo, L. (2025). From Genes to Healing: The Protective Mechanisms of Poria cocos Polysaccharide in Endometrial Health. Current Issues in Molecular Biology, 47(3), 139. https://doi.org/10.3390/cimb47030139







