Development for Probiotics Based Insulin Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Construction of the Plasmid Harboring Insulin-CBT1 for Expression in E. coli
2.3. Purification of Insulin-CBT1 Protein from E. coli
2.4. Cell Proliferation and Damage Recovery Assays
2.5. Immunocytochemistry Using ImageXpress® Micro Confocal Microscopy
2.6. Western Blot Analysis
2.7. Chromatographic Characterization
2.8. Fluorescence Quenching Analysis
2.9. Circular Dichroism (CD)
2.10. Prediction of a 3D Model Using Protein Homology/Analogy Recognition Engine 2 (Phyre2)
2.11. LC-MS/MS Analysis and Database Searches
2.12. Adipocyte Differentiation and Glucose Uptake by 3T3-L1 Cells in Response to Insulin Analogs
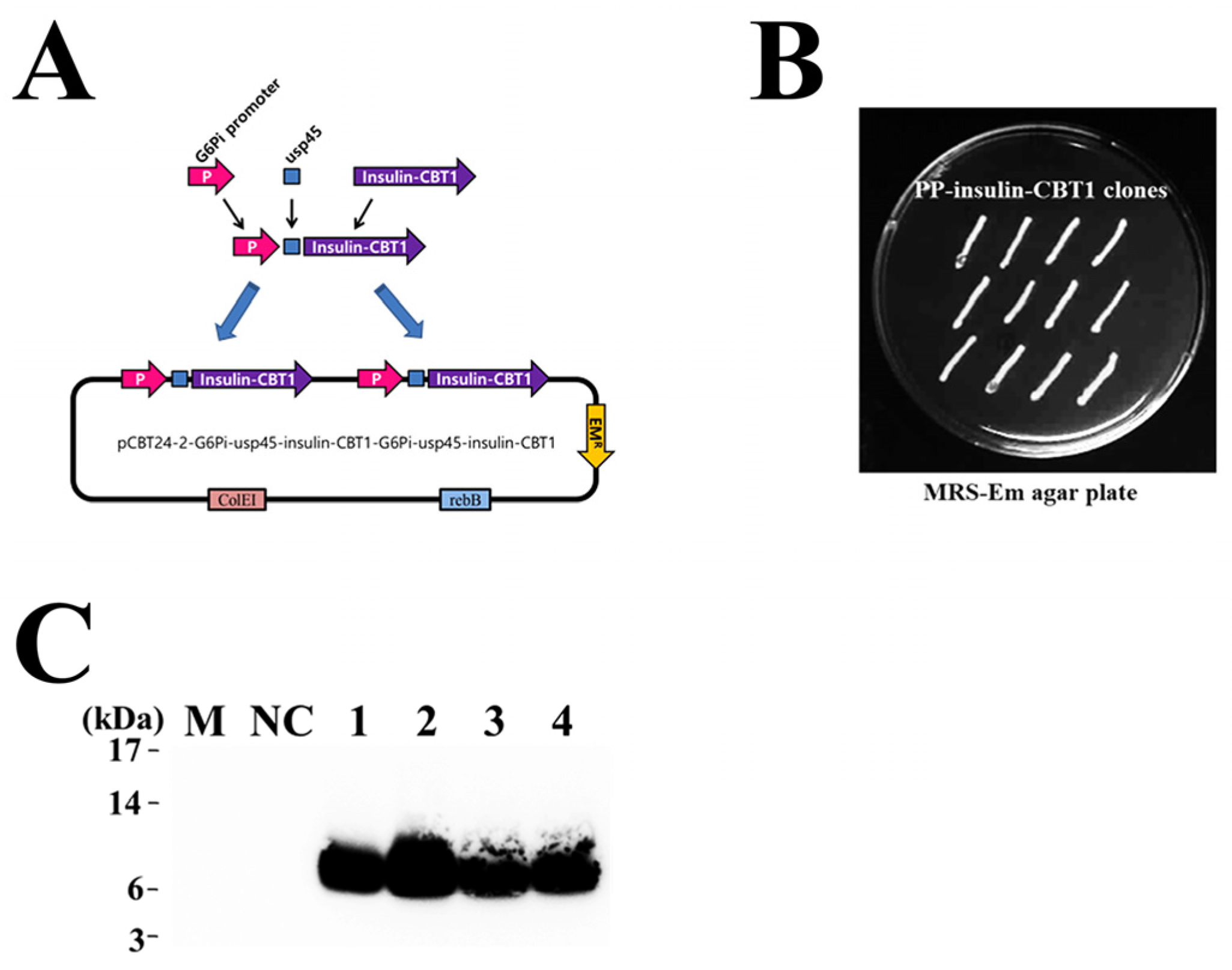
2.13. Construction of the Plasmid Harboring Insulin-CBT1 Gene for Use in the Pediococcus pentosaceus SL4 DDS
2.14. Transformation of Pediococcus pentosaceus SL4 and Detection of Insulin-CBT1 Proteins in the Culture Supernatant
3. Results and Discussion
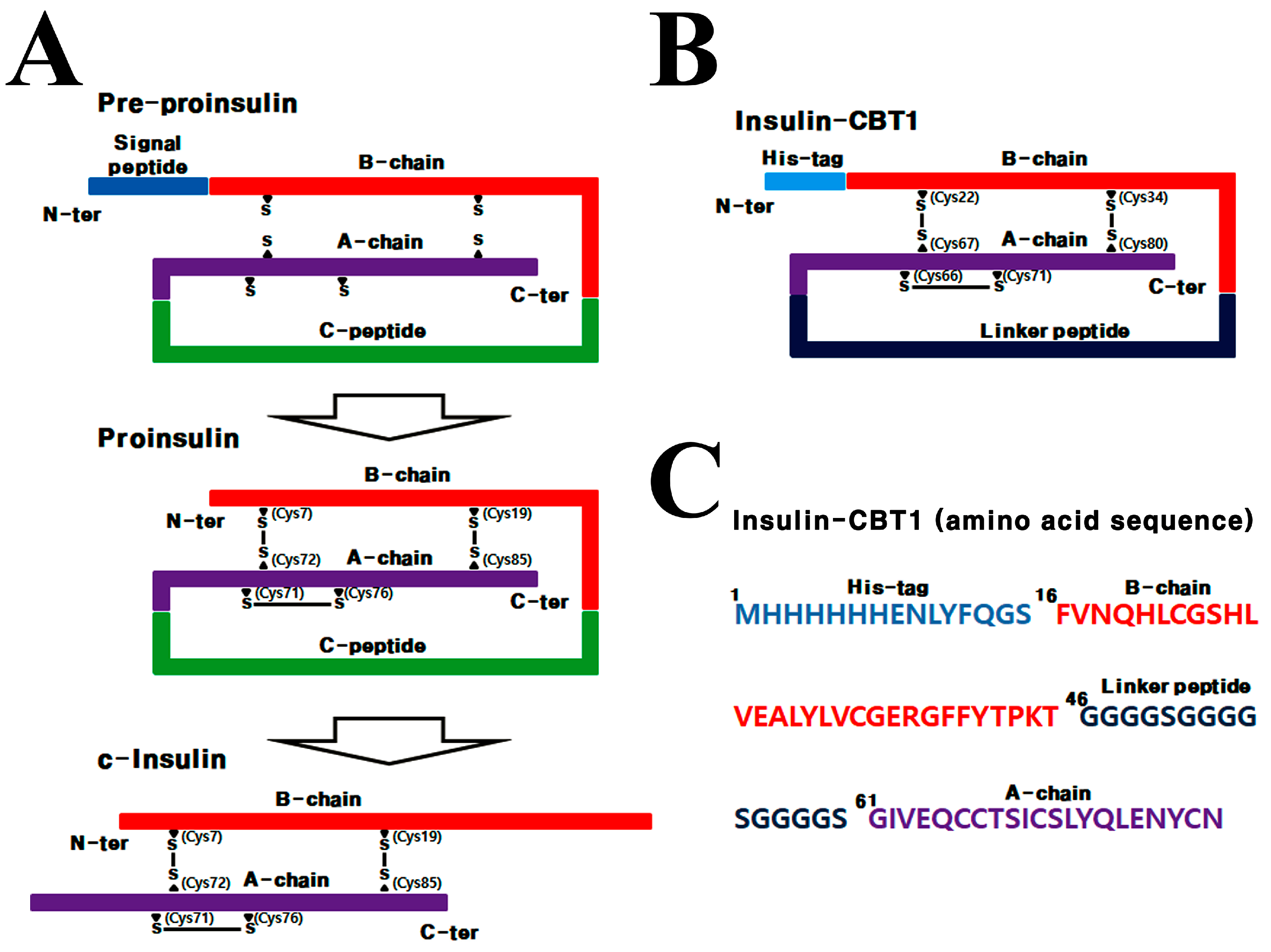
3.1. Design Strategy for Single-Chain Insulin (Insulin-CBT1) Expressed in E. coli
3.2. Biophysical Differences Between Insulin-CBT1 and c-Insulin
3.3. Disulfide Bond Pairing in Insulin-CBT1
3.4. Biological Activity of Insulin-CBT1 in MIN6 Cells (Pancreatic Beta-Cells)
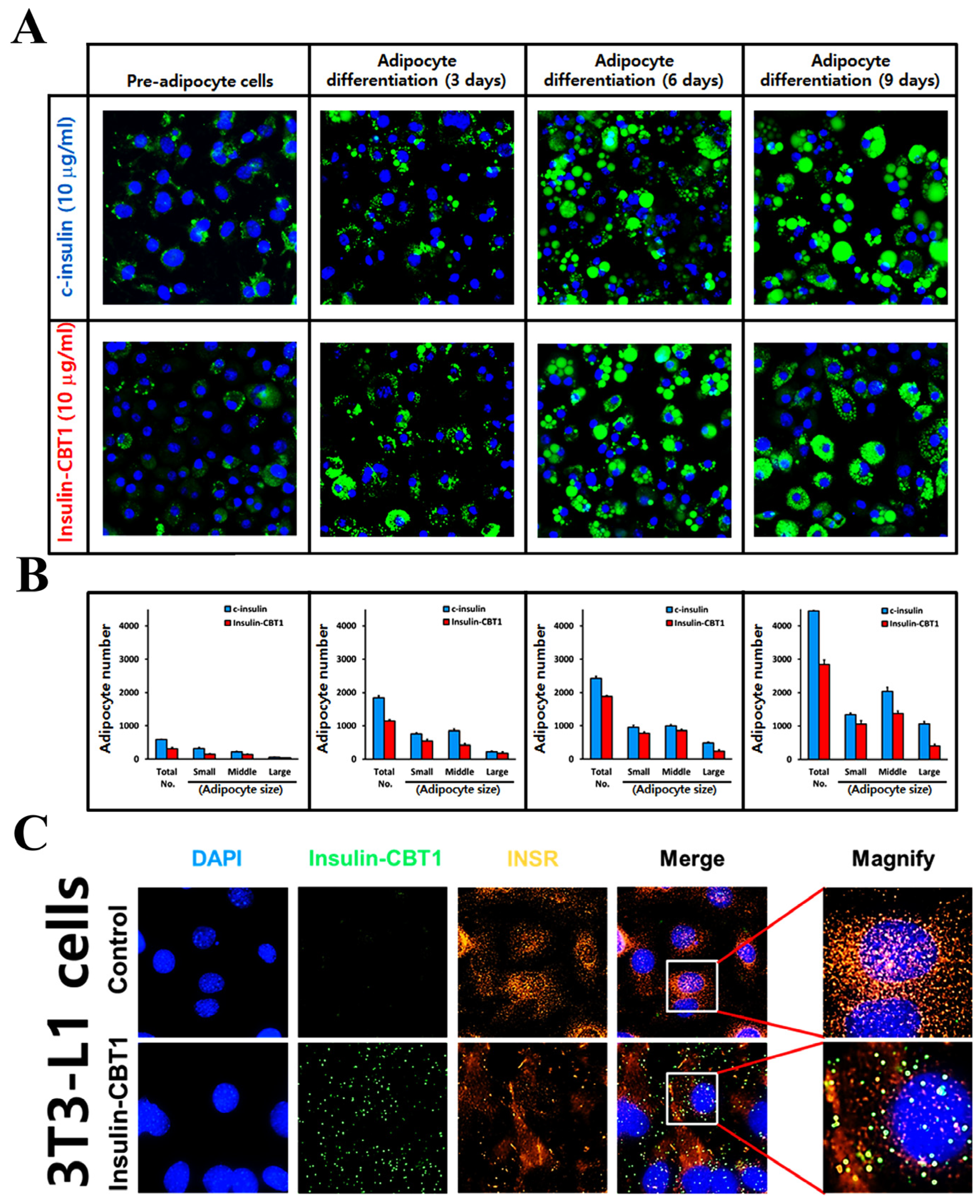
3.5. Biological Activity of Insulin-CBT1 in 3T3-L1 Cells (Pre-Adipocytic Cells)
3.6. Selection of Pediococcus pentosaceus SL4 Transformant and Detection of Insulin-CBT1 in the Culture Supernatant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kahn, C.R. The molecular mechanism of insulin action. Annu. Rev. Med. 1985, 36, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Brange, J.; Owens, D.R.; Kang, S.; Volund, A. Monomeric insulins and their experimental and clinical implications. Diabetes Care 1990, 13, 923–954. [Google Scholar] [CrossRef]
- Landgraf, W.; Sandow, J. Recombinant human insulins—Clinical efficacy and safety in diabetes therapy. Eur. J. Endocrinol. 2016, 12, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Masoud, S.; Tarlan, A.; Nafiseh, V.; Hossein, H.F.; Morteza, G. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar]
- Redwan, E.-R.M.; Matar, S.M.; El-Aziz, G.A.; Serour, E.A. Synthesis of the human insulin gene: Protein expression, scaling up and bioactivity. Prep. Biochem. Biotechnol. 2008, 38, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Romanik-Chruścielewska, A.; Mikiewicz, D.; Łukasiewicz, N.; Sokołowska, I.; Antosik, J.; Sobolewska-Ruta, A.; Bierczyńska-Krzysik, A.; Zaleski, P.; Płucienniczak, A. Expression and purification of recombinant human insulin from E. coli 20 strain. Protein Expr. Purif. 2019, 157, 63–69. [Google Scholar] [CrossRef]
- Aslam, F.; Latif, K.; Waseem, R.; Naz, S.; Iftikhar, S. Recombinant human proinsulin expression in E. coli by altering 5′ untranslated and translated region. Gene Technol. 2019, 8, 1. [Google Scholar]
- Goeddel, D.V.; Kleid, D.G.; Bolivar, F.; Heyneker, H.L.; Yansura, D.G.; Crea, R.; Hirose, T.; Kraszewski, A.; Itakura, K.; Riggs, A.D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc. Natl. Acad. Sci. USA 1979, 76, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Yousefi, R. Human αB-crystallin as fusion protein and molecular chaperone increases the expression and folding efficiency of recombinant insulin. PLoS ONE 2018, 13, e0206169. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L. Do insulin-treated diabetic patients use an injection-meal-interval in daily life? Diabet. Med. 1995, 12, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Desmet, M.; Rutters, A.; Schmitt, H.; Satter, E. [Lys (B28), Pro (B29)] human insulin (LysPro): Patients treated with LysPro versus human regular insulin-quality of life assessment. Diabetes 1994, 43 (Suppl. S1), 167. [Google Scholar]
- Fullerton, B.; Siebenhofer, A.; Jeitler, K.; Horvath, K.; Semlitsch, T.; Berghold, A.; Plank, J.; Pieber, T.R.; Gerlach, F.M. Short-acting insulin analogues versus regular human insulin for adults with type 1 diabetes mellitus. Cochrane Database Syst. Rev. 2016, 2016, CD012161. [Google Scholar] [CrossRef]
- Mosekilde, E.; Jensen, K.S.; Binder, C.; Pramming, S.; Thorsteinson, B. Modeling absorption kinetics of subcutaneous injected soluble insulin. J. Pharmacokinet. Biopharm. 1989, 17, 67–87. [Google Scholar] [CrossRef]
- Brange, J. The new era of biotech insulin analogues. Diabetologia 1997, 40 (Suppl. S2), S48–S53. [Google Scholar] [CrossRef]
- Schwartz, G.P.; Thompson Burke, G.; Katsoyannis, P.G. A superactive insulin: [B10-Aspartic acid] insulin (human). Proc. Natl. Acad. Sci. USA 1987, 84, 6408–6411. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.S.; Jorgensen, L.N.; Ipsen, M.; Voldsgaard, A.I.; Parving, H.H. Long-term comparison of human insulin analogue B10Asp and soluble human insulin in IDDM patients on a basal/bolus insulin regimen. Diabetologia 1995, 38, 592–598. [Google Scholar] [CrossRef]
- Rosskamp, R.H.; Park, G. Long-acting insulin analogs. Diabetes Care 1999, 22 (Suppl. S2), B109–B113. [Google Scholar] [PubMed]
- Jorgensen, S.; Vaag, A.; Langkjaer, L.; Hougaard, P.; Markussen, J. NovoSol Basal: Pharmacokinetics of a novel soluble long acting insulin analog. Br. Med. J. 1989, 299, 415–459. [Google Scholar] [CrossRef]
- Bahr, M.; Kolter, T.; Seipke, G.; Eckel, J. Growth promoting and metabolic activity of the human insulin analogue [GlyA21, ArgB31, ArgB32]insulin (HOE 901) in muscle cells. Eur. J. Pharmacol. 1997, 320, 259–265. [Google Scholar] [CrossRef]
- Kurtzhals, P.; Schaffer, L.; Sorensen, A.; Kristensen, C.; Jonassen, I.; Schmid, C.; Trub, T. Correlations of receptor binding and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000, 49, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Pais, J.M.; Varas, L.; Valdes, J.; Cabello, C.; Rodriguez, L.; Mansur, M. Modeling of mini-proinsulin production in Pichia pastoris using the AOX promoter. Biotechnol. Lett. 2003, 25, 251–255. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Z.-H.; Zhang, Y.-S.; Yao, S.-Y.; Xu, Y.-G.; Tang, Y.-H.; Zhu, S.-Q.; Cui, D.-F.; Feng, Y.-M. Human insulin from a precursor overexpressed in the methylotrophic yeast Pichia pastoris and a simple procedure for purifying the expression product. Biotechnol. Bioeng. 2001, 73, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Markussen, J.; Fiil, N.; Hansen, M.T.; Norris, K.; Ammerer, G.; Thim, L.; Voigt, H.O. DNA-Sequence Encoding Biosynthetic Insulin Precursors and Process for Preparing the Insulin Precursors and Human Insulin. US4916212A, 10 April 1990. [Google Scholar]
- Peavy, D.E.; Brunner, M.R.; Duckworth, W.C.; Hooker, C.S.; Frank, B.H. Receptor binding and biological potency of several split forms (conversion intermediates) of human proinsulin. Studies in cultured IM-9 lymphocytes and in vivo and in vitro in rats. J. Biol. Chem. 1985, 260, 13989–13994. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.; Andersen, A.S.; Hach, M.; Wiberg, F.C.; Schaffer, L.; Kjeldsen, T. A single-chain insulin-like growth factor I/insulin hybrid binds with high affinity to the insulin receptor. Biochem. J. 1995, 305, 981–986. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; Calder, P.C.; et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Quigley, E.M. Gut microbiota and the role of probiotics in therapy. Curr. Opin. Pharmacol. 2011, 11, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hong, S.-P.; Lim, S.-D. Physiological characteristics and anti-diabetic effect of Pediococcus pentosaceus KI62. Food Sci. Anim. Resour. 2021, 41, 274–287. [Google Scholar] [CrossRef] [PubMed]
- An, B.C.; Ryu, Y.; Yoon, Y.S.; Choi, O.; Park, H.J.; Kim, T.Y.; Kim, S.I.; Kim, B.K.; Chung, M.J. Colorectal cancer therapy using a pediococcus pentosaceus SL4 drug delivery system secreting lactic acid bacteria-derived protein p8. Mol. Cells 2019, 42, 755–762. [Google Scholar]
- Lane, M.D.; Reed, B.C.; Clements, P.R. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Prog. Clin. Biol. Res. 1981, 66 Pt A, 523–542. [Google Scholar]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb. Cell Fact. 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- An, B.C.; Hong, S.; Park, H.J.; Kim, B.-K.; Ahn, J.Y.; Ryu, Y.; An, J.H.; Chung, M.J. Anti-colorectal cancer effects of probiotic-derived p8 protein. Genes 2019, 10, 624. [Google Scholar] [CrossRef]
- Liu, Y.D.; Chou, R.Y.-T.; Dillon, T.M.; Poppe, L.; Spahr, C.; Shi, S.D.H.; Flynn, G.C. Protected hinge in the immunoglobulin G2-A2 disulfide isoform. Protein Sci. 2014, 23, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Roskamp, K.W.; Montelongo, D.M.; Anorma, C.D.; Bandak, D.N.; Chua, J.A.; Malecha, K.T.; Martin, R.W. Multiple Aggregation Pathways in Human γS-Crystallin and Its Aggregation-Prone G18V Variant. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Bekard, I.B.; Dunstan, D.E. Tyrosine autofluorescence as a measure of bovine insulin fibrillation. Biophys. J. 2009, 97, 2521–2531. [Google Scholar] [CrossRef]
- Jelvehgari, M.; Zakeri-Milani, P.; Siahi-Shadbad, M.R.; Loveymi, B.D.; Nokhodchi, A.; Azari, Z.; Valizadeh, H. Development of pH-sensitive insulin nanoparticles using Eudragit L100-55 and chitosan with different molecular weights. AAPS Open 2010, 11, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Chusuei, C.C.; Suarez, V. Nontoxic carbon dots potently inhibit human insulin fibrillation. Chem. Mater. 2015, 27, 1764–1771. [Google Scholar] [CrossRef]
- Jafari, E.; Gheysarzadeh, A.; Mahnam, K.; Shahmohammadi, R.; Ansari, A.; Bakhtyari, H.; Mofid, M.R. In silico interaction of insulin-like growth factor binding protein 3 with insulin-like growth factor 1. Res. Pharm. Sci. 2018, 13, 332–342. [Google Scholar]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B.; De Bakker, P.I.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- An, B.C.; Lee, S.S.; Jung, H.S.; Kim, J.Y.; Lee, Y.; Lee, K.W.; Lee, S.Y.; Tripathi, B.N.; Chung, B.Y. An additional cysteine in a typical 2-Cys peroxiredoxin of Pseudomonas promotes functional switching between peroxidase and molecular chaperone. FEBS Lett. 2015, 589 Pt B, 2831–2840. [Google Scholar] [CrossRef]
- Joo, J.I.; Kim, D.H.; Yun, J.W. Extract of Chaga mushroom (Inonotus obliquus) stimulates 3T3-L1 adipocyte differentiation. Phytother. Res. 2010, 24, 1592–1599. [Google Scholar] [CrossRef]
- Tordjman, K.M.; Leingang, K.A.; James, D.E.; Mueckler, M.M. Differential regulation of two distinct glucose transporter species expressed in 3T3-L1 adipocytes: Effect of chronic insulin and tolbutamide treatment. Proc. Natl. Acad. Sci. USA 1989, 86, 7761–7765. [Google Scholar] [CrossRef] [PubMed]
- Parkin, C.G.; Schwenke, S.; Ossege, A.K.; Gruchmann, T. Use of an integrated tool for interpretation of blood glucose data improves correctness of glycemic risk assessment in individuals with type 1 and type 2 diabetes. J. Diabetes Sci. Technol. 2017, 11, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ye, M.; Cao, Y.; Wang, C. Losartan improves palmitate-induced insulin resistance in 3T3-L1 adipocytes through upregulation of Src phosphorylation. Exp. Clin. Endocrinol. Diabetes 2017, 125, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Villa-Komaroff, L.; Broome, S.; Naber, S.P.; Efstratiadis, A.; Lomedico, P.; Tizard, R.; Chick, W.L.; Gilbert, W. The synthesis of insulin in bacteria: A model for the production of medically useful proteins in prokaryotic cells. Birth Defects Orig. Artic. Ser. 1980, 16, 53–68. [Google Scholar] [PubMed]
- Kanatsuna, N.; Papadopoulos, G.K.; Moustakas, A.K.; Lenmark, A. Etiopathogenesis of insulin autoimmunity. Anat. Res. Int. 2012, 2012, 457546. [Google Scholar] [CrossRef]
- Hua, Q.-X.; Mayer, J.P.; Jia, W.; Zhang, W.; Weiss, M.A. The folding nucleus of the insulin superfamily: A flexible peptide model foreshadows the native state. J. Biol. Chem. 2006, 281, 28131–281042. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Peng, Y.-C.; Yang, K.-M. Cellular signaling pathways regulating β-cell proliferation as a promising therapeutic target in the treatment of diabetes. Exp. Ther. Med. 2018, 16, 3275–3285. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Bernal-Mizrachi, E.; Alejandro, E.U.; Han, Z.; Kalynyak, T.B.; Li, H.; Beith, J.L.; Gross, J.; Warnock, G.L.; Townsend, R.R.; et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc. Natl. Acad. Sci. USA 2006, 103, 19575–19580. [Google Scholar] [CrossRef]
- Bonner-Weir, S. Life and death of the pancreatic β cells. Trends Endocrinol. Metab. 2000, 11, 375–378. [Google Scholar] [CrossRef]
- Luo, C.; Yu, L.-T.; Yang, M.-Q.; Li, X.; Zhang, Z.-Y.; Alfred, M.O.; Liu, J.-L.; Wang, M. Recombinant Reg3β protein protects against streptozotocin-induced β-cell damage and diabetes. Sci. Rep. 2016, 6, 35640. [Google Scholar] [CrossRef]
- Draznin, B.; Goodman, M.; Leitner, J.W.; Sussman, K.E. Feedback inhibition of insulin on insulin secretion in isolated pancreatic islets. Endocrinology 1986, 118, 1054–1058. [Google Scholar] [CrossRef]
- Belfiore, A.; Malaguarnera, R. Insulin receptor and cancer. Endocr. Relat. Cancer 2011, 18, R125–R147. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.J.; Leitner, J.W.; Watson, P.; Nesterova, A.; Reusch, J.E.-B.; Goalstone, M.L.; Draznin, B. Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J. Biol. Chem. 2001, 276, 28430–28435. [Google Scholar] [CrossRef] [PubMed]
- Song, E.-K.; Lee, Y.-R.; Kim, Y.-R.; Yeom, J.-H.; Yoo, C.-H.; Kim, H.-K.; Park, H.-M.; Kang, H.-S.; Kim, J.-S.; Kim, U.-H.; et al. NAADP mediates insulin-stimulated glucose uptake and insulin sensitization by PPARγ in adipocytes. Cell Rep. 2012, 2, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Chen, Y.; Chi, Z.; Wang, Y. Insulin and its single-chain analogue. Appl. Microbiol. Biotechnol. 2019, 103, 8737–8751. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.P.; Holder, A.L.; Catchpole, B. Recombinant canine single chain insulin analogues: Insulin receptor binding capacity and ability to stimulate glucose uptake. Vet. J. 2014, 202, 436–442. [Google Scholar] [CrossRef]
- Mikiewicz, D.; Bierczyńska-Krzysik, A.; Sobolewska, A.; Stadnik, D.; Bogiel, M.; Pawłowska, M.; Wójtowicz-Krawiec, A.; Baran, P.A.; Łukasiewicz, N.; Romanik-Chruścielewska, A.; et al. Soluble insulin analogs combining rapid- and long-acting hypoglycemic properties—From an efficient E. coli expression system to a pharmaceutical formulation. PLoS ONE 2017, 12, e0172600. [Google Scholar] [CrossRef] [PubMed]
- Brange, J.; Ribel, U.; Hansen, J.F.; Dodson, G.; Hansen, M.T.; Havelund, S.; Melberg, S.G.; Norris, F.; Norris, K.; Snel, L.; et al. Monomeric insulins obtained by protein engineering and their medical implications. Nature 1988, 333, 679–682. [Google Scholar] [CrossRef]
- Brange, J.; Dodson, G.G.; Xiao, B. Designing insulin for diabetes therapy by protein engineering. Curr. Opin. Struct. Biol. 1991, 1, 934–940. [Google Scholar] [CrossRef]
- Markussen, J.; Hougaard, P.; Ribel, U.; Sorrenson, A.; Sorrenson, E. Soluble, prolonged-acting insulin derivatives. II. Degree of protraction and crystallizability of insulins substituted in positions A17, B8, B13, B27 and B30. Protein Eng. 1987, 1, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Glidden, M.D.; Yang, Y.; Smith, N.A.; Phillips, N.B.; Carr, K.; Wickramasinghe, N.P.; Ismail-Beigi, F.; Lawrence, M.C.; Smith, B.J.; Weiss, M.A. Solution structure of an ultra-stable single-chain insulin analog connects protein dynamics to a novel mechanism of receptor binding. J. Biol. Chem. 2018, 293, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.X.; Nakagawa, S.H.; Jia, W.; Huang, K.; Phillips, N.B.; Hu, S.Q.; Weiss, M.A. Design of an active ultra-stable single-chain insulin analog: Synthesis, structure, and therapeutic implications. J. Biol. Chem. 2008, 283, 14703–14716. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, A.; Liu, M.; Zhang, Y.; Arvan, P. Single-Chain Insulins as Receptor Agonists. Mol. Endocrinol. 2009, 23, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, M. Long-waited dream of oral insulin: Where did we reach? Asian. J. Pharm. Clin. Res. 2011, 4, 15–20. [Google Scholar]
- Fuller, R. Probiotics in human medicine. Gut 1991, 32, 439–442. [Google Scholar] [CrossRef]
- Saarela, M.; Mogensen, G.; Fonden, R.; Matto, J.; Mattila-Sandholm, T. Probiotic bacteria: Safety, functional and technological properties. J. Biotechnol. 2000, 84, 197–215. [Google Scholar] [CrossRef]

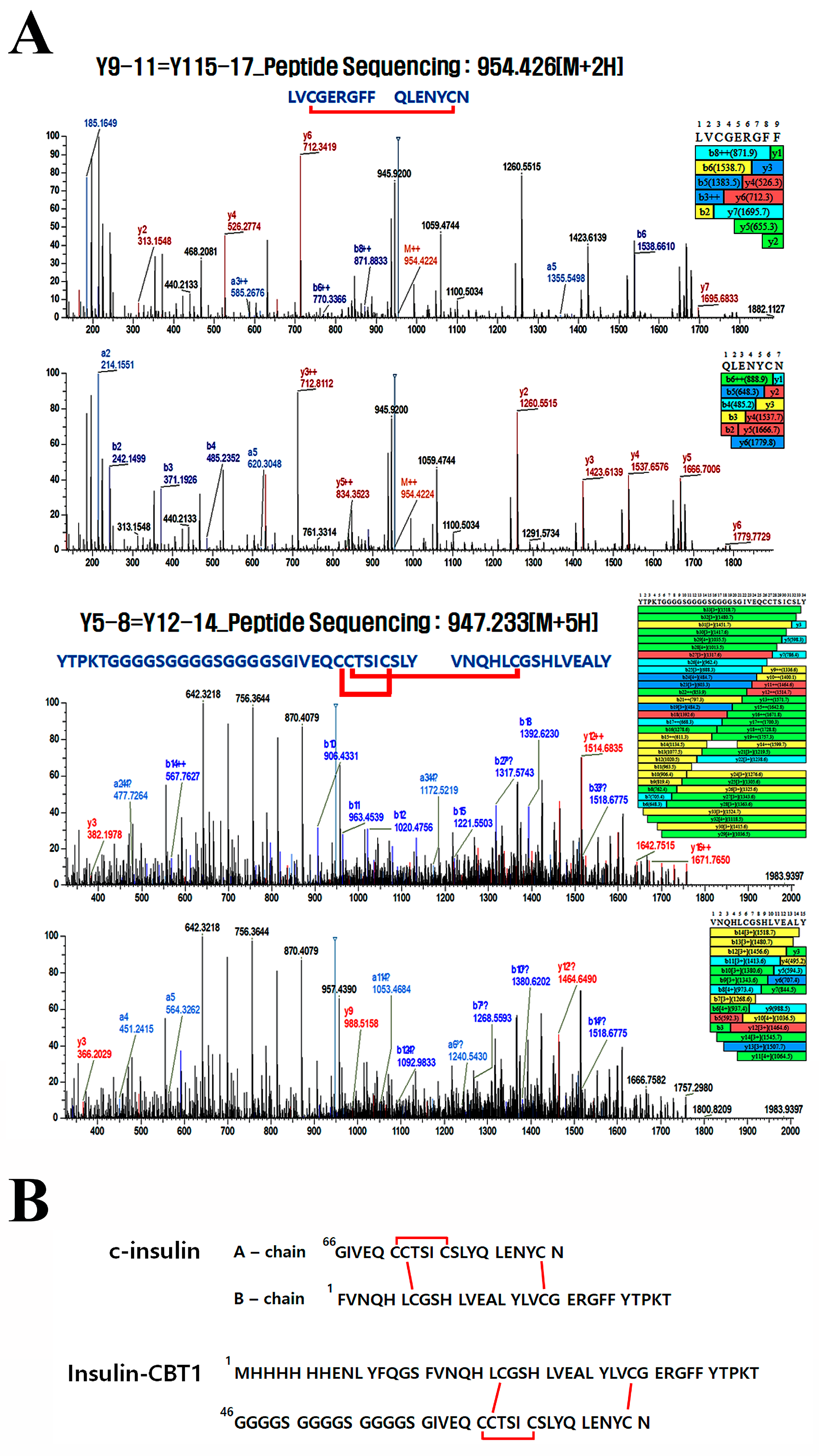


| Theoretical Mass (Y) | Practical Mass | |||||
|---|---|---|---|---|---|---|
| Fragment No. | Residue No. | S=S Bond | m/z | Charge | m /z | RT |
| Y9–11 = Y15– 17 | 32–40 = 75–81 | Cys34=Cys80 | 636.620 | 3 | 636.619 | 25.43 |
| Y12–14 = Y15–17 | 41–74 = 75–81 | Cys66=Cys71 or Cys67=Cys80 | 983.922 | 4 | 983.916 | 26.47 |
| Y5–8 = Y12–14 | 17–31 = 41–74 | Cys66=Cys71 or Cys67=Cys22 | 947.233 | 5 | 947.231 | 28.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.C.; Lee, J.; Won, H.Y.; Ryu, Y.; Chung, M.J. Development for Probiotics Based Insulin Delivery System. Curr. Issues Mol. Biol. 2025, 47, 137. https://doi.org/10.3390/cimb47030137
An BC, Lee J, Won HY, Ryu Y, Chung MJ. Development for Probiotics Based Insulin Delivery System. Current Issues in Molecular Biology. 2025; 47(3):137. https://doi.org/10.3390/cimb47030137
Chicago/Turabian StyleAn, Byung Chull, Jusung Lee, Hye Yeon Won, Yongku Ryu, and Myung Jun Chung. 2025. "Development for Probiotics Based Insulin Delivery System" Current Issues in Molecular Biology 47, no. 3: 137. https://doi.org/10.3390/cimb47030137
APA StyleAn, B. C., Lee, J., Won, H. Y., Ryu, Y., & Chung, M. J. (2025). Development for Probiotics Based Insulin Delivery System. Current Issues in Molecular Biology, 47(3), 137. https://doi.org/10.3390/cimb47030137





