Immunomodulatory Effects of Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition
Abstract
1. Introduction
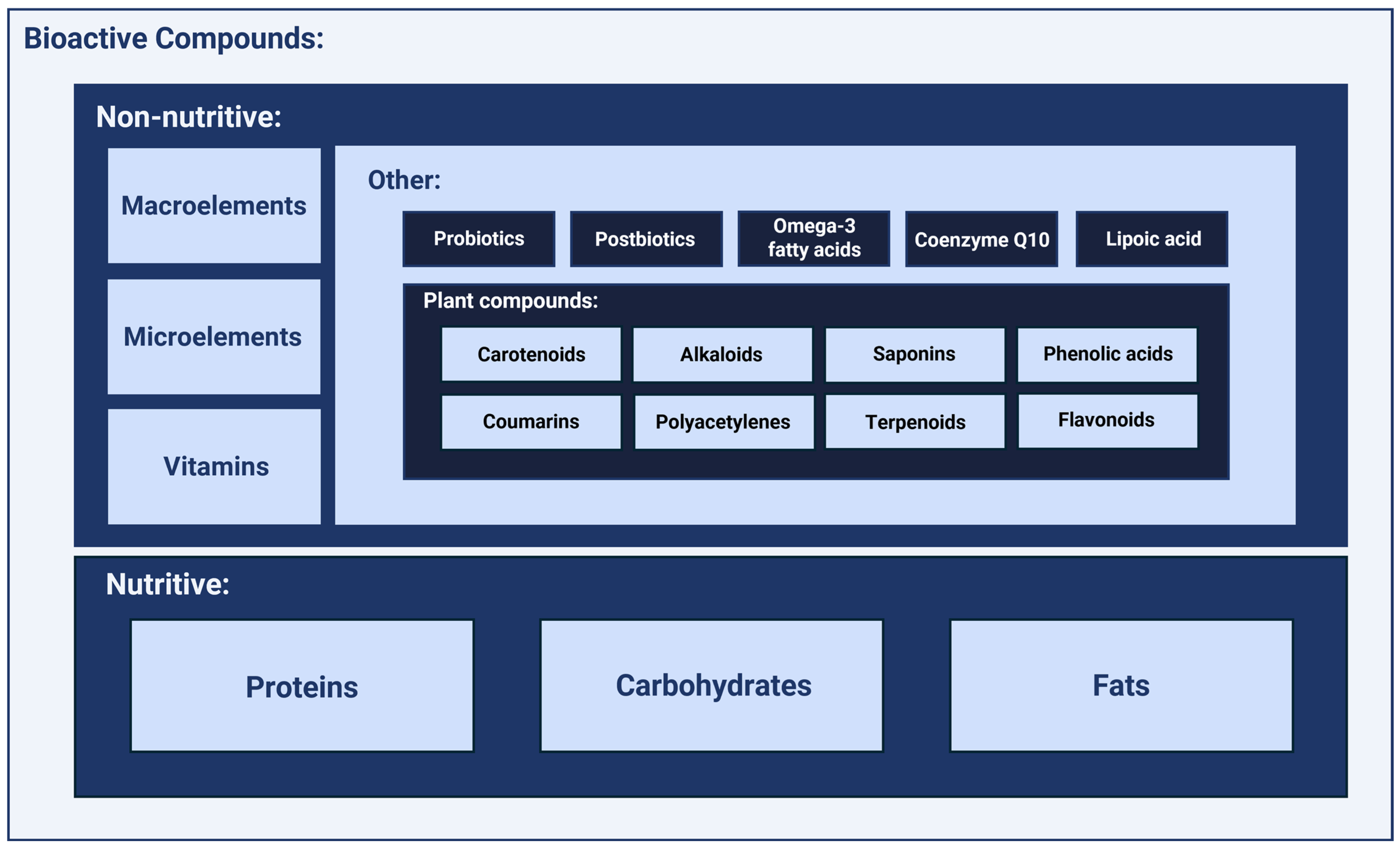
2. Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition
2.1. Macroelements
2.1.1. Magnesium
2.1.2. Calcium
2.1.3. Potassium
2.1.4. Sodium
2.1.5. Phosphorus
2.1.6. Sulfur
2.2. Microelements
2.2.1. Zinc
2.2.2. Selenium
2.2.3. Iron
2.2.4. Iodine
2.2.5. Copper
2.2.6. Cobalt
2.2.7. Chromium
2.2.8. Manganese
2.3. Vitamins
2.3.1. Vitamin D
2.3.2. Vitamin E
2.3.3. Vitamin C
2.3.4. Vitamin B1
2.3.5. Vitamin B6
2.3.6. Vitamin B12
2.3.7. Folic Acid
2.3.8. Other B Vitamins
| Nutrient | Daily Requirement | Serum Concentration |
|---|---|---|
| Macroelements | ||
| Calcium (Ca) | 1000–1200 mg | 2.2–2.6 mmol/L (8.8–10.4 mg/dL) |
| Magnesium (Mg) | 300–400 mg | 0.75–1.0 mmol/L (1.8–2.4 mg/dL) |
| Potassium (K) | 3500–4700 mg | 3.5–5.0 mmol/L |
| Sodium (Na) | 1500–2300 mg | 135–145 mmol/L |
| Phosphorus (P) | 700 mg | 0.81–1.45 mmol/L (2.5–4.5 mg/dL) |
| Chloride (Cl) | 2300 mg | 96–106 mmol/L |
| Microelements | ||
| Iron (Fe) | 8–18 mg (pregnant women: 27 mg) | 10–30 µmol/L (60–170 µg/dL) |
| Zinc (Zn) | 8–11 mg | 10–18 µmol/L (65–110 µg/dL) |
| Copper (Cu) | 0.9–1.2 mg | 11–22 µmol/L (70–140 µg/dL) |
| Selenium (Se) | 55 µg | 0.89–1.19 µmol/L (70–90 µg/L) |
| Iodine (I) | 150 µg (pregnant women: 220 µg) | 100–200 µg/L |
| Chromium (Cr) | 25–35 µg | 0.1–0.2 µg/L |
| Manganese (Mn) | 1.8–2.3 mg | 4–15 µg/L |
| Molybdenum (Mo) | 45 µg | 0.1–0.3 µg/L |
| Vitamins | ||
| Vitamin A | 700–900 µg (RAE) | 1.2–2.8 µmol/L (40–80 µg/dL) |
| Vitamin D | 15–20 µg (600–800 IU) | 75–125 nmol/L (30–50 ng/mL) |
| Vitamin E | 15 mg (22.4 IU) | 12–46 µmol/L |
| Vitamin K | 90–120 µg | 0.2–3.2 nmol/L |
| Vitamin C | 75–90 mg | 23–85 µmol/L |
| Vitamin B1 (thiamine) | 1.1–1.2 mg | 66–200 nmol/L |
| Vitamin B2 (riboflavin) | 1.1–1.3 mg | 136–370 nmol/L |
| Vitamin B3 (niacin) | 14–16 mg | 0.5–8.45 µmol/L |
| Vitamin B6 (pyridoxine) | 1.3–1.7 mg | 20–125 nmol/L |
| Vitamin B12 (cobalamin) | 2.4 µg | 148–740 pmol/L |
| Folic acid | 400 µg | 7–40 nmol/L |
| Biotin (vitamin H) | 30 µg | 200–500 ng/L |
| Pantothenic acid | 5 mg | 1–10 µmol/L |
2.4. Additional Immunomodulatory Compounds
2.4.1. Omega-3 Fatty Acids
2.4.2. Prebiotics
2.4.3. Probiotics
2.4.4. Postbiotics
2.4.5. Alpha-Lipoic Acid
2.4.6. Coenzyme Q10
2.4.7. Plant Compounds and Their Derivatives
Alkaloids
Phenolic Acids
Coumarins
Polyacetylenes
Saponins
Terpenoids
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed] [PubMed Central]
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum Magnesium: Time for a Standardized and Evidence-Based Reference Range. Magnes. Res. 2021, 34, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Aaseth, J.O. Magnesium: A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 10322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Folsom, A.R.; Brancati, F.L. Is Low Magnesium Concentration a Risk Factor for Coronary Heart Disease? The Atherosclerosis Risk in Communities (ARIC) Study. Am. Heart J. 1998, 136, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. Int. Rev. J. 2013, 4, 378S–383S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nielsen, F.H. Magnesium, Inflammation, and Obesity in Chronic Disease. Nutr. Rev. 2010, 68, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trapani, V.; Rosanoff, A.; Baniasadi, S.; Barbagallo, M.; Castiglioni, S.; Guerrero-Romero, F.; Iotti, S.; Mazur, A.; Micke, O.; Pourdowlat, G.; et al. The Relevance of Magnesium Homeostasis in COVID-19. Eur. J. Nutr. 2021, 61, 625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beto, J.A. The role of calcium in human aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez de Victoria, E. El calcio, esencial para la salud Calcium, essential for health. Nutr. Hosp. 2016, 33 (Suppl. S4), 341. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, D.O. Calcium’s Role in Orchestrating Cancer Apoptosis: Mitochondrial-Centric Perspective. Int. J. Mol. Sci. 2023, 24, 8982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terrell, K.; Choi, S.; Choi, S. Calcium’s Role and Signaling in Aging Muscle, Cellular Senescence, and Mineral Interactions. Int. J. Mol. Sci. 2023, 24, 17034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, E.; Sharma, S. Physiology, Calcium. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Young, W. Role of calcium in central nervous system injuries. J. Neurotrauma 1992, 9 (Suppl. 1), S9–S25. [Google Scholar] [PubMed]
- Sharma, A.; Schray, A.; Bartolovic, M.; Roesch-Ely, D.; Aschenbrenner, S.; Weisbrod, M. Relationship between serum calcium and neuropsychological performance might indicate etiological heterogeneity underlying cognitive deficits in schizophrenia and depression. Psychiatry Res. 2017, 252, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review. Dietary Reference Intakes for Vitamin, D. In Calcium Dietary Reference Intakes for Calcium Vitamin D.; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [PubMed]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2021, 14, 180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feske, S.; Wulff, H.; Skolnik, E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium-Calcineurin-NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanham-New, S.A.; Lambert, H.; Frassetto, L. Potassium. Adv. Nutr. Int. Rev. J. 2012, 3, 820–821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Udensi, U.K.; Tchounwou, P.B. Potassium Homeostasis, Oxidative Stress, and Human Disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McLean, R.M.; Wang, N.X. Potassium. Adv. Food Nutr. Res. 2021, 96, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Potassium and health. Adv. Nutr. Int. Rev. J. 2013, 4, 368S–377S. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, R.; Slentz, C.; Davenport, C.A.; Johnson, J.; Lin, P.H.; Muehlbauer, M.; D’Alessio, D.; Svetkey, L.P.; Edelman, D. Effects of potassium supplements on glucose metabolism in African Americans with prediabetes: A pilot trial. Am. J. Clin. Nutr. 2017, 106, 1431–1438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Elia, L. Potassium Intake and Human Health. Nutrients 2024, 16, 833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oberleithner, H.; Callies, C.; Kusche-Vihrog, K.; Schillers, H.; Shahin, V.; Riethmüller, C.; Macgregor, G.A.; de Wardener, H.E. Potassium softens vascular endothelium and increases nitric oxide release. Proc. Natl. Acad. Sci. USA 2009, 106, 2829–2834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, K.; Su, T.; Li, M.; Xu, B.; Xu, M.; Lu, J.; Liu, J.; Bi, Y.; Ning, G. Serum potassium level is associated with metabolic syndrome: A population-based study. Clin. Nutr. 2013, 33, 521–527. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences; Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board. Committee to Review the Dietary Reference Intakes for Sodium and Potassium. Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press (US): Washington, DC, USA, 2019. [Google Scholar] [PubMed]
- McGuire, S. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Departments of Agriculture and Health and Human Services, 2015. Adv. Nutr. Int. Rev. J. 2016, 7, 202–204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Vodnala, S.K.; Eil, R.; Kishton, R.J.; Sukumar, M.; Yamamoto, T.N.; Ha, N.H.; Lee, P.H.; Shin, M.; Patel, S.J.; Yu, Z.; et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 2019, 363, eaau0135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 2020, 145, 187–241. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and potassium in health and disease. In Interrelations Between Essential Metal Ions and Human Diseases; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; Volume 13, pp. 29–47. [Google Scholar] [CrossRef] [PubMed]
- Bie, P. Mechanisms of sodium balance: Total body sodium, surrogate variables, and renal sodium excretion. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R945–R962. [Google Scholar] [CrossRef] [PubMed]
- Strazzullo, P.; Leclercq, C. Sodium. Adv. Nutr. Int. Rev. J. 2014, 5, 188–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gagnon, K.B.; Delpire, E. Sodium Transporters in Human Health and Disease. Front. Physiol. 2021, 11, 588664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Lilly, M.N.; Shapiro, J.I. Targeting Na/K-ATPase Signaling: A New Approach to Control Oxidative Stress. Curr. Pharm. Des. 2018, 24, 359–364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. WHO Global Report on Sodium Intake Reduction, 1st ed.; World Health Organization: Geneva, Switzerland, 2023; ISBN 978-92-4-006998-5. [Google Scholar]
- Sodium Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 12 November 2024).
- World Health Organization. World Health Organization Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150483-6. [Google Scholar]
- Bhat, S.; Marklund, M.; Henry, M.E.; Appel, L.J.; Croft, K.D.; Neal, B.; Wu, J.H.Y. A Systematic Review of the Sources of Dietary Salt Around the World. Adv. Nutr. 2020, 11, 677–686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilck, N.; Balogh, A.; Markó, L.; Bartolomaeus, H.; Müller, D.N. The role of sodium in modulating immune cell function. Nat. Rev. Nephrol. 2019, 15, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Quast, T.; Schröder, A.; Hucke, S.; Klotz, L.; Jantsch, J.; Gerzer, R.; Hemmersbach, R.; Kolanus, W. Salt-dependent chemotaxis of macrophages. PLoS ONE 2013, 8, e73439. [Google Scholar] [CrossRef]
- Junger, W.G.; Liu, F.C.; Loomis, W.H.; Hoyt, D.B. Hypertonic saline enhances cellular immune function. Circ. Shock. 1994, 42, 190–196. [Google Scholar] [PubMed]
- Shapiro, L.; Dinarello, C.A. Osmotic regulation of cytokine synthesis in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 12230–12234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bird, R.P.; Eskin, N.A.M. The emerging role of phosphorus in human health. Adv. Food Nutr. Res. 2021, 96, 27–88. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.H. Overview of the Vital Roles of Macro Minerals in the Human Body. J. Trace Elem. Miner. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Elser, J.J. Phosphorus: A limiting nutrient for humanity? Curr. Opin. Biotechnol. 2012, 23, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2020, 108, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press (US): Washington, DC, USA, 1997. [Google Scholar] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar] [CrossRef]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Dietary phosphorus in bone health and quality of life. Nutr. Rev. 2012, 70, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Uribarri, J. Contributions to total phosphorus intake: All sources considered. Semin. Dial. 2012, 26, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Vorland, C.J.; Stremke, E.R.; Moorthi, R.N.; Hill Gallant, K.M. Effects of Excessive Dietary Phosphorus Intake on Bone Health. Curr. Osteoporos. Rep. 2017, 15, 473–482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.Y.; Kao, T.W.; Peng, T.C.; Chen, Y.Y.; Yang, H.F.; Wu, C.J.; Chen, W.L. Examining the association between serum phosphate levels and leukocyte telomere length. Sci. Rep. 2020, 10, 5438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuro-OM Molecular Mechanisms Underlying Accelerated Aging by Defects in the FGF23-Klotho System. Int. J. Nephrol. 2018, 2018, 9679841. [CrossRef] [PubMed] [PubMed Central]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- University of Hawai’i at Mānoa Food Science and Human Nutrition Program. Human Nutrition: 2020 Edition. USA, 2020. Available online: https://pressbooks.oer.hawaii.edu/humannutrition2/ (accessed on 15 December 2024).
- Weiss, M.; Steiner, D.F.; Philipson, L.H. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar] [PubMed]
- Mitchell, S.C. Nutrition and sulfur. Adv. Food Nutr. Res. 2021, 96, 123–174. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar] [PubMed] [PubMed Central]
- Dong, Z.; Gao, X.; Chinchilli, V.M.; Sinha, R.; Muscat, J.; Winkels, R.M.; Richie, J.P., Jr. Association of sulfur amino acid consumption with cardiometabolic risk factors: Cross-sectional findings from NHANES III. eClinicalMedicine 2020, 19, 100248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albalat, E.; Telouk, P.; Albarède, F. Sulfur Isotope Measurement in Human Serum; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2015; Application Note. [Google Scholar]
- Hewlings, S.; Kalman, D. Sulfur in human health. EC Nutr. 2019, 14, 785–791. [Google Scholar]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadler, R.A.; Mallard, B.A.; Shandilya, U.K.; Hachemi, M.A.; Karrow, N.A. The Immunomodulatory Effects of Selenium: A Journey from the Environment to the Human Immune System. Nutrients 2024, 16, 3324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Li, C.; Wu, Q.; An, P.; Huang, L.; Wang, J.; Chen, C.; Chen, X.; Zhang, F.; Ma, L.; et al. Iron-dependent histone 3 lysine 9 demethylation controls B cell proliferation and humoral immune responses. Nat. Commun. 2019, 10, 2935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Aspects Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.S.; Li, Q.; Wang, H.Y.; Wang, R.F. JMJD3 as an epigenetic regulator in development and disease. Int. J. Biochem. Cell Biol. 2015, 67, 148–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polak, E.; Stępień, A.E.; Gol, O.; Tabarkiewicz, J. Potential Immunomodulatory Effects from Consumption of Nutrients in Whole Foods and Supplements on the Frequency and Course of Infection: Preliminary Results. Nutrients 2021, 13, 1157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuenca-Micó, O.; Delgado-González, E.; Anguiano, B.; Vaca-Paniagua, F.; Medina-Rivera, A.; Rodríguez-Dorantes, M.; Aceves, C. Effects of Molecular Iodine/Chemotherapy in the Immune Component of Breast Cancer Tumoral Microenvironment. Biomolecules 2021, 11, 1501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, J.; Liu, X.; Li, X.; Li, H.; Shi, L.; Xia, X.; He, B.L.; Meyer, T.F.; Li, X.; Sun, H.; et al. Copper regulates the host innate immune response against bacterial infection via activation of ALPK1 kinase. Proc. Natl. Acad. Sci. USA 2024, 121, e2311630121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, J.; Dong, L.; Liu, Y.M.; Hu, Y.; Jiang, C.; Liu, K.; Liu, L.; Song, Y.H.; Sun, M.; Xiang, X.C.; et al. Nickle-cobalt alloy nanocrystals inhibit activation of inflammasomes. Natl. Sci. Rev. 2023, 10, nwad179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Cui, L.; Chen, B.; Xiong, Q.; Zhan, Y.; Ye, J.; Yin, Q. Effect of chromium supplementation on hs-CRP, TNF-α and IL-6 as risk factor for cardiovascular diseases: A meta-analysis of randomized-controlled trials. Complement. Ther. Clin. Pract. 2021, 42, 101291. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Estêvão, M.D.; Qorbani, M.; Heydari, H.; Hosseini, A.S.; Fazelian, S.; Belančić, A.; Persad, E.; Rezamand, G.; Heshmati, J. The effect of chromium intake on oxidative stress parameters: A systematic review and meta-analysis. J. Trace Elements Med. Biol. 2021, 69, 126879. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, M.; Shang, Y.; Dou, M.; Gao, S.; Yang, H.; Zhang, F. Effects of co-supplementation of chromium and magnesium on metabolic profiles, inflammation, and oxidative stress in impaired glucose tolerance. Diabetes Vasc. Dis. Res. 2024, 21, 14791641241228156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mokgobu, M.I.; Cholo, M.C.; Anderson, R.; Steel, H.C.; Motheo, M.P.; Hlatshwayo, T.N.; Tintinger, G.R.; Theron, A.J. Oxidative induction of pro-inflammatory cytokine formation by human monocyte-derived macrophages following exposure to manganese in vitro. J. Immunotoxicol. 2014, 12, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Occupancy of CD11b/CD18 (Mac-1) divalent ion binding site(s) induces leukocyte adhesion. J. Immunol. 1991, 147, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Bohnsack, J.F.; Zhou, X.N. Divalent cation substitution reveals CD18- and very late antigen-dependent pathways that mediate human neutrophil adherence to fibronectin. J. Immunol. 1992, 149, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Q.; Mu, Q.; Xia, Z.; Min, J.; Wang, F. Manganese homeostasis at the host-pathogen interface and in the host immune system. Semin. Cell Dev. Biol. 2021, 115, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, Y.; Lv, M.; Zhang, R.; Guo, Z.; Wei, X.; Du, X.; Yang, J.; Li, T.; Wan, Y.; et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 2018, 48, 675–687.e7. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, H.M. The role of manganese superoxide dismutase in inflammation defense. Enzym. Res. 2011, 2011, 387176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monteith, A.J.; Miller, J.M.; Beavers, W.N.; Juttukonda, L.J.; Skaar, E.P. Increased Dietary Manganese Impairs Neutrophil Extracellular Trap Formation Rendering Neutrophils Ineffective at Combating Staphylococcus aureus. Infect. Immun. 2022, 90, e0068521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urena-Torres, P.; Souberbielle, J.C. Pharmacologic role of vitamin D natural products. Curr. Vasc. Pharmacol. 2014, 12, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2020, 143, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the Immune System: Genomic and Non-Genomic Actions. Mini-Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mustacich, D.J.; Bruno, R.S.; Traber, M.G. Vitamin E. Vitam. Horm. 2007, 76, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free. Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cannon, J.G.; Meydani, S.N.; Fielding, R.A.; Fiatarone, M.A.; Meydani, M.; Farhangmehr, M.; Orencole, S.F.; Blumberg, J.B.; Evans, W.J. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am. J. Physiol. Integr. Comp. Physiol. 1991, 260 Pt 2, R1235–R1240. [Google Scholar] [CrossRef] [PubMed]
- Capó, X.; Martorell, M.; Sureda, A.; Riera, J.; Drobnic, F.; Tur, J.A.; Pons, A. Effects of Almond- and Olive Oil-Based Docosahexaenoic- and Vitamin E-Enriched Beverage Dietary Supplementation on Inflammation Associated to Exercise and Age. Nutrients 2016, 8, 619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahalingam, D.; Radhakrishnan, A.K.; Amom, Z.; Ibrahim, N.; Nesaretnam, K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 2010, 65, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Han, S.N.; Wu, D. Vitamin E and immune response in the aged: Molecular mechanisms and clinical implications. Immunol. Rev. 2005, 205, 269–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanson, M.G.; Ozenci, V.; Carlsten, M.C.; Glimelius, B.L.; Frödin, J.E.; Masucci, G.; Malmberg, K.J.; Kiessling, R.V. A short-term dietary supplementation with high doses of vitamin E increases NK cell cytolytic activity in advanced colorectal cancer patients. Cancer Immunol. Immunother. 2006, 56, 973–984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li-Weber, M.; Weigand, M.A.; Giaisi, M.; Süss, D.; Treiber, M.K.; Baumann, S.; Ritsou, E.; Breitkreutz, R.; Krammer, P.H. Vitamin E inhibits CD95 ligand expression and protects T cells from activation-induced cell death. J. Clin. Investig. 2002, 110, 681–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2018, 71, 487–494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Bin Emran, T.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gasmi, A.; Shanaida, M.; Oleshchuk, O.; Semenova, Y.; Mujawdiya, P.K.; Ivankiv, Y.; Pokryshko, O.; Noor, S.; Piscopo, S.; Adamiv, S.; et al. Natural Ingredients to Improve Immunity. Pharmaceuticals 2023, 16, 528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vishwakarma, S.; Panigrahi, C.; Barua, S.; Sahoo, M.; Mandliya, S. Food nutrients as inherent sources of immunomodulation during COVID-19 pandemic. Leb. Wiss. Technol. 2022, 158, 113154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polegato, B.F.; Pereira, A.G.; Azevedo, P.S.; Costa, N.A.; Zornoff, L.A.M.; Paiva, S.A.R.; Minicucci, M.F. Role of Thiamin in Health and Disease. Nutr. Clin. Pr. 2019, 34, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elmadfa, I.; Meyer, A.L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1100–1115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batista, K.S.; Cintra, V.M.; Lucena, P.A.F.; Manhães-de-Castro, R.; Toscano, A.E.; Costa, L.P.; Queiroz, M.E.B.S.; de Andrade, S.M.; Guzman-Quevedo, O.; Aquino, J.S. The role of vitamin B12 in viral infections: A comprehensive review of its relationship with the muscle-gut-brain axis and implications for SARS-CoV-2 infection. Nutr. Rev. 2021, 80, 561–578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Visser, M.E.; Durao., S.; Sinclair., D.; Irlam, J.H.; Siegfried, N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst. Rev. 2017, 5, CD003650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obeid, R. High Plasma Vitamin B12 and Cancer in Human Studies: A Scoping Review to Judge Causality and Alterna-tive Explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurowska, K.; Kobylińska, M.; Antosik, K. Folic acid—Importance for human health and its role in COVID-19 therapy. Rocz. Państwowego Zakładu Hig. 2023, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska-Sachadyn, A.; Borzyszkowska, J.; Krzemiński, M.; Janowicz, A.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Limon, J. Folate/homocysteine me-tabolism and lung cancer risk among smokers. PLoS ONE 2019, 14, e0214462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munteanu, C.; Schwartz, B. B Vitamins, Glucoronolactone and the Immune System: Bioavailability, Doses and Efficiency. Nutrients 2023, 16, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perry, C.A.; Butterick, T.A. Biotin. Adv. Nutr. Int. Rev. J. 2024, 15, 100251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Institutes of Health, Office of Dietary Supplements. Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals [Internet]. 2023. Available online: https://ods.od.nih.gov (accessed on 15 December 2024).
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A Review of Dietary Zinc Recommendations and the Implications for Assessing Dietary Zinc Adequacy. Nutr. Res. Rev. 2008, 21, 79–86. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calder, P.C. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rousseaux, A.; Brosseau, C.; Bodinier, M. Immunomodulation of B Lymphocytes by Prebiotics, Probiotics and Synbiotics: Application in Pathologies. Nutrients 2023, 15, 269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alli, S.R.; Gorbovskaya, I.; Liu, J.C.W.; Kolla, N.J.; Brown, L.; Müller, D.J. The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies. Int. J. Mol. Sci. 2022, 23, 4494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Salek Farrokhi, A.; Darabi, N. Probiotics importance and their immunomodulatory properties. J. Cell. Physiol. 2018, 234, 8008–8018. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in Human Health: A Narrative Review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaźmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Połom, K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front. Cell. Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Sharma, K.; Ranabhat, D.; Thapa, R.; Aryal, D.; et al. Potential Therapeutic Applications of Plant-Derived Alkaloids against Inflammatory and Neurodegenerative Diseases. Evid Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trushina, E.N.; Vybornov, V.D.; Riger, N.A.; Mustafina, O.K.; Solntseva, T.N.; Timonin, A.N.; Zilova, I.S.; Rajabkadiev, R.M. Immunomodulating effects of using L-carnitine and coenzyme Q10 in the nutrition of junior athletes. Vopr. Pitan. 2019, 88, 40–49. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shi, L.J.; Li, S.G. The Immunomodulatory Effect of Alpha-Lipoic Acid in Autoimmune Diseases. BioMed Res. Int. 2019, 2019, 8086257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, H.; Yang, X.; Cao, Y.; Long, X.; Shang, H.; Jia, Z. Role of lipoic acid in multiple sclerosis. CNS Neurosci. Ther. 2021, 28, 319–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poles, J.; Karhu, E.; McGill, M.; McDaniel, H.R.; Lewis, J.E. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: A narrative review. J. Clin. Transl. Res. 2021, 7, 333–376. [Google Scholar] [PubMed] [PubMed Central]
- Mantle, D.; Hargreaves, I.P. Coenzyme Q10 and Autoimmune Disorders: An Overview. Int. J. Mol. Sci. 2024, 25, 4576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Fernández Vega, A.; de la Mata, M.; Delgado Pavón, A.; de Miguel, M.; Pérez Calero, C.; Villanueva Paz, M.; Cotán, D.; et al. Coenzyme q10 therapy. Mol. Syndromol. 2014, 5, 187–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faggian, M.; Bernabè, G.; Pauletto, A.; Loschi, F.; Tezze, C.; Merlo, R.; Merlo, L.; Sut, S.; Ferrarese, I.; Brun, P.; et al. Nutraceutical formulation for immune system modulation: Active constituents, in vitro antibacterial and immunomodulatory activity, and metabolomics analysis. Phytother. Res. 2023, 37, 5883–5896. [Google Scholar] [CrossRef] [PubMed]
- Fakhrolmobasheri, M.; Hosseini, M.S.; Shahrokh, S.G.; Mohammadi, Z.; Kahlani, M.J.; Majidi, S.E.; Zeinalian, M. Coenzyme Q10 and Its Therapeutic Potencies Against COVID-19 and Other Similar Infections: A Molecular Review. Adv. Pharm. Bull. 2021, 13, 233–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Pavon, A.D.; Alcocer-Gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M.; et al. Clinical applications of coenzyme Q10. Front. Biosci. 2014, 19, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Khan, M.I.; Mahesh, P.; Kumar, S.R.; Kumar, S.S. Preclinical and Clinical Role of Coenzyme Q10 Supplementation in Various Pathological States. Drug Res. 2022, 72, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ye, J.; Ji, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, ume 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. BioFactors 2019, 46, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leváková, L.; Lacko-Bartošová, M. Phenolic acids and antioxidant activity of wheat species: A review. Agriculture 2017, 63, 92–101. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Alvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rettie, A.E.; Tai, G. The pharmocogenomics of warfarin: Closing in on personalized medicine. Mol. Interv. 2006, 6, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemical—Isolation, Characterisation and Role in Human Health; BoD–Books on Demand: Norderstedt, Germany, 2015; pp. 533–538. [Google Scholar]
- Vianna, D.R.; Hamerski, L.; Figueiró, F.; Bernardi, A.; Visentin, L.C.; Pires, E.N.; Teixeira, H.F.; Salbego, C.G.; Eifler-Lima, V.L.; Battastini, A.M.; et al. Selective cytotoxicity and apoptosis induction in glioma cell lines by 5-oxygenated-6,7-methylenedioxycoumarins from Pterocaulon species. Eur. J. Med. Chem. 2012, 57, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharmacy, Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; BakarMohamad, A. Antioxidant activity of coumarins. Syst. Rev. Pharm. 2016, 8, 24–30. [Google Scholar] [CrossRef]
- Lin, T.H.; Chang, K.H.; Chiu, Y.J.; Weng, Z.K.; Sun, Y.C.; Lin, W.; Lee-Chen, G.J.; Chen, C.M. Neuroprotective Action of Coumarin Derivatives through Activation of TRKB-CREB-BDNF Pathway and Reduction of Caspase Activity in Neuronal Cells Expressing Pro-Aggregated Tau Protein. Int. J. Mol. Sci. 2022, 23, 12734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, P.S.; Kumar, A.; Kaur, K.; Jaitak, V. Recent Developments in Coumarin Derivatives as Neuroprotective Agents. Curr. Med. Chem. 2024, 31, 5702–5738. [Google Scholar] [CrossRef] [PubMed]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Coumarins: The Antimicrobial agents. Syst. Rev. Pharm. 2017, 8, 62–70. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry 2022, 201, 113288. [Google Scholar] [CrossRef] [PubMed]
- Rezende, A.M.A.; Albuquerque, A.L.S.; E Silva, M.J.T.C.; Cruvinel, W.D.M.; Gomes, C.M.; Borges, L.L.; Taft, C.A.; Da Silva, V.B. Drug-Like Properties Therapeutical Potential of Calendula officinalis, L. Active Ingredients. In Progress in Hydrogen Energy, Fuel Cells, Nano-Biotechnology and Advanced, Bioactive Compounds. Engineering Materials; Taft, C.A., de Lazaro, S.R., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Tiwari, P.; Sharma, B.; Guerrero-Perilla, C.; Coy-Barrera, E. Chapter 23—Analysis of polyacetylenes. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 707–722. [Google Scholar]
- El-Houri, R.B.; Kotowska, D.; Christensen, K.B.; Bhattacharya, S.; Oksbjerg, N.; Wolber, G.; Kristiansen, K.; Christensen, L.P. Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct. 2015, 6, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Oleszek, M.; Oleszek, W. Saponins in Food. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer: Singapore, 2020; pp. 1–40. [Google Scholar]
- Mohlakoana, M.; Moteetee, A. Southern African Soap Plants and Screening of Selected Phytochemicals and Quantitative Analysis of Saponin Content. Resources 2021, 10, 96. [Google Scholar] [CrossRef]
- Yıldırım, I.; Kutlu, T. Anti-cancer agents: Saponin and tannin. Int. J. Biol. Chem. 2015, 9, 332–340. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Ghanadian, S.M.; Bahrami, G.; Mansouri, K.; Iranpanah, A.; Farzaei, M.H.; Mojarrab, M. Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways. Metabolites 2023, 13, 323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, K.A.; Kim, H.S.; Kim, D.H.; Hyun, J.W. The role of a ginseng saponin metabolite as a DNA methyltransferase inhibitor in colorectal cancer cells. Int. J. Oncol. 2013, 43, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.D.; Kang, K.A.; Zhang, R.; Lim, C.M.; Kim, H.S.; Kim, D.H.; Jeon, Y.J.; Lee, C.H.; Park, J.; Chang, W.Y.; et al. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ. Toxicol. Pharmacol. 2010, 30, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules 2023, 29, 113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Aziz, M.; Ashour, A.; Melad, A.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 8, 282–288. [Google Scholar] [CrossRef]
- Deng, R.; Chen, X.; Zhao, S.; Zhang, Q.; Shi, Y. The effects and mechanisms of natural products on Helicobacter pylori eradication. Front. Cell. Infect. Microbiol. 2024, 14, 1360852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Algal Terpenoids: A Potential Source of Antioxidants for Cancer Therapy. In Terpenes and Terpenoids-Recent Advances; Perveen, S., Al-Taweel, A.M., Eds.; Intechopen: London, UK, 2021; ISBN 9781838819163. [Google Scholar]
- Weathers, P.J.; Elkholy, S.; Wobbe, K.K. Artemisinin: The biosynthetic pathway and its regulation in Artemisia annua, a terpenoid-rich species. Vitr. Cell. Dev. Biol. Plant 2006, 42, 309–317. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar] [PubMed]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.; Emami, S.A.; Tayarani-Najaran, Z. Artemisia: A promising plant for the treatment of cancer. Bioorg. Med. Chem. 2020, 28, 115180. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V. Lycopene and heart health. Mol. Nutr. Food Res. 2012, 56, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Park, M.-K.; Kim, S.-K.; Cho, Y.-H. Antioxidant Capacity and Anti-Inflammatory Activity of Lycopene in Watermelon. Int. J. Food Sci. Technol. 2014, 49, 2083–2091. [Google Scholar] [CrossRef]
- Tounta, D.; Spanos, G.; Tesseromatis, C. Alzheimer-Dementia: Drugs and Herbal preparations. J. Med. Plants Stud. 2021, 9, 63–74. [Google Scholar]
- More, M.P.; Motule, A.S.; Dongare, P.N.; Patinge, P.A.; Jawarkar, R.D.; Bakal, R.L.; Manwar, J.V. Pharmacognosy, phytochemistry, pharmacology and clinical application of Ginkgo biloba. GSC Biol. Pharm. Sci. 2021, 16, 229–240. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, Q.; Chen, W.; Bai, Y.; Hu, D.; Xie, X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol. Med. 2019, 25, 57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishida, S.; Satoh, H. Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clin. Chim. Acta 2003, 339, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eccles, R. Menthol: Effects on nasal sensation of airflow and the drive to breathe. Curr. Allergy Asthma Rep. 2003, 3, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Millqvist, E.; Ternesten-Hasséus, E.; Bende, M. Inhalation of menthol reduces capsaicin cough sensitivity and influences inspiratory flows in chronic cough. Respir. Med. 2012, 107, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Liu, S.C.; Chao, P.Z.; Lee, F.P. Menthol inhibiting parasympathetic function of tracheal smooth muscle. Int. J. Med. Sci. 2016, 13, 923–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Q.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q.; Zhang, H. Randomized controlled trials of the effects of capsaicin or menthol on irritable bowel syndrome: A systematic review and meta-analysis. Food Funct. 2024, 15, 11865–11874. [Google Scholar] [CrossRef] [PubMed]
- Rozza, A.L.; Meira de Faria, F.; Souza Brito, A.R.; Pellizzon, C.H. The gastroprotective effect of menthol: Involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS ONE 2014, 9, e86686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amato, A.; Liotta, R.; Mulè, F. Effects of menthol on circular smooth muscle of human colon: Analysis of the mechanism of action. Eur. J. Pharmacol. 2014, 740, 295–301. [Google Scholar] [CrossRef] [PubMed]
| Immune Function Roles | Non-Nutritive Bioactive Compounds |
|---|---|
| Maintaining the structural and functional integrity of innate barriers (e.g., skin, respiratory tract, and digestive tract) | Vitamins: A, D, E, C, B6, B12, folate; iron, zinc, omega-3 fatty acids, probiotics, prebiotics, sodium butyrate, phenolic acids |
| Differentiation, proliferation, functioning, and movement of innate immune cells | Vitamins: A, D, E, C, B6, B12, folate; zinc, iron, copper, selenium, magnesium, calcium, sodium, iodine, manganese |
| Roles in inflammation, antioxidant effects, and effects in oxidative burst | Vitamins: A, D, E, C, B6; zinc, iron, copper, selenium, magnesium, potassium, iodine, cobalt, chromium, manganese, omega-3 fatty acids, probiotics, prebiotics, sodium butyrate, coenzyme Q10, alkaloids, phenolic acids, coumarins, polyacetylenes, saponins, terpenoids |
| Differentiation, proliferation, and normal functioning of T cells | Vitamins: A, D, C, E, B6, B12, folate; zinc, iron, copper, selenium |
| Antibody production and development | Vitamins: A, D, C, E, B6, B12; folate, zinc, copper, selenium, magnesium, probiotics, prebiotics |
| Responses to antigen | Vitamins: A, D, E, folate, zinc, magnesium |
| Activity of the complement system | Vitamins: A, D, E, C, B6, B12, folate; iron, zinc, selenium, magnesium, probiotics, prebiotics, coenzyme Q10 |
| Antimicrobial effects | Vitamins: A, D, C; zinc, iron, copper, selenium, probiotics, prebiotics, coenzyme Q10, manganese, coumarins, polyacetylenes, saponins, terpenoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska-Baran, K.; Treichel, P.; Dardzińska, A.; Majcherczak, A.; Pilichowicz, A.; Szota, M.; Szymczak, B.; Alska, E.; Przybyszewska, J.; Bartuzi, Z. Immunomodulatory Effects of Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition. Curr. Issues Mol. Biol. 2025, 47, 89. https://doi.org/10.3390/cimb47020089
Napiórkowska-Baran K, Treichel P, Dardzińska A, Majcherczak A, Pilichowicz A, Szota M, Szymczak B, Alska E, Przybyszewska J, Bartuzi Z. Immunomodulatory Effects of Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition. Current Issues in Molecular Biology. 2025; 47(2):89. https://doi.org/10.3390/cimb47020089
Chicago/Turabian StyleNapiórkowska-Baran, Katarzyna, Paweł Treichel, Anita Dardzińska, Agata Majcherczak, Anastazja Pilichowicz, Maciej Szota, Bartłomiej Szymczak, Ewa Alska, Justyna Przybyszewska, and Zbigniew Bartuzi. 2025. "Immunomodulatory Effects of Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition" Current Issues in Molecular Biology 47, no. 2: 89. https://doi.org/10.3390/cimb47020089
APA StyleNapiórkowska-Baran, K., Treichel, P., Dardzińska, A., Majcherczak, A., Pilichowicz, A., Szota, M., Szymczak, B., Alska, E., Przybyszewska, J., & Bartuzi, Z. (2025). Immunomodulatory Effects of Selected Non-Nutritive Bioactive Compounds and Their Role in Optimal Nutrition. Current Issues in Molecular Biology, 47(2), 89. https://doi.org/10.3390/cimb47020089








