Epigenetic Mechanisms in CRSwNP: The Role of MicroRNAs as Potential Biomarkers and Therapeutic Targets
Abstract
1. Introduction
2. Background on Epigenetics
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Alternative Polyadenylation (APA)
2.4. Non-Coding RNAs (ncRNAs)
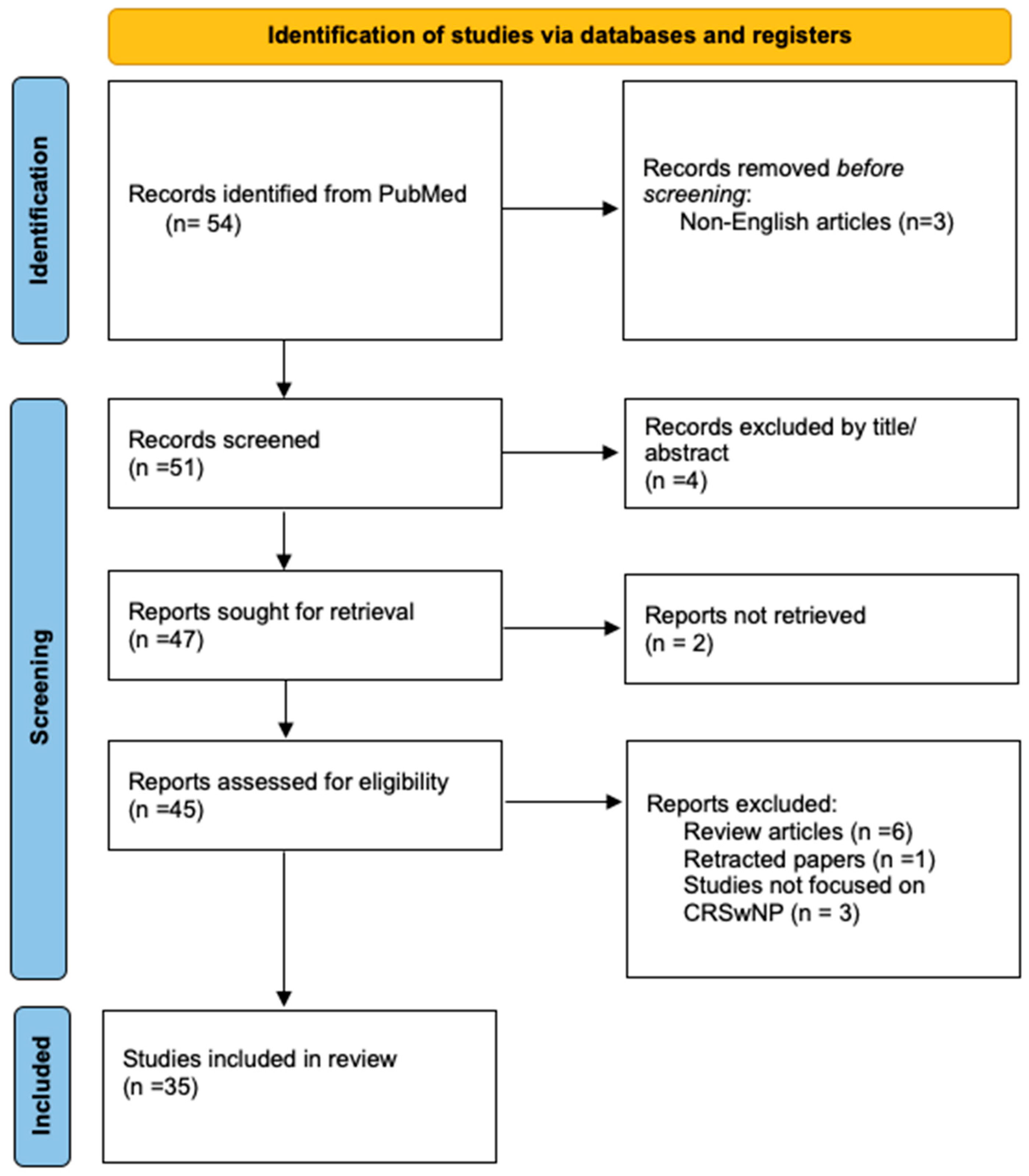
3. Methodology
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
3.3. Data Extraction and Presentation
4. The Role of miRNAs in the Pathophysiology of CRSwNP
4.1. Epithelial–Mesenchymal Transition (EMT) in CRSwNP
4.2. Th2 Inflammation and Immune Dysregulation
4.3. Cytokine Regulation and Immune Balance
4.4. Vascular Remodeling and Structural Changes
5. Decoding Molecular Pathways in CRSwNP: The Role of miRNA Profiling and Beyond
5.1. Subtype-Specific Insights from miRNA Profiling
5.2. Regulatory Networks: ceRNA Mechanisms in CRSwNP
5.3. Exosomal miRNA Profiling: Systemic Insights and Therapeutic Potential
| Study | Methodology | Findings | Clinical Implications |
|---|---|---|---|
| Xuan et al., 2019 [49] | miRNA profiling using miRCURY™ LNA Array in nasal mucosa tissues of CRSwNP patients (n = 19) and healthy controls (n = 10) | Identified 5 upregulated miRNAs (e.g., miR-210-5p) and 19 downregulated miRNAs (e.g., miR-32-3p); pathways regulated include mucin-type O-glycan biosynthesis, TGF-β signaling, TRP channels, and MAPK signaling | The upregulation of miRNAs linked to mucin-type O-glycan biosynthesis suggests a role in excessive mucus production in CRSwNP, while the downregulation of miRNAs targeting TGF-β and MAPK signaling pathways highlights their involvement in tissue remodeling and inflammation. These miRNAs may serve as targets for modulating these pathways in future therapeutic approaches |
| Chen et al., 2022 [50] | Constructed a ceRNA network using bioinformatics and experimental validation; analyzed lncRNA, miRNA, and mRNA expression profiles from Gene Expression Omnibus datasets (42 CRSwNP tissues compared with 28 control samples) | Identified 565 DE-lncRNAs, 23 DE-miRNAs, and 1799 DE-mRNAs; constructed a ceRNA network centered on the MIAT/miR-125a/IRF4 axis; IRF4 was linked to immune cell infiltration, including eosinophils and M2 macrophages; MIAT and IRF4 correlated positively with dendritic cells and M2 macrophages | The MIAT/miR-125a/IRF4 axis plays a critical role in CRSwNP pathogenesis by regulating immune cell infiltration and inflammatory responses. IRF4 and MIAT show high predictive value for CRSwNP diagnosis (AUC = 0.944), offering potential as diagnostic biomarkers and therapeutic targets. Interventions targeting this axis may mitigate eosinophil-driven inflammation and tissue remodeling in CRSwNP |
| Li and Liu, 2022 [51] | Profiling of mRNAs, miRNAs, and lncRNAs; construction of ceRNA network linking miRNA–mRNA–lncRNA interactions in eosinophilic CRSwNP (12 eosinophilic CRSwNP nasal polyp samples and 12 normal control nasal samples were included) | Identified 358 differentially expressed miRNAs (DEmiRs), 964 mRNAs (DEmRs), and 15 lncRNAs (DElncRs); key pathways enriched include cytokine–cytokine receptor interaction, chemokine signaling, complement cascades, and S. aureus infection pathways. CFI and MIAT were highlighted as hub elements in ceRNA networks | Identified miRNA–mRNA–lncRNA interactions reveal potential mechanisms behind inflammation, eosinophil infiltration, and NP formation. Specific hubs like MSC-AS1 (broad regulation) and CFI-associated networks suggest targets for future therapeutic interventions targeting ceRNA dynamics in eosinophilic CRSwNP. |
| Xia et al., 2015 [48] | RT-qPCR analysis of 7 inflammation-associated miRNAs (miR-181b, miR-26b, miR-155, miR-146a, miR-125b, miR-124, miR-92a) in 40 CRS patients and 5 controls | miR-125b, miR-155, and miR-146a were upregulated in CRS and NP; miR-92a, miR-26b, and miR-181b were downregulated. In CRS without NP, miR-125b and miR-155 were significantly upregulated, while miR-92a, miR-26b, and miR-181b were downregulated. miR-124 showed no significant change | miR-181b, as a regulator of NF-κB signaling via importin-β3, emerges as a potential therapeutic target. miR-125b and miR-155 could serve as biomarkers for distinguishing CRS subtypes, while miR-92a and miR-26b are associated with decreased inflammation |
| Ma et al., 2015 [47] | miRNA microarray analysis of mature dendritic cells (DCs) from peripheral blood of CRS patients (n = 30) and controls (n = 7); RT-qPCR validation of selected miRNAs | Identified 31 differentially expressed miRNAs in CRS patients, with miR-125b-5p, miR-210-3p, and miR-150-5p upregulated, and miR-708-5p and miR-126-3p downregulated. Subgroup-specific miRNAs were identified, e.g., miR-1290 was upregulated in CRSwNP but downregulated in CRSsNP. Shared miRNAs across subgroups suggest core regulatory roles in CRS | Highlights miR-125b-5p as a regulator in both epithelial and peripheral immune cell functions, linking epithelial and systemic immune responses in CRS. Subgroup-specific miRNAs provide insights into phenotypic differences in CRS, aiding in patient stratification. miR-126-3p’s regulation of Th2 function and miR-708-5p’s involvement in airway inflammation suggest therapeutic potential in targeting immune dysregulation |
| Bu et al., 2021 [46] | Whole-transcriptome sequencing of nasal tissues from ECRSwNP (n = 10), non-ECRSwNP (n = 5), and controls (n = 9); integrated analysis of miRNA and mRNA expression; real-time PCR validation of target genes and miRNAs | Identified 3884 DE-mRNAs in ECRSwNP vs. control, 5009 DE-mRNAs in non-ECRSwNP vs. control, and 998 DE-mRNAs in non-ECRSwNP vs. ECRSwNP; miRNA families miR-154, miR-221, and miR-223 associated with both ECRSwNP and non-ECRSwNP; let-7 and miR-34/449 families associated with non-ECRSwNP. Pathway analysis revealed type 2 inflammation (eosinophil migration/chemotaxis) in ECRSwNP and type 1/type 3 inflammation (neutrophil activation, IFN-γ production) in non-ECRSwNP | Overall, this study highlights the potential for miRNA-mRNA networks to distinguish CRSwNP subtypes. miR-223’s association with eosinophilia and miR-221’s involvement in airway remodeling suggest subtype-specific biomarkers and therapeutic targets. The let-7 and miR-34/449 families offer insights into non-ECRSwNP pathogenesis and could guide phenotype-specific treatments |
| He et al. 2022 [52] | Plasma exosomal miRNAs were isolated from five patients with CRSwNP and five controls. Differential expression was assessed via miRNA sequencing and transcripts per million (TPM). KEGG and GO analyses were used for functional prediction of target genes. | Identified 159 differentially expressed exosomal miRNAs (93 upregulated, 66 downregulated). Notable pathways included axon guidance, extracellular matrix (ECM) receptor interaction, Hippo signaling, and Wnt/β-catenin signaling. Exosomal miRNAs may regulate epithelial–mesenchymal transition (EMT), mucosal remodeling, and inflammation. | Differential plasma exosomal miRNAs may serve as non-invasive biomarkers for CRSwNP diagnosis, monitoring persistence/recurrence, and treatment efficacy. Exosomes carrying engineered miRNAs could be a therapeutic avenue targeting EMT and inflammation. |
6. miRNAs as Potential Biomarkers
7. MiRNAs as Potential Therapeutic Targets
7.1. Targeting EMT
7.2. Inflammatory Pathways and Immune Modulation
7.3. Emerging Therapeutic Pathways
7.4. The Role of Small Extracellular Vesicles (sEVs) and Exosomes
8. Current Gaps and Future Research Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRSwNP | chronic rhinosinusitis with nasal polyps |
| miRNAs | microRNAs |
| EMT | epithelial–mesenchymal transition |
| sEVs | small extracellular vesicles |
| CRS | chronic rhinosinusitis |
| Th2 | type 2 helper T-cell |
| IL | interleukin |
| COPD | chronic obstructive pulmonary disease |
| HDAC | histone deacetylase |
| APA | alternative polyadenylation |
| UTR | 3’ untranslated region |
| ncRNAs | non-coding RNAs |
| TGF-β1 | transforming growth factor beta 1 |
| PTEN | phosphatase and tensin homolog |
| PI3K/AKT | phosphoinositide 3-kinase/protein kinase B signaling pathway |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| COX2 | cyclooxygenase-2 |
| SOCS1 | suppressor of cytokine signaling 1 |
| SIRT1 | sirtuin 1 |
| TP53INP1 | tumor protein P53 inducible nuclear protein 1 |
| MMP-9 | matrix metalloproteinase 9 |
| VE-Cadherin | vascular endothelial cadherin |
| EGR2 | early growth response protein 2 |
| STAT3/GDF15 | signal transducer and activator of transcription 3/growth differentiation Factor 15 |
| QKI | quaking protein |
| NOTCH1 | notch receptor 1 |
| MYB | myeloblastosis transcription factor |
| CCNO | Cyclin O |
| FOXJ1 | forkhead box protein J1 |
| ZEB1 | zinc finger E-box binding homeobox 1 |
| ERK | extracellular signal-regulated kinase |
| MAPK | mitogen-activated protein kinase |
| GLP-1R | glucagon-like peptide-1 receptor |
| AHR | aryl hydrocarbon receptor |
| HSP47 | heat shock protein 47 |
| T2 | type 2 inflammation |
| TNF-α | tumor necrosis factor alpha |
| LPS | lipopolysaccharide |
| Sp1 | specificity Protein 1 |
| ceRNA | competitive endogenous RNA |
| DCs | dendritic cells |
| lncRNAs | long non-coding RNAs |
| MIAT | myocardial infarction associated transcript |
| IRF4 | interferon regulatory factor 4 |
| CFI | complement factor I |
| MSC-AS1 | mesenchymal stem cell antisense RNA 1 |
| CRSsNP | chronic rhinosinusitis without nasal polyps |
| DE-miRNA | differentially expressed microRNA |
| DE-mRNA | differentially expressed messenger RNA |
| DE-lncRNA | differentially expressed long non-coding RNA |
| ECRSwNP | eosinophilic chronic rhinosinusitis with nasal polyps |
| non-ECRSwNP | non-eosinophilic chronic rhinosinusitis with nasal polyps |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| TPM | transcripts per million |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene ontology |
| ECM | extracellular matrix |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppilla-Salmi, S.; Bernal-Sprekelsen, L.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Won, H.K.; Kim, Y.C.; Kang, M.G.; Park, H.K.; Lee, S.E.; Kim, M.H.; Yang, M.S.; Chang, Y.S.; Cho, S.H.; Shong, W.J. Age-related prevalence of chronic rhinosinusitis and nasal polyps and their relationships with asthma onset. Ann. Allergy Asthma Immunol. 2018, 120, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Åkerlund, A.; Melén, I.; Holmberg, K.; Bende, M. Prevalence of Nasal Polyps in Adults: The Skovde Population-Based Study. Ann. Otol. Rhinol. Laryngol. 2003, 112, 625–629. [Google Scholar] [CrossRef]
- Brar, T.; Marks, L.; Lal, D. Insights into the epigenetics of chronic rhinosinusitis with and without nasal polyps: A systematic review. Front. Allergy 2023, 4, 1165271. [Google Scholar] [CrossRef]
- Nepali, K.; Liou, J.P. Recent developments in epigenetic cancer therapeutics: Clinical advancement and emerging trends. J. Biomed. Sci. 2021, 28, 27. [Google Scholar] [CrossRef]
- Oakley, G.M.; Curtin, K.; Orb, Q.; Schaefer, C.; Orlandi, R.R.; Alt, J.A. Familial risk of chronic rhinosinusitis with and without nasal polyposis: Genetics or environment. Int. Forum Allergy Rhinol. 2015, 5, 276–282. [Google Scholar] [CrossRef]
- Bohman, A.; Oscarsson, M.; Holmberg, K.; Johansson, L.; Millqvist, E.; Nasic, S.; Torinsson-Naluai, A.; Bende, M. Heredity of nasal polyps. Rhinology 2015, 53, 25–28. [Google Scholar] [CrossRef]
- Brar, T.; Marino, M.J.; Lal, D. Unified Airway Disease: Genetics and Epigenetics. Otolaryngol. Clin. North Am. 2023, 56, 23–38. [Google Scholar] [CrossRef]
- Sarnowski, C.; Laprise, C.; Malerba, G.; Moffatt, M.F.; Dizier, M.H.; Morin, A.; Vincent, Q.B.; Rhode, K.; Esparza-Gordillo, J.; Margaritte-Jeannine, P.; et al. DNA methylation within melatonin receptor 1A (MTNR1A) mediates paternally transmitted genetic variant effect on asthma plus rhinitis. J. Allergy Clin. Immunol. 2016, 138, 748–753. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.; Bai, W. The Role of Epigenetics in the Chronic Sinusitis with Nasal Polyp. Curr. Allergy Asthma Rep. 2021, 21, 1. [Google Scholar] [CrossRef]
- Cardenas, A.; Sordillo, J.E.; Rifas-Shiman, S.L.; Chung, W.; Liang, L.; Coull, B.A.; Hivert, M.F.; Lai, P.S.; Forno, E.; Celedon, J.C.; et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat. Commun. 2019, 10, 3095. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiao, J.; Wang, M.; Gao, Y.; Li, Y.; Wang, Y.; Zhang, Y.; Whang, X.; Zhang, L. Hypomethylation of the IL8 promoter in nasal epithelial cells of patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2019, 144, 993–1003.e12. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Han, X.; Jiao, J.; Wang, M.; Li, Y.; Wang, Y.; Zhang, L. Histone deacetylase activity is a novel target for epithelial barrier defects in patients with eosinophilic chronic rhinosinusitis with nasal polyps. Clin. Exp. Allergy 2023, 53, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Zhipu, N.; Zitao, H.; Jichao, S.; Cuida, M. Research advances in roles of microRNAs in nasal polyp. Front. Genet. 2022, 13, 1043888. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, Y.N.; Liu, Z. MicroRNA in Chronic Rhinosinusitis and Allergic Rhinitis. Curr. Allergy Asthma Rep. 2014, 14, 415. [Google Scholar] [CrossRef]
- Fossiez, F.; Djossou, O.; Chomarat, P.; Flores-Romo, L.; Ait-Yahia, S.; Maat, C.; Pin, J.J.; Garrone, P.; Garcia, E.; Saeland, S.; et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef]
- Lee, K.; Tai, J.; Lee, S.H.; Kim, T.H. Advances in the Knowledge of the Underlying Airway Remodeling Mechanisms in Chronic Rhinosinusitis Based on the Endotypes: A Review. Int. J. Mol. Sci. 2021, 22, 910. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, C.; Ma, C.; Cao, Y.; Hu, G.; Li, H. TGF-β1 induces epithelial-to-mesenchymal transition in chronic rhinosinusitis with nasal polyps through microRNA-182. Asian Pac. J. Allergy Immunol. 2024, 42, 61–73. [Google Scholar]
- Chen, J.; Liu, D.; Yang, J.; Jin, C.; Zhao, C.; Cheng, J. Epidermal growth factor activates a hypoxia-inducible factor 1α–microRNA-21 axis to inhibit aquaporin 4 in chronic rhinosinusitis. Ann. N. Y. Acad. Sci. 2022, 1518, 299–314. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhu, G.; Yuan, W.; Xiao, Z.-A. TGF-β1 Induces Epithelial-Mesenchymal Transition of Chronic Sinusitis with Nasal Polyps through MicroRNA-21. Int. Arch. Allergy Immunol. 2019, 179, 304–319. [Google Scholar] [CrossRef]
- Shin, J.M.; Park, J.H.; Yang, H.W.; Moon, J.W.; Lee, H.M.; Park, I.H. miR-29b Regulates TGF-β1-Induced Epithelial–Mesenchymal Transition by Inhibiting Heat Shock Protein 47 Expression in Airway Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 11535. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Cheng, H.; Mo, Q.; Zhou, X.; Xie, M. miR-155-5p downregulation inhibits epithelial-to-mesenchymal transition by targeting SIRT1 in human nasal epithelial cells. Mol. Med. Rep. 2020, 22, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, R.; Liang, W.; Qiu, H.; Yuan, T.; Wang, W.; Deng, H.; Kong, W.; Chen, J.; Bai, Y.; et al. Small extracellular vesicles facilitate epithelial-mesenchymal transition in chronic rhinosinusitis with nasal polyps via the miR-375-3p/QKI axis. Rhinology 2024, 62, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, Y.; You, B.; You, Y.; Yan, Y.; Zhang, J.; Pei, Y.; Zhang, W.; Chen, J. miR-30a-5p Inhibits Epithelial-to-Mesenchymal Transition by Targeting CDK6 in Nasal Polyps. Am. J. Rhinol. Allergy 2021, 35, 152–163. [Google Scholar] [CrossRef]
- Jian, B.; Yin, P. STAT1 mediates the PI3K/AKT pathway through promoting microRNA-18a in nasal polyps. Immunopharmacol. Immunotoxicol. 2022, 44, 194–205. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, K.; Tu, Y.; Li, P.; Hao, D.; Yu, P.; Wan, Y.; Shi, L. miR-200a-3p regulates epithelial–mesenchymal transition and inflammation in chronic rhinosinusitis with nasal polyps by targeting ZEB1 via ERK/p38 pathway. Int. Forum Allergy Rhinol. 2024, 14, 41–56. [Google Scholar] [CrossRef]
- Gata, A.; Neagoe, I.B.; Leucuta, D.C.; Budisan, L.; Raduly, L.; Trombitas, V.E.; Albu, S. MicroRNAs: Potential Biomarkers of Disease Severity in Chronic Rhinosinusitis with Nasal Polyps. Medicina 2023, 59, 550. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, Y.N.; Li, H.B.; Hu, C.Y.; Wang, N.; Cao, P.P.; Liao, B.; Lu, X.; Cui, Y.H.; Liu, Z. Overexpression of miR-125b, a Novel Regulator of Innate Immunity, in Eosinophilic Chronic Rhinosinusitis with Nasal Polyps. Am. J. Respir. Crit. Care Med. 2012, 185, 140–151. [Google Scholar] [CrossRef]
- Liu, R.; Du, J.; Zhou, J.; Zhong, B.; Ba, L.; Zhang, J.; Liu, Y.; Liu, S. Elevated microRNA-21 Is a Brake of Inflammation Involved in the Development of Nasal Polyps. Front. Immunol. 2021, 12, 530488. [Google Scholar] [CrossRef]
- Luan, G.; Wang, M.; Yuan, J.; Bu, X.; Wang, Y.; Ying, S.; Wang, C.; Zhang, L. MicroRNA-21-5p promotes mucosal type 2 inflammation via regulating GLP1R/IL-33 signaling in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2022, 150, 1460–1475. [Google Scholar] [CrossRef]
- Zhang, J.; Han, L.; Chen, F. Let-7a-5p regulates the inflammatory response in chronic rhinosinusitis with nasal polyps. Diagn. Pathol. 2021, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lv, H.; Dou, X.; Cao, Z. Nuclear Factor κB/MicroRNA-155 Upregulates the Expression Pattern of Cytokines in Regulating the Relapse of Chronic Sinusitis with Nasal Polyps and the Underlying Mechanism of Glucocorticoid. Med Sci. Monit. 2020, 26, e923618-1–e923618-13. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.L.C.; Tamashiro, E.; Santos, A.R.D.; Martins, R.B.; Faria, F.M.; Silva, L.E.C.M.; Torrieri, R.; Ruy, P.C.; Silva, W.A., Jr.; Arruda, E.; et al. miRNA-205-5p can be related to T2-polarity in Chronic Rhinosinusitis with Nasal Polyps. Rhinology 2021, 59, 567–576. [Google Scholar] [CrossRef]
- Luo, X.Q.; Shao, J.B.; Xie, R.D.; Zeng, L.; Li, X.X.; Qiu, S.Q.; Geng, X.R.; Yang, L.T.; Li, L.J.; Liu, D.B.; et al. Micro RNA-19a interferes with IL-10 expression in peripheral dendritic cells of patients with nasal polyposis. Oncotarget 2017, 8, 48915–48921. [Google Scholar] [CrossRef]
- Ma, Z.; Shen, Y.; Zeng, Q.; Liu, J.; Yang, L.; Fu, R.; Guohua, H. MiR-150-5p regulates EGR2 to promote the development of chronic rhinosinusitis via the DC-Th axis. Int. Immunopharmacol. 2018, 54, 188–197. [Google Scholar] [CrossRef]
- Li, L.; Feng, J.; Zhang, D.; Yong, J.; Wang, Y.; Yao, J.; Huang, R. Differential expression of miR-4492 and IL-10 is involved in chronic rhinosinusitis with nasal polyps. Exp. Ther. Med. 2019, 18, 3968–3976. [Google Scholar] [CrossRef]
- Liu, C.C.; Xia, M.; Zhang, Y.J.; Jin, P.; Zhao, L.; Zhang, J.; Li, T.; Zhou, X.M.; Tu, X.X.; Kong, F.; et al. Micro124-mediated AHR expression regulates the inflammatory response of chronic rhinosinusitis (CRS) with nasal polyps. Biochem. Biophys. Res. Commun. 2018, 500, 145–151. [Google Scholar] [CrossRef]
- Qing, X.; Zhang, Y.; Peng, Y.; He, G.; Liu, A.; Liu, H. Mir-142-3p Regulates Inflammatory Response by Contributing to Increased TNF-α in Chronic Rhinosinusitis with Nasal Polyposis. Ear Nose Throat J. 2021, 100, NP50–NP56. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Yan, Y.; Zhang, J.; Zhou, Y.; Pei, Y.; Yao, L.; You, B.; Chen, J. Exosomal miR-22-3p Derived from Chronic Rhinosinusitis with Nasal Polyps Regulates Vascular Permeability by Targeting VE-Cadherin. BioMed Res. Int. 2020, 2020, 1237678. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Wang, Q.; Jayasinghe, U.; Luo, X.; Wei, Q.; Wang, J.; Xiong, H.; Chen, C.; Xu, B.; et al. Exosome: Emerging biomarker in breast cancer. Oncotarget 2017, 8, 41717–41733. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, D.; Zhao, Y.; Liu, H.; Yang, T.; Li, A. MiR-214 Expression Is Elevated in Chronic Rhinosinusitis Mucosa and Regulates Lipopolysaccharide-Mediated Responses in Undifferentiated Human Nasal Epithelial Cell Culture. Am. J. Rhinol. Allergy 2023, 37, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Yu, D.; Gao, J.; Ruan, B.; Long, R. Downregulation of miR-29b-3p promotes α-tubulin deacetylation by targeting the interaction of matrix metalloproteinase-9 with integrin β1 in nasal polyps. Int. J. Mol. Med. 2021, 48, 126. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Díaz, B.; Fernandez, G.; Fuentes, M.; Martínez-Antón, A.; Alobid, I.; Roca-Ferrer, J.; Picado, C.; Tubita, V.; Mullol, J. Integrated mRNA and microRNA transcriptome profiling during differentiation of human nasal polyp epithelium reveals an altered ciliogenesis. Allergy 2020, 75, 2548–2561. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, X.; Qu, X.; Lv, C. Transcription Factor Specificity Protein 1 Regulates Inflammation and Fibrin Deposition in Nasal Polyps Via the Regulation of microRNA-125b and the Wnt/β-catenin Signaling Pathway. Inflammation 2022, 45, 1118–1132. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, F.; Fu, Y.; Zhu, X.; Wang, Z.; Wu, S.; Guo, L.; Zhang, Q.; Mou, X.; Liu, Y. MicroRNA-155-5p regulates the Th1/Th2 cytokines expression and the apoptosis of group 2 innate lymphoid cells via targeting TP53INP1 in allergic rhinitis. Int. Immunopharmacol. 2021, 101, 108317. [Google Scholar] [CrossRef]
- Bu, X.; Wang, M.; Luan, G.; Wang, Y.; Wang, C.; Zhang, L. Integrated miRNA and mRNA expression profiling reveals dysregulated miRNA-mRNA regulatory networks in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2021, 11, 1207–1219. [Google Scholar] [CrossRef]
- Ma, Z.X.; Tan, X.; Shen, Y.; Ke, X.; Yang, Y.C.; He, X.B.; Wang, Z.H.; Dai, Y.B.; Hong, S.L.; Hu, G.H. MicroRNA expression profile of mature dendritic cell in chronic rhinosinusitis. Inflamm. Res. 2015, 64, 885–893. [Google Scholar] [CrossRef]
- Xia, G.; Bao, L.; Gao, W.; Liu, S.; Ji, K.; Li, J. Differentially Expressed miRNA in Inflammatory Mucosa of Chronic Rhinosinusitis. J. Nanosci. Nanotechnol. 2015, 15, 2132–2139. [Google Scholar] [CrossRef]
- Xuan, L.; Luan, G.; Wang, Y.; Lan, F.; Zhang, X.; Hao, Y.; Zheng, M.; Whang, X.; Zhang, L. MicroRNAs regulating mucin type O-glycan biosynthesis and transforming growth factor β signaling pathways in nasal mucosa of patients with chronic rhinosinusitis with nasal polyps in Northern China. Int. Forum Allergy Rhinol. 2019, 9, 106–113. [Google Scholar] [CrossRef]
- Chen, J.-C.; Xing, Q.-L.; Yang, H.-W.; Yang, F.; Luo, Y.; Kong, W.-J.; Wang, Y.J. Construction and analysis of a ceRNA network and patterns of immune infiltration in chronic rhinosinusitis with nasal polyps: Based on data mining and experimental verification. Sci. Rep. 2022, 12, 9735. [Google Scholar] [CrossRef]
- Li, K.; Liu, F. Analysis of competing endogenous RNA (ceRNA) crosstalk in eosinophilic chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2022, 12, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, J.; Han, D.; Li, Y.; Wang, T.; Wei, H.; Pan, Y.; Zhang, H. Differential expression profile of plasma exosomal microRNAs in chronic rhinosinusitis with nasal polyps. Exp. Biol. Med. 2022, 247, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, L. Biomarkers guiding biological therapeutic strategies in chronic rhinosinusitis with nasal polyps: An emerging challenge. Expert Rev. Clin. Immunol. 2023, 19, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, B.; Zhang, L. The epithelium-derived inflammatory mediators of chronic rhinosinusitis with nasal polyps. Expert Rev. Clin. Immunol. 2020, 16, 293–310. [Google Scholar] [CrossRef]
- Nakayama, T.; Haruna, S.I. A review of current biomarkers in chronic rhinosinusitis with or without nasal polyps. Expert Rev. Clin. Immunol. 2023, 19, 883–892. [Google Scholar] [CrossRef]
- Danielides, G.; Lygeros, S.; Kanakis, M.; Naxakis, S. Periostin as a biomarker in chronic rhinosinusitis: A contemporary systematic review. Int. Forum Allergy Rhinol. 2022, 12, 1535–1550. [Google Scholar] [CrossRef]
- Liu, P.; Liu, M.; Sun, Y.; Lei, W.; Xu, Y. Assessing the predictive potential of ADAM8 for disease control in chronic rhinosinusitis with nasal polyps. Front. Allergy 2024, 5, 1488441. [Google Scholar] [CrossRef]
- Lygeros, S.; Danielides, G.; Kyriakopoulos, G.C.; Tsapardoni, F.; Grafanaki, K.; Stathopoulos, C.; Danielides, V. Expression profiles of MMP-9 and EMMPRIN in chronic rhinosinusitis with nasal polyps. Acta Otorhinolaryngol. Ital. 2023, 43, 400–408. [Google Scholar] [CrossRef]
- Lygeros, S.; Danielides, G.; Kyriakopoulos, G.C.; Grafanaki, K.; Tsapardoni, F.; Stathopoulos, C.; Danielides, V. Evaluation of MMP-12 expression in chronic rhinosinusitis with nasal polyposis. Rhinology 2022, 60, 39–46. [Google Scholar] [CrossRef]
- Gata, A.; Leucuta, D.C.; Budisan, L.; Raduly, L.; Trombitas, V.E.; Berindan-Neagoe, I.; Albu, S. MicroRNA-125b Is a Potential Predictor of Surgical Outcomes in Chronic Rhinosinusitis with Nasal Polyps. Am. J. Rhinol. Allergy 2024, 38, 92–101. [Google Scholar] [CrossRef]
- Mimmi, S.; Lombardo, N.; Maisano, D.; Piazzetta, G.; Pelaia, C.; Pelaia, G.; Greco, M.; Foti, D.; Dattilo, V.; Iaccino, E. Spotlight on a Short-Time Treatment with the IL-4/IL-13 Receptor Blocker in Patients with CRSwNP: microRNAs Modulations and Preliminary Clinical Evidence. Genes 2022, 13, 2366. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Regenberg, A.; Luong, A.U.; Wise, S.K.; Toskala, E.; Lam, K.K.; Levy, J.M.; Franzese, C.B.; Smith, K.; Kim, J. Biologics for chronic rhinosinusitis with nasal polyps: Economics and ethics. Int. Forum Allergy Rhinol. 2021, 11, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sha, J.; Meng, C.; Zhu, D. The Role of Small Extracellular Vesicles and MicroRNAs in the Diagnosis and Treatment of Allergic Rhinitis and Nasal Polyps. Wei, H., editor. Mediat. Inflamm. 2022, 2022, 4428617. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Rasul, M.F.; Abdullah, S.R.; Hidayat, H.J.; Faraj, G.S.H.; Ali, F.A.; Salihi, A.; Baniahmad, A.; Ghafouri-Fard, S.; Rahman, M.; et al. Targeting miRNA by CRISPR/Cas in cancer: Advantages and challenges. Mil. Med. Res. 2023, 10, 32. [Google Scholar] [CrossRef]
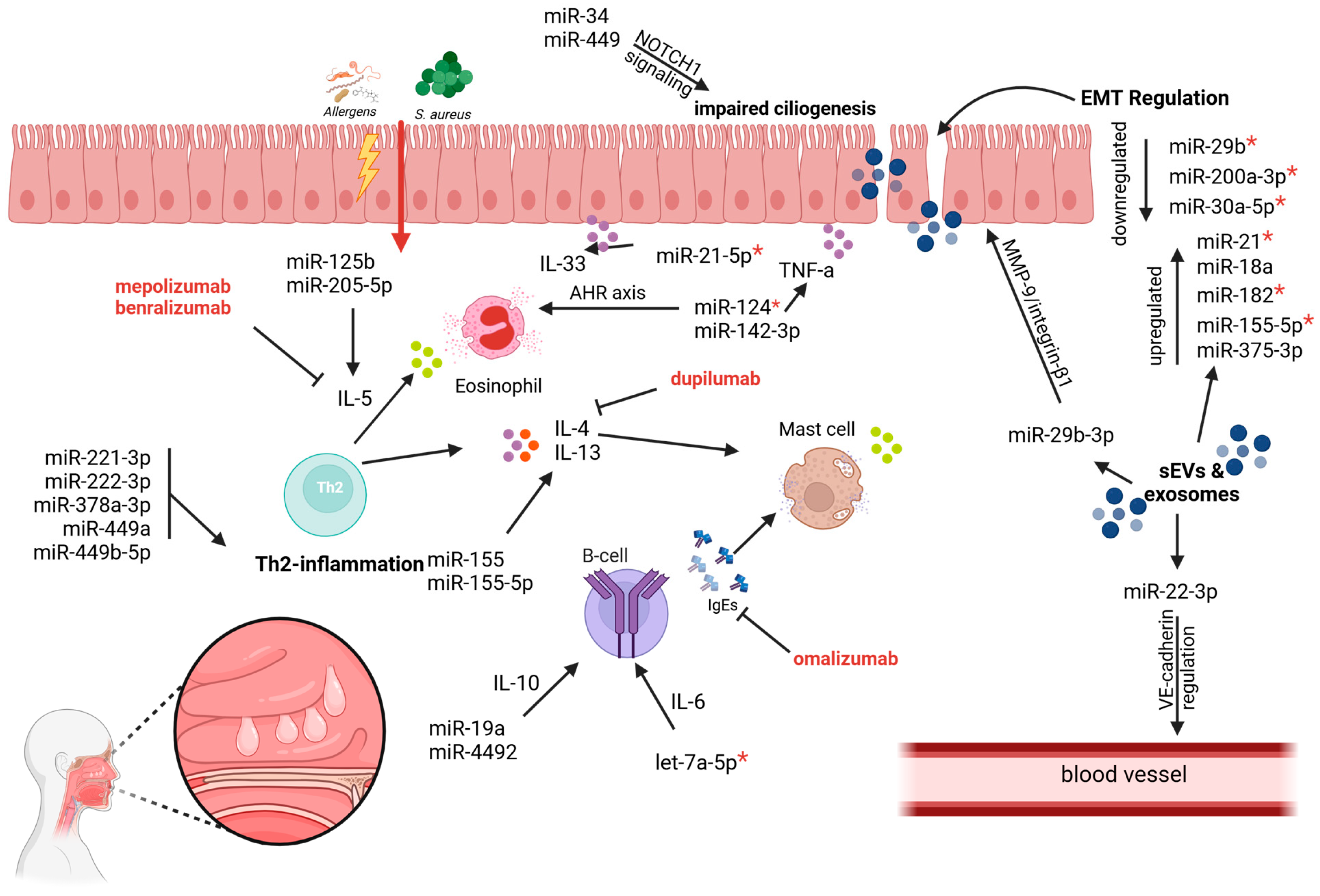
| MicroRNA | Expression Status | Target Genes/Pathways | Functional Role in CRSwNP | Reference(s) |
|---|---|---|---|---|
| miR-18a | upregulated | PTEN regulation, activation of PI3K/AKT pathway | contributes to epithelial–mesenchymal transition (EMT) | Jian and Yin, 2022 [25] |
| miR-21 | upregulated | PDCD4, NF-κB, PTEN, Akt | facilitates EMT via the TGF-β1-miR-21-PTEN-Akt axis; exhibits anti-inflammatory effects through IL-10 regulation | Li, 2019 [20] Liu, 2021 [29] |
| EGF/HIF-1α/miR-21/AQP4 axis | Promotes epithelial cell proliferation and survival, promotes EMT; contributes to inflammation and tissue remodeling | Chen et al., 2022 [19] | ||
| miR-125b | upregulated | Wnt/β-catenin signaling pathway | increased inflammation and fibrin deposition contributing to NP development | Song et al., 2022 [44] |
| upregulated | IFN signaling pathway, elevating IL-5 | Leads to eosinophil infiltration and inflammation in eosinophilic CRSwNP | Gata et al., 2023 [27] Zhang et al., 2012 [28] | |
| miR-142-3p | upregulated | TNF-α, LPS-TLR signaling | drives inflammatory responses by amplifying the LPS-TLR-TNF-α pathway | Qing et al., 2021 [38] |
| miR-182 | upregulated | TGF-β1 induced EMT pathways | regulation of EMT in response to inflammation | Jiang et al., 2024 [18] |
| miR-30a-5p | downregulated | TGF-β1 induced EMT pathways | promotes EMT in nasal polyps | Zhang et al., 2021 [24] |
| miR-19a | upregulated | IL-10 | suppresses IL-10 production, exacerbating inflammation and immune dysregulation | Luo, 2017 [34] |
| miR-155 | upregulated | NF-κB pathway, upregulated COX2, downregulated SOCS1 | expression of inflammatory cytokines TNF-α, IL-1, IL-4, and IL-5 amplifies inflammation | Du et al., 2020 [32] |
| miR-155-5p | upregulated | SIRT1, TP53INP1 | promotes EMT and Th2 cytokine secretion and ILC2 survival, aggravating inflammation | Zhu, 2021 [45] Yang, 2020 [22] |
| miR-29b-3p | downregulated | MMP-9, integrin β1 | contributes to polyp formation through α-tubulin deacetylation and increased matrix metalloproteinase activity | Liu, 2021 [42] |
| miR-22-3p | upregulated | VE-cadherin | elevates vascular permeability, fostering tissue edema, and nasal polyp growth | Zhang, 2020 [39] |
| miR-150-5p | upregulated | EGR2 | elevated IL-17, activation and proliferation of CD4+ T cells into Th17, dysregulation of dendritic cells (DC) leading to inflammation in CRS | Ma et al., 2018 [35] |
| miR-214 | upregulated | STAT3/GDF15 pathway and SIRT1 | regulates LPS-mediated inflammation and MUC5AC expression | Whang et al., 2023 [41] |
| miR-375-3p | upregulated | miR-375-3p in sEVs inhibits KH domain containing RNA binding (QKI) | Promotes EMT by suppressing QKI | Wang et al., 2024 [23] |
| miR-4492 | downregulated | miR-4492/IL-10 interaction in the Jak/STAT signaling pathway | Inflammation and tissue remodeling in nasal polyps | Li et al., 2019 [36] |
| miR-34 | downregulated | NOTCH1 signaling pathway and reduced expression of ciliogenesis regulators MYB, CCNO, FOXJ1 | Impaired ciliogenesis and defective cilia structure leads to impaired mucociliary clearance in the epithelium of CRSwNP | Callejas et al., 2020 [43] |
| miR-449 | downregulated | |||
| miR-200a-3p | downregulated | ZEB1, ERK/p38 signaling pathway, MAPK signaling pathway | suppresses EMT and inflammation by regulating ZEB1; protects nasal epithelial cells from tissue remodeling | Wu et al., 2024 [26] |
| miR-205-5p | upregulated | promotes IL-5 and eosinophilia | enhances T2 inflammation | Silveira et al., 2021 [33] |
| miR-221-3p | upregulated | Implicated in cell cycle regulation and apoptosis | Implicated to inflammation in a minor extend | |
| miR-222-3p | ||||
| miR-378a-3p | ||||
| miR-449a | ||||
| miR-449b-5p | ||||
| miR-21-5p | upregulated | Glucagon-like peptide-1 receptor (GLP-1R)/IL-33 signaling | aggravates Th2-inflammation by promoting eosinophil infiltration and IL-33 expression | Luan et al., 2022 [30] |
| let-7a-5p | downregulated | IL-6, Ras-MAPK signaling pathway | Negatively regulates TNF-α, IL-1β, and IL-6 expression; reduces inflammation by inhibiting Ras-MAPK signaling pathway | Zhang et al., 2021 [31] |
| miR-124 | downregulated | Negative regulation of aryl hydrocarbon receptor (AHR) expression, and TNF-a | reduces inflammation by inhibiting AHR-mediated inflammatory signaling | Liu et al., 2018 [37] |
| miR-29b | downregulated | TGF-β1/miR-29b/HSP47 axis | promotes EMT and tissue remodeling | Shin et al., 2021 [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatsounia, A.; Schinas, G.; Danielides, G.; Grafanaki, K.; Mastronikolis, N.; Stathopoulos, C.; Lygeros, S. Epigenetic Mechanisms in CRSwNP: The Role of MicroRNAs as Potential Biomarkers and Therapeutic Targets. Curr. Issues Mol. Biol. 2025, 47, 114. https://doi.org/10.3390/cimb47020114
Gatsounia A, Schinas G, Danielides G, Grafanaki K, Mastronikolis N, Stathopoulos C, Lygeros S. Epigenetic Mechanisms in CRSwNP: The Role of MicroRNAs as Potential Biomarkers and Therapeutic Targets. Current Issues in Molecular Biology. 2025; 47(2):114. https://doi.org/10.3390/cimb47020114
Chicago/Turabian StyleGatsounia, Alkmini, Georgios Schinas, Gerasimos Danielides, Katerina Grafanaki, Nicholas Mastronikolis, Constantinos Stathopoulos, and Spyridon Lygeros. 2025. "Epigenetic Mechanisms in CRSwNP: The Role of MicroRNAs as Potential Biomarkers and Therapeutic Targets" Current Issues in Molecular Biology 47, no. 2: 114. https://doi.org/10.3390/cimb47020114
APA StyleGatsounia, A., Schinas, G., Danielides, G., Grafanaki, K., Mastronikolis, N., Stathopoulos, C., & Lygeros, S. (2025). Epigenetic Mechanisms in CRSwNP: The Role of MicroRNAs as Potential Biomarkers and Therapeutic Targets. Current Issues in Molecular Biology, 47(2), 114. https://doi.org/10.3390/cimb47020114








