Comprehensive Pan-Cancer Analysis of ZNF668 Reveals the Prognostic and Immunological Significance of ZNF668
Abstract
1. Introduction
2. Materials and Methods
2.1. ZNF668 Expression and Subcellular Localization Analysis
2.2. Diagnostic and Prognostic Significance of ZNF668
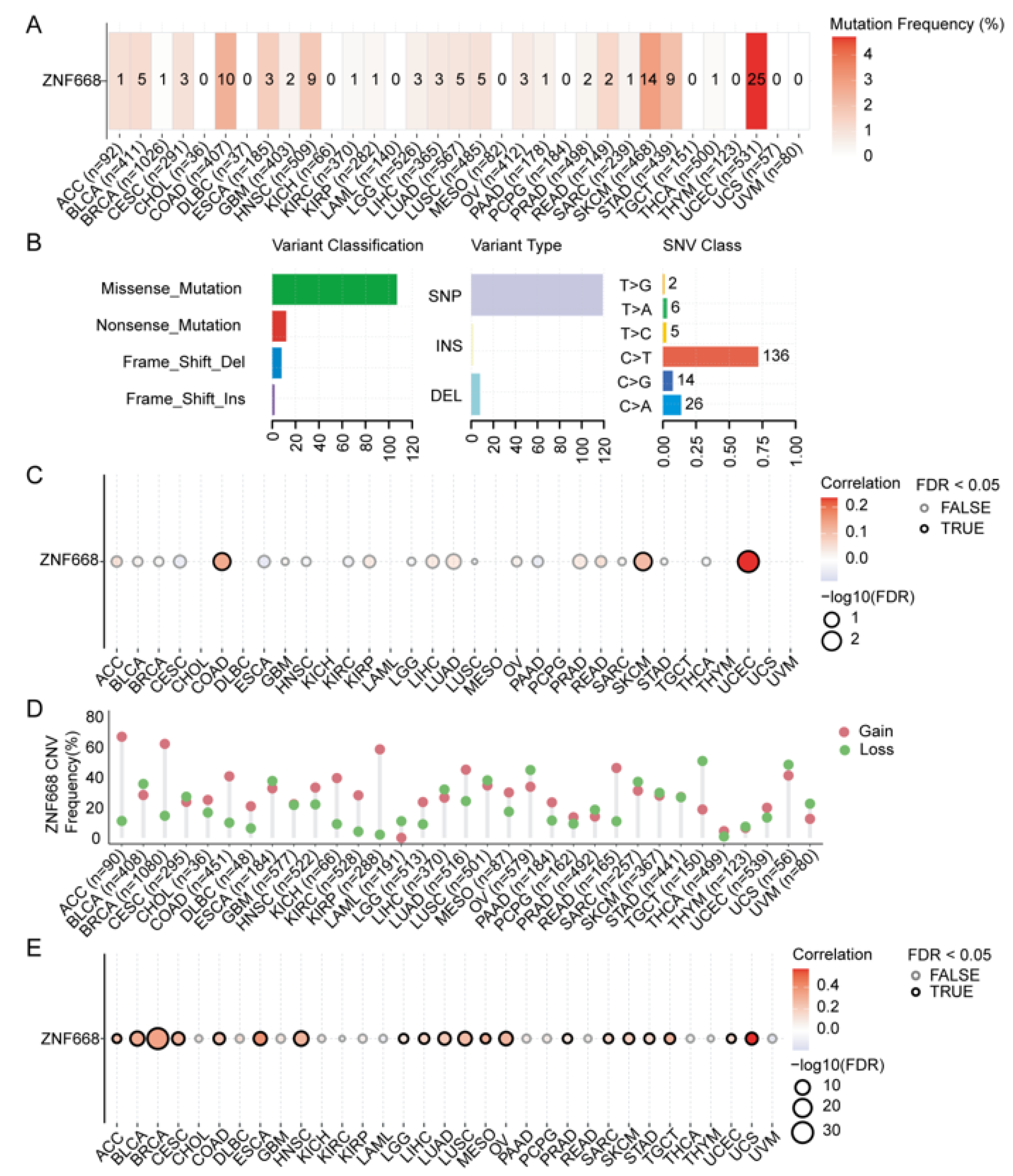
2.3. Mutation Status of ZNF668
2.4. Copy Number Variation (CNV) Analysis of ZNF668
2.5. Single Nucleotide Variant (SNV) Analysis of ZNF668
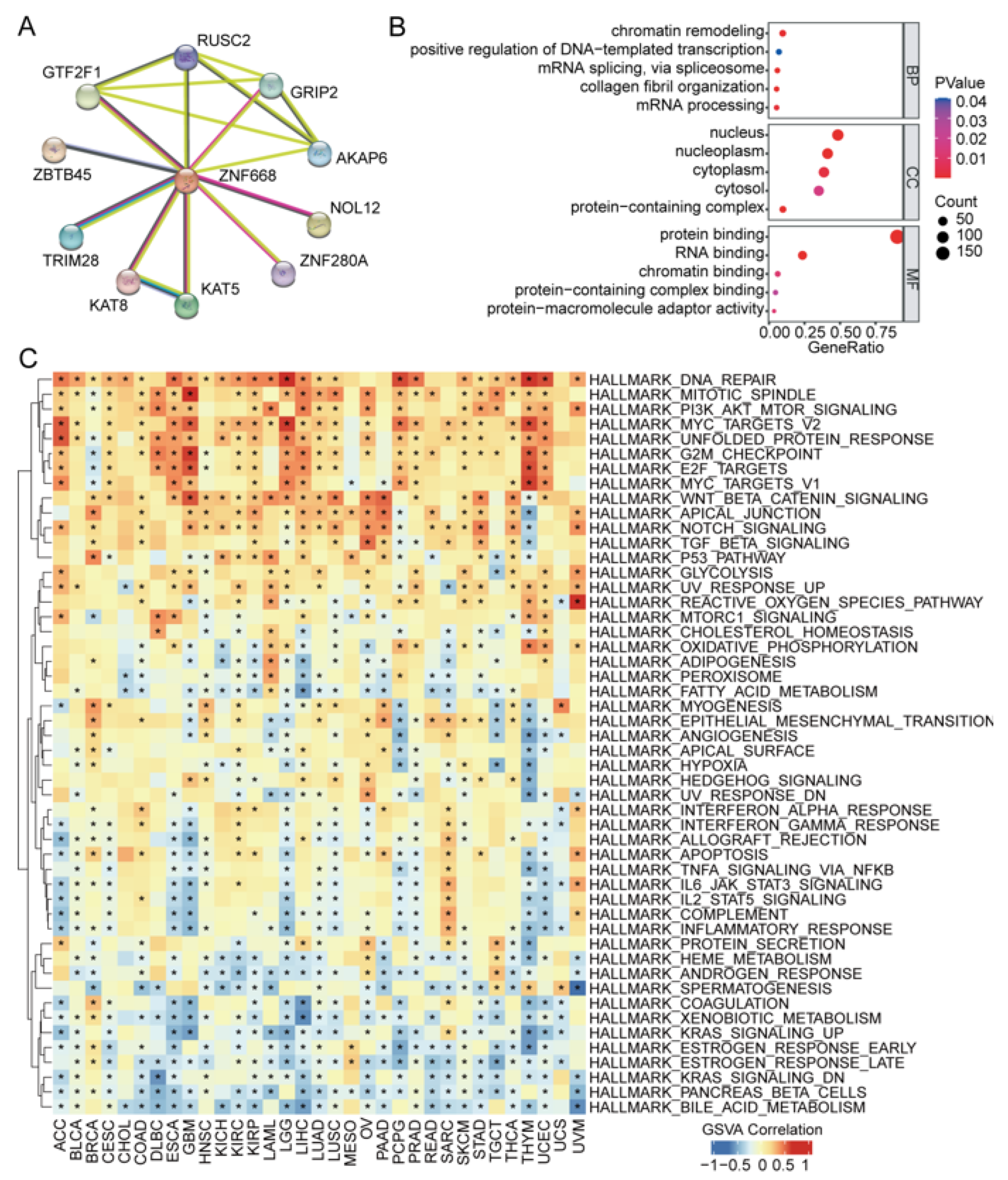
2.6. Functional Enrichment and Protein–Protein Interaction (PPI) Network Analysis of ZNF668 in Cancers
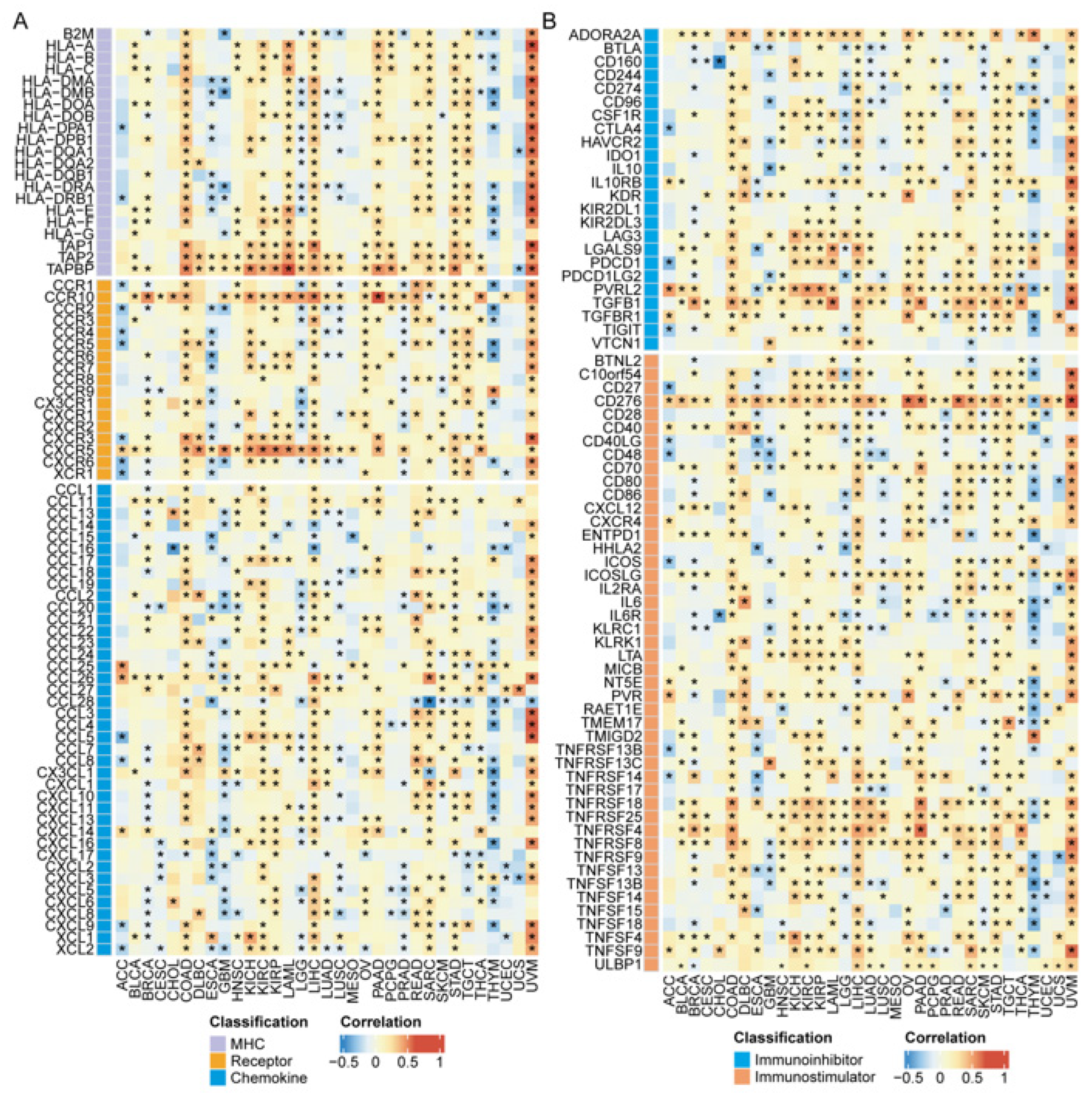
2.7. The Role of ZNF668 in the Tumor Immune Microenvironment
2.8. Drug Sensitivity Analysis
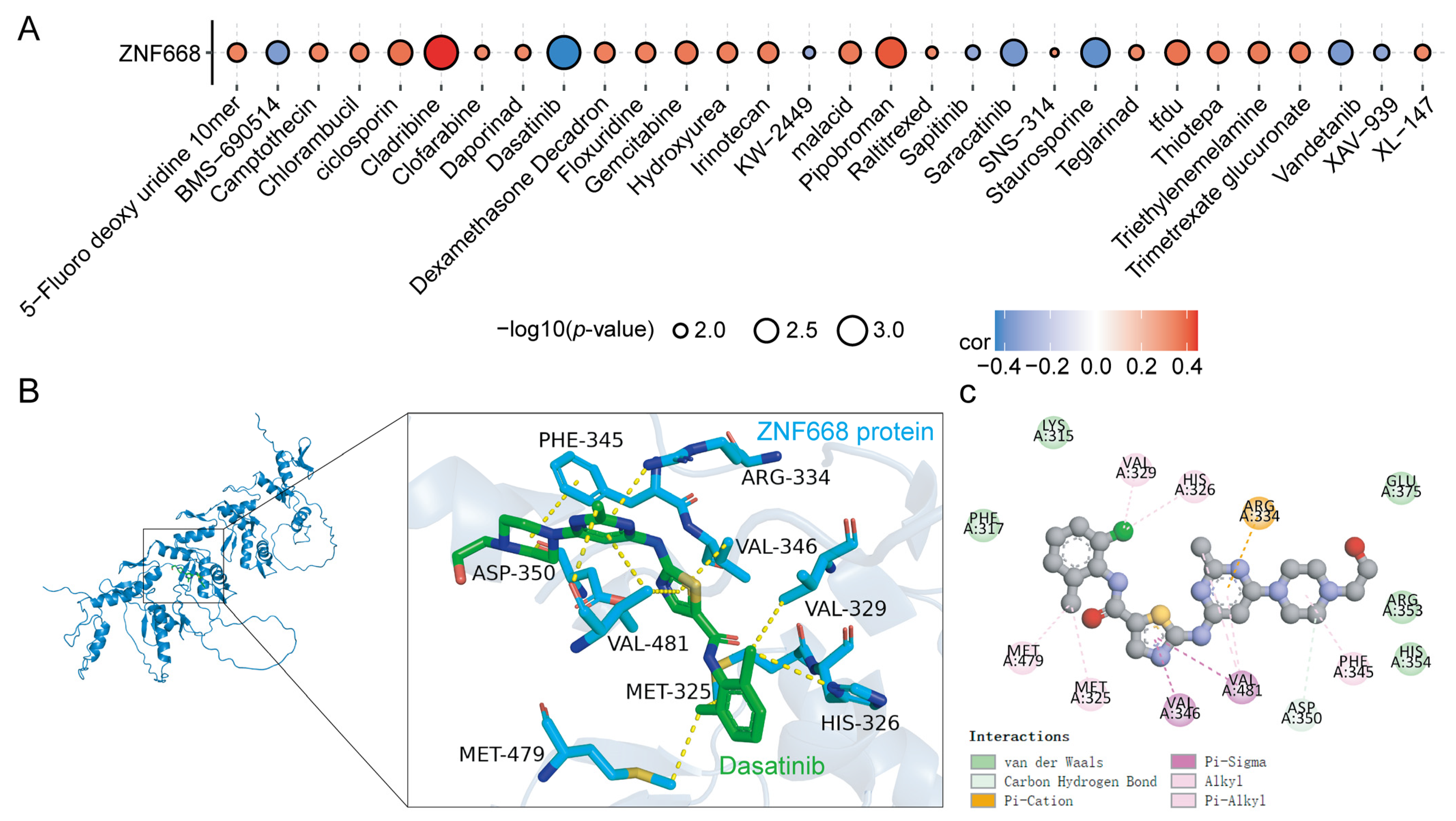
2.9. Docking Validation of Drug Molecule Components and ZNF668
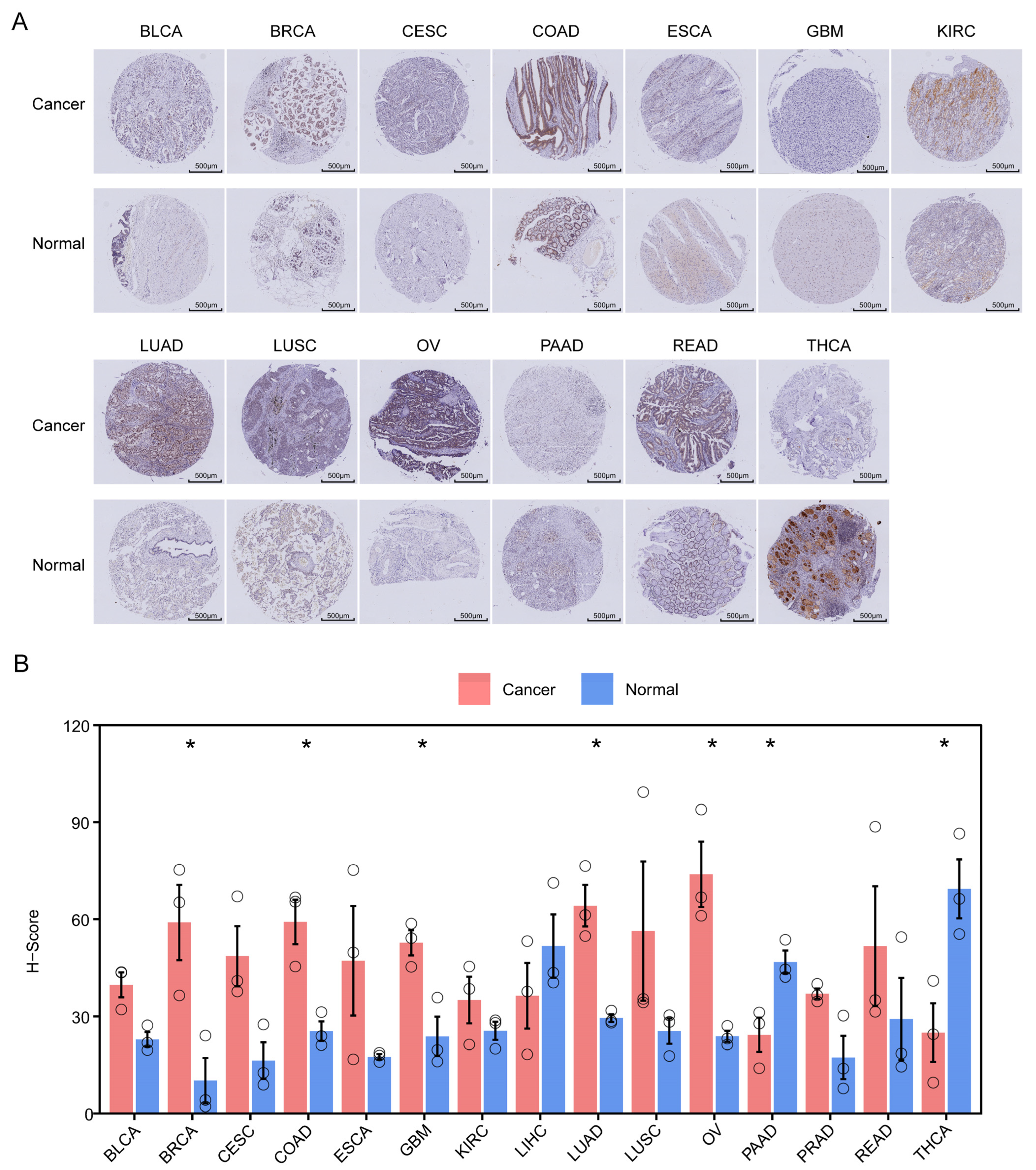
2.10. Immunohistochemistry (IHC) and Evaluation
2.11. Statistical Analysis
3. Results
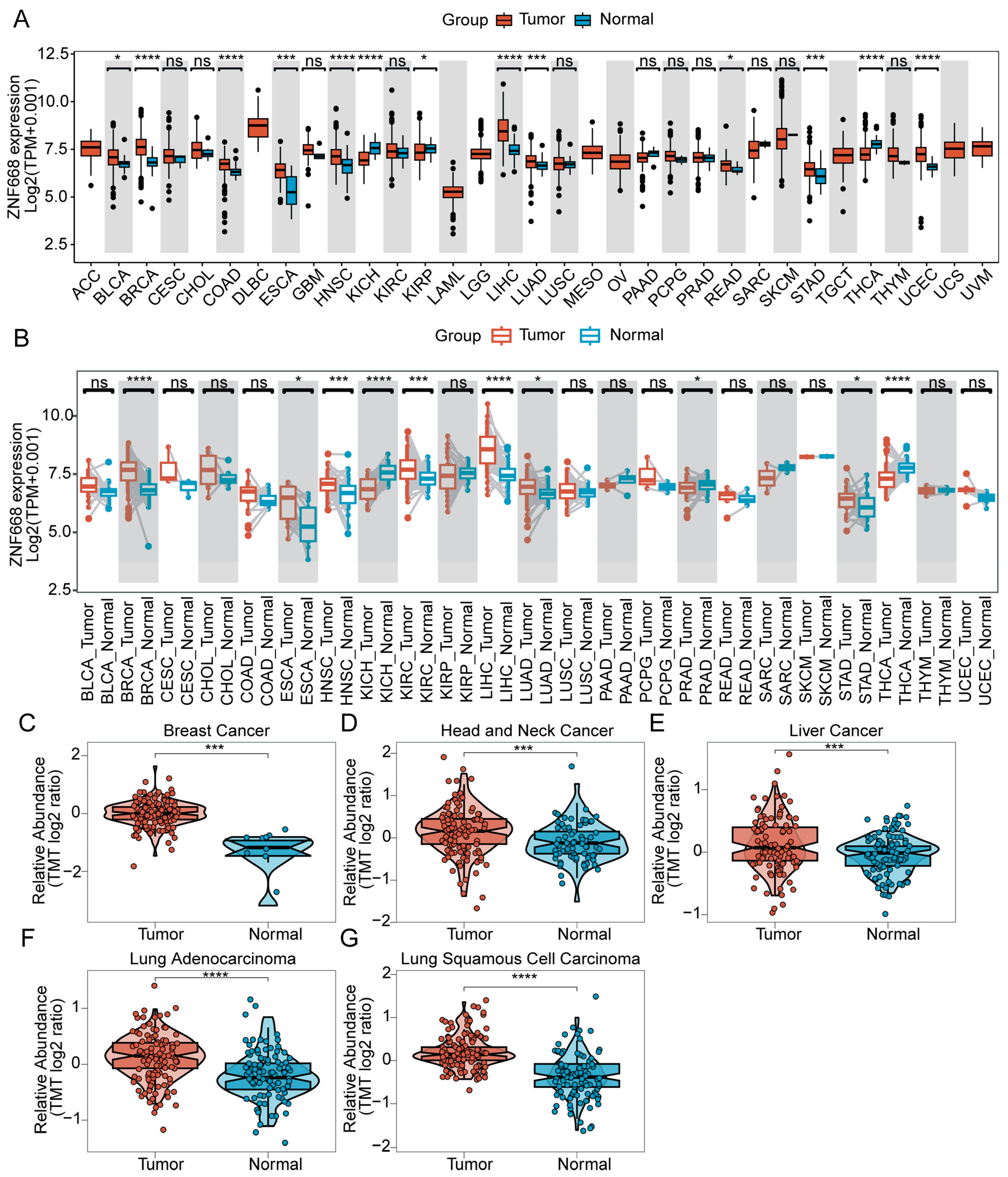
3.1. ZNF668 mRNA and Protein Expression in Pan-Cancer
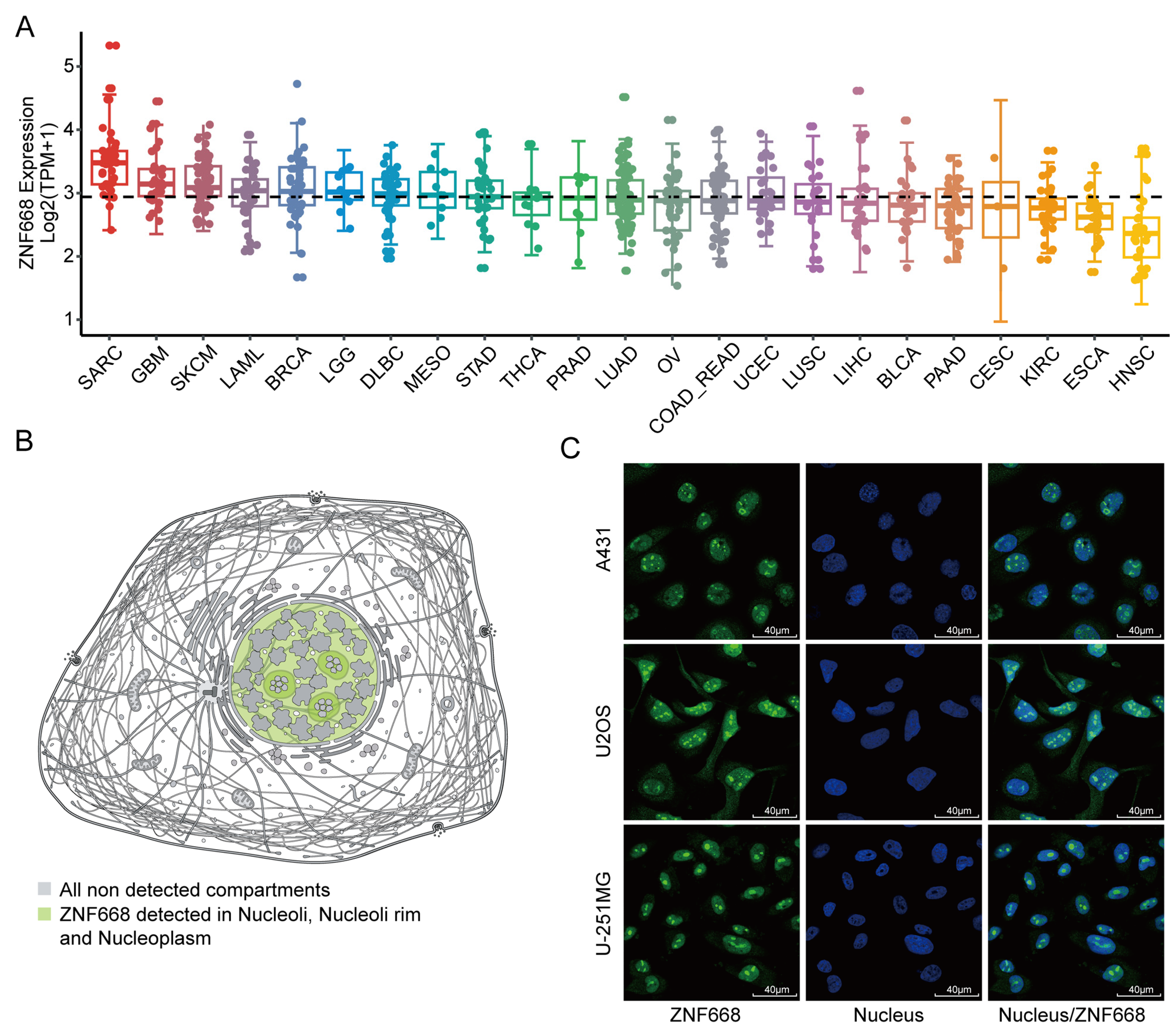
3.2. Expression Profiles and Subcellular Localization of ZNF668 in Pan-Cancer Cell Lines
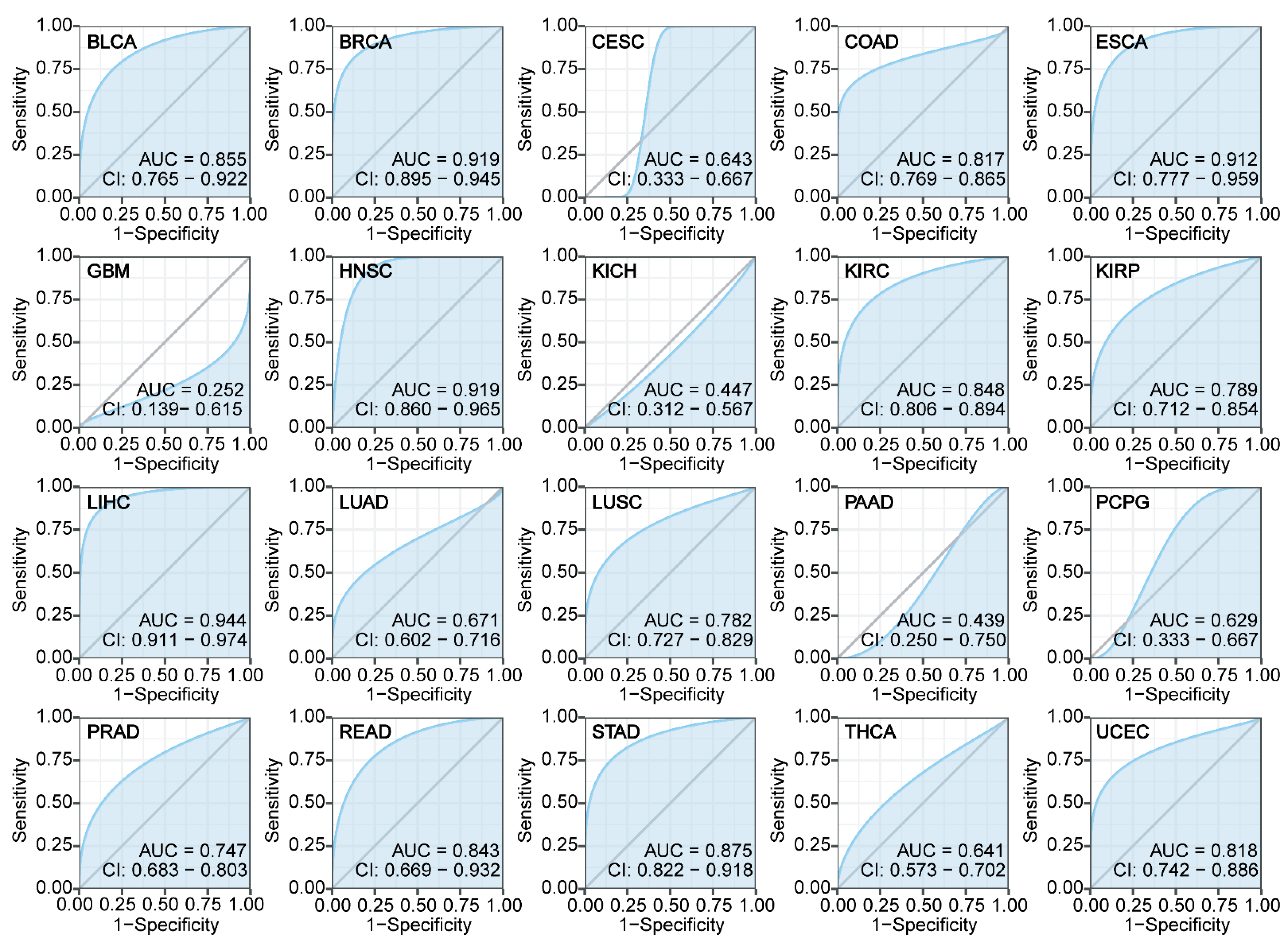
3.3. Diagnostic Performance of ZNF668
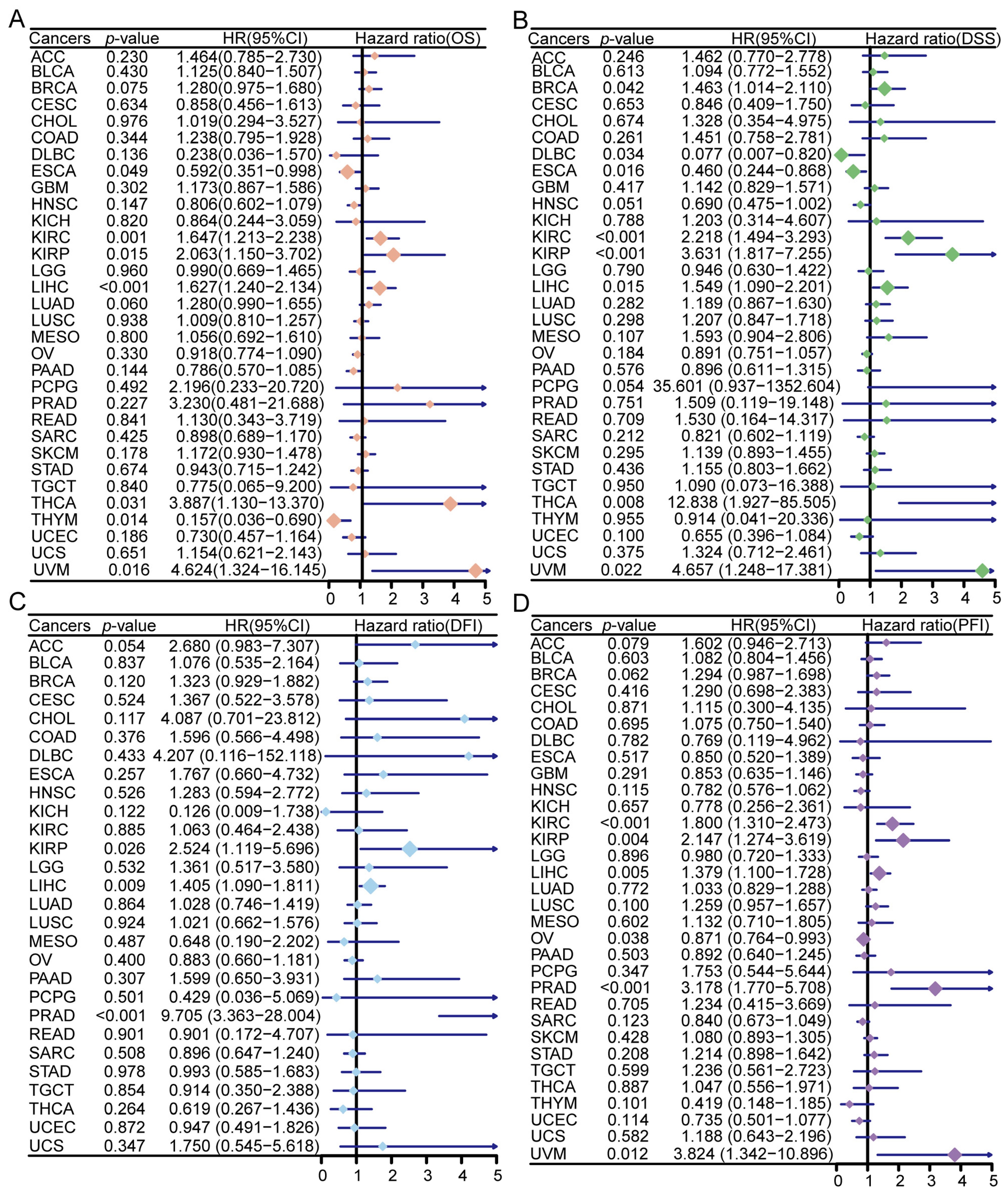
3.4. Prognostic Significance of ZNF668 Expression
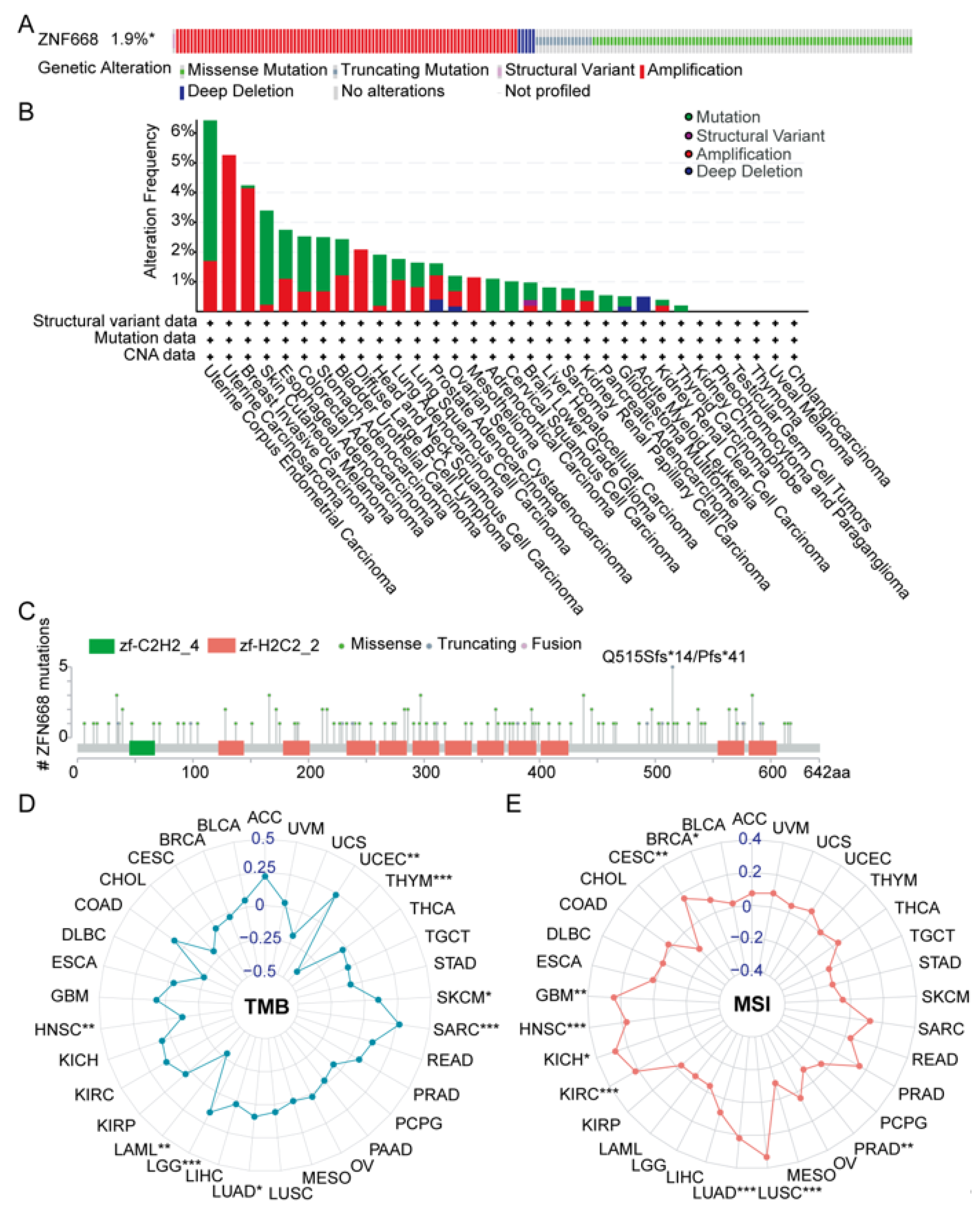
3.5. Genetic Alteration Features of ZNF668
3.6. Landscape of Genetic Alterations in ZNF668
3.7. ZNF668 Interaction Network and Functional Enrichment Analysis
3.8. Association of ZNF668 Expression with Immune Regulatory Molecules
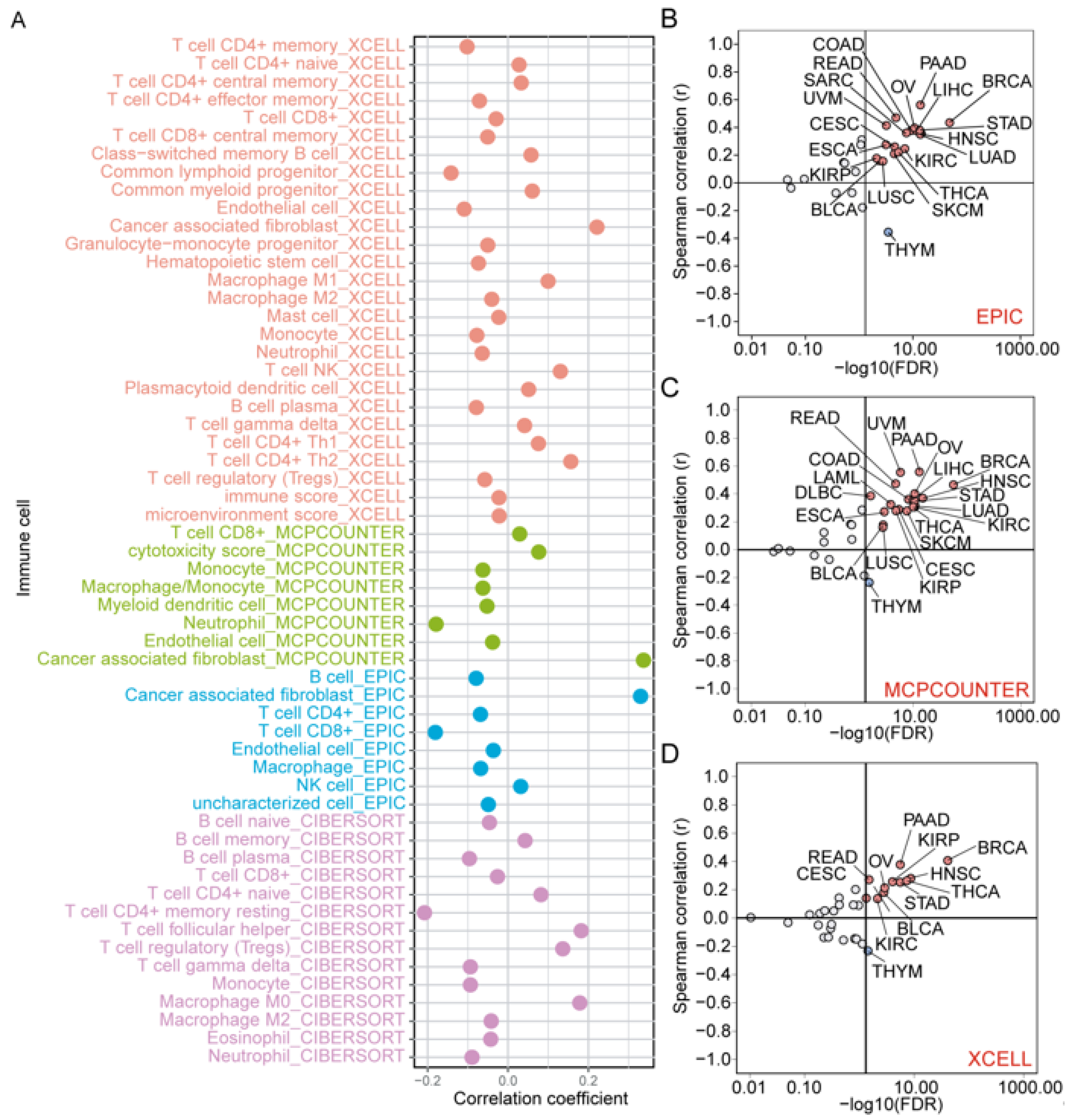
3.9. Correlation of ZNF668 Expression with Immune Cell Infiltration
3.10. Drug Sensitivity Analysis and Molecular Docking
3.11. Validation of ZNF668 Expression by TMA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global cancer burden growing, amidst mounting need for services. Saudi Med. J. 2024, 45, 326–327. [Google Scholar]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, J.; Chen, S.; Wang, X.; Xi, Q.; Shen, H.; Zhang, R. Immune checkpoint inhibitors: Breakthroughs in cancer treatment. Cancer Biol. Med. 2024, 21, 451–472. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Kim, S.J.; Khadka, D.; Seo, J.H. Interplay between Solid Tumors and Tumor Microenvironment. Front. Immunol. 2022, 13, 882718. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Rakhra, G.; Rakhra, G. Zinc finger proteins: Insights into the transcriptional and post transcriptional regulation of immune response. Mol. Biol. Rep. 2021, 48, 5735–5743. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wu, Q. The Multifaceted Roles of Zinc Finger Proteins in Pluripotency and Reprogramming. Int. J. Mol. Sci. 2025, 26, 5106. [Google Scholar] [CrossRef]
- Zhao, J.; Wen, D.; Zhang, S.; Jiang, H.; Di, X. The role of zinc finger proteins in malignant tumors. FASEB J. 2023, 37, e23157. [Google Scholar] [CrossRef]
- Jen, J.; Wang, Y.C. Zinc finger proteins in cancer progression. J. Biomed. Sci. 2016, 23, 53. [Google Scholar] [CrossRef]
- Liu, S.; Sima, X.; Liu, X.; Chen, H. Zinc Finger Proteins: Functions and Mechanisms in Colon Cancer. Cancers 2022, 14, 5242. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, T.; Luo, X.; Li, C.; Li, Q.; Peng, W.; Li, L.; Li, S.; Wang, Z.; Tang, L.; et al. Zinc-finger protein 545 inhibits cell proliferation as a tumor suppressor through inducing apoptosis and is disrupted by promoter methylation in breast cancer. PLoS ONE 2014, 9, e110990. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Li, J. Zinc finger transcription factor ZNF24 inhibits colorectal cancer growth and metastasis by suppressing MMP2 transcription. Genes Dis. 2025, 12, 101529. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Wang, D.; Qian, X.; Du, Y.N.; Sanchez-Solana, B.; Chen, K.; Kanigicherla, M.; Jenkins, L.M.; Luo, J.; Eng, S.; Park, B.; et al. cProSite: A web based interactive platform for online proteomics, phosphoproteomics, and genomics data analysis. J. Biotechnol. Biomed. 2023, 6, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Nusinow, D.P.; Szpyt, J.; Ghandi, M.; Rose, C.M.; McDonald, E.R.; Kalocsay, M.; Jané-Valbuena, J.; Gelfand, E.; Schweppe, D.K.; Jedrychowski, M.; et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell 2020, 180, 387–402.e16. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Reinhold, W.C.; Sunshine, M.; Liu, H.; Varma, S.; Kohn, K.W.; Morris, J.; Doroshow, J.; Pommier, Y. CellMiner: A web-based suite of genomic and pharmacologic tools to explore transcript and drug patterns in the NCI-60 cell line set. Cancer Res. 2012, 72, 3499–3511. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Hou, Y.; Ning, W.; Huhe, M.; Shu, C. Genome-Wide Detection of Leukemia Biomarkers from lincRNA-Protein-Coding Gene Interaction Networks in the Three-Dimensional Chromatin Structure. Curr. Issues Mol. Biol. 2025, 47, 384. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, G.; Wu, J.; Zhou, H.; Zhang, Y.; Miao, Y.; Feng, Y.; Yu, J. Zinc finger protein 668 suppresses non-small cell lung cancer invasion and migration by downregulating Snail and upregulating E-cadherin and zonula occludens-1. Oncol. Lett. 2018, 15, 3806–3813. [Google Scholar] [CrossRef]
- Okuno, Y.; Hattori-Kato, M.; Tanaka, H.; Tonooka, A.; Takeuchi, T. Relationship between the Reduced Expression of Zinc Finger Protein 668 in Bladder Cancer and Its Invasiveness. Int. J. Mol. Sci. 2023, 24, 8668. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Hauge, A.; Rofstad, E.K. Antifibrotic therapy to normalize the tumor microenvironment. J. Transl. Med. 2020, 18, 207. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Maia, A.; Schöllhorn, A.; Schuhmacher, J.; Gouttefangeas, C. CAF-immune cell crosstalk and its impact in immunotherapy. Semin. Immunopathol. 2023, 45, 203–214. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N.; Kafka, A.; Salamon, I.; Bukovac, A. Mismatch Repair Pathway, Genome Stability and Cancer. Front. Mol. Biosci. 2020, 7, 122. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Capucetti, A.; Albano, F.; Bonecchi, R. Multiple Roles for Chemokines in Neutrophil Biology. Front. Immunol. 2020, 11, 1259. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell. Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef]
- Yang, H.; Beutler, B.; Zhang, D. Emerging roles of spliceosome in cancer and immunity. Protein Cell 2022, 13, 559–579. [Google Scholar] [CrossRef]
- Ivanova, O.M.; Anufrieva, K.S.; Kazakova, A.N.; Malyants, I.K.; Shnaider, P.V.; Lukina, M.M.; Shender, V.O. Non-canonical functions of spliceosome components in cancer progression. Cell Death Dis. 2023, 14, 77. [Google Scholar] [CrossRef]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional Addiction in Cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef]
- Nair, S.S.; Kumar, R. Chromatin remodeling in cancer: A gateway to regulate gene transcription. Mol. Oncol. 2012, 6, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Ihara, T.; Suda, H.; Mikami, H.; Semba, K. Gene amplification: Mechanisms and involvement in cancer. Biomol. Concepts 2013, 4, 567–582. [Google Scholar] [CrossRef]
- Haubeiss, S.; Schmid, J.O.; Mürdter, T.E.; Sonnenberg, M.; Friedel, G.; van der Kuip, H.; Aulitzky, W.E. Dasatinib reverses cancer-associated fibroblasts (CAFs) from primary lung carcinomas to a phenotype comparable to that of normal fibroblasts. Mol. Cancer 2010, 9, 168. [Google Scholar] [CrossRef]
- Kadota, H.; Yuge, R.; Shimizu, D.; Miyamoto, R.; Otani, R.; Hiyama, Y.; Takigawa, H.; Hayashi, R.; Urabe, Y.; Kitadai, Y.; et al. Anti-Programmed Cell Death-1 Antibody and Dasatinib Combination Therapy Exhibits Efficacy in Metastatic Colorectal Cancer Mouse Models. Cancers 2022, 14, 6146. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Wang, Y.; Wu, Y.; Li, Y.; Wang, B.; Liu, G.; Gong, Q.; Luo, K.; Jing, J. Stimuli-responsive polymer-dasatinib prodrug to reprogram cancer-associated fibroblasts for boosted immunotherapy. J. Control. Release 2025, 381, 113606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Guo, J.; Zhong, H.; Huang, W.; Chen, S.; He, C. Comprehensive Pan-Cancer Analysis of ZNF668 Reveals the Prognostic and Immunological Significance of ZNF668. Curr. Issues Mol. Biol. 2025, 47, 997. https://doi.org/10.3390/cimb47120997
Hu X, Guo J, Zhong H, Huang W, Chen S, He C. Comprehensive Pan-Cancer Analysis of ZNF668 Reveals the Prognostic and Immunological Significance of ZNF668. Current Issues in Molecular Biology. 2025; 47(12):997. https://doi.org/10.3390/cimb47120997
Chicago/Turabian StyleHu, Xiaoyan, Jiali Guo, Hua Zhong, Wenxin Huang, Size Chen, and Canfeng He. 2025. "Comprehensive Pan-Cancer Analysis of ZNF668 Reveals the Prognostic and Immunological Significance of ZNF668" Current Issues in Molecular Biology 47, no. 12: 997. https://doi.org/10.3390/cimb47120997
APA StyleHu, X., Guo, J., Zhong, H., Huang, W., Chen, S., & He, C. (2025). Comprehensive Pan-Cancer Analysis of ZNF668 Reveals the Prognostic and Immunological Significance of ZNF668. Current Issues in Molecular Biology, 47(12), 997. https://doi.org/10.3390/cimb47120997




