Contribution of 18F-Fluorodeoxyglucose to the Identification of Dubious Lesions Caused by SARS-CoV-2
Abstract
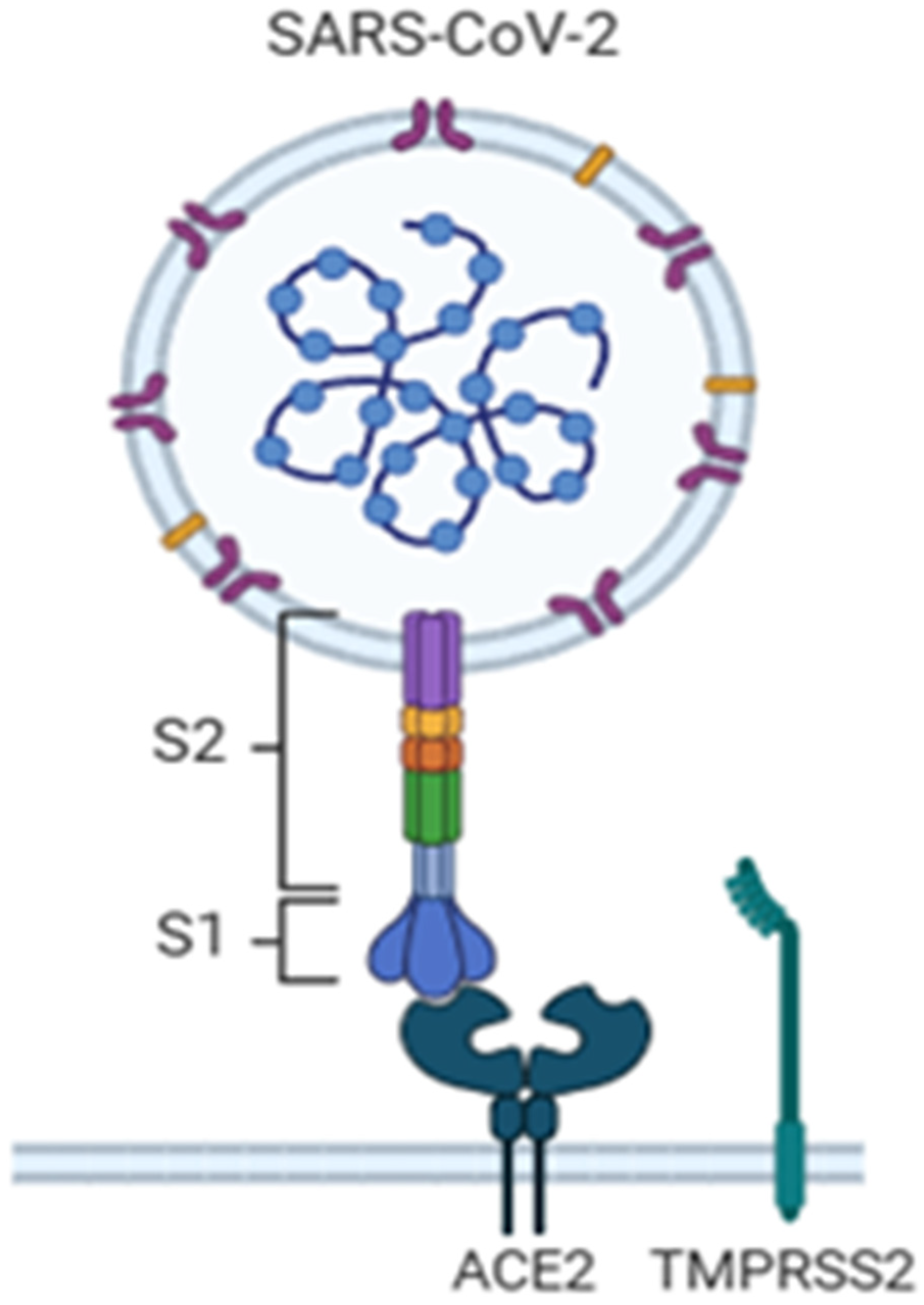
1. Introduction
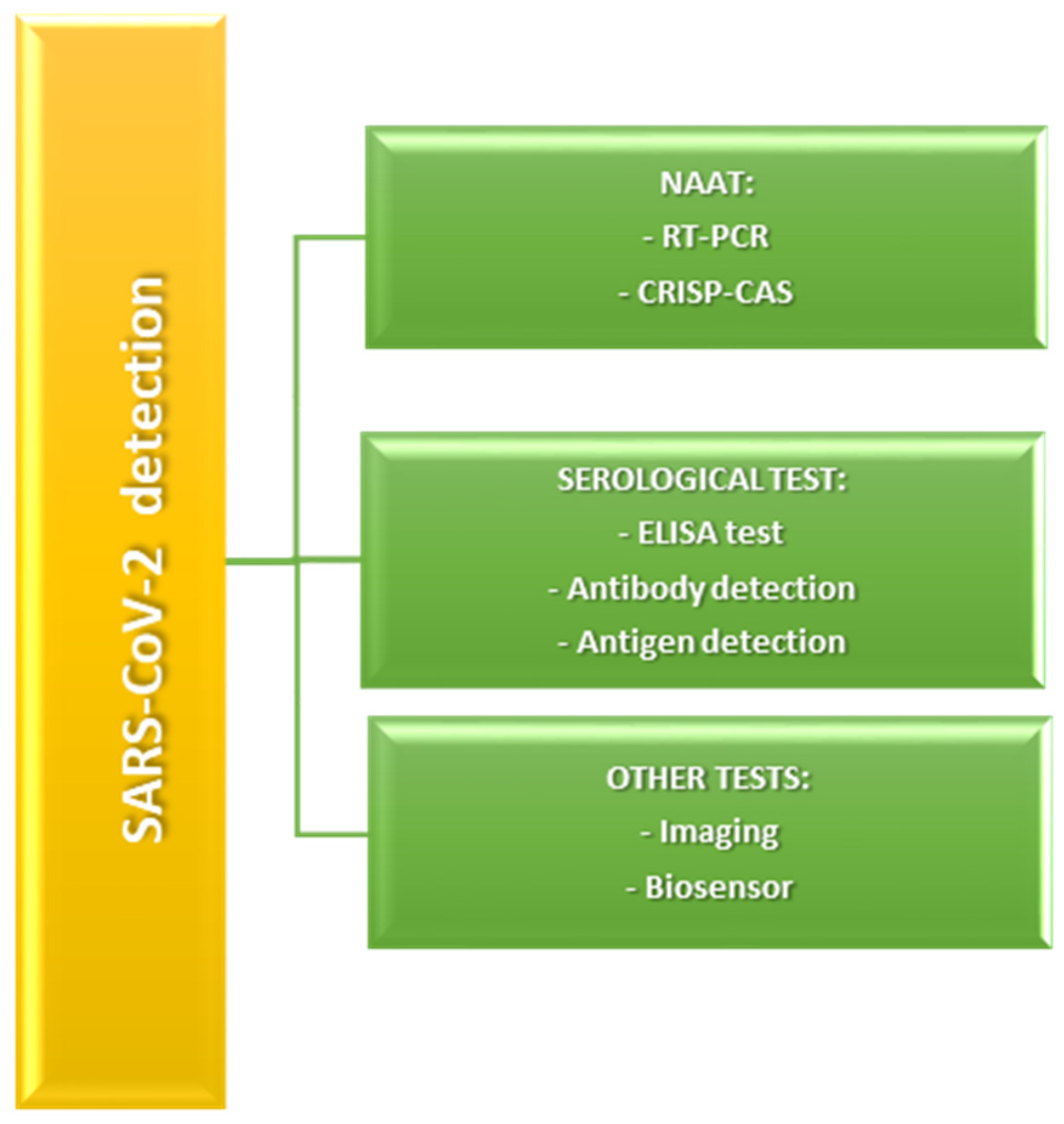
2. Techniques Applied for the Detection of COVID-19 Infection
3. Discussion
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome COronaVirus 2 |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| [18F]FDG | FluoroDeoxyGlucose (18F) |
| PET | Positron Emission Tomography |
| CT | Computed Tomography |
| COVID-19 | COronaVIrus Disease 19 |
| RNA | RiboNucleic Acid |
| S | Spike protein |
| M | Membrane protein |
| E | Envelope protein |
| N | Nucleocapsid protein |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| (rt)-RT PCR | Reverse Transcription—Real Time Polymerase Chain Reaction |
| GGOs | Ground-Glass Opacities |
| NAATs | Nucleic Acid Amplification Tests |
| DNA | DeoxyriboNucleic Acid |
| cDNA | Complementary DNA |
| RPA | Recombinase-Aided Amplification |
| MCDA | Multiple Cross Displacement Amplification |
| LAMP | Loop-Mediated Isothermal Amplification |
| NEAR | Nicking and Extension Amplification Reaction |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeat |
| LFICS | Lateral Flow ImmunoChromatographic Strip |
| ILFA | Lateral Flow Immunochromatographic Assay |
| CLIA | ChemiLuminescence ImmunoAssay |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| EC | ElectroChemical |
| FET | Field-Effect Transistor |
| SPR | Surface Plasmon Resonance |
| SN2 | Substitution Nucleophilic Bimolecular |
| TMPRSS2 | TransMembrane PRoteaSe Serine 2 |
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Declares Public Health Emergency on Novel Coronavirus; Pan American Health Organization (PAHO): Washington, DC, USA, 2020; Available online: https://www.paho.org/en/news/30-1-2020-who-declares-public-health-emergency-novel-coronavirus (accessed on 30 January 2020).
- Manigandan, S.; Wu, M.-T.; Ponnusamy, V.K.; Raghavendra, V.B.; Pugazhendhi, A.; Brindhadevi, K. A Systematic Review on Recent Trends in Transmission, Diagnosis, Prevention and Imaging Features of COVID-19. Process Biochem. 2020, 98, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Sharma, A.; Kumar, S.; Singh, G.; Barnwal, R.P. SARS-CoV-2: Insights into Its Structural Intricacies and Functional Aspects for Drug and Vaccine Development. Int. J. Biol. Macromol. 2021, 179, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The Origin, Transmission and Clinical Therapies on Coronavirus Disease 2019 (COVID-19) Outbreak—An Update on the Status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Tang, X. Guidelines for the Diagnosis and Treatment of Coronavirus Disease 2019 (COVID-19) in China. Glob. Health Med. 2020, 2, 66–72. [Google Scholar] [CrossRef]
- Katal, S.; Aghaghazvini, L.; Gholamrezanezhad, A. Chest-CT Findings of COVID-19 in Patients with Pre-Existing Malignancies; A Pictorial Review. Clin. Imaging 2020, 67, 121–129. [Google Scholar] [CrossRef]
- Şendur, H.N. Debate of Chest CT and RT-PCR Test for the Diagnosis of COVID-19. Radiology 2020, 297, E341–E342. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Momozono, Y.; Ishikawa, M.; Yamada, T.; Yamane, H.; Haradahira, T.; Maeda, M.; Kojima, M. Metabolic Pathway of 2-Deoxy-2-Fluoro-D-Glucose Studied by F-19 NMR. Life Sci. 1986, 39, 737–742. [Google Scholar] [CrossRef]
- Cheng, M.P.; Papenburg, J.; Desjardins, M.; Kanjilal, S.; Quach, C.; Libman, M.; Dittrich, S.; Yansouni, C.P. Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus 2: A Narrative Review. Ann. Intern. Med. 2020, 172, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Yaqinuddin, A. Loop Mediated Isothermal Amplification (LAMP) Assays as a Rapid Diagnostic for COVID-19. Med. Hypotheses 2020, 141, 109786. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.; Li, Y.; Zheng, H.; Qu, W.; Wang, B.; Luo, H. Diagnostic Assays for COVID-19: A Narrative Review. J. Bio-X Res. 2020, 3, 123–134. [Google Scholar] [CrossRef]
- Das, D.; Lin, C.-W.; Chuang, H.-S. LAMP-Based Point-of-Care Biosensors for Rapid Pathogen Detection. Biosensors 2022, 12, 1068. [Google Scholar] [CrossRef]
- Diao, B.; Wen, K.; Chen, J.; Liu, Y.; Yuan, Z.; Han, C.; Chen, J.; Pan, Y.; Chen, L.; Dan, Y.; et al. Diagnosis of Acute Respiratory Syndrome Coronavirus 2 Infection by Detection of Nucleocapsid Protein. 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.03.07.20032524v2 (accessed on 13 March 2020).
- Nguyen, N.N.T.; McCarthy, C.; Lantigua, D.; Camci-Unal, G. Development of Diagnostic Tests for Detection of SARS-CoV-2. Diagnostics 2020, 10, 905. [Google Scholar] [CrossRef]
- Xia, S.; Chen, X. Single-Copy Sensitive, Field-Deployable, and Simultaneous Dual-Gene Detection of SARS-CoV-2 RNA via Modified RT–RPA. Cell Discov. 2020, 6, 37. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, R.H.; Gong, F.; Wei, X.; Dong, Y.; Chen, R.; Yue Liang, M.; Tang, C.; Lu, L. Accuracy of serological tests for COVID-19: A systematic review and meta-analysis. Front. Public Health 2022, 10, 923525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lambert-Niclot, S.; Cuffel, A.; Le Pape, S.; Vauloup-Fellous, C.; Morand-Joubert, L.; Roque-Afonso, A.-M.; Le Goff, J.; Delaugerre, C. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-CoV-2 Antigen in Nasopharyngeal Swabs. J. Clin. Microbiol. 2020, 58, e00977-20. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Cömert, Z.; Polat, K. A Novel Medical Diagnosis Model for COVID-19 Infection Detection Based on Deep Features and Bayesian Optimization. Appl. Soft Comput. 2020, 97, 106580. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Multiplex Reverse Transcription Loop-Mediated Isothermal Amplification Combined with Nanoparticle-Based Lateral Flow Biosensor for the Diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene-Based Field-Effect Transistor Biosensors for the Rapid Detection and Analysis of Viruses: A Perspective in View of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, H.S. Diagnostic Methods and Potential Portable Biosensors for Coronavirus Disease 2019. Biosens. Bioelectron. 2020, 165, 112349. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Polidori, T.; Rucci, C.; Guido, G.; Bracci, B.; De Dominicis, C.; Laghi, A. Chest CT Features of COVID-19 in Rome, Italy. Radiology 2020, 296, E79–E85. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hossain, M.Z.; Shinozuka, K.; Shimizu, N.; Kitada, S.; Suzuki, T.; Ichige, R.; Kuwana, A.; Kobayashi, H. Graphene Field-Effect Transistor Biosensor for Detection of Biotin with Ultrahigh Sensitivity and Specificity. Biosens. Bioelectron. 2020, 165, 112363. [Google Scholar] [CrossRef]
- Dong, D.; Tang, Z.; Wang, S.; Hui, H.; Gong, L.; Lu, Y.; Xue, Z.; Liao, H.; Chen, F.; Yang, F.; et al. The Role of Imaging in the Detection and Management of COVID-19: A Review. IEEE Rev. Biomed. Eng. 2021, 14, 16–29. [Google Scholar] [CrossRef]
- Treglia, G. Diagnostic Performance of 18F-FDG PET/CT in Infectious and Inflammatory Diseases According to Published Meta-Analyses. Contrast Media Mol. Imaging 2019, 2019, 3018349. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Bonacina, M.; Meroni, R.; Kirienko, M.; Galli, F.; Dalto, S.C.; Erba, P.A.; Bombardieri, E. Increased Incidence of Interstitial Pneumonia Detected on [18F]-FDG-PET/CT in Asymptomatic Cancer Patients during COVID-19 Pandemic in Lombardy: A Casualty or COVID-19 Infection? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 777–785. [Google Scholar] [CrossRef]
- Cabrera Villegas, A.; Romero Robles, L.G.; Boulvard Chollet, X.L.E.; Albornoz Almada, M.C.; Mangas Losada, M.; Garrastachu, P.; Cañete Sánchez, F.M.; Ramírez Lasanta, R.; Delgado Bolton, R.C. [18F]-FDG PET/CT in Oncologic Patients with Unsuspected Asymptomatic Infection with SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 786–793. [Google Scholar] [CrossRef]
- Dietz, M.; Chironi, G.; Claessens, Y.-E.; Farhad, R.L.; Rouquette, I.; Serrano, B.; Nataf, V.; Hugonnet, F.; Paulmier, B.; Berthier, F.; et al. COVID-19 Pneumonia: Relationship between Inflammation Assessed by Whole-Body FDG PET/CT and Short-Term Clinical Outcome. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 260–268. [Google Scholar] [CrossRef]
- Bahloul, A.; Boursier, C.; Jeulin, H.; Imbert, L.; Mandry, D.; Karcher, G.; Marie, P.-Y.; Verger, A. CT Abnormalities Evocative of Lung Infection Are Associated with Lower 18F-FDG Uptake in Confirmed COVID-19 Patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Charters, P.F.P.; Little, D.; Rodrigues, J.C.L.; Graham, R.N.; Redman, S.L. 18 FDG-PET/CT Findings in COVID-19: A Single Centre Retrospective Radiological Review. BJR|Case Rep. 2020, 6, 20200091. [Google Scholar] [CrossRef] [PubMed]
- Halsey, R.; Priftakis, D.; Mackenzie, S.; Wan, S.; Davis, L.M.; Lilburn, D.; Thornton, A.; Papathanasiou, N.; Gnanasegaran, G.; Bomanji, J. COVID-19 in the Act: Incidental 18F-FDG PET/CT Findings in Asymptomatic Patients and Those with Symptoms Not Primarily Correlated with COVID-19 during the United Kingdom Coronavirus Lockdown. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 269–281. [Google Scholar] [CrossRef]
- Maurea, S.; Mainolfi, C.G.; Bombace, C.; Annunziata, A.; Attanasio, L.; Petretta, M.; Del Vecchio, S.; Cuocolo, A. FDG-PET/CT Imaging during the COVID-19 Emergency: A Southern Italian Perspective. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2691–2697. [Google Scholar] [CrossRef]
- Mucientes Rasilla, J.; Jimeno Pernett, R.; Cardona Arboniés, J. Diagnóstico de neumonía COVID-19 en pacientes asintomáticos tras la realización de un PET/TC oncológico. Rev. Española Med. Nucl. E Imagen Mol. 2020, 39, 299–302. [Google Scholar] [CrossRef]
- Olivari, L.; Riccardi, N.; Rodari, P.; Buonfrate, D.; Diodato, S.; Formenti, F.; Angheben, A.; Salgarello, M. Accidental Diagnosis of COVID-19 Pneumonia after 18F FDG PET/CT: A Case Series. Clin. Transl. Imaging 2020, 8, 393–400. [Google Scholar] [CrossRef]
- Romeih, M.; Mahrous, M.R.; El Kassas, M. Incidental Radiological Findings Suggestive of COVID-19 in Asymptomatic Patients. World J. Radiol. 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Hossein, H.; Ali, K.M.; Hosseini, M.; Sarveazad, A.; Safari, S.; Yousefifard, M. Value of Chest Computed Tomography Scan in Diagnosis of COVID-19; A Systematic Review and Meta-Analysis. Clin. Transl. Imaging 2020, 8, 469–481. [Google Scholar] [CrossRef]
- Xu, X.; Yu, C.; Qu, J.; Zhang, L.; Jiang, S.; Huang, D.; Chen, B.; Zhang, Z.; Guan, W.; Ling, Z.; et al. Imaging and Clinical Features of Patients with 2019 Novel Coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidis, E.; Exarhos, D.; Housianakou, I.; Bournazos, A.; Datseris, I. FDG Uptake in Axillary Lymph Nodes after Vaccination against Pandemic (H1N1). Eur. Radiol. 2010, 20, 1251–1253. [Google Scholar] [CrossRef]
- Kirienko, M.; Padovano, B.; Serafini, G.; Marchianò, A.; Gronchi, A.; Seregni, E.; Alessi, A. CT, [18F]FDG-PET/CT and Clinical Findings before and during Early COVID-19 Onset in a Patient Affected by Vascular Tumour. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1769–1770. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.Y.; Chen, Y.X.; Fang, J.Y. 2019 Novel Coronavirus Infection and Gastrointestinal Tract. J. Dig. Dis. 2020, 21, 125–126. [Google Scholar] [CrossRef]
- Castelnovo, L.; Capelli, F.; Tamburello, A.; Faggioli, P.M.; Mazzone, A. Symmetric Cutaneous Vasculitis in COVID-19 Pneumonia. Acad. Dermatol. Venereol. 2020, 34, e362–e363. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Whittaker, E. Kawasaki-like Disease: Emerging Complication during the COVID-19 Pandemic. Lancet 2020, 395, 1741–1743. [Google Scholar] [CrossRef]
- Farrah, T.E.; Basu, N.; Dweck, M.; Calcagno, C.; Fayad, Z.A.; Dhaun, N. Advances in Therapies and Imaging for Systemic Vasculitis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1520–1541. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Millon, A.; Fayad, Z.A. Molecular Imaging in Atherosclerosis: FDG PET. Curr. Atheroscler. Rep. 2012, 14, 429–437. [Google Scholar] [CrossRef]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In Vivo 18F-Fluorodeoxyglucose Positron Emission Tomography Imaging Provides a Noninvasive Measure of Carotid Plaque Inflammation in Patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef]
- Rondina, M.T.; Lam, U.T.; Pendleton, R.C.; Kraiss, L.W.; Wanner, N.; Zimmerman, G.A.; Hoffman, J.M.; Hanrahan, C.; Boucher, K.; Christian, P.E.; et al. 18F-FDG PET in the Evaluation of Acuity of Deep Vein Thrombosis. Clin. Nucl. Med. 2012, 37, 1139–1145. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Amati, E.; Perbellini, O.; Rotta, G.; Bernardi, M.; Chieregato, K.; Sella, S.; Rodeghiero, F.; Ruggeri, M.; Astori, G. High-Throughput Immunophenotypic Characterization of Bone Marrow- and Cord Blood-Derived Mesenchymal Stromal Cells Reveals Common and Differentially Expressed Markers: Identification of Angiotensin-Converting Enzyme (CD143) as a Marker Differentially Expressed between Adult and Perinatal Tissue Sources. Stem Cell Res. Ther. 2018, 9, 10. [Google Scholar] [CrossRef]
- Bao, W.; Min, D.; Twigg, S.M.; Shackel, N.A.; Warner, F.J.; Yue, D.K.; McLennan, S.V. Monocyte CD147 Is Induced by Advanced Glycation End Products and High Glucose Concentration: Possible Role in Diabetic Complications. Am. J. Physiol.-Cell Physiol. 2010, 299, C1212–C1219. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, X.N.; Pan, H.X.; Su, H.; Huang, B.; Yang, M.; Luo, D.J.; Weng, M.X.; Ma, L.; Nie, X. Pathological changes of the spleen in ten patients with coronavirus disease 2019(COVID-19) by postmortem needle autopsy. Zhonghua Bing Li Xue Za Zhi 2020, 49, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Galougahi, M.; Yousefi-Koma, A.; Bakhshayeshkaram, M.; Raad, N.; Haseli, S. 18FDG PET/CT Scan Reveals Hypoactive Orbitofrontal Cortex in Anosmia of COVID-19. Acad. Radiol. 2020, 27, 1042–1043. [Google Scholar] [CrossRef]
- Bonnini, S.; Borghesi, M. Relationship between mental health and socio-economic, demographic and environmental factors in the COVID-19 lockdown period—A multivariate regression analysis. Mathematics 2022, 10, 3237. [Google Scholar] [CrossRef]
- Tana, M.; Porreca, E.; Ricci, F.; Mattoli, M.V.; Pizzi, A.D.; Ciliberti, F.; Spagnolo, P.; Tana, C. 18F-FDG PET-CT Imaging in Sarcoidosis Molecular Mechanisms and Applications. J. Biol. Regul. Homeost. Agents 2024, 38, 1–10. [Google Scholar]
- Palamidas, D.A.; Chatzis, L.; Papadaki, M.; Gissis, I.; Kambas, K.; Andreakos, E.; Goules, A.V.; Tzioufas, A.G. Current insights into tissue injury of giant cell arteritis: From acute inflammatory responses towards inappropriate tissue remodeling. Cells 2024, 13, 430. [Google Scholar] [CrossRef]
- Di Bella, S.; Sanson, G.; Monticelli, J.; Zerbato, V.; Principe, L.; Giuffrè, M.; Pipitone, G.; Luzzati, R. Clostridioides difficile infection: History, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin. Microbiol. Rev. 2024, 37, e00135-23. [Google Scholar] [CrossRef]
- Marinaccio, L.; Stefanucci, A.; Scioli, G.; Della Valle, A.; Zengin, G.; Cichelli, A.; Mollica, A. Peptide Human Neutrophil Elastase Inhibitors from Natural Sources: An Overview. Int. J. Mol. Sci. 2022, 23, 2924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Authors (Year) | Study Type | Patient Population/Sample Size | Main PET/CT Findings | Key Conclusions |
|---|---|---|---|---|
| Setti et al., 2021 [30] | Retrospective, oncologic patients | 65 asymptomatic cancer patients | Incidental interstitial pneumonia detected on PET/CT during COVID-19 pandemic; increased [18F]FDG uptake in GGOs. | PET/CT may incidentally detect COVID-19 in asymptomatic individuals. |
| Cabrera-Villegas et al., 2021 [31] | Retrospective | 12 oncologic patients | FDG uptake in bilateral pulmonary GGOs, compatible with viral pneumonia. | PET/CT can reveal unexpected COVID-19-related findings in cancer patients. |
| Dietz et al., 2021 [32] | Prospective observational | 30 hospitalized COVID-19 patients | Correlation between whole-body inflammatory activity and disease severity. | Higher [18F]FDG uptake associated with poorer short-term outcomes. |
| Bahloul et al., 2021 [33] | Observational | 20 confirmed COVID-19 patients | Lower pulmonary FDG uptake in confirmed COVID-19 cases than expected. | Suggests variability in inflammatory response; PET/CT role may depend on disease stage. |
| Halsey et al., 2021 [35] | Retrospective | Mixed (oncologic + inflammatory cases) | Incidental FDG uptake in lungs, lymph nodes in asymptomatic COVID-19 cases. | PET/CT may detect subclinical inflammation before symptoms. |
| Olivari et al., 2020 [38] | Case series | 4 patients | Bilateral GGOs with increased [18F]FDG uptake. | Early pulmonary metabolic changes visible before severe symptoms. |
| Mucientes Rasilla et al., 2020 [37] | Case report | 1 oncologic patient | Incidental pneumonia with FDG-avid GGOs. | PET/CT can contribute to early identification of COVID-19 lesions. |
| Maurea et al., 2020 [36] | Retrospective, multicentric | 10 PET/CT centers in Italy | FDG uptake in lungs and lymph nodes in COVID-19-positive patients. | Confirms systemic inflammatory involvement detectable via PET/CT. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamura, C.; Marinaccio, L.; Dimiccoli, V.; Mollica, A.; Stefanucci, A. Contribution of 18F-Fluorodeoxyglucose to the Identification of Dubious Lesions Caused by SARS-CoV-2. Curr. Issues Mol. Biol. 2025, 47, 984. https://doi.org/10.3390/cimb47120984
Altamura C, Marinaccio L, Dimiccoli V, Mollica A, Stefanucci A. Contribution of 18F-Fluorodeoxyglucose to the Identification of Dubious Lesions Caused by SARS-CoV-2. Current Issues in Molecular Biology. 2025; 47(12):984. https://doi.org/10.3390/cimb47120984
Chicago/Turabian StyleAltamura, Claudia, Lorenza Marinaccio, Vincenzo Dimiccoli, Adriano Mollica, and Azzurra Stefanucci. 2025. "Contribution of 18F-Fluorodeoxyglucose to the Identification of Dubious Lesions Caused by SARS-CoV-2" Current Issues in Molecular Biology 47, no. 12: 984. https://doi.org/10.3390/cimb47120984
APA StyleAltamura, C., Marinaccio, L., Dimiccoli, V., Mollica, A., & Stefanucci, A. (2025). Contribution of 18F-Fluorodeoxyglucose to the Identification of Dubious Lesions Caused by SARS-CoV-2. Current Issues in Molecular Biology, 47(12), 984. https://doi.org/10.3390/cimb47120984








