Oxytocin Deficiency in Childhood and Adolescence: Clinical Features, Diagnostic Challenges and Therapeutic Perspectives
Abstract
1. Introduction
2. Physiology of Oxytocin and Vasopressin in Development
2.1. Sites of Synthesis and Release
2.2. Receptors and Cross-Reactivity
2.3. Developmental Aspects
2.4. Peripheral Versus Central Actions in Pediatric Physiology
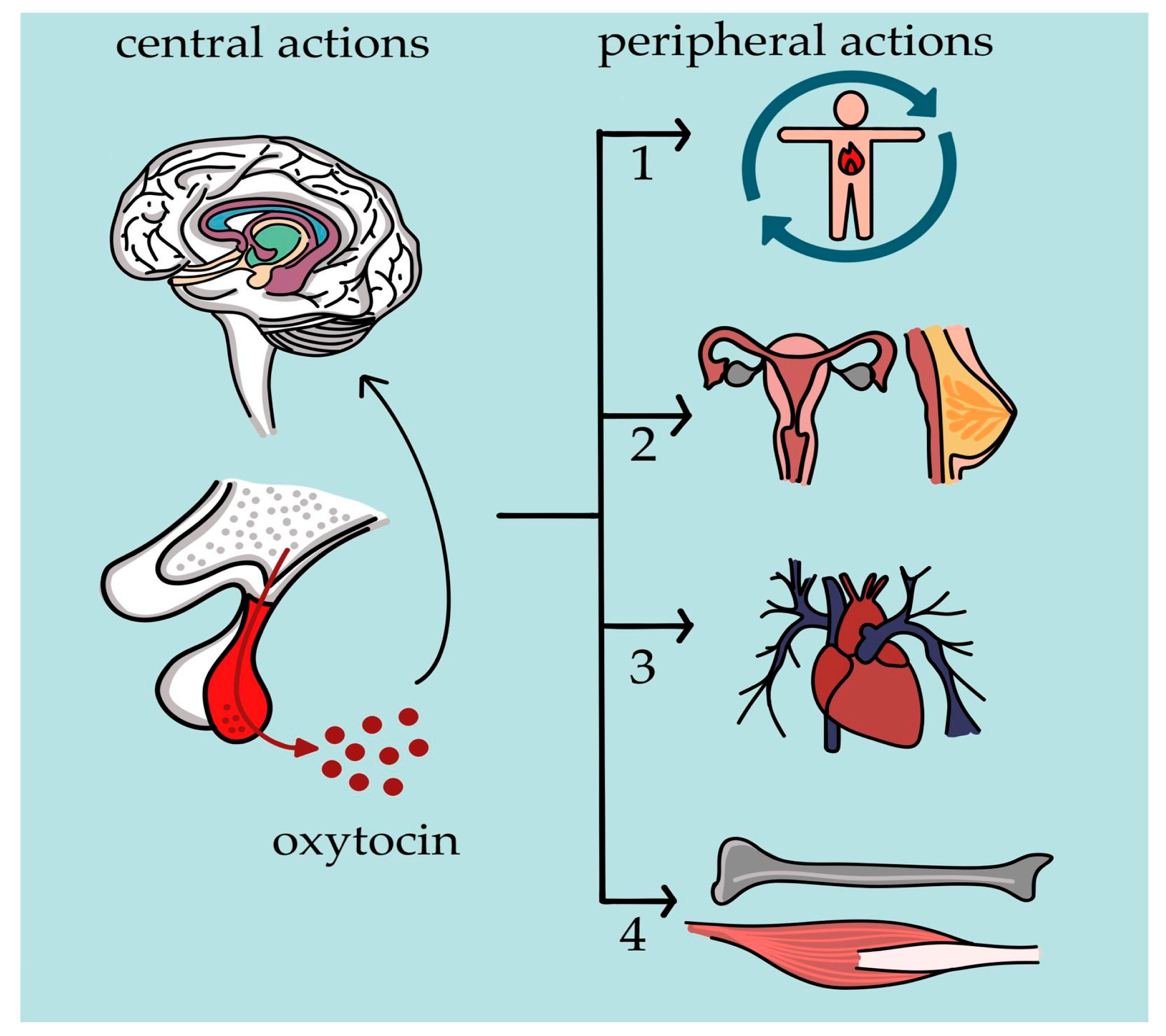
3. Etiology of Oxytocin Deficiency in Pediatric Populations
3.1. Hypothalamic-Pituitary Axis
3.1.1. Congenital Causes
3.1.2. Acquired Causes
3.1.3. Secondary Deficiency and Neurohypophyseal Dysfunction
3.2. Genetic and Epigenetic Alterations of the Oxytocin Receptor
3.3. Nutritional Factors
4. Clinical Manifestations of Oxytocin Deficiency in Pediatrics
4.1. Metabolic Features
4.2. Neurobehavioral and Psychiatric Features
4.3. Endocrine and Growth-Related Features
4.4. Insights from Animal and Translational Models
5. Diagnostic Challenges in Identifying Oxytocin Deficiency
Preliminary Clinical Indicators of Pediatric Oxytocin Deficiency
6. Therapeutic Perspectives
6.1. Oxytocin Administration Strategies
6.2. Clinical Trials and Applications in Pediatric Disorders
| Prader–Willi Syndrome | Autism Spectrum Disorder | Hypothalamic Obesity Post-Craniopharyngioma | ||
|---|---|---|---|---|
| Population | Infants under six months | Children and adolescents | Children/adolescents 3–18 years | Children and adolescents |
| Trial design and size | Early-phase, small RCT [146,147] | Small RCTs, heterogeneous samples [148,149,150,158,159,160] | Phase III studies and smaller RCTs [37,100,153,155,161,162,163,164] | Pilot RCTs [157] |
| Dose and duration (intranasal administration) | 4 IU every other day–8 IU daily. | 18–40 IU daily | 8–80 IU daily (24 IU in most studies), intermittent dosing; 5–24 weeks | 24 IU daily, short term |
| Main findings | Improved feeding (suction, swallowing), social engagement, motor abilities (crawling); benefits are greater with early treatment | Reduced hyperphagia, anxiety, and improved social behaviors; higher doses or older age associated with irritability/aggression; personalized dosing recommended | Mixed efficacy; the largest Phase III trial showed no significant benefit; subgroup benefits in younger or intellectually disabled children; biomarkers (low baseline OXT, OXTR genotype) may predict response | Reduced impulsivity, anxiety, hyperphagia; no significant body-mass-index or metabolic improvements |
6.3. Safety and Efficacy Considerations
6.4. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder |
| AVP | Arginine vasopressin |
| CDI | Central diabetes insipidus |
| GPCRs | G protein-coupled receptors |
| OXT | Oxytocin |
| OXT-D | Oxytocin deficiency |
| OXTR | Oxytocin receptor |
| PWS | Prader–Willi syndrome |
| RCT | Randomized controlled trial |
| SYS | Schaaf–Yang syndrome |
References
- Camerino, C. The Long Way of Oxytocin from the Uterus to the Heart in 70 Years from Its Discovery. Int. J. Mol. Sci. 2023, 24, 2556. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin Receptor-Deficient Mice Developed Late-Onset Obesity. NeuroReport 2008, 19, 951–955. [Google Scholar] [CrossRef]
- Camerino, C. Low Sympathetic Tone and Obese Phenotype in Oxytocin-deficient Mice. Obesity 2009, 17, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Camerino, C. The New Frontier in Oxytocin Physiology: The Oxytonic Contraction. Int. J. Mol. Sci. 2020, 21, 5144. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.; Zyga, O.; Pineo-Cavanaugh, P.L.; Jeng, M.; Fischbein, N.J.; Partap, S.; Katznelson, L.; Parker, K.J. Socio-Behavioral Dysfunction in Disorders of Hypothalamic-Pituitary Involvement: The Potential Role of Disease-Induced Oxytocin and Vasopressin Signaling Deficits. Neurosci. Biobehav. Rev. 2022, 140, 104770. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Leow, M.K.-S.; Magkos, F. Oxytocin in Metabolic Homeostasis: Implications for Obesity and Diabetes Management. Obes. Rev. 2019, 20, 22–40. [Google Scholar] [CrossRef]
- Torres, N.; Martins, D.; Santos, A.J.; Prata, D.; Veríssimo, M. How Do Hypothalamic Nonapeptides Shape Youth’s Sociality? A Systematic Review on Oxytocin, Vasopressin and Human Socio-Emotional Development. Neurosci. Biobehav. Rev. 2018, 90, 309–331. [Google Scholar] [CrossRef]
- Jin, Y.; Song, D.; Yan, Y.; Quan, Z.; Qing, H. The Role of Oxytocin in Early-Life-Stress-Related Neuropsychiatric Disorders. Int. J. Mol. Sci. 2023, 24, 10430. [Google Scholar] [CrossRef]
- Bhargava, R.; Daughters, K.L.; Rees, A. Oxytocin Therapy in Hypopituitarism: Challenges and Opportunities. Clin. Endocrinol. 2019, 90, 257–264. [Google Scholar] [CrossRef]
- Taylor, A.E.; Lee, H.; Buisman-Pijlman, F.T.A. Oxytocin Treatment in Pediatric Populations. Front. Behav. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and Vasopressin: Linking Pituitary Neuropeptides and Their Receptors to Social Neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and Vasopressin in the Human Brain: Social Neuropeptides for Translational Medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Rigney, N.; De Vries, G.J.; Petrulis, A.; Young, L.J. Oxytocin, Vasopressin, and Social Behavior: From Neural Circuits to Clinical Opportunities. Endocrinology 2022, 163, bqac111. [Google Scholar] [CrossRef] [PubMed]
- Maejima, Y.; Sakuma, K.; Santoso, P.; Gantulga, D.; Katsurada, K.; Ueta, Y.; Hiraoka, Y.; Nishimori, K.; Tanaka, S.; Shimomura, K.; et al. Oxytocinergic Circuit from Paraventricular and Supraoptic Nuclei to Arcuate POMC Neurons in Hypothalamus. FEBS Lett. 2014, 588, 4404–4412. [Google Scholar] [CrossRef] [PubMed]
- Aulinas, A.; Lawson, E.A. The Oxytocin System and Implications for Oxytocin Deficiency in Hypothalamic-Pituitary Disease. Endocr. Rev. 2025, 46, 518–548. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Leng, G. Dendritic Peptide Release and Peptide-Dependent Behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Żera, T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int. J. Mol. Sci. 2021, 22, 11465. [Google Scholar] [CrossRef]
- Opacka-Juffry, J.; Mohiyeddini, C. Experience of Stress in Childhood Negatively Correlates with Plasma Oxytocin Concentration in Adult Men. Stress 2012, 15, 1–10. [Google Scholar] [CrossRef]
- Lahaye, E.; Fetissov, S.O. Functional Role of Immunoglobulin G as an Oxytocin-Carrier Protein. Peptides 2024, 177, 171221. [Google Scholar] [CrossRef]
- Jones, P.M.; Robinson, I.C.A.F. Differential Clearance of Neurophysin and Neurohypophysial Peptides from the Cerebrospinal Fluid in Conscious Guinea Pigs. Neuroendocrinology 1982, 34, 297–302. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Cid-Jofré, V.; Moreno, M.; Reyes-Parada, M.; Renard, G.M. Role of Oxytocin and Vasopressin in Neuropsychiatric Disorders: Therapeutic Potential of Agonists and Antagonists. Int. J. Mol. Sci. 2021, 22, 12077. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R.; Hegoburu, C.; Van Den Burg, E. New Opportunities in Vasopressin and Oxytocin Research: A Perspective from the Amygdala. Annu. Rev. Neurosci. 2015, 38, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Pittman, D.Q.J. A Neuro-Endocrine-Immune Symphony: Neuroendocrinology Briefing 39. J. Neuroendocrinol. 2011, 23, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, H.-P.; Tian, S.; Wang, L.; Wang, S.C.; Zhang, F.; Wang, Y.-F. Oxytocin-Secreting System: A Major Part of the Neuroendocrine Center Regulating Immunologic Activity. J. Neuroimmunol. 2015, 289, 152–161. [Google Scholar] [CrossRef]
- Hausmann, H.; Richters, A.; Kreienkamp, H.J.; Meyerhof, W.; Mattes, H.; Lederis, K.; Zwiers, H.; Richter, D. Mutational Analysis and Molecular Modeling of the Nonapeptide Hormone Binding Domains of the [Arg8]Vasotocin Receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 6907–6912. [Google Scholar] [CrossRef]
- Postina, R.; Kojro, E.; Fahrenholz, F. Separate Agonist and Peptide Antagonist Binding Sites of the Oxytocin Receptor Defined by Their Transfer into the V2 Vasopressin Receptor. J. Biol. Chem. 1996, 271, 31593–31601. [Google Scholar] [CrossRef]
- Cao, C.; Wang, L.; Wu, J.; Li, G.; Fang, R.; Liu, P.; Luo, S.; Elhai, J.D. Association between the OXTR Rs53576 Genotype and Latent Profiles of Post-Traumatic Stress Disorder and Depression Symptoms in a Representative Sample of Earthquake Survivors. Anxiety Stress Coping 2020, 33, 140–147. [Google Scholar] [CrossRef]
- Birnbaumer, M.; Gilbert, S.; Rosenthal, W. An Extracellular Congenital Nephrogenic Diabetes Insipidus Mutation of the Vasopressin Receptor Reduces Cell Surface Expression, Affinity for Ligand, and Coupling to the Gs/Adenylyl Cyclase System. Mol. Endocrinol. 1994, 8, 886–894. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Maejima, Y.; Suyama, S.; Yoshida, M.; Arai, T.; Katsurada, K.; Kumari, P.; Nakabayashi, H.; Kakei, M.; Yada, T. Peripheral Oxytocin Activates Vagal Afferent Neurons to Suppress Feeding in Normal and Leptin-Resistant Mice: A Route for Ameliorating Hyperphagia and Obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R360–R369. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kumari, P.; Wang, L.; Hidema, S.; Nishimori, K.; Yada, T. Relay of Peripheral Oxytocin to Central Oxytocin Neurons via Vagal Afferents for Regulating Feeding. Biochem. Biophys. Res. Commun. 2019, 519, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.K.; Lumpkin, M.D.; McCANN, S.M. Evidence for a Physiological Role for Oxytocin in the Control of Prolactin Secretion. Endocrinology 1986, 119, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Kotwica, G.; Staszkiewicz, J.; Skowroński, M.T.; Siawrys, G.; Bogacka, I.; Franczak, A.; Kurowicka, B.; Kraziński, B.; Okrasa, S. Effects of Oxytocin Alone and in Combination with Selected Hypothalamic Hormones on ACTH, Beta-Endorphin, LH and PRL Secretion by Anterior Pituitary Cells of Cyclic Pigs. Reprod. Biol. 2006, 6, 115–131. [Google Scholar]
- Hammock, E.A.D. Developmental Perspectives on Oxytocin and Vasopressin. Neuropsychopharmacology 2015, 40, 24–42. [Google Scholar] [CrossRef]
- Sinding, C.; Czernichow, P.; Seif, S.M.; Robinson, A.G. Quantitative Changes in Neurohypophyseal Peptides in the Developing Brain. Peptides 1980, 1, 45–50. [Google Scholar] [CrossRef]
- Zheng, J.-J.; Li, S.-J.; Zhang, X.-D.; Miao, W.-Y.; Zhang, D.; Yao, H.; Yu, X. Oxytocin Mediates Early Experience–Dependent Cross-Modal Plasticity in the Sensory Cortices. Nat. Neurosci. 2014, 17, 391–399. [Google Scholar] [CrossRef]
- Johnson, Z.V.; Young, L.J. Oxytocin and Vasopressin Neural Networks: Implications for Social Behavioral Diversity and Translational Neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef]
- McCormack, S.E.; Blevins, J.E.; Lawson, E.A. Metabolic Effects of Oxytocin. Endocr. Rev. 2020, 41, 121–145. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by Oxytocin and Vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef]
- Heinrichs, M.; Domes, G. Neuropeptides and Social Behaviour: Effects of Oxytocin and Vasopressin in Humans. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 170, pp. 337–350. ISBN 978-0-444-53201-5. [Google Scholar]
- Carter, C.; Grippo, A.; Pournajafinazarloo, H.; Ruscio, M.; Porges, S. Oxytocin, Vasopressin and Sociality. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 170, pp. 331–336. ISBN 978-0-444-53201-5. [Google Scholar]
- Bittel, D.C.; Butler, M.G. Prader–Willi Syndrome: Clinical Genetics, Cytogenetics and Molecular Biology. Expert Rev. Mol. Med. 2005, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Assinder, S.J. The Importance of Experimental Investigation of the Peripheral Oxytocin System. In Oxytocin; Werry, E.L., Reekie, T.A., Kassiou, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2384, pp. 1–27. ISBN 978-1-0716-1758-8. [Google Scholar]
- Kabasakalian, A.; Ferretti, C.J.; Hollander, E. Oxytocin and Prader-Willi Syndrome. In Behavioral Pharmacology of Neuropeptides: Oxytocin; Hurlemann, R., Grinevich, V., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2017; Volume 35, pp. 529–557. ISBN 978-3-319-63738-9. [Google Scholar]
- Butler, M.G. Prader-Willi Syndrome: Current Understanding of Cause and Diagnosis. Am. J. Med. Genet. 1990, 35, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Purba, J.S.; Hofman, M.A. Alterations in the Hypothalamic Paraventricular Nucleus and Its Oxytocin Neurons (Putative Satiety Cells) in Prader-Willi Syndrome: A Study of Five Cases. J. Clin. Endocrinol. Metab. 1995, 80, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Althammer, F.; Muscatelli, F.; Grinevich, V.; Schaaf, C.P. Oxytocin-Based Therapies for Treatment of Prader-Willi and Schaaf-Yang Syndromes: Evidence, Disappointments, and Future Research Strategies. Transl. Psychiatry 2022, 12, 318. [Google Scholar] [CrossRef]
- Camerino, C. The Pivotal Role of Oxytocin’s Mechanism of Thermoregulation in Prader-Willi Syndrome, Schaaf-Yang Syndrome, and Autism Spectrum Disorder. Int. J. Mol. Sci. 2024, 25, 2066. [Google Scholar] [CrossRef]
- McCarthy, J.; Lupo, P.J.; Kovar, E.; Rech, M.; Bostwick, B.; Scott, D.; Kraft, K.; Roscioli, T.; Charrow, J.; Schrier Vergano, S.A.; et al. Schaaf-Yang Syndrome Overview: Report of 78 Individuals. Am. J. Med. Genet. Part A 2018, 176, 2564–2574. [Google Scholar] [CrossRef]
- Negishi, Y.; Ieda, D.; Hori, I.; Nozaki, Y.; Yamagata, T.; Komaki, H.; Tohyama, J.; Nagasaki, K.; Tada, H.; Saitoh, S. Schaaf-Yang Syndrome Shows a Prader-Willi Syndrome-like Phenotype during Infancy. Orphanet J. Rare Dis. 2019, 14, 277. [Google Scholar] [CrossRef]
- John, S.; Jaeggi, A.V. Oxytocin Levels Tend to Be Lower in Autistic Children: A Meta-Analysis of 31 Studies. Autism 2021, 25, 2152–2161. [Google Scholar] [CrossRef]
- Parker, K.J.; Garner, J.P.; Libove, R.A.; Hyde, S.A.; Hornbeak, K.B.; Carson, D.S.; Liao, C.-P.; Phillips, J.M.; Hallmayer, J.F.; Hardan, A.Y. Plasma Oxytocin Concentrations and OXTR Polymorphisms Predict Social Impairments in Children with and without Autism Spectrum Disorder. Proc. Natl. Acad. Sci. USA 2014, 111, 12258–12263. [Google Scholar] [CrossRef]
- Prodam, F.; Caputo, M.; Mele, C.; Marzullo, P.; Aimaretti, G. Insights into Non-Classic and Emerging Causes of Hypopituitarism. Nat. Rev. Endocrinol. 2021, 17, 114–129. [Google Scholar] [CrossRef]
- Mann, A.; Kalitsi, J.; Jani, K.; Martins, D.; Kapoor, R.R.; Paloyelis, Y. The Oxytocin System in Patients with Craniopharyngioma: A Systematic Review. Front. Neuroendocrinol. 2025, 76, 101170. [Google Scholar] [CrossRef]
- Gebert, D.; Auer, M.K.; Stieg, M.R.; Freitag, M.T.; Lahne, M.; Fuss, J.; Schilbach, K.; Schopohl, J.; Stalla, G.K.; Kopczak, A. De-Masking Oxytocin-Deficiency in Craniopharyngioma and Assessing Its Link with Affective Function. Psychoneuroendocrinology 2018, 88, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Daubenbüchel, A.M.M.; Hoffmann, A.; Eveslage, M.; Özyurt, J.; Lohle, K.; Reichel, J.; Thiel, C.M.; Martens, H.; Geenen, V.; Müller, H.L. Oxytocin in Survivors of Childhood-Onset Craniopharyngioma. Endocrine 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Brandi, M.; Gebert, D.; Kopczak, A.; Auer, M.K.; Schilbach, L. Oxytocin Release Deficit and Social Cognition in Craniopharyngioma Patients. J. Neuroendocrinol. 2020, 32, e12842. [Google Scholar] [CrossRef] [PubMed]
- Vaiani, E.; Felizzia, G.; Lubieniecki, F.; Braier, J.; Belgorosky, A. Paediatric Langerhans Cell Histiocytosis Disease: Long-Term Sequelae in the Hypothalamic Endocrine System. Horm. Res. Paediatr. 2021, 94, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.-W.; Phipps, K.; Aquilina, K.; Gaze, M.N.; Hayward, R.; Spoudeas, H.A. Neuroendocrine Morbidity After Pediatric Optic Gliomas: A Longitudinal Analysis of 166 Children Over 30 Years. J. Clin. Endocrinol. Metab. 2015, 100, 3787–3799. [Google Scholar] [CrossRef]
- Murai, A.; Shinojima, N.; Ikuta, G.; Ozono, K.; Ueda, Y.; Mabe, H.; Nakamura, K.; Iwata, N.; Fujisawa, H.; Nagamatsu, F.; et al. Two Children with Lymphocytic Hypophysitis Presenting with Positive Anti-Rabphilin-3A Antibody. Endocr. J. 2023, 70, 703–709. [Google Scholar] [CrossRef]
- Refardt, J.; Winzeler, B.; Christ-Crain, M. Diabetes Insipidus: An Update. Endocrinol. Metab. Clin. N. Am. 2020, 49, 517–531. [Google Scholar] [CrossRef]
- Ebstein, R.P.; Knafo, A.; Mankuta, D.; Chew, S.H.; Lai, P.S. The Contributions of Oxytocin and Vasopressin Pathway Genes to Human Behavior. Horm. Behav. 2012, 61, 359–379. [Google Scholar] [CrossRef]
- Kohlhoff, J.; Cibralic, S.; Hawes, D.J.; Eapen, V. Oxytocin Receptor Gene (OXTR) Polymorphisms and Social, Emotional and Behavioral Functioning in Children and Adolescents: A Systematic Narrative Review. Neurosci. Biobehav. Rev. 2022, 135, 104573. [Google Scholar] [CrossRef]
- LoParo, D.; Waldman, I.D. The Oxytocin Receptor Gene (OXTR) Is Associated with Autism Spectrum Disorder: A Meta-Analysis. Mol. Psychiatry 2015, 20, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Chander, R.J.; Mather, K.A.; Cleary, R.; Grainger, S.A.; Thalamuthu, A.; Numbers, K.; Kochan, N.A.; Armstrong, N.J.; Brodaty, H.; Henry, J.D.; et al. The Influence of Rs53576 Polymorphism in the Oxytocin Receptor (OXTR) Gene on Empathy in Healthy Adults by Subtype and Ethnicity: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2022, 33, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.C.; Ferdinand, R.F.; Van Lang, N.D.J.; Bongers, I.L.; Van Der Ende, J.; Verhulst, F.C. Developmental Trajectories of Depressive Symptoms from Early Childhood to Late Adolescence: Gender Differences and Adult Outcome. Child Psychol. Psychiatry 2007, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Andreou, D.; Comasco, E.; Åslund, C.; Nilsson, K.W.; Hodgins, S. Maltreatment, the Oxytocin Receptor Gene, and Conduct Problems Among Male and Female Teenagers. Front. Hum. Neurosci. 2018, 12, 112. [Google Scholar] [CrossRef]
- McQuaid, R.J.; McInnis, O.A.; Stead, J.D.; Matheson, K.; Anisman, H. A Paradoxical Association of an Oxytocin Receptor Gene Polymorphism: Early-Life Adversity and Vulnerability to Depression. Front. Neurosci. 2013, 7, 128. [Google Scholar] [CrossRef]
- Coriale, G.; Ceccanti, M.; Fiore, M.; Tarani, F.; Micangeli, G.; Menghi, M.; Minutillo, A.; Berretta, P.; Ferraguti, G.; Iannitelli, A.; et al. Delay in the Fine-Tuning of Locomotion in Infants with Meconium Positive to Biomarkers of Alcohol Exposure: A Pilot Study. Riv. Psichiatr. 2024, 59, 52–59. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Krasileva, K.; Green, S.A.; Sherman, L.E.; Ponting, C.; McCarron, R.; Lowe, J.K.; Geschwind, D.H.; Bookheimer, S.Y.; Dapretto, M. Additive Effects of Oxytocin Receptor Gene Polymorphisms on Reward Circuitry in Youth with Autism. Mol. Psychiatry 2017, 22, 1134–1139. [Google Scholar] [CrossRef]
- Imaizumi, J.; Kamada, S.; Taniguchi, M.; Sugimoto, T.; Maeda, T.; Arakaki, R.; Yamamoto, S.; Shirakawa, A.; Mineda, A.; Yoshida, A.; et al. Developmental Changes in Hypothalamic and Serum Oxytocin Levels in Prenatally Normally Nourished and Undernourished Rats. Nutrients 2023, 15, 2768. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; Van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From Basics to Birth and Beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central Nervous System Control of Food Intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Altirriba, J.; Poher, A.-L.; Rohner-Jeanrenaud, F. Chronic Oxytocin Administration as a Treatment Against Impaired Leptin Signaling or Leptin Resistance in Obesity. Front. Endocrinol. 2015, 6, 119. [Google Scholar] [CrossRef]
- Schorr, M.; Marengi, D.A.; Pulumo, R.L.; Yu, E.; Eddy, K.T.; Klibanski, A.; Miller, K.K.; Lawson, E.A. Oxytocin and Its Relationship to Body Composition, Bone Mineral Density, and Hip Geometry Across the Weight Spectrum. J. Clin. Endocrinol. Metab. 2017, 102, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A. Understanding Oxytocin in Human Physiology and Pathophysiology: A Path towards Therapeutics. Compr. Psychoneuroendocrinol. 2024, 19, 100242. [Google Scholar] [CrossRef] [PubMed]
- Plessow, F.; Eddy, K.T.; Lawson, E.A. The Neuropeptide Hormone Oxytocin in Eating Disorders. Curr. Psychiatry Rep. 2018, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Kerem, L.; Hadjikhani, N.; Holsen, L.; Lawson, E.A.; Plessow, F. Oxytocin Reduces the Functional Connectivity between Brain Regions Involved in Eating Behavior in Men with Overweight and Obesity. Int. J. Obes. 2020, 44, 980–989. [Google Scholar] [CrossRef]
- Russell, J.; Maguire, S.; Hunt, G.E.; Kesby, A.; Suraev, A.; Stuart, J.; Booth, J.; McGregor, I.S. Intranasal Oxytocin in the Treatment of Anorexia Nervosa: Randomized Controlled Trial during Re-Feeding. Psychoneuroendocrinology 2018, 87, 83–92. [Google Scholar] [CrossRef]
- Maguire, S.; Kesby, A.; Brownlow, R.; Hunt, G.E.; Kim, M.; McAulay, C.; Grisham, J.R.; McGregor, I.S.; Suraev, A.; Kevin, R.C.; et al. A Phase II Randomised Controlled Trial of Intranasal Oxytocin in Anorexia Nervosa. Psychoneuroendocrinology 2024, 164, 107032. [Google Scholar] [CrossRef]
- Blevins, J.E.; Graham, J.L.; Morton, G.J.; Bales, K.L.; Schwartz, M.W.; Baskin, D.G.; Havel, P.J. Chronic Oxytocin Administration Inhibits Food Intake, Increases Energy Expenditure, and Produces Weight Loss in Fructose-Fed Obese Rhesus Monkeys. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 308, R431–R438. [Google Scholar] [CrossRef]
- van der Klaauw, A.A.; Farooqi, I.S. The Hunger Genes: Pathways to Obesity. Cell 2015, 161, 119–132. [Google Scholar] [CrossRef]
- Paparella, R.; Ferraguti, G.; Fiore, M.; Menghi, M.; Micangeli, G.; Tarani, F.; Ligotino, A.; Messina, M.P.; Ceccanti, M.; Minni, A.; et al. Serum Lipocalin-2 Levels as a Biomarker in Pre- and Post-Pubertal Klinefelter Syndrome Patients: A Pilot Study. Int. J. Mol. Sci. 2024, 25, 2214. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of Obesity and Diabetes Using Oxytocin or Analogs in Patients and Mouse Models. PLoS ONE 2013, 8, e61477. [Google Scholar] [CrossRef]
- Gajdosechova, L.; Krskova, K.; Olszanecki, R.; Zorad, S. Differential Regulation of Oxytocin Receptor in Various Adipose Tissue Depots and Skeletal Muscle Types in Obese Zucker Rats. Horm. Metab. Res. 2015, 47, 600–604. [Google Scholar] [CrossRef]
- Amri, E.-Z.; Pisani, D.F. Control of Bone and Fat Mass by Oxytocin. Horm. Mol. Biol. Clin. Investig. 2016, 28, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Paparella, R.; Panvino, F.; Tarani, F.; D’Agostino, B.; Leonardi, L.; Ferraguti, G.; Venditti, S.; Colloridi, F.; Pucarelli, I.; Tarani, L.; et al. An Overview of Oxidative Stress in Sex Chromosome Aneuploidies in Pediatric Populations. Antioxidants 2025, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.; Colaianni, G.; Mori, G.; Grano, M.; Zallone, A. Human Osteoclasts Express Oxytocin Receptor. Biochem. Biophys. Res. Commun. 2002, 297, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Breuil, V.; Trojani, M.-C.; Ez-Zoubir, A. Oxytocin and Bone: Review and Perspectives. Int. J. Mol. Sci. 2021, 22, 8551. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Fiore, M.; Tonchev, A.B.; Aloe, L. Neuroadipology: A Novel Component of Neuroendocrinology. Cell Biol. Int. 2010, 34, 1051–1053. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin Is an Age-Specific Circulating Hormone That Is Necessary for Muscle Maintenance and Regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef]
- Colaianni, G.; Sun, L.; Di Benedetto, A.; Tamma, R.; Zhu, L.-L.; Cao, J.; Grano, M.; Yuen, T.; Colucci, S.; Cuscito, C.; et al. Bone Marrow Oxytocin Mediates the Anabolic Action of Estrogen on the Skeleton. J. Biol. Chem. 2012, 287, 29159–29167. [Google Scholar] [CrossRef]
- Feixiang, L.; Yanchen, F.; Xiang, L.; Yunke, Z.; Jinxin, M.; Jianru, W.; Zixuan, L. The Mechanism of Oxytocin and Its Receptors in Regulating Cells in Bone Metabolism. Front. Pharmacol. 2023, 14, 1171732. [Google Scholar] [CrossRef]
- Paparella, R.; Panvino, F.; Gambuti, L.; Cerrito, A.; Pallante, A.; Micangeli, G.; Menghi, M.; Pisani, F.; Bruni, O.; Ardizzone, I.; et al. Evaluation of Sleep Disorders in Children and Adolescents Affected by Klinefelter Syndrome. Eur. J. Pediatr. 2025, 184, 129. [Google Scholar] [CrossRef] [PubMed]
- Chaulagain, R.P.; Shrestha, Y.; Shrestha, H.; Bhandari, R.; Gurung, P. The Neurobiological Impact of Oxytocin in Mental Health Disorders: A Comprehensive Review. Ann. Med. Surg. 2025, 87, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Onaka, T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int. J. Mol. Sci. 2021, 23, 150. [Google Scholar] [CrossRef] [PubMed]
- Panvino, F.; Paparella, R.; Tarani, F.; Lombardi, C.; Ferraguti, G.; Pisani, F.; Fiore, M.; Pancheva, R.; Ardizzone, I.; Tarani, L. Neurotrophins in Neurodevelopmental Disorders: A Narrative Review of the Literature. Int. J. Mol. Sci. 2025, 26, 8335. [Google Scholar] [CrossRef]
- Panvino, F.; Paparella, R.; Gambuti, L.; Cerrito, A.; Menghi, M.; Micangeli, G.; Petrella, C.; Fiore, M.; Tarani, L.; Ardizzone, I. Klinefelter Syndrome: A Genetic Disorder Leading to Neuroendocrine Modifications and Psychopathological Vulnerabilities in Children—A Literature Review and Case Report. Children 2024, 11, 509. [Google Scholar] [CrossRef]
- Sikich, L.; Kolevzon, A.; King, B.H.; McDougle, C.J.; Sanders, K.B.; Kim, S.-J.; Spanos, M.; Chandrasekhar, T.; Trelles, M.D.P.; Rockhill, C.M.; et al. Intranasal Oxytocin in Children and Adolescents with Autism Spectrum Disorder. N. Engl. J. Med. 2021, 385, 1462–1473. [Google Scholar] [CrossRef]
- Sasaki, T.; Hashimoto, K.; Oda, Y.; Ishima, T.; Kurata, T.; Takahashi, J.; Kamata, Y.; Kimura, H.; Niitsu, T.; Komatsu, H.; et al. Decreased Levels of Serum Oxytocin in Pediatric Patients with Attention Deficit/Hyperactivity Disorder. Psychiatry Res. 2015, 228, 746–751. [Google Scholar] [CrossRef]
- Patwardhan, A.; Choe, K.Y. The Social Salience Network Hypothesis of Autism: Disrupted Network Activity, Oxytocin Signaling, and Implications for Social Symptoms. Prog. Neurobiol. 2025, 251, 102787. [Google Scholar] [CrossRef]
- Andari, E.; Nishitani, S.; Kaundinya, G.; Caceres, G.A.; Morrier, M.J.; Ousley, O.; Smith, A.K.; Cubells, J.F.; Young, L.J. Epigenetic Modification of the Oxytocin Receptor Gene: Implications for Autism Symptom Severity and Brain Functional Connectivity. Neuropsychopharmacology 2020, 45, 1150–1158. [Google Scholar] [CrossRef]
- Preckel, K.; Kanske, P. Amygdala and Oxytocin Functioning as Keys to Understanding and Treating Autism: Commentary on an RDoC Based Approach. Neurosci. Biobehav. Rev. 2018, 94, 45–48. [Google Scholar] [CrossRef]
- Rubin, L.H.; Carter, C.S.; Drogos, L.; Pournajafi-Nazarloo, H.; Sweeney, J.A.; Maki, P.M. Peripheral Oxytocin Is Associated with Reduced Symptom Severity in Schizophrenia. Schizophr. Res. 2010, 124, 13–21. [Google Scholar] [CrossRef]
- Strauss, G.P.; Keller, W.R.; Koenig, J.I.; Gold, J.M.; Ossenfort, K.L.; Buchanan, R.W. Plasma Oxytocin Levels Predict Olfactory Identification and Negative Symptoms in Individuals with Schizophrenia. Schizophr. Res. 2015, 162, 57–61. [Google Scholar] [CrossRef]
- Matsushita, H.; Latt, H.M.; Koga, Y.; Nishiki, T.; Matsui, H. Oxytocin and Stress: Neural Mechanisms, Stress-Related Disorders, and Therapeutic Approaches. Neuroscience 2019, 417, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mellentin, A.I.; Finn, S.W.; Skøt, L.; Thaysen-Petersen, D.; Mistarz, N.; Fink-Jensen, A.; Nielsen, D.G. The Effectiveness of Oxytocin for Treating Substance Use Disorders:A Systematic Review of Randomized Placebo-Controlled Trials. Neurosci. Biobehav. Rev. 2023, 151, 105185. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Tempesta, B.; Micioni Di Bonaventura, M.V.; Gaetani, S. From Autism to Eating Disorders and More: The Role of Oxytocin in Neuropsychiatric Disorders. Front. Neurosci. 2016, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D. Emotional Deprivation in Children: Growth Faltering and Reversible Hypopituitarism. Front. Endocrinol. 2020, 11, 596144. [Google Scholar] [CrossRef]
- Samson, W.K.; Alexander, B.D.; Skala, K.D.; Huang, F.-L.S.; Fulton, R.J. Ricin-Cytotoxin Conjugate Administration Reveals a Physiologically Relevant Role for Oxytocin in the Control of Gonadotropin Secretiona. Ann. N. Y. Acad. Sci. 1992, 652, 411–422. [Google Scholar] [CrossRef]
- Sclafani, A.; Rinaman, L.; Vollmer, R.R.; Amico, J.A. Oxytocin Knockout Mice Demonstrate Enhanced Intake of Sweet and Nonsweet Carbohydrate Solutions. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R1828–R1833. [Google Scholar] [CrossRef]
- Nishimori, K.; Takayanagi, Y.; Yoshida, M.; Kasahara, Y.; Young, L.; Kawamata, M. New Aspects of Oxytocin Receptor Function Revealed by Knockout Mice: Sociosexual Behaviour and Control of Energy Balance. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 170, pp. 79–90. ISBN 978-0-444-53201-5. [Google Scholar]
- Kasahara, Y.; Takayanagi, Y.; Kawada, T.; Itoi, K.; Nishimori, K. Impaired Thermoregulatory Ability of Oxytocin-Deficient Mice during Cold-Exposure. Biosci. Biotechnol. Biochem. 2007, 71, 3122–3126. [Google Scholar] [CrossRef]
- Gigliucci, V.; Busnelli, M.; Santini, F.; Paolini, C.; Bertoni, A.; Schaller, F.; Muscatelli, F.; Chini, B. Oxytocin Receptors in the Magel2 Mouse Model of Autism: Specific Region, Age, Sex and Oxytocin Treatment Effects. Front. Neurosci. 2023, 17, 1026939. [Google Scholar] [CrossRef]
- Bertoni, A.; Schaller, F.; Tyzio, R.; Gaillard, S.; Santini, F.; Xolin, M.; Diabira, D.; Vaidyanathan, R.; Matarazzo, V.; Medina, I.; et al. Oxytocin Administration in Neonates Shapes Hippocampal Circuitry and Restores Social Behavior in a Mouse Model of Autism. Mol. Psychiatry 2021, 26, 7582–7595. [Google Scholar] [CrossRef] [PubMed]
- Peñagarikano, O.; Lázaro, M.T.; Lu, X.-H.; Gordon, A.; Dong, H.; Lam, H.A.; Peles, E.; Maidment, N.T.; Murphy, N.P.; Yang, X.W.; et al. Exogenous and Evoked Oxytocin Restores Social Behavior in the Cntnap2 Mouse Model of Autism. Sci. Transl. Med. 2015, 7, 271ra8. [Google Scholar] [CrossRef] [PubMed]
- Tabak, B.A.; Leng, G.; Szeto, A.; Parker, K.J.; Verbalis, J.G.; Ziegler, T.E.; Lee, M.R.; Neumann, I.D.; Mendez, A.J. Advances in Human Oxytocin Measurement: Challenges and Proposed Solutions. Mol. Psychiatry 2023, 28, 127–140. [Google Scholar] [CrossRef]
- Baskaran, C.; Plessow, F.; Silva, L.; Asanza, E.; Marengi, D.; Eddy, K.T.; Sluss, P.M.; Johnson, M.L.; Misra, M.; Lawson, E.A. Oxytocin Secretion Is Pulsatile in Men and Is Related to Social-Emotional Functioning. Psychoneuroendocrinology 2017, 85, 28–34. [Google Scholar] [CrossRef]
- Shafer, S.L.; Ririe, D.G.; Miller, S.; Curry, R.S.; Hsu, D.T.; Sullivan, G.M.; Eisenach, J.C. Plasma Pharmacokinetics of Intravenous and Intranasal Oxytocin in Nonpregnant Adults. Br. J. Anaesth. 2025, 134, 1513–1522. [Google Scholar] [CrossRef]
- Hering, A.; Jieu, B.; Jones, A.; Muttenthaler, M. Approaches to Improve the Quantitation of Oxytocin in Human Serum by Mass Spectrometry. Front. Chem. 2022, 10, 889154. [Google Scholar] [CrossRef]
- Lefevre, A.; Mottolese, R.; Dirheimer, M.; Mottolese, C.; Duhamel, J.-R.; Sirigu, A. A Comparison of Methods to Measure Central and Peripheral Oxytocin Concentrations in Human and Non-Human Primates. Sci. Rep. 2017, 7, 17222. [Google Scholar] [CrossRef]
- Szeto, A.; McCabe, P.M.; Nation, D.A.; Tabak, B.A.; Rossetti, M.A.; McCullough, M.E.; Schneiderman, N.; Mendez, A.J. Evaluation of Enzyme Immunoassay and Radioimmunoassay Methods for the Measurement of Plasma Oxytocin. Psychosom. Med. 2011, 73, 393–400. [Google Scholar] [CrossRef]
- Valstad, M.; Alvares, G.A.; Egknud, M.; Matziorinis, A.M.; Andreassen, O.A.; Westlye, L.T.; Quintana, D.S. The Correlation between Central and Peripheral Oxytocin Concentrations: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2017, 78, 117–124. [Google Scholar] [CrossRef]
- Sailer, C.O.; Winzeler, B.; Urwyler, S.A.; Schnyder, I.; Refardt, J.; Eckert, A.; Varghese, N.; Fassnacht, M.; Chifu, I.; Lawson, E.A.; et al. Oxytocin Levels in Response to Pituitary Provocation Tests in Healthy Volunteers. Eur. J. Endocrinol. 2021, 185, 355–364. [Google Scholar] [CrossRef]
- Asla, Q.; Garrido, M.; Urgell, E.; Terzan, S.; Santos, A.; Fernández, M.; Varghese, N.; Atila, C.; Calabrese, A.; Biagetti, B.; et al. Oxytocin Levels in Response to CRH Administration in Hypopituitarism and Hypothalamic Damage: A Randomized, Crossover, Placebo-Controlled Trial. Sci. Rep. 2025, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Atila, C.; Holze, F.; Murugesu, R.; Rommers, N.; Hutter, N.; Varghese, N.; Sailer, C.O.; Eckert, A.; Heinrichs, M.; Liechti, M.E.; et al. Oxytocin in Response to MDMA Provocation Test in Patients with Arginine Vasopressin Deficiency (Central Diabetes Insipidus): A Single-Centre, Case-Control Study with Nested, Randomised, Double-Blind, Placebo-Controlled Crossover Trial. Lancet Diabetes Endocrinol. 2023, 11, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Aulinas, A.; O’Malley, T.; Maehler, P.; Adler, G.K.; Grinspoon, S.K.; Lawson, E.A. Oxytocin Response to Controlled Dietary Sodium and Angiotensin II among Healthy Individuals. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E671–E675. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, J.; Hazra, R.; Ahern, T.H.; Guo, J.-D.; McDonald, A.J.; Mascagni, F.; Muller, J.F.; Young, L.J.; Rainnie, D.G. Neuroanatomical Evidence for Reciprocal Regulation of the Corticotrophin-Releasing Factor and Oxytocin Systems in the Hypothalamus and the Bed Nucleus of the Stria Terminalis of the Rat: Implications for Balancing Stress and Affect. Psychoneuroendocrinology 2011, 36, 1312–1326. [Google Scholar] [CrossRef]
- MacLean, E.L.; Carranza, E.; Gnanadesikan, G.E.; King, K.M.; Allen, A.M.; Linde-Krieger, L.B.; Feldman, R.; White-Traut, R.C.; Hammock, E.A.D.; Carter, C.S.; et al. Neurophysin I Is an Analytically Robust Surrogate Biomarker for Oxytocin. Psychoneuroendocrinology 2024, 161, 106951. [Google Scholar] [CrossRef]
- Atila, C.; Nikaj, A.; Leibnitz, S.; Liechti, M.E.; Christ-Crain, M. Neurophysin I: A Reliable, Novel, and Robust Biomarker for Oxytocin. Eur. J. Endocrinol. 2025, 192, 502–510. [Google Scholar] [CrossRef]
- Lyle, A.N.; Pokuah, F.; Dietzen, D.J.; Wong, E.C.C.; Pyle-Eilola, A.L.; Fuqua, J.S.; Woodworth, A.; Jones, P.M.; Akinbami, L.J.; Garibaldi, L.R.; et al. Current State of Pediatric Reference Intervals and the Importance of Correctly Describing the Biochemistry of Child Development: A Review. JAMA Pediatr. 2022, 176, 699. [Google Scholar] [CrossRef]
- Fleseriu, M.; Christ-Crain, M.; Langlois, F.; Gadelha, M.; Melmed, S. Hypopituitarism. Lancet 2024, 403, 2632–2648. [Google Scholar] [CrossRef]
- Prata, D.; Silva, M. Neuroimaging Genetics of Oxytocin: A Transcriptomics-Informed Systematic Review. Neurosci. Biobehav. Rev. 2022, 142, 104912. [Google Scholar] [CrossRef]
- Xiao, S.; Fischer, H.; Ebner, N.C.; Rukh, G.; Dang, J.; Westberg, L.; Schiöth, H.B. Oxytocin Pathway Gene Variation and Corticostriatal Resting-State Functional Connectivity. Compr. Psychoneuroendocrinol. 2024, 20, 100255. [Google Scholar] [CrossRef]
- Striepens, N.; Kendrick, K.M.; Hanking, V.; Landgraf, R.; Wüllner, U.; Maier, W.; Hurlemann, R. Elevated Cerebrospinal Fluid and Blood Concentrations of Oxytocin Following Its Intranasal Administration in Humans. Sci. Rep. 2013, 3, 3440. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the Field of Intranasal Oxytocin Research: Lessons Learned and Future Directions for Clinical Research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, D.C.; Hanson, L.R.; Carson, D.S.; Tunstall, B.J.; Lee, M.R.; Tzabazis, A.Z.; Jacobs, D.; Frey, W.H. Nasal Oxytocin for the Treatment of Psychiatric Disorders and Pain: Achieving Meaningful Brain Concentrations. Transl. Psychiatry 2021, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.D.; Maloumby, R.; Beiderbeck, D.I.; Lukas, M.; Landgraf, R. Increased Brain and Plasma Oxytocin after Nasal and Peripheral Administration in Rats and Mice. Psychoneuroendocrinology 2013, 38, 1985–1993. [Google Scholar] [CrossRef]
- Ishii, D.; Kageyama, M.; Umeda, S. Cerebral and Extracerebral Distribution of Radioactivity Associated with Oxytocin in Rabbits after Intranasal Administration: Comparison of TTA-121, a Newly Developed Oxytocin Formulation, with Syntocinon. PLoS ONE 2021, 16, e0261451. [Google Scholar] [CrossRef]
- Kou, J.; Zhang, Y.; Zhou, F.; Sindermann, C.; Montag, C.; Becker, B.; Kendrick, K.M. A Randomized Trial Shows Dose-Frequency and Genotype May Determine the Therapeutic Efficacy of Intranasal Oxytocin. Psychol. Med. 2022, 52, 1959–1968. [Google Scholar] [CrossRef]
- Ricchiuti, G.; Tuerlinckx, E.; Taillieu, A.; Prinsen, J.; Steyaert, J.; Boets, B.; Alaerts, K. Toward Effective Oxytocin Interventions in Autism: Overcoming Challenges and Harnessing Opportunities. J. Psychopharmacol. 2025, 39, 179–186. [Google Scholar] [CrossRef]
- Xiao, S.; Ebner, N.C.; Manzouri, A.; Li, T.-Q.; Cortes, D.S.; Månsson, K.N.T.; Fischer, H. Age-Dependent Effects of Oxytocin in Brain Regions Enriched with Oxytocin Receptors. Psychoneuroendocrinology 2024, 160, 106666. [Google Scholar] [CrossRef]
- Barton, S.; Pruin, A.; Schulze, J.; Kiebs, M.; Scheele, D.; Hurlemann, R. Dose-Response Effects of Exogenous Oxytocin on Social Cognition: A Systematic Review. Neurosci. Biobehav. Rev. 2025, 178, 106350. [Google Scholar] [CrossRef]
- Zaman, R.U.; Mulla, N.S.; Braz Gomes, K.; D’Souza, C.; Murnane, K.S.; D’Souza, M.J. Nanoparticle Formulations That Allow for Sustained Delivery and Brain Targeting of the Neuropeptide Oxytocin. Int. J. Pharm. 2018, 548, 698–706. [Google Scholar] [CrossRef]
- Tauber, M.; Boulanouar, K.; Diene, G.; Çabal-Berthoumieu, S.; Ehlinger, V.; Fichaux-Bourin, P.; Molinas, C.; Faye, S.; Valette, M.; Pourrinet, J.; et al. The Use of Oxytocin to Improve Feeding and Social Skills in Infants With Prader–Willi Syndrome. Pediatrics 2017, 139, e20162976. [Google Scholar] [CrossRef] [PubMed]
- Valette, M.; Diene, G.; Glattard, M.; Cortadellas, J.; Molinas, C.; Faye, S.; Benvegnu, G.; Boulanouar, K.; Payoux, P.; Salles, J.-P.; et al. Early Oxytocin Treatment in Infants with Prader–Willi Syndrome Is Safe and Is Associated with Better Endocrine, Metabolic and Behavioral Outcomes. Orphanet J. Rare Dis. 2025, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Damen, L.; Grootjen, L.N.; Juriaans, A.F.; Donze, S.H.; Huisman, T.M.; Visser, J.A.; Delhanty, P.J.D.; Hokken-Koelega, A.C.S. Oxytocin in Young Children with Prader-Willi Syndrome: Results of a Randomized, Double-blind, Placebo-controlled, Crossover Trial Investigating 3 Months of Oxytocin. Clin. Endocrinol. 2021, 94, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Kuppens, R.J.; Donze, S.H.; Hokken-Koelega, A.C.S. Promising Effects of Oxytocin on Social and Food-related Behaviour in Young Children with Prader–Willi Syndrome: A Randomized, Double-blind, Controlled Crossover Trial. Clin. Endocrinol. 2016, 85, 979–987. [Google Scholar] [CrossRef]
- Einfeld, S.L.; Smith, E.; McGregor, I.S.; Steinbeck, K.; Taffe, J.; Rice, L.J.; Horstead, S.K.; Rogers, N.; Hodge, M.A.; Guastella, A.J. A Double-blind Randomized Controlled Trial of Oxytocin Nasal Spray in Prader Willi Syndrome. Am. J. Med. Genet. Part A 2014, 164, 2232–2239. [Google Scholar] [CrossRef]
- Shalma, N.M.; Alsharabasy, M.A.; Taha, A.M.; Alsawareah, A.; Manirambona, E.; Ahmed, S.K.; Mohamed, M.R.; Taha, N.A.; Abd-ElGawad, M. The Efficacy of Intranasal Oxytocin in Patients with Prader-Willi Syndrome: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102711. [Google Scholar] [CrossRef]
- Rice, L.J.; Einfeld, S.L.; Hu, N.; Carter, C.S. A Review of Clinical Trials of Oxytocin in Prader–Willi Syndrome. Curr. Opin. Psychiatry 2018, 31, 123–127. [Google Scholar] [CrossRef]
- Daniels, N.; Moerkerke, M.; Steyaert, J.; Bamps, A.; Debbaut, E.; Prinsen, J.; Tang, T.; Van Der Donck, S.; Boets, B.; Alaerts, K. Effects of Multiple-Dose Intranasal Oxytocin Administration on Social Responsiveness in Children with Autism: A Randomized, Placebo-Controlled Trial. Mol. Autism 2023, 14, 16. [Google Scholar] [CrossRef]
- Yamasue, H.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Effect of Intranasal Oxytocin on the Core Social Symptoms of Autism Spectrum Disorder: A Randomized Clinical Trial. Mol. Psychiatry 2020, 25, 1849–1858. [Google Scholar] [CrossRef]
- Guastella, A.J.; Boulton, K.A.; Whitehouse, A.J.O.; Song, Y.J.; Thapa, R.; Gregory, S.G.; Pokorski, I.; Granich, J.; DeMayo, M.M.; Ambarchi, Z.; et al. The Effect of Oxytocin Nasal Spray on Social Interaction in Young Children with Autism: A Randomized Clinical Trial. Mol. Psychiatry 2023, 28, 834–842. [Google Scholar] [CrossRef]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal Oxytocin Treatment for Social Deficits and Biomarkers of Response in Children with Autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Özyurt, J.; Lohle, K.; Reichel, J.; Thiel, C.M.; Müller, H.L. First Experiences with Neuropsychological Effects of Oxytocin Administration in Childhood-Onset Craniopharyngioma. Endocrine 2017, 56, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Roof, E.; Deal, C.L.; McCandless, S.E.; Cowan, R.L.; Miller, J.L.; Hamilton, J.K.; Roeder, E.R.; McCormack, S.E.; Roshan Lal, T.R.; Abdul-Latif, H.D.; et al. Intranasal Carbetocin Reduces Hyperphagia, Anxiousness, and Distress in Prader-Willi Syndrome: CARE-PWS Phase 3 Trial. J. Clin. Endocrinol. Metab. 2023, 108, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.; Driscoll, D.J. Oxytocin Treatment in Children with Prader–Willi Syndrome: A Double-blind, Placebo-controlled, Crossover Study. Am. J. Med. Genet. Part A 2017, 173, 1243–1250. [Google Scholar] [CrossRef]
- Hollander, E.; Levine, K.G.; Ferretti, C.J.; Freeman, K.; Doernberg, E.; Desilva, N.; Taylor, B.P. Intranasal Oxytocin versus Placebo for Hyperphagia and Repetitive Behaviors in Children with Prader-Willi Syndrome: A Randomized Controlled Pilot Trial. J. Psychiatr. Res. 2021, 137, 643–651. [Google Scholar] [CrossRef]
- Le, J.; Zhang, L.; Zhao, W.; Zhu, S.; Lan, C.; Kou, J.; Zhang, Q.; Zhang, Y.; Li, Q.; Chen, Z.; et al. Infrequent Intranasal Oxytocin Followed by Positive Social Interaction Improves Symptoms in Autistic Children: A Pilot Randomized Clinical Trial. Psychother. Psychosom. 2022, 91, 335–347. [Google Scholar] [CrossRef]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The Effect of Oxytocin Nasal Spray on Social Interaction Deficits Observed in Young Children with Autism: A Randomized Clinical Crossover Trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Ebstein, R.P.; Yu, R. Intranasal Oxytocin in the Treatment of Autism Spectrum Disorders: A Multilevel Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 122, 18–27. [Google Scholar] [CrossRef]
- Audunsdottir, K.; Sartorius, A.M.; Kang, H.; Glaser, B.D.; Boen, R.; Nærland, T.; Alaerts, K.; Kildal, E.S.M.; Westlye, L.T.; Andreassen, O.A.; et al. The Effects of Oxytocin Administration on Social and Routinized Behaviors in Autism: A Preregistered Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2024, 167, 107067. [Google Scholar] [CrossRef]
- Dimitri, P. The Management of Hypothalamic Obesity in Craniopharyngioma. Best Pract. Res. Clin. Endocrinol. Metab. 2025, 39, 102018. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Qiu, Y. Oxytocin Lipidation Expanding Therapeutics for Long-Term Reversal of Autistic Behaviors in Rats. Int. J. Pharm. 2025, 672, 125299. [Google Scholar] [CrossRef]
- Moy, S.S.; Teng, B.L.; Nikolova, V.D.; Riddick, N.V.; Simpson, C.D.; Van Deusen, A.; Janzen, W.P.; Sassano, M.F.; Pedersen, C.A.; Jarstfer, M.B. Prosocial Effects of an Oxytocin Metabolite, but Not Synthetic Oxytocin Receptor Agonists, in a Mouse Model of Autism. Neuropharmacology 2019, 144, 301–311. [Google Scholar] [CrossRef]
- Wiśniewski, K. Design of Oxytocin Analogs. Methods Mol. Biol. 2019, 2001, 235–271. [Google Scholar] [CrossRef]
- Nance, M.G.; Sullivan, K.M.; Puglia, M.H. The Impact of the Early Environment on Oxytocin Receptor Epigenetics and Potential Therapeutic Implications. Pediatr. Res. 2025, 97, 1290–1304. [Google Scholar] [CrossRef]
| Etiology | Pathophysiological Mechanism | Clinical Correlates | |
|---|---|---|---|
| Congenital causes | Prader–Willi syndrome | Loss of paternally expressed genes on 15q11–q13 → impaired development and signaling of hypothalamic OXT neurons | Neonatal hypotonia, hyperphagia, obesity, and emotional dysregulation |
| Schaaf–Yang syndrome (MAGEL2 variants) | Altered neurodevelopment and OXT neuron differentiation, similar to PWS | Hypotonia, feeding difficulties, autism-like features, and social dysfunction | |
| Williams syndrome | Dysregulated OXT/AVP pathways in neurodevelopmental circuits | Hypersociability, anxiety, impaired social cognition | |
| Fragile X syndrome | Synaptic and neuropeptide dysregulation impacting OXT/AVP release | Social anxiety, cognitive delay, autistic traits | |
| Autism spectrum disorder | OXTR gene polymorphisms and promoter methylation → reduced receptor expression and oxytocinergic tone | Impaired social interaction, communication deficits | |
| Acquired causes | Craniopharyngioma | Tumor and/or treatment-induced injury to paraventricular and supraoptic nuclei | Blunted OXT response to stimuli, social and emotional dysfunction |
| Germ cell tumors, hypothalamic gliomas, Langerhans cell histiocytosis | Structural damage to hypothalamic nuclei | Secondary neuroendocrine deficits, behavioral changes | |
| Autoimmune/inflammatory hypophysitis (lymphocytic, IgG4-related) | Immune-mediated hypothalamic–posterior pituitary injury | Possible secondary OXT loss; anxiety, altered stress responses | |
| Traumatic brain injury or neurosurgical lesions | Disruption of axonal transport and neuronal connectivity in OXT pathways | Emotional lability, altered feeding and social regulation | |
| Secondary neurohypophyseal dysfunction (central diabetes insipidus, panhypopituitarism) | Involvement of the posterior pituitary adjacent to OXT-secreting neurons | Variable OXT deficiency, often subclinical; impaired socio-emotional adaptation | |
| Genetic and epigenetic causes | OXTR gene polymorphisms (e.g., rs53576, rs2254298) | Altered receptor expression, ligand affinity, and signaling efficiency | Variability in social cognition, stress response, attachment behaviors, and autism spectrum disorder features |
| OXTR epigenetic modifications (DNA methylation, histone modification) | Reduced OXTR expression due to promoter methylation and environmental influences (stress, malnutrition) | Lower peripheral OXT, diminished social sensitivity, and emotional dysregulation | |
| Nutritional factors | Prenatal and early-life undernutrition | Disrupted hypothalamic OXT and OXTR expression via developmental reprogramming | Increased fat mass, reduced lean body mass, and altered metabolic and emotional regulation |
| Chronic energy deprivation (e.g., anorexia nervosa, oligomenorrheic athletes) | Suppressed OXT secretion as an adaptive response to conserve energy | Low OXT correlates with leptin reduction, low bone density, and impaired socio-emotional functioning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparella, R.; Bei, A.; Bernabei, I.; Fiorentini, C.; Iafrate, N.; Lucibello, R.; Marchetti, L.; Pastore, F.; Maglione, V.; Niceta, M.; et al. Oxytocin Deficiency in Childhood and Adolescence: Clinical Features, Diagnostic Challenges and Therapeutic Perspectives. Curr. Issues Mol. Biol. 2025, 47, 982. https://doi.org/10.3390/cimb47120982
Paparella R, Bei A, Bernabei I, Fiorentini C, Iafrate N, Lucibello R, Marchetti L, Pastore F, Maglione V, Niceta M, et al. Oxytocin Deficiency in Childhood and Adolescence: Clinical Features, Diagnostic Challenges and Therapeutic Perspectives. Current Issues in Molecular Biology. 2025; 47(12):982. https://doi.org/10.3390/cimb47120982
Chicago/Turabian StylePaparella, Roberto, Arianna Bei, Irene Bernabei, Cinzia Fiorentini, Norma Iafrate, Roberta Lucibello, Lavinia Marchetti, Francesca Pastore, Vittorio Maglione, Marcello Niceta, and et al. 2025. "Oxytocin Deficiency in Childhood and Adolescence: Clinical Features, Diagnostic Challenges and Therapeutic Perspectives" Current Issues in Molecular Biology 47, no. 12: 982. https://doi.org/10.3390/cimb47120982
APA StylePaparella, R., Bei, A., Bernabei, I., Fiorentini, C., Iafrate, N., Lucibello, R., Marchetti, L., Pastore, F., Maglione, V., Niceta, M., Fiore, M., Caronti, B., Vitali, M., Pucarelli, I., & Tarani, L. (2025). Oxytocin Deficiency in Childhood and Adolescence: Clinical Features, Diagnostic Challenges and Therapeutic Perspectives. Current Issues in Molecular Biology, 47(12), 982. https://doi.org/10.3390/cimb47120982







