Correlation Between A Disintegrin and Metalloproteinase (ADAM) Family Proteins (8, 10, 17, 22) and Link with Neuroplasticity in Autism Spectrum Disorder
Abstract
1. Introduction
1.1. A Disintegrin and Metalloproteinase Protein 8 (ADAM 8)
1.2. A Disintegrin and Metalloproteinase Protein 10 (ADAM 10)
1.3. A Disintegrin and Metalloproteinase Protein 17 (ADAM 17)
1.4. A Disintegrin and Metalloproteinase Protein 22 (ADAM 22)
2. Materials and Methods
2.1. Participants
2.2. Behavioral Assessment
2.3. Blood Sample Collection and Measurement of Plasma Levels by ELISA
2.4. Statistical Analysis
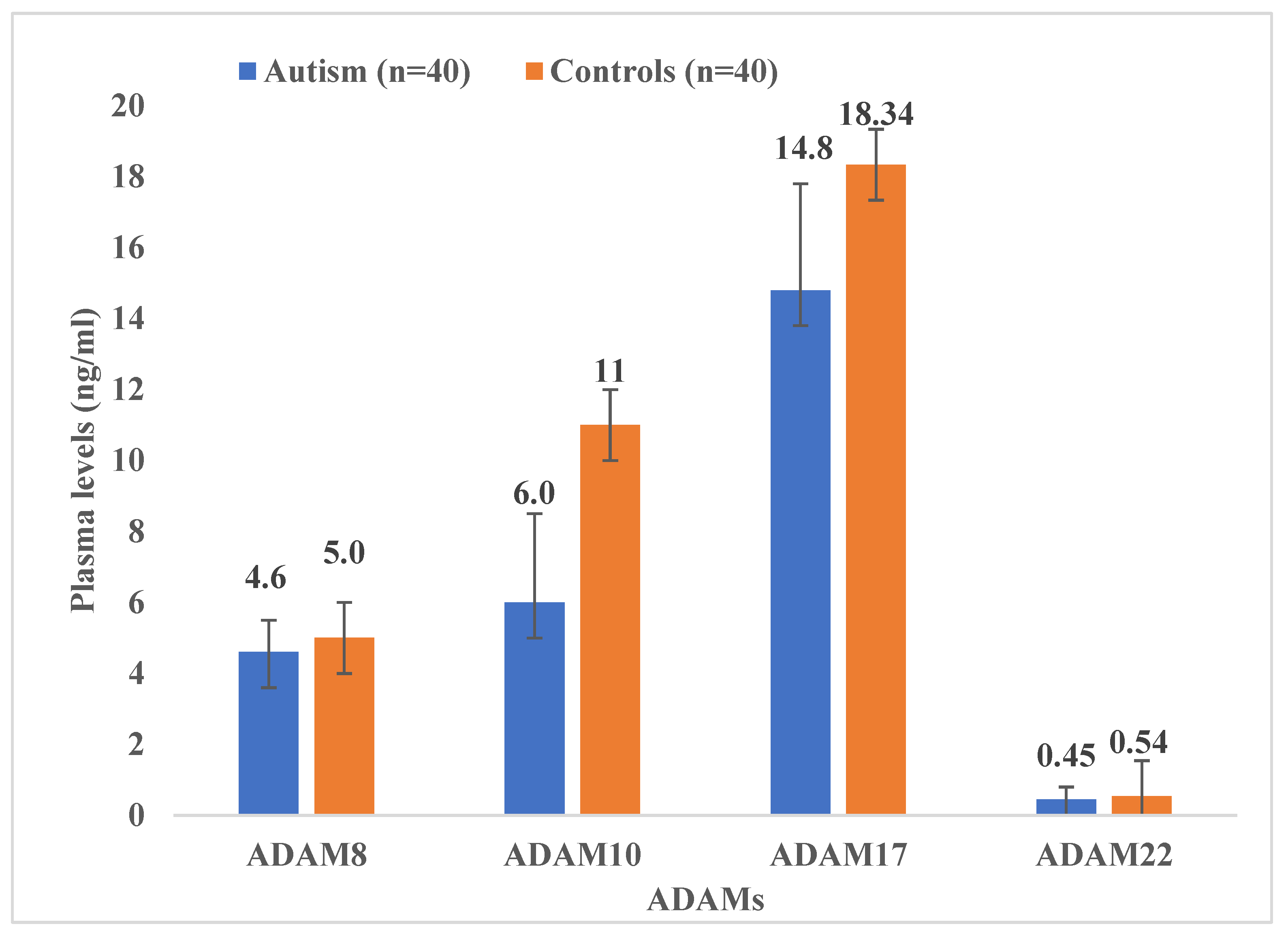
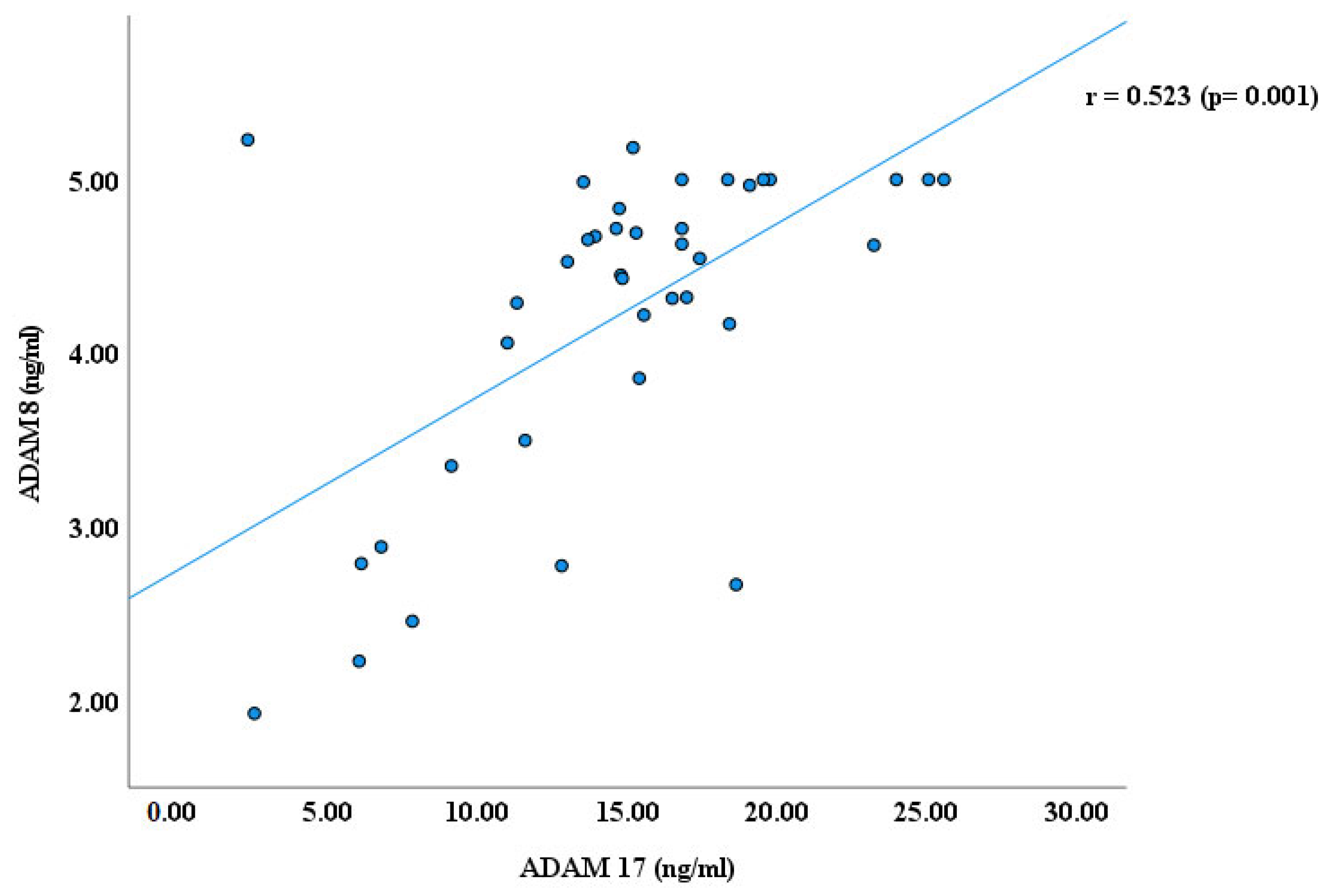
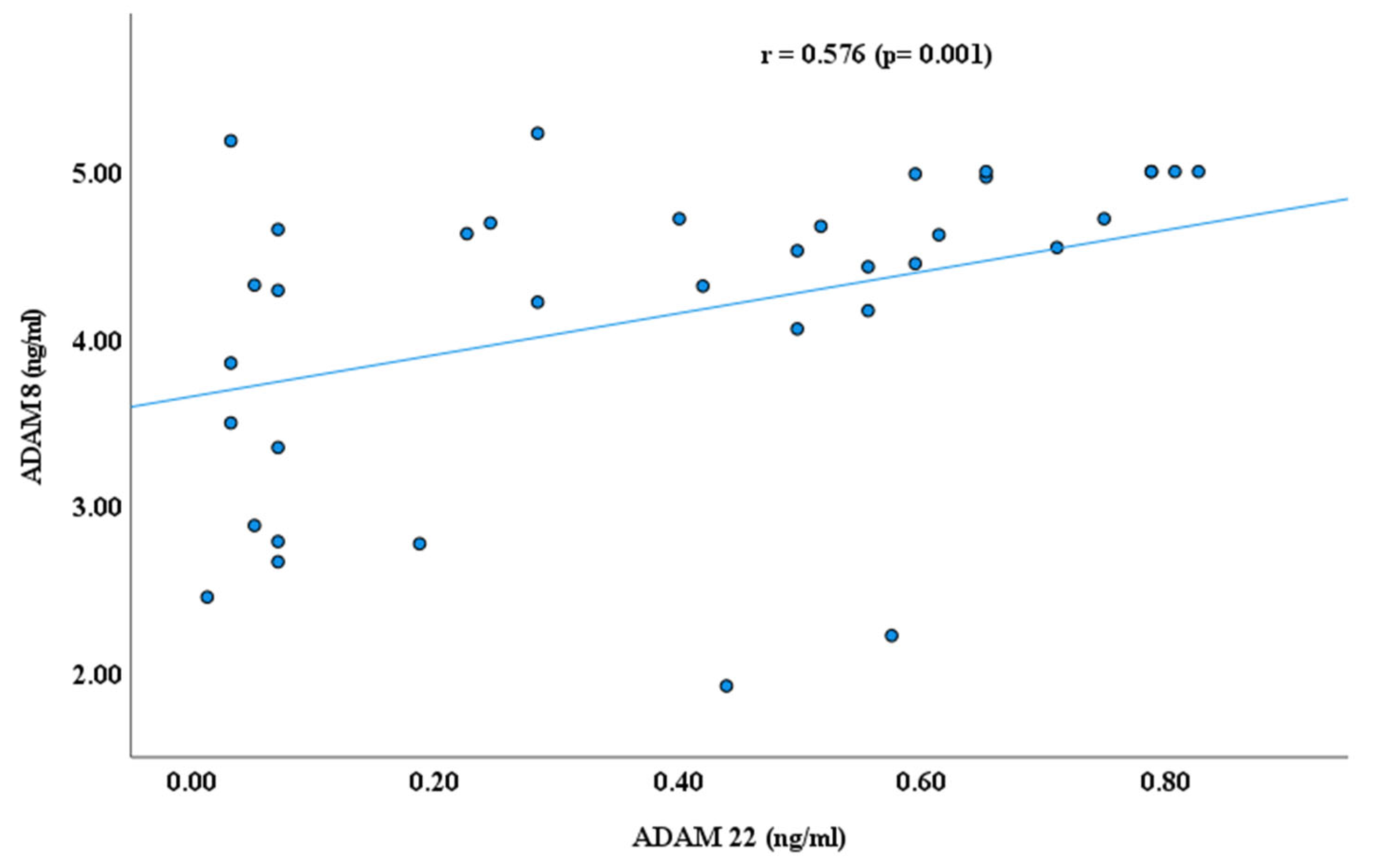
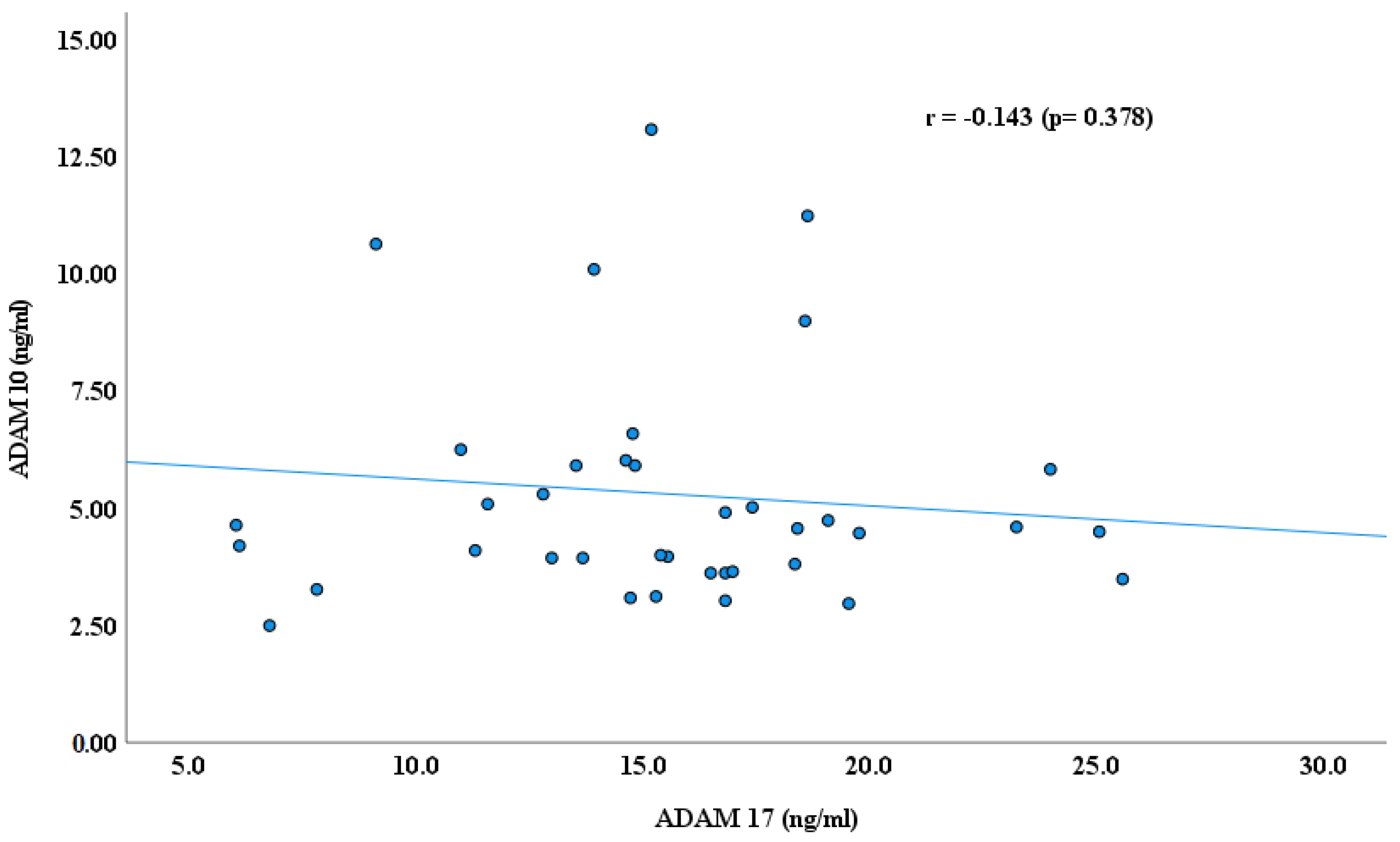
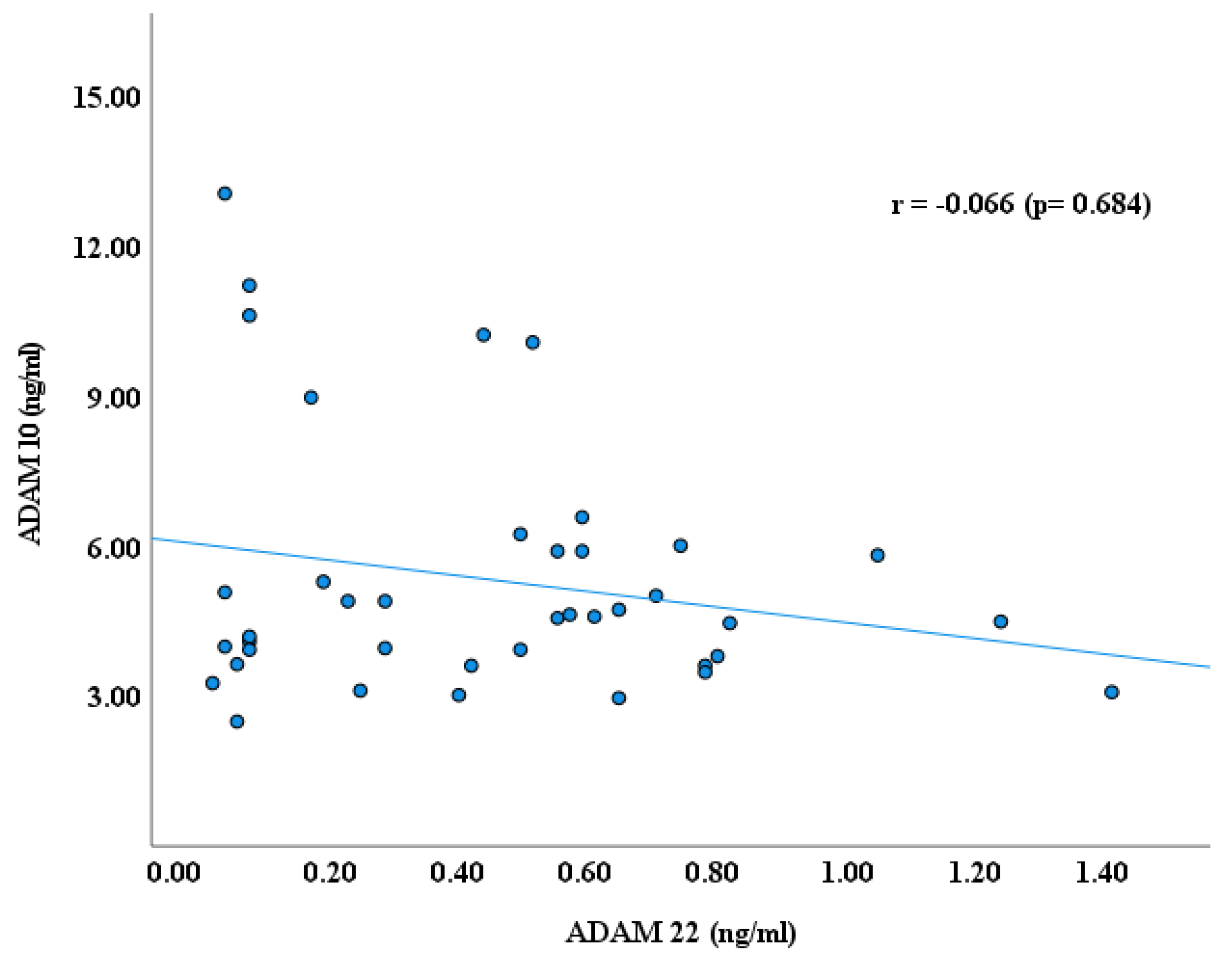
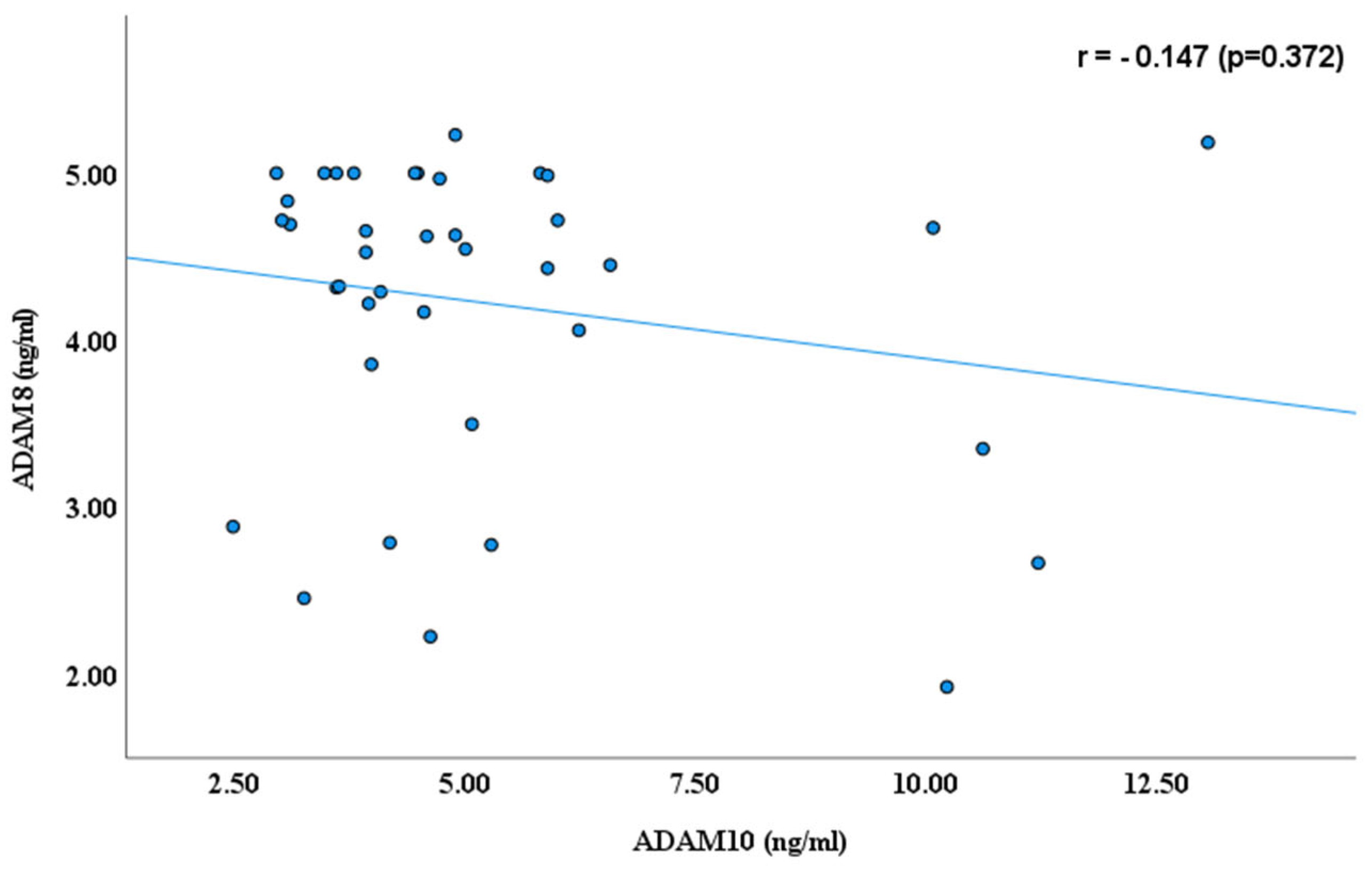
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| DSM–5 | Diagnostic and Statistical Manual of Mental Disorders–5 |
| ADAM | A disintegrin and metalloproteinase |
| ADAMs | A disintegrin and metalloproteinases |
| CNS | Central Nervous System |
| KACST | King Abdulaziz City for Science and Technology |
| APA | American Psychiatric Association |
| ARTC | Autism Research and Treatment Center |
| TNF-α | Tumor Necrosis Factor Alpha |
| NrCAM | Neural Glial-Related Cell Adhesion Molecules |
| pCS | p-cresyl sulfate |
| CARS | Childhood Autism Rating Scale |
| SRS | Social Responsiveness Scale |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| SPSS | Statistical Package for the Social Sciences |
| APP | Amyloid precursor protein |
| IL-1β | Interleukin-1 beta |
| TGF-α | Transforming Growth Factor Alpha |
| sAPPα | Soluble Amyloid Precursor Protein Alpha |
| CNTNAP2 | Contactin-associated protein-like 2 |
References
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association, Washington DSM-5; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Mostafa, G.A.; ElGebaly, H.H.; Shehab, A.A.; Mohamed, M.A.E. Up-regulated serum levels of TAM receptor tyrosine kinases in a group of Egyptian autistic children. J. Neuroimmunol. 2022, 364, 577811. [Google Scholar] [CrossRef]
- Alayadhi, L.Y.; Halepoto, D.M.; Alhowikan, A.M.; Elamin, N.E.; Halepota, A.T. Low Plasma Levels of Contactin-Associated Protein-Like 2 in Children with Autism Spectrum Disorder: Links to Neural Development. Neuropsychiatr. Dis. Treat. 2024, 20, 2423–2431. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Zhang, S.; Han, F. Neuroplasticity of children in autism spectrum disorder. Front. Psychiatry 2024, 15, 1362288. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. ADAM proteins, their ligands, and clinical implications. Neurology 2012, 78, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.; Saftig, P. Ectodomain shedding and ADAMs in development. Development 2012, 139, 3693–3709. [Google Scholar] [CrossRef] [PubMed]
- Hsia, H.E.; Tüshaus, J.; Brummer, T.; Zheng, Y.; Scilabra, S.D.; Lichtenthaler, S.F. Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cell. Mol. Life Sci. 2019, 76, 3055–3081. [Google Scholar] [CrossRef]
- Cho, C. Testicular and epididymal ADAMs: Expression and function during fertilization. Nat. Rev. Urol. 2012, 9, 550–560. [Google Scholar] [CrossRef]
- Primakoff, P.; Myles, D.G. The ADAM gene family: Surface proteins with adhesion and protease activity. Trends Genet. 2000, 16, 83–87. [Google Scholar] [CrossRef]
- Matsuno, O.; Miyazaki, E.; Nureki, S.; Ueno, T.; Ando, M.; Ito, K.; Kumamoto, T.; Higuchi, Y. Elevated soluble ADAM8 in bronchoalveolar lavage fluid in patients with eosinophilic pneumonia. Int Arch Allergy Immunol. 2007, 142, 285–290. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.; Abualnaja, A.; AlZarroug, A.; Alharbi, T.; Alhowikan, A.M.; Halepoto, D.M.; Al-Mazidi, S.A. Disintegrin and Metalloproteinase Protein 8 (ADAM 8) in Autism Spectrum Disorder: Links to Neuroinflammation. Neuropsychiatr. Dis. Treat. 2023, 19, 1771–1780. [Google Scholar] [CrossRef]
- Liu, J.; Xu, L.; Lu, J.; Shen, X.; Li, D.; Bai, L.; Li, X.; Yu, Z.; Li, H. Roles of Adam8 in Neuroinflammation in experimental ischemic Stroke: Insights from single-cell and ribosome-bound mRNA sequencing. Exp. Neurol. 2025, 388, 115207. [Google Scholar] [CrossRef]
- Erbek, S.S.; Hizal, E.; Erinanc, H.; Erbek, S. Expression of a disintegrin and metalloproteinase 8 by inflammatory cells in nasal polyps. Am. J. Rhinol. Allergy 2013, 27, 151. [Google Scholar] [CrossRef]
- Lundgren, J.L.; Ahmed, S.; Schedin-Weiss, S.; Gouras, G.K.; Winblad, B.; Tjernberg, L.O.; Frykman, S. ADAM10 and BACE1 are localized to synaptic vesicles. J. Neurochem. 2015, 135, 606–615. [Google Scholar] [CrossRef]
- Zheng, Y.; Verhoeff, T.A.; Perez Pardo, P.; Garssen, J.; Kraneveld, A.D. The gut-brain axis in autism spectrum disorder: A focus on the metalloproteases ADAM10 and ADAM17. Int. J. Mol. Sci. 2020, 22, 118. [Google Scholar] [CrossRef]
- Zheng, Y.; Prince, N.Z.; PeraltaMarzal, L.N.; Ahmed, S.; Garssen, J.; Perez Pardo, P.; Kraneveld, A.D. The Autism Spectrum Disorder-Associated Bacterial Metabolite p-Cresol Derails the Neuroimmune Response of Microglial Cells Partially via Reduction of ADAM17 and ADAM10. Int. J. Mol. Sci. 2022, 23, 11013. [Google Scholar] [CrossRef] [PubMed]
- Musardo, S.; Marcello, E.; Gardoni, F.; Di Luca, M. ADAM10 in synaptic physiology and pathology. Neurodegener. Dis. 2014, 13, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Endres, K.; Deller, T. Regulation of alpha-secretase ADAM10 in vitro and in vivo: Genetic, epigenetic, and protein-based mechanisms. Front. Mol. Neurosci. 2017, 10, 56. [Google Scholar] [CrossRef]
- Luo, L.; Li, G.; Wang, Y. PLD promotes dendritic spine development by inhibiting ADAM10-mediated N-cadherin cleavage. Sci. Rep. 2017, 7, 6035. [Google Scholar] [CrossRef]
- Oliveira Monteiro, M.P.A.; Salheb Oliveira, D.S.; Manzine, P.R.; Crispim Nascimento, C.M.; dos Santos Orlandi, A.A.; de Oliveira Gomes, G.A.; dos Santos Orlandi, F.; Zazzetta, M.S.; Pott-Junior, H.; Cominetti, M.R. ADAM10 plasma levels predict worsening in cognition of older adults: A 3-year follow-up study. Alzheimers Res. Ther. 2021, 13, 18. [Google Scholar] [CrossRef]
- Lam, S.; Shiu, S.W.; Wong, Y.; Tan, K.C. Effect of type 2 diabetes on A disintegrin and metalloprotease 10. J. Diabetes 2022, 14, 394–400. [Google Scholar] [CrossRef]
- Al-Mazidi, S.; Alhowikan, A.; Elamin, N.; Abualnaja, A.; Al-Mnaizel, A.; Alharbi, T.; Al-Ayadhi, L. Cognitive function and retinal biomarkers as novel approach to diagnosing and assessing autism spectrum disorder. Sci. Rep. 2025, 15, 17946. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.J.; López-Soto, E.J.; Li, L.; Liu, H.; Nedelcu, D.; Lipscombe, D.; Hu, Z.; Kaplan, J.M. Retrograde Synaptic Inhibition Is Mediated by α-Neurexin Binding to the α2δ Subunits of N-Type Calcium Channels. Neuron. 2017, 95, 326–340. [Google Scholar] [CrossRef]
- Winkler, M.; Biswas, S.; Berger, S.M.; Küchler, M.; Preisendörfer, L.; Choo, M.; Früh, S.; Rem, P.D.; Enkel, T.; Arnold, B.; et al. Pianp deficiency links GABAB receptor signaling and hippocampal and cerebellar neuronal cell composition to autism-like behavior. Mol. Psychiatry 2020, 25, 2979–2993. [Google Scholar] [CrossRef]
- Karkkainen, I.; Rybnikova, E.; Pelto-Huikko, M.; Huovila, A.P. Metalloprotease–disintegrin (ADAM) genes are widely and differentially expressed in the adult CNS. Mol. Cell Neurosci. 2000, 15, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Z.; Li, Z.; Zhou, M.; Liu, B.; Pan, L.; Ma, Z.; Zheng, Y. ADAM17 is critical for multipolar exit and radial migration of neuronal intermediate progenitor cells in mice cerebral cortex. PLoS ONE 2013, 8, e65703. [Google Scholar] [CrossRef] [PubMed]
- Zunke, F.; Rose-John, S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim. Biophys. Acta 2017, 1864 Pt B, 2059–2070. [Google Scholar] [CrossRef]
- Ray, B.; Sokol, D.K.; Maloney, B.; Lahiri, D.K. Finding novel distinctions between the sAPP-mediated anabolic biochemical pathways in Autism Spectrum Disorder and Fragile X Syndrome plasma and brain tissue. Sci. Rep. 2016, 6, 26052. [Google Scholar] [CrossRef]
- Al-Mazidi, S.H.; El-Ansary, A.; Abualnaja, A.; AlZarroug, A.; Alharbi, T.; Al-Ayadhi, L.Y. Exploring the Potential Role of ADAM 17 and ADAM 22 in the Etiology of Autism Spectrum Disorders. Brain Sci. 2023, 13, 972. [Google Scholar] [CrossRef]
- Fukata, Y.; Hirano, Y.; Miyazaki, Y.; Yokoi, N.; Fukata, M. Trans-synaptic LGI1-ADAM22-MAGUK in AMPA and NMDA re-ceptor regulation. Neuropharmacology 2021, 194, 108628. [Google Scholar] [CrossRef]
- Sagane, K.; Ohya, Y.; Hasegawa, Y.; Tanaka, I. Metalloproteinase-like, disintegrin-like, cysteine-rich proteins MDC2 and MDC3: Novel human cellular disintegrins highly expressed in the brain. Biochem. J. 1998, 334, 93–98. [Google Scholar] [CrossRef]
- Sagane, K.; Hayakawa, K.; Kai, J.; Hirohashi, T.; Takahashi, E.; Miyamoto, N.; Ino, M.; Oki, T.; Yamazaki, K.; Nagasu, T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Pereira Vatanabe, I.; Peron, R.; Mantellatto Grigoli, M.; Pelucchi, S.; De Cesare, G.; Magalhaes, T.; Manzine, P.R.; Figueredo Balthazar, M.L.; Di Luca, M.; Marcello, E.; et al. ADAM10 Plasma and CSF Levels Are Increased in Mild Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2416. [Google Scholar] [CrossRef] [PubMed]
- Dreymueller, D.; Pruessmeyer, J.; Groth, E.; Ludwig, A. The role of ADAM-mediated shedding in vascular biology. Eur. J. Cell Biol. 2012, 91, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Bernd, C.; Kieseier, B.C.; Pischel, H.; Neuen-Jacob, E.; Tourtellotte, W.W.; Hartung, H.P. ADAM-10 and ADAM-17 in the inflamed human CNS. Glia 2003, 42, 398–405. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halepoto, D.M.; Al-Ayadhi, L.; Alhowikan, A.; Elamin, N.; Abdulmaged, D.; Halepota, A.; Al-Mazidi, S. Correlation Between A Disintegrin and Metalloproteinase (ADAM) Family Proteins (8, 10, 17, 22) and Link with Neuroplasticity in Autism Spectrum Disorder. Curr. Issues Mol. Biol. 2025, 47, 980. https://doi.org/10.3390/cimb47120980
Halepoto DM, Al-Ayadhi L, Alhowikan A, Elamin N, Abdulmaged D, Halepota A, Al-Mazidi S. Correlation Between A Disintegrin and Metalloproteinase (ADAM) Family Proteins (8, 10, 17, 22) and Link with Neuroplasticity in Autism Spectrum Disorder. Current Issues in Molecular Biology. 2025; 47(12):980. https://doi.org/10.3390/cimb47120980
Chicago/Turabian StyleHalepoto, Dost Muhammad, Laila Al-Ayadhi, Abdulrahman Alhowikan, Nadra Elamin, Durria Abdulmaged, Aurangzeb Halepota, and Sarah Al-Mazidi. 2025. "Correlation Between A Disintegrin and Metalloproteinase (ADAM) Family Proteins (8, 10, 17, 22) and Link with Neuroplasticity in Autism Spectrum Disorder" Current Issues in Molecular Biology 47, no. 12: 980. https://doi.org/10.3390/cimb47120980
APA StyleHalepoto, D. M., Al-Ayadhi, L., Alhowikan, A., Elamin, N., Abdulmaged, D., Halepota, A., & Al-Mazidi, S. (2025). Correlation Between A Disintegrin and Metalloproteinase (ADAM) Family Proteins (8, 10, 17, 22) and Link with Neuroplasticity in Autism Spectrum Disorder. Current Issues in Molecular Biology, 47(12), 980. https://doi.org/10.3390/cimb47120980






