A Review of Differential Plant Responses to Drought, Heat, and Combined Drought + Heat Stress
Abstract
1. Introduction
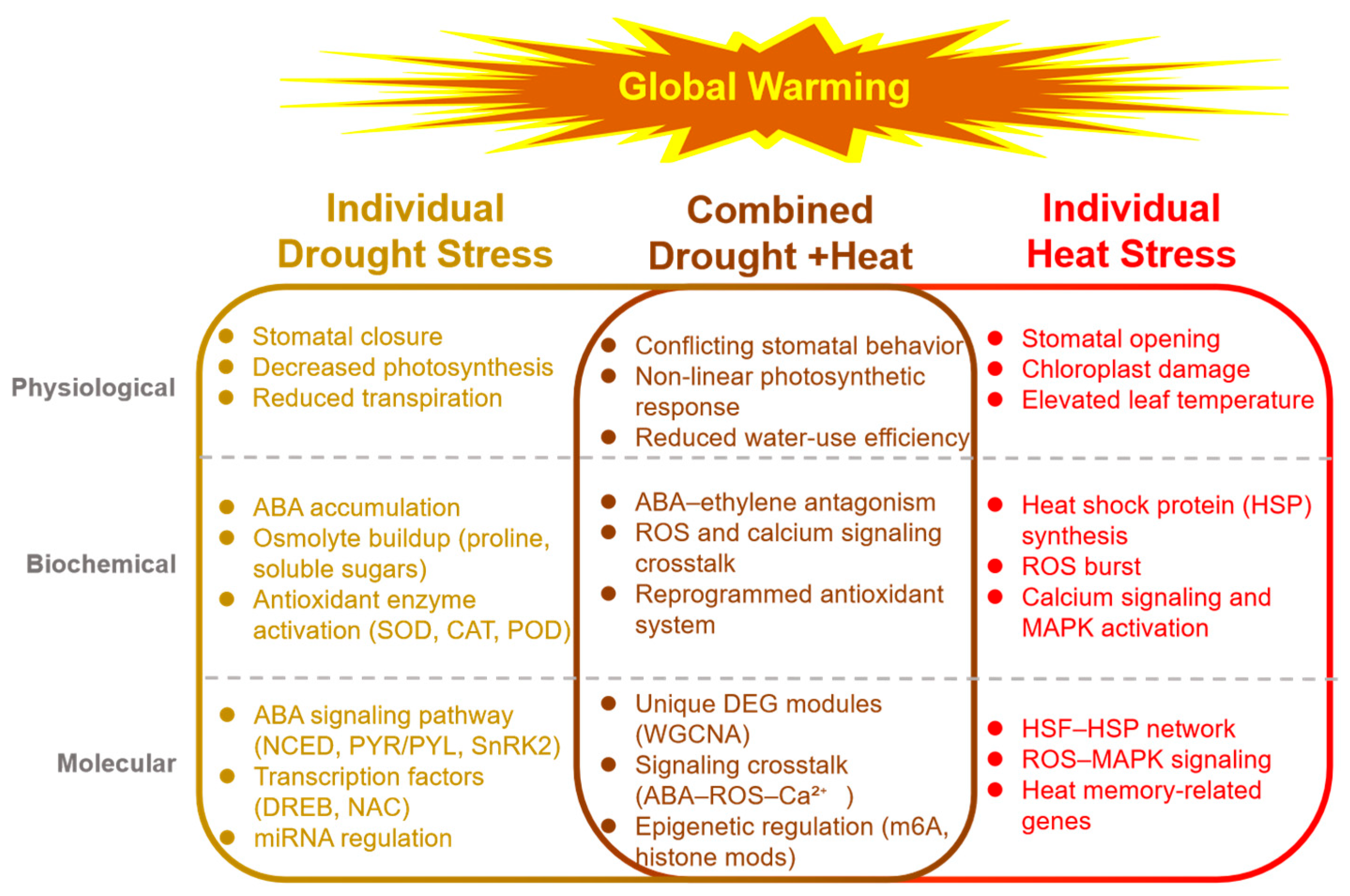
2. Comparative Analysis of Physiological Responses
2.1. Individual Stress Responses
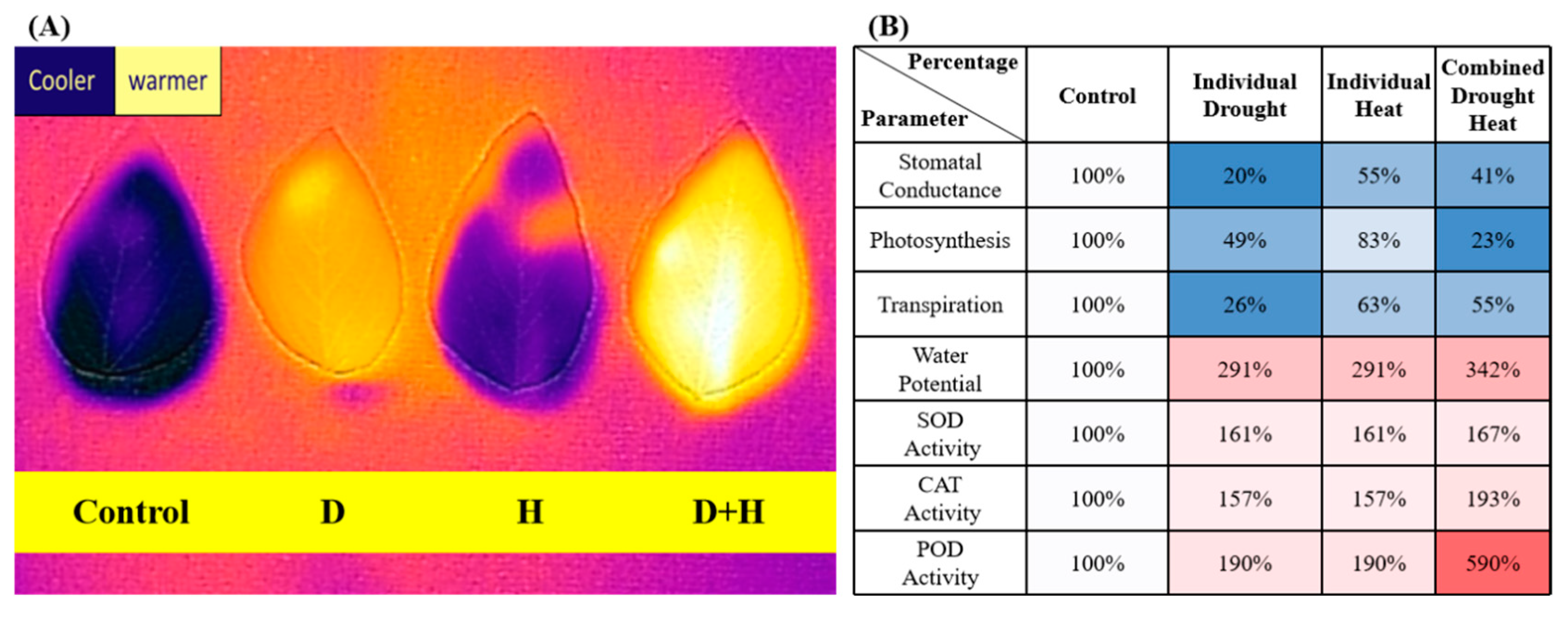
2.2. Combined Stress Responses
2.2.1. Photosynthetic and Stomatal Responses
2.2.2. Water Status and Leaf Damage
2.2.3. Reproductive Impacts
2.2.4. Whole-Plant Water-Use Efficiency
3. Comparative Analysis of Biochemical Responses
3.1. Individual Stress Responses
3.1.1. Osmolyte and Hormonal Reprogramming
3.1.2. Soluble Sugar and Invertase Networks
3.1.3. Antioxidant and Oxidative Metabolism
3.2. Combined Stress Responses: Metabolite Accumulation and Hormonal Crosstalk
3.2.1. Proline and Polyamine Metabolism
3.2.2. Hormonal Regulation: ABA and JA
3.2.3. GABA and TCA Cycle Reconfiguration
3.2.4. Integration of Metabolic and Hormonal Pathways
4. Comparative Analysis of Molecular Responses
4.1. Individual Stress Responses
4.1.1. Ca2+-Mediated Membrane-to-Nucleus Signaling
4.1.2. Reactive Oxygen Species (ROS) Wave and MAPK Phosphorylation
4.1.3. Hormone Biosynthesis Genes: Differential Rate-Limiting Steps
4.1.4. Metabolic Gene Modules: Proline vs. GABA Shunt
4.2. Combined Stress Responses: Decoding Signaling Hubs and Transcriptional Trade-Offs
4.2.1. Hormone Signaling Networks: From Antagonistic Circuits to Convergent Nodes
4.2.2. Sugar Sensing and Invertase-Mediated Metabolic Checkpoints
4.2.3. Heat Shock Factors and ROS-Dependent Transcriptional Hubs
4.2.4. Epigenetic Priming and Chromatin Memory
5. Future Perspectives
5.1. From Linear Pathways to Resilient Networks: Exploiting WGCNA and Machine Learning-Guided Systems Genetics
5.2. Toward a Unified Multi-Omics Space: From Correlation to Causality
5.3. Genome Editing 2.0: Multiplexed, Precise, and Cis-Regulatory
5.4. Breeding by Design: Integrating Genomic Selection with Mechanistic Priors
5.5. Current Challenges in Stress Response Research
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous drought and heat stress in plants: Molecular mechanisms and future prospects. Front. Plant Sci. 2013, 4, 616. [Google Scholar]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Mittler, R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef]
- Chaves, M.M.; Marôco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. J. Exp. Bot. 2003, 54, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Xu, C. Physiological and Molecular Responses in Soybean to Drought and Heat Stresses. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2018. [Google Scholar]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar]
- Piveta, L.B.; Roma-Burgos, N.; Noldin, J.A.; Viana, V.E.; de Oliveira, C.; Lamego, F.P.; de Avila, L.A. Molecular and Physiological Responses of Rice and Weedy Rice to Heat and Drought Stress. Agriculture 2020, 11, 9. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, B.; Gomez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Ristic, Z.; Bukovnik, U.; Momčilović, I.; Fu, J.; Prasad, P.V. Heat-induced accumulation of chloroplast protein synthesis elongation factor (EF-Tu) in wheat. J. Exp. Bot. 2007, 58, 3153–3164. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Zhong, W.; Cheng, K.; Zhou, L.; Zhang, J.; Zhang, F.; Feng, G.; Zhang, X.; Ma, Q.; Su, Y.; Jin, S.; et al. Metabolite-derived spectral modelling can differentiate heat and drought stress under hot-dry environments. Remote Sens. Environ. 2025, 328, 114884. [Google Scholar] [CrossRef]
- Yáñez, M.A.; Flores, S.; Hormazábal-Abarza, F.; Pollmann, S.; Gundel, P.E.; Cabrera-Ariza, A.; Santelices-Moya, R.; Morales-Quintana, L.; Ramos, P. Antarctic endophytic fungi enhance strawberry resilience to drought and heat stress by modulating aquaporins and dehydrins. Plant Stress 2025, 16, 100805. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.-D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Zhang, R. High temperature effects on electron and proton circuits of photosynthesis. J. Integr. Plant Biol. 2010, 52, 712–722. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, B.; Li, S.; Cai, N.; Xu, Y. JA regulates the impact of combined heat stress and drought stress on the GA-mediated response of Pinus yunnanensis. Ind. Crops Prod. 2025, 235, 121826. [Google Scholar] [CrossRef]
- Gao, Q.; Deng, D.; Zeng, R.; Liu, Y.; Jiang, J.; Shen, Q.; Ma, Y.; Fang, W.; Zhu, X. GABA is a key player regulating the TCA cycle and polyamine metabolism under combined heat-drought stress in tea plants. Plant Stress 2025, 18, 101006. [Google Scholar] [CrossRef]
- Pressman, E.; Peet, M.M.; Pharr, D.M. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in developing anthers. Ann. Bot. 2002, 90, 631–636. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, C. Multi-omics integration reveals distinct molecular signatures of combined drought and heat stress in plants. Plant Sci. 2021, 307, 111203. [Google Scholar]
- Findurová, H.; Urban, O.; Veselá, B.; Nezval, J.; Pech, R.; Špunda, V.; Klem, K. Acclimation of barley plants to elevated CO2 concentration and high light intensity does not increase their protection against drought, heat, and their combination. Plant Stress 2024, 14, 100687. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Boyer, J.S. Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Ann. Bot. 2004, 94, 675–689. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- McLaughlin, J.E.; Boyer, J.S. Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Ann. Bot. 2004, 94, 75–86. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135. [Google Scholar] [CrossRef]
- Sack, L.; John, G.P.; Buckley, T.N. ABA accumulation in dehydrating leaves is associated with decline in cell volume, not turgor pressure. Plant Physiol. 2018, 176, 489–495. [Google Scholar] [CrossRef]
- Ismail, M.R.; Davies, W.J. Root restriction affects leaf growth and stomatal response: The role of xylem sap aba. Sci. Hortic. 1998, 74, 257–268. [Google Scholar] [CrossRef]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.J.M.; Wasternack, C.; Mur, L.A.J. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef]
- Tang, G.Q.; Luscher, M.; Sturm, A. Antisense repression and vacuolar and cell wall invertase in transgenic carrot alter searly plant development and sucrose partitioning. Plant Cell. 1999, 11, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.; Pressman, E.; Ophir, R.; Shaked, A.L.R.; Freedman, M.; Shen, S.; Firon, N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J. Exp. Bot. 2009, 60, 3891–3908. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Lone, W.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. Genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2012, 1819, 104–119, Corrigendum to Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2018, 1861, 60. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Zhao, Q.; Mishra, B.K.; Liu, J.; Mohan, B.; Thingujam, D.; Pajerowska-Mukhtar, K.M.; Mukhtar, M.S. Systems biology approaches reveal complex regulatory networks in Arabidopsis under abiotic stress. Front. Plant Sci. 2023, 14, 1043. [Google Scholar]
- Latijnhouwers, M.; Xu, X.M.; Møller, I.M. Arabidopsis stromal 70-kDa heat shock proteins are essential for chloroplast development. Planta 2010, 232, 567–578. [Google Scholar] [CrossRef]
- Hu, S.; Ye, H.; Cui, Y.; Jiang, L. Heat shock protein 90 functions in chloroplast development and abiotic stress response in Arabidopsis. J. Exp. Bot. 2015, 66, 283–298. [Google Scholar] [CrossRef]
- Rütgers, M.; Muranaka, L.S.; Mühlhaus, T.; Sommer, F.; Thoms, S.; Schurig, J.; Willmund, F.; Schulz-Raffelt, M.; Schroda, M. Crosstalk between Hsp90 protein folding machinery and Hsp70 chaperone sustains photosynthetic efficiency. Biochim. Biophys. Acta 2017, 1864, 592–603. [Google Scholar] [CrossRef]
- Liu, H.-C.; Liao, H.-T.; Charng, Y.-Y. The Role of Class A1 Heat Shock Factors (HSFA1s) in Arabidopsis Thermotolerance. Int. J. Mol. Sci. 2011, 12, 5329–5349. [Google Scholar]
- Raturi, V.; Zinta, G. Hsfa1 heat shock factors integrate warm temperature and heat signals in plants. Trends Plant Sci. 2024, 29, 3. [Google Scholar] [CrossRef]
- Miller, M.J.; Barrett-Wilt, G.A.; Vierstra, H.R.D. Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 16512–16517. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, A.T.; Garelis, N.E.; Peterson, B.K.; Abid Ali, F.; Renault, L.; Gannon, J.; Gahlon, H.L.; Kotecha, A.; Zhou, J.C. Cryo-EM Structures of the Eukaryotic Replicative Helicase Bound to DNA Reveal a Dynamic Trimeric State. Nat. Struct. Mol. Biol. 2022, 29, 320–328. [Google Scholar]
- Zhang, X.; Lv, Z.; Yu, J.; Wang, Y.; Li, Y.; Xia, H.; Liang, D. Genome-wide identification of NF-Y genes in kiwifruit genome and the role of AcNF-YC22 in response to heat stress. Plant Physiol. Biochem. 2025, 110762. [Google Scholar] [CrossRef]
- Pirnajmedin, F.; Jakūn, K.; Majidi, M.M. Adaptive strategies to drought stress in grasses of the poaceae family under climate change: Physiological, genetic and molecular perspectives: A review. Plant Physiol. Biochem. 2024, 213, 11. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Polyamines as regulators of abiotic stress tolerance mechanisms in plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of aba in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Wei, Y.S.; Javed, T.; Liu, T.T.; Ali, A.; Gao, S.J. Mechanisms of abscisic acid (aba)-mediated plant defense responses: An updated review. Plant Stress 2025, 15, 100724. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide: The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2002, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Galli, M.; Gallavotti, A. Mechanisms of temperature-regulated growth and thermotolerance in crop species. Curr. Opin. Plant Biol. 2022, 65, 102134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, H.; Wang, J.; Wang, X.; Xu, B.; Yao, X.; Sun, L.; Yang, R.; Wang, J.; Sun, A.; et al. Jasmonic acid enhances osmotic stress responses by MYC2-mediated inhibition of protein phosphatase 2C1 and response regulators 26 transcription factor in tomato. Plant J. 2023, 113, 546–561. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-L.; Yao, J.; Mei, C.-S.; Tong, X.-H.; Zeng, L.-J.; Li, Q.; Xiao, L.-T.; Sun, T.-P.; Li, J.; Deng, X.-W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, 1192–1197. [Google Scholar] [CrossRef]
- Hou, X.; Ding, L.; Yu, H. Crosstalk between GA and JA signaling mediates plant growth and defense. Plant Cell Rep. 2013, 32, 1067–1074. [Google Scholar] [CrossRef]
- Fromm, H. GABA signaling in plants: Targeting the missing pieces of the puzzle. J. Exp. Bot. 2020, 71, 6238–6245. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell. 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Liu, H.-C.; Charng, Y.-Y. Acquired Thermotolerance Independent of Heat Shock Factor A1 (HsfA1), the Master Regulator of the Heat Stress Response. Plant Signal. Behav. 2012, 7, 796–798. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371, Correction in Nature 2015, 519, 378. [Google Scholar] [CrossRef] [PubMed]
- Volkov, R.A.; Panchuk, I.I.; Mullineaux, P.M.; Schoffl, F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.; Axel de Zélicourt Boudsocq, M.; Neubauer, J.; Colcombet, J. Identification and characterization of an aba-activated map kinase cascade in arabidopsis thaliana. Plant J. Cell Mol. Biol. 2015, 82, 232–244. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2000, 123, 553–652. [Google Scholar] [CrossRef]
- Rehschuh, R.; Ruehr, N.K. Diverging responses of water and carbon relations during and after heat and hot drought stress in Pinus sylvestris. Tree Physiol. 2022, 42, 1532–1548. [Google Scholar] [CrossRef]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcriptional regulation of ABA-responsive genes. Plant Signal. Behav. 2011, 6, 1173–1175. [Google Scholar]
- Qi, T.; Huang, H.; Wu, D.; Yan, J.; Qi, Y.; Song, S.; Xie, D. Arabidopsis DELLA and JAZ proteins bind the WD-Repeat/bHLH/MYB complex to modulate gibberellin and jasmonate signaling synergy. Plant Cell 2014, 26, 1118–1133. [Google Scholar] [CrossRef]
- Frerigmann, H.; Hoecker, U.; Gigolashvili, T. New insights on the regulation of glucosinolate biosynthesis via COP1 and DELLA proteins in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 680255. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Gong, M.; Qin, F.; Xiao, D.; Zhan, J.; Wang, A.; He, L. Regulatory mechanism of GA3 on tuber growth by DELLA-dependent pathway in yam (Dioscorea opposita). Plant Mol. Biol. 2021, 106, 433–448. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signalling in plants: Conserved and novel mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Zinselmeier, C.; Jeong, B.R.; Boyer, J.S. Starch and the control of kernel number in maize at low water potentials. Plant Physiol. 1999, 121, 25–35. [Google Scholar] [CrossRef]
- Barratt, D.H.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S.; McLaughlin, J.E. Functional reversion to identify controlling genes in multigenic responses: Analysis of floral abortion. J. Exp. Bot. 2007, 58, 267–277. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.Y.; Zhang, Z.H.; Chen, W.T.; Liu, M.K.; Li, Z.C.; Mugweru, J.; Wang, Z.C.; Wang, J.S. Whole-genome identification and expression characterization of the HSF gene family of Triticum aestivum under heat and drought stress. Plant Sci. 2026, 362, 112780, Corrigendum to Plant Sci. 2026, 362, 112836. [Google Scholar]
- Juneja, S.; Saini, R.; Adhikary, A.; Yadav, R.; Khan, S.A.; Nayyar, H.; Kumar, S. Drought priming evokes essential regulation of Hsp gene families, Hsfs and their related miRNAs and induces heat stress tolerance in chickpea. Plant Stress 2023, 10, 100189. [Google Scholar] [CrossRef]
- Xu, D.; Ni, Y.; Zhang, X.; Guo, Y. Multiomic analyses of two sorghum cultivars reveals the change of membrane lipids in their responses to water deficit. Plant Physiol. Biochem. 2022, 176, 44–56. [Google Scholar] [CrossRef]
- Lämke, J.; Bäurle, I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017, 18, 124. [Google Scholar] [CrossRef]
- Brzezinka, K.; Altmann, S.; Baurle, I. BRUSHY1/TONSOKU/MGOUN3 is required for heat stress memory. Plant Cell Environ. 2019, 42, 765–775. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, A.; Li, G.; Zhang, Y. Integrated bioinformatics and multi-omics analyses reveal possible molecular mechanisms for seed starch content differences between glycine max and cicer arietinum. Agronomy 2024, 14, 17. [Google Scholar] [CrossRef]
- Hong, Y.; Ni, S.J.; Zhang, G.P. Transcriptome and metabolome analysis reveals regulatory networks and key genes controlling barley malting quality in responses to drought stress. Plant Physiol. Biochem. 2020, 152, 1–11. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Transcriptomic and metabolomic profiling in rice under combined drought and heat stress. J. Plant Physiol. 2022, 266, 153–165. [Google Scholar]
- Wang, X.; Guo, X.; Lv, L.; Zhao, A.; Zhao, W.; Liu, Y.; Li, Z. Integrated analysis of transcriptome and metabolome reveals adaptive responses to drought and heat stress in wheat. BMC Plant Biol. 2019, 25, 57. [Google Scholar]
- Lehretz, G.G.; Sonnewald, S.; Lugassi, N.; Granot, D.; Sonnewald, U. Future-proofing potato for drought and heat tolerance by overexpression of hexokinase and SP6A. Front. Plant Sci. 2020, 11, 614534–614543. [Google Scholar] [CrossRef]
- Ravelombola, W.; Shi, A. Genome-Wide Association Study (GWAS) and Genomic Selection (GS) for Drought Tolerance in Cowpea at Early Vegetative Stage. In Proceedings of the American Society for Horticultural Science, Las Vegas, NV, USA, 21–25 July 2019. [Google Scholar]
- Voss-Fels, K.P.; Stahl, A.; Wittkop, B.; Lichthardt, C.; Nagler, S.; Rose, T.; Chen, T.-W.; Zetzsche, H.; Seddig, S.; Baig, M.M.; et al. Breeding improves wheat productivity under contrasting agrochemical input levels. Nat. Plants 2019, 5, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Richardson, R.E.; You, F. Advancing microplastic analysis in the era of artificial intelligence: From current applications to the promise of generative ai. Nexus 2024, 1, 100043. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Geng, Z.; Huang, X.; Huang, S.; Song, L.; Chen, R.; Chen, Z.; Du, L.; Xu, C. A Review of Differential Plant Responses to Drought, Heat, and Combined Drought + Heat Stress. Curr. Issues Mol. Biol. 2025, 47, 975. https://doi.org/10.3390/cimb47120975
Li N, Geng Z, Huang X, Huang S, Song L, Chen R, Chen Z, Du L, Xu C. A Review of Differential Plant Responses to Drought, Heat, and Combined Drought + Heat Stress. Current Issues in Molecular Biology. 2025; 47(12):975. https://doi.org/10.3390/cimb47120975
Chicago/Turabian StyleLi, Nankai, Zhi Geng, Xiaodong Huang, Shunqi Huang, Lulu Song, Ruirui Chen, Ziping Chen, Liji Du, and Congshan Xu. 2025. "A Review of Differential Plant Responses to Drought, Heat, and Combined Drought + Heat Stress" Current Issues in Molecular Biology 47, no. 12: 975. https://doi.org/10.3390/cimb47120975
APA StyleLi, N., Geng, Z., Huang, X., Huang, S., Song, L., Chen, R., Chen, Z., Du, L., & Xu, C. (2025). A Review of Differential Plant Responses to Drought, Heat, and Combined Drought + Heat Stress. Current Issues in Molecular Biology, 47(12), 975. https://doi.org/10.3390/cimb47120975





