Zingerone Targets LKB1/AMPK to Block FcεRI-Dependent Mast Cell Degranulation and Anaphylaxis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Culture and Activation of Mouse Bone Marrow-Derived Mast Cells and RBL-2H3 Cells
2.4. CCK-8 Assay
2.5. Measurement of Intracellular Ca2+ Level
2.6. Immunoblotting
2.7. Immunofluorescence Assay
- Image Acquisition and Processing:
- Quantification of Fluorescence Intensity:
2.8. Transfection of siRNA
2.9. IgE-Mediated Local and Systemic Anaphylaxis in Mice
2.10. Statistic Analysis
3. Results
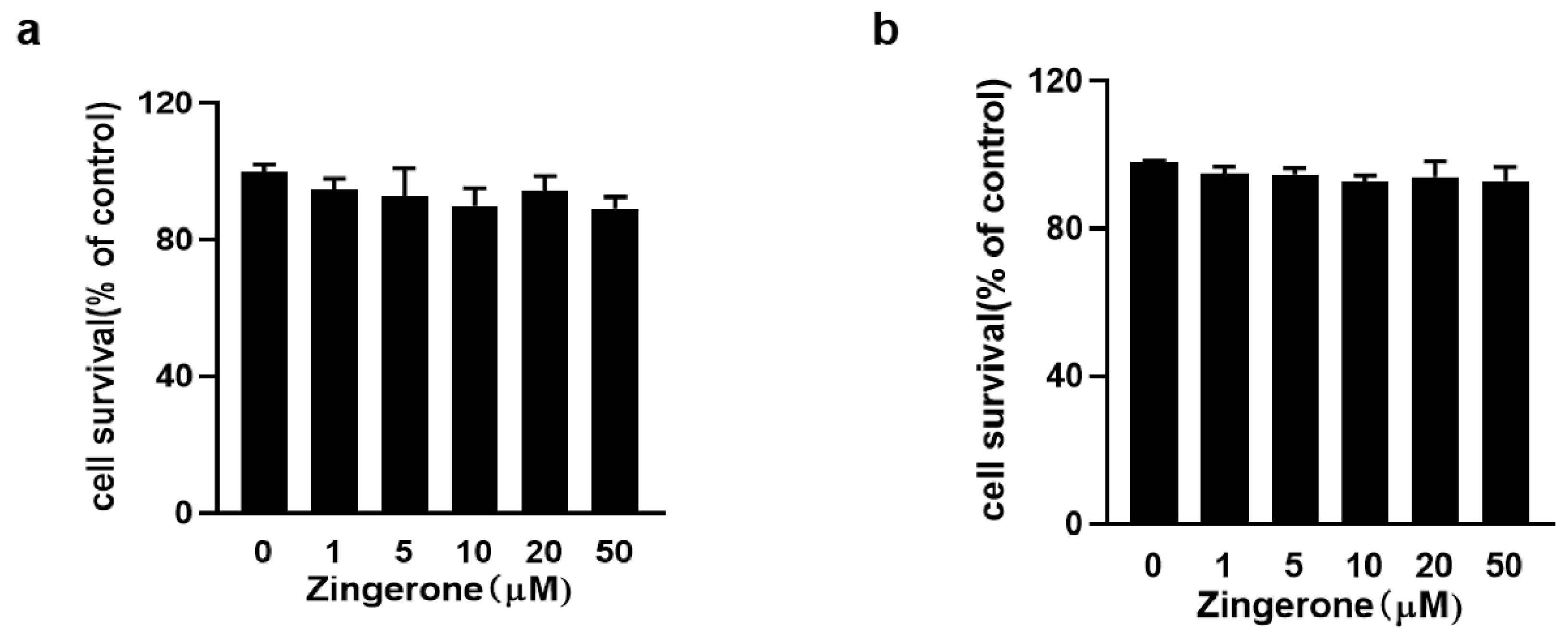
3.1. Assessment of Zingerone Cytotoxicity by CCK-8 Assay
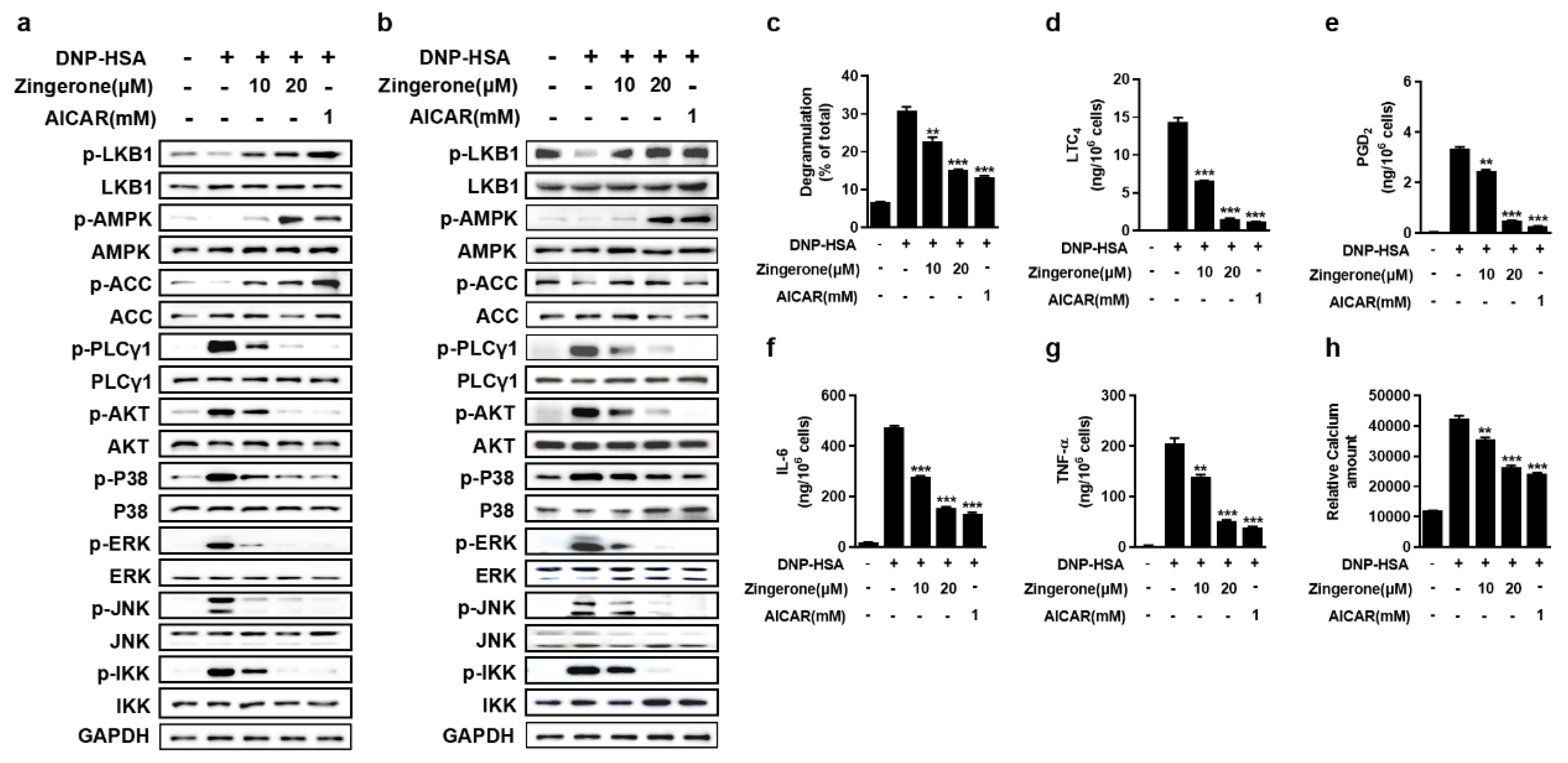
3.2. Zingerone Suppresses IgE/Ag-Induced Mast Cell Activation Through Potentiation of the LKB1-AMPK Signaling Axis
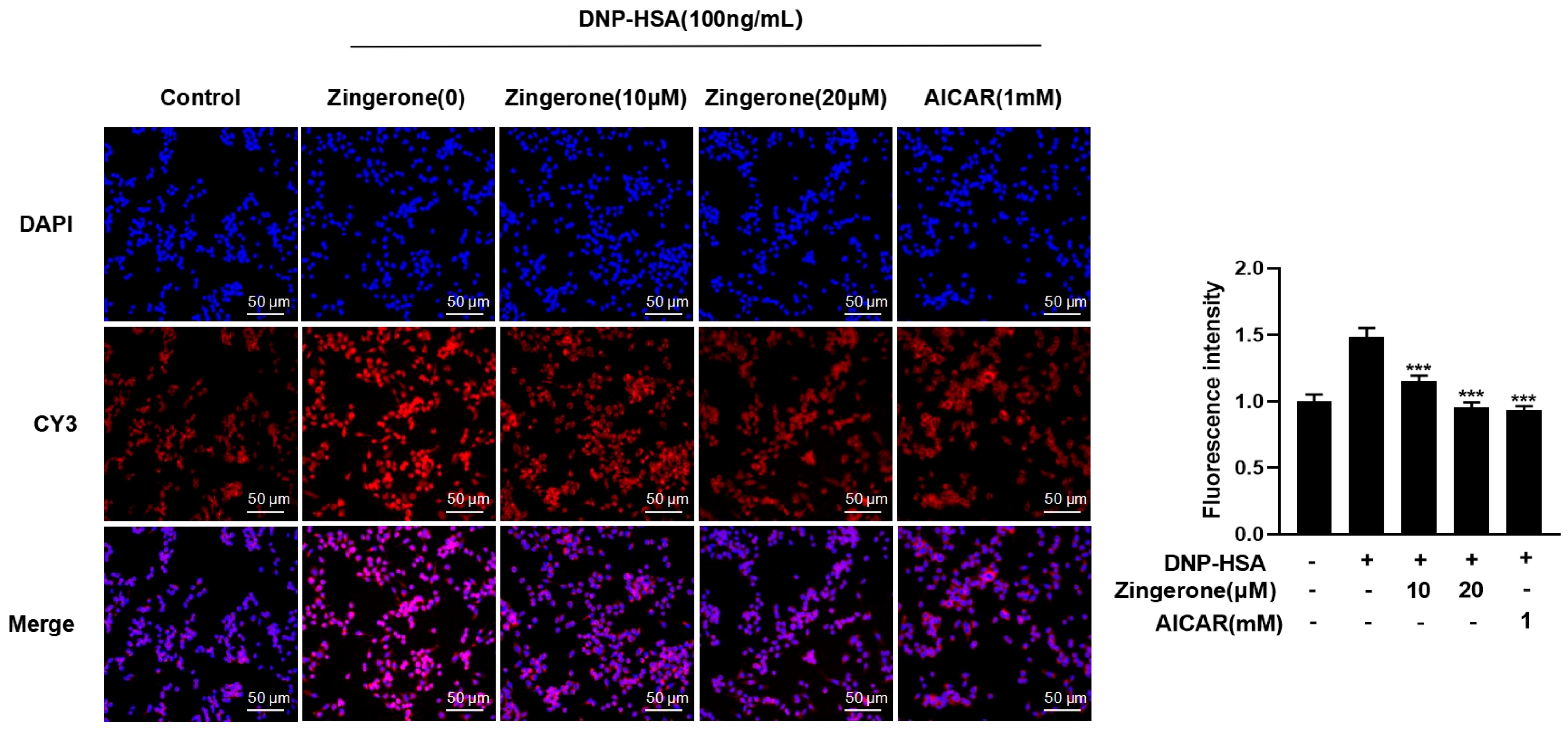
3.3. Zingerone Regulates the Subcellular Localization of AMPK
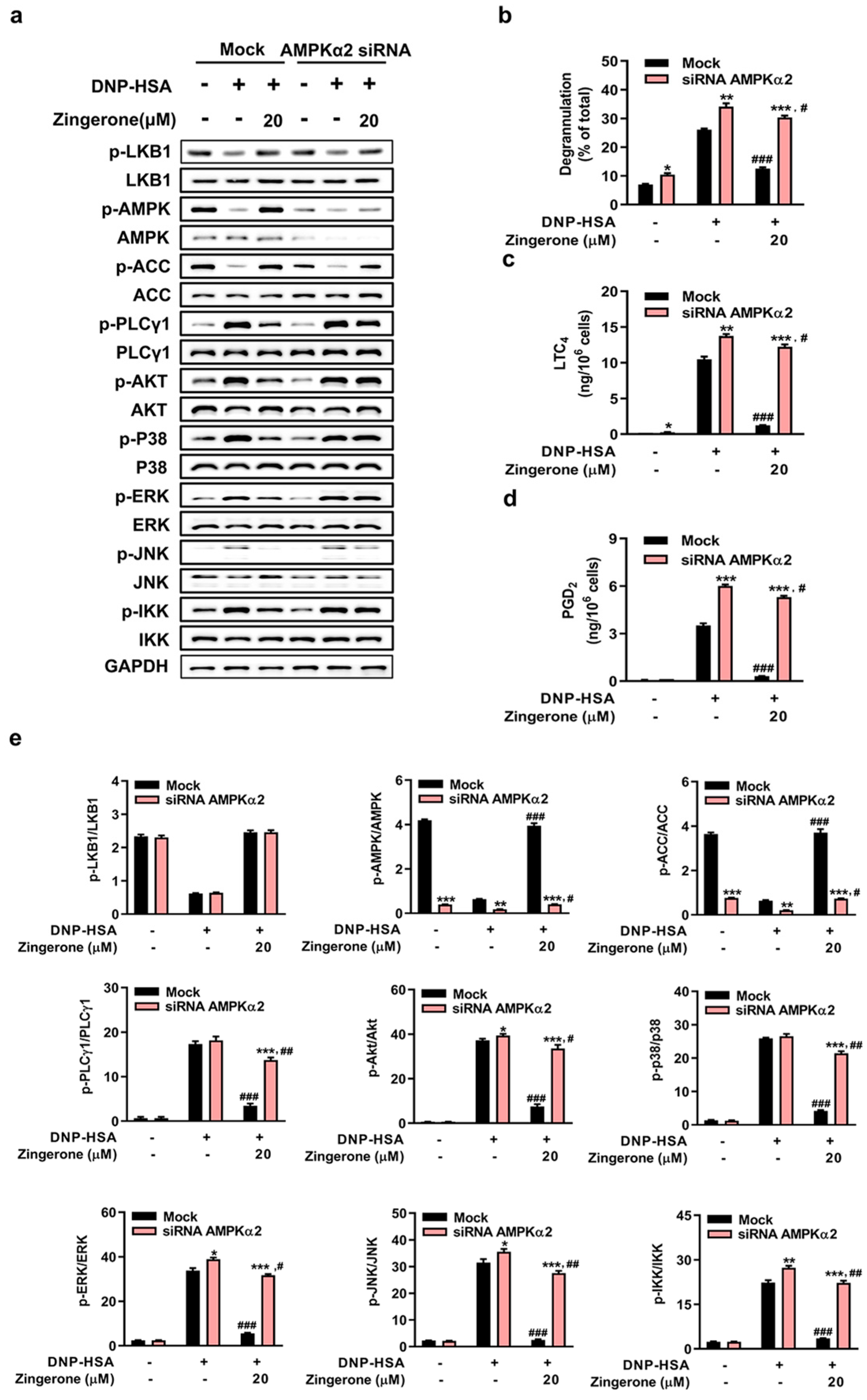
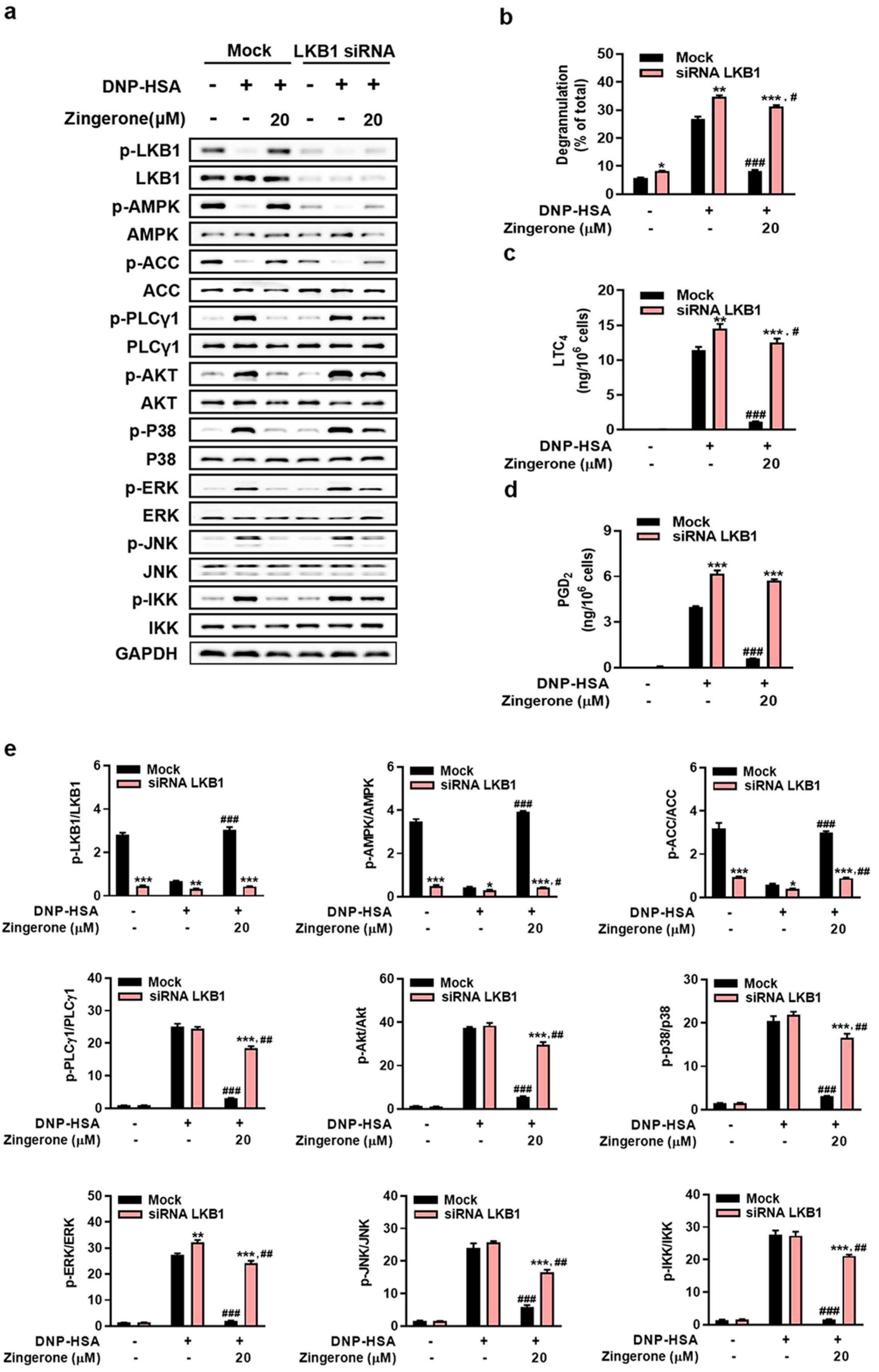
3.4. Genetic Silencing of AMPK or LKB1 Abrogates Zingerone’s Suppression of IgE/Ag-Induced Mast Cell Activation
3.5. Zingerone Suppresses IgE-Mediated Passive Cutaneous Anaphylaxis (PCA) in Mice
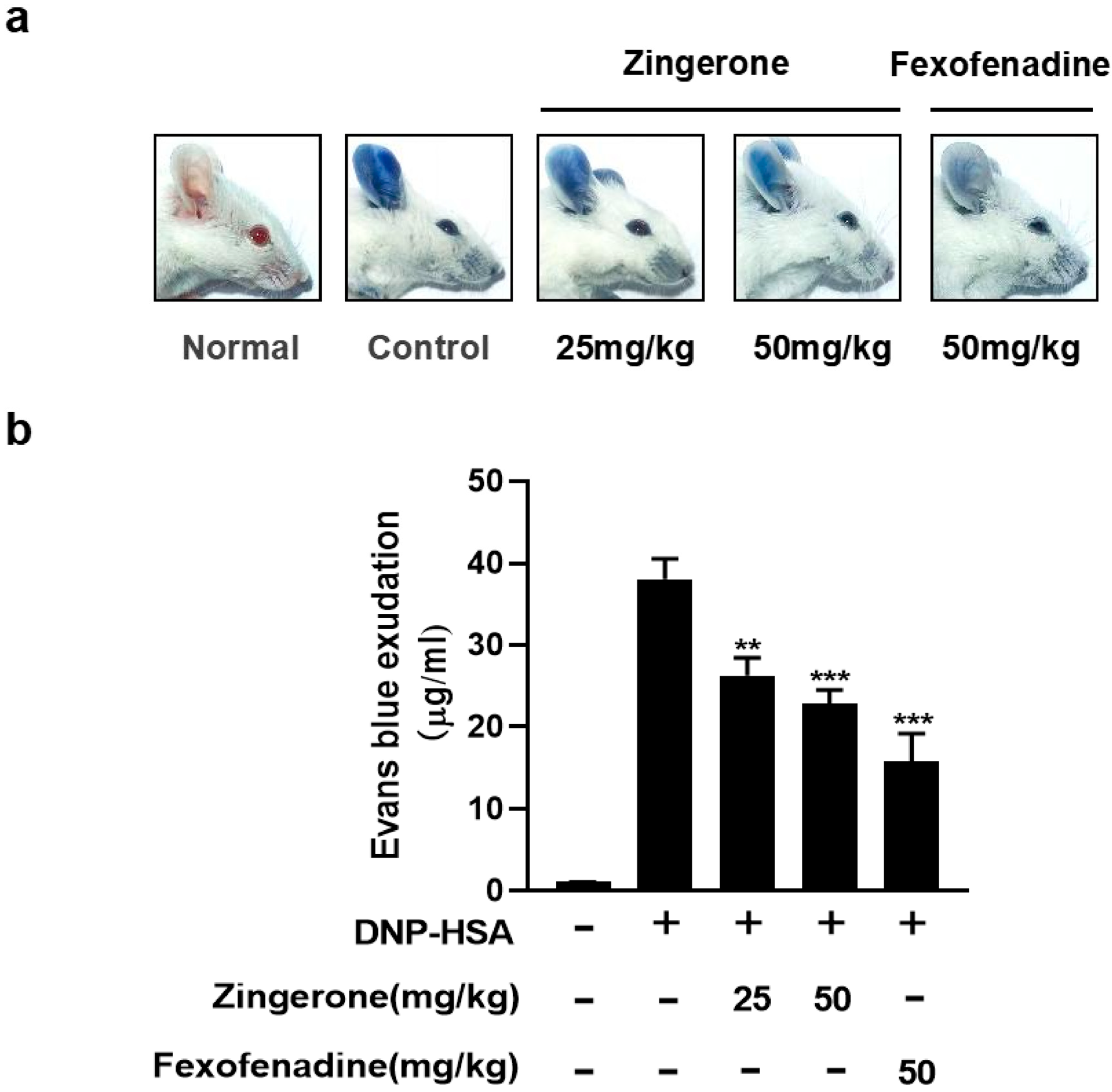
3.6. Zingerone Inhibits IgE-Induced Passive Systemic Allergic Reaction (PSA) in Mice
4. Discussion
4.1. Zingerone as a Putative LKB1 Activator and Its Downstream Signaling
4.2. Upstream Regulation of LKB1: Insights into Potential Mechanisms
4.3. Distinct Mechanism and Therapeutic Positioning Among Ginger Components
4.4. Therapeutic Niche and Clinical Translation
4.5. Pharmacokinetic Considerations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golden, D.B.K.; Wang, J.; Waserman, S.; Akin, C.; Campbell, R.L.; Ellis, A.K.; Greenhawt, M.; Lang, D.M.; Ledford, D.K.; Lieberman, J.; et al. Anaphylaxis: A 2023 practice parameter update. Ann. Allergy Asthma Immunol. 2024, 132, 124–176. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.S.C.; Gershwin, M.E.; Song, J. New Mechanistic Advances in FcεRI-Mast Cell-Mediated Allergic Signaling. Clin. Rev. Allergy Immunol. 2022, 63, 431–446. [Google Scholar] [CrossRef]
- Yang, B.G.; Kim, A.R.; Lee, D.; An, S.B.; Shim, Y.A.; Jang, M.H. Degranulation of Mast Cells as a Target for Drug Development. Cells 2023, 12, 1506. [Google Scholar] [CrossRef]
- Lundequist, A.; Pejler, G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 2011, 68, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Nakamura, T.; Maehara, T.; Hayashi, A.; Aritake, K.; Murata, T. Mast cell-derived prostaglandin D(2) limits the subcutaneous absorption of honey bee venom in mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2300284120. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Siraganian, R.P. Mast cell signal transduction from the high-affinity IgE receptor. Curr. Opin. Immunol. 2003, 15, 639–646. [Google Scholar] [CrossRef]
- Sutton, B.J.; Davies, A.M. Structure and dynamics of IgE-receptor interactions: FcεRI and CD23/FcεRII. Immunol. Rev. 2015, 268, 222–235. [Google Scholar] [CrossRef]
- Hardie, D.G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef]
- Faubert, B.; Vincent, E.E.; Poffenberger, M.C.; Jones, R.G. The AMP-activated protein kinase (AMPK) and cancer: Many faces of a metabolic regulator. Cancer Lett. 2015, 356, 165–170. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Hwang, S.L.; Li, X.; Lu, Y.; Jin, Y.; Jeong, Y.T.; Kim, Y.D.; Lee, I.K.; Taketomi, Y.; Sato, H.; Cho, Y.S.; et al. AMP-activated protein kinase negatively regulates FcεRI-mediated mast cell signaling and anaphylaxis in mice. J. Allergy Clin. Immunol. 2013, 132, 729–736.e12. [Google Scholar] [CrossRef]
- Hwang, S.L.; Lu, Y.; Li, X.; Kim, Y.D.; Cho, Y.S.; Jahng, Y.; Son, J.K.; Lee, Y.J.; Kang, W.; Taketomi, Y.; et al. ERK1/2 antagonize AMPK-dependent regulation of FcεRI-mediated mast cell activation and anaphylaxis. J. Allergy Clin. Immunol. 2014, 134, 714–721.e7. [Google Scholar] [CrossRef]
- Li, X.; Lee, Y.J.; Jin, F.; Park, Y.N.; Deng, Y.; Kang, Y.; Yang, J.H.; Chang, J.H.; Kim, D.Y.; Kim, J.A.; et al. Sirt1 negatively regulates FcεRI-mediated mast cell activation through AMPK- and PTP1B-dependent processes. Sci. Rep. 2017, 7, 6444, Erratum in Sci. Rep. 2020, 10, 3552. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Li, X.; Deng, Y.; Timilshina, M.; Huang, B.; Kim, D.Y.; Chang, J.H.; Ichinose, H.; Baek, S.H.; Murakami, M.; et al. The orphan nuclear receptor NR4A1 promotes FcεRI-stimulated mast cell activation and anaphylaxis by counteracting the inhibitory LKB1/AMPK axis. Allergy 2019, 74, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.M. Zingerone ameliorates non-alcoholic fatty liver disease in rats by activating AMPK. J. Food Biochem. 2022, 46, e14149. [Google Scholar] [CrossRef]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef]
- Amin, I.; Hussain, I.; Rehman, M.U.; Mir, B.A.; Ganaie, S.A.; Ahmad, S.B.; Mir, M.U.R.; Shanaz, S.; Muzamil, S.; Arafah, A.; et al. Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. J. Food Biochem. 2021, 45, e13241. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Khalili, H.; Fatemi, I.; Malayeri, A.; Siahpoosh, A.; Goudarzi, M. Zingerone Mitigates Carrageenan-Induced Inflammation Through Antioxidant and Anti-inflammatory Activities. Inflammation 2021, 44, 186–193. [Google Scholar] [CrossRef]
- Han, L.K.; Morimoto, C.; Zheng, Y.N.; Li, W.; Asami, E.; Okuda, H.; Saito, M. Effects of zingerone on fat storage in ovariectomized rats. Yakugaku Zasshi 2008, 128, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, J.H.; Li, X.; Hwangbo, K.; Hwang, S.L.; Taketomi, Y.; Murakami, M.; Chang, Y.C.; Kim, C.H.; Son, J.K.; et al. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem. Pharmacol. 2011, 82, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, C.; Luo, J.; Hua, S.; Li, D.; Peng, L.; Liu, H.; Song, L. The protective role of Zingerone in a murine asthma model via activation of the AMPK/Nrf2/HO-1 pathway. Food Funct. 2021, 12, 3120–3131. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Hillary, V.E.; Antony, P.J.; Zhong, L.L.D.; Yogesh, D.; Krishnakumar, N.M.; Ceasar, S.A.; Gan, R.Y. A systematic review on anti-diabetic plant essential oil compounds: Dietary sources, effects, molecular mechanisms, and safety. Crit. Rev. Food Sci. Nutr. 2023, 64, 6526–6545. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Pyla, R.; Hartney, T.J.; Segar, L. AICAR promotes endothelium-independent vasorelaxation by activating AMP-activated protein kinase via increased ZMP and decreased ATP/ADP ratio in aortic smooth muscle. J. Basic. Clin. Physiol. Pharmacol. 2022, 33, 759–768. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, H.W.; Kim, M.Y.; Kim, T.W.; Kim, E.N.; Kim, Y.; Chung, S.; Kim, Y.S.; Choi, B.S.; Kim, Y.S.; et al. Cinacalcet-mediated activation of the CaMKKβ-LKB1-AMPK pathway attenuates diabetic nephropathy in db/db mice by modulation of apoptosis and autophagy. Cell Death Dis. 2018, 9, 270. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, X.; Khuri, F.R.; Sun, S.Y.; Vertino, P.M.; Zhou, W. LKB1 is necessary for Akt-mediated phosphorylation of proapoptotic proteins. Cancer Res. 2008, 68, 7270–7277. [Google Scholar] [CrossRef]
- Dispenza, M.C.; Bochner, B.S.; MacGlashan, D.W., Jr. Targeting the FcεRI Pathway as a Potential Strategy to Prevent Food-Induced Anaphylaxis. Front. Immunol. 2020, 11, 614402. [Google Scholar] [CrossRef]
- Shamsabadi, S.; Nazer, Y.; Ghasemi, J.; Mahzoon, E.; Baradaran Rahimi, V.; Ajiboye, B.O.; Askari, V.R. Promising influences of zingerone against natural and chemical toxins: A comprehensive and mechanistic review. Toxicon 2023, 233, 107247. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, Q.; Wang, Q.L.; Adu-Frimpong, M.; Wei, C.M.; Weng, W.; Bao, R.; Wang, Y.P.; Yu, J.N.; Xu, X.M. Improvement of Oral Bioavailability and Anti-Tumor Effect of Zingerone Self-Microemulsion Drug Delivery System. J. Pharm. Sci. 2021, 110, 2718–2727. [Google Scholar] [CrossRef]
- Ren, J.; Xiang, B.; Song, L.; René, D.J.; Luo, Y.; Wen, G.; Gu, H.; Yang, Z.; Zhang, Y. Kaixinsan regulates neuronal mitochondrial homeostasis to improve the cognitive function of Alzheimer’s disease by activating CaMKKβ-AMPK-PGC-1α signaling axis. Phytomedicine 2024, 135, 156170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Zhang, H.; Mao, C.; Liang, J.; Li, X. Zingerone Targets LKB1/AMPK to Block FcεRI-Dependent Mast Cell Degranulation and Anaphylaxis. Curr. Issues Mol. Biol. 2025, 47, 963. https://doi.org/10.3390/cimb47110963
Zheng D, Zhang H, Mao C, Liang J, Li X. Zingerone Targets LKB1/AMPK to Block FcεRI-Dependent Mast Cell Degranulation and Anaphylaxis. Current Issues in Molecular Biology. 2025; 47(11):963. https://doi.org/10.3390/cimb47110963
Chicago/Turabian StyleZheng, Defeng, Hui Zhang, Can Mao, Jinqiang Liang, and Xian Li. 2025. "Zingerone Targets LKB1/AMPK to Block FcεRI-Dependent Mast Cell Degranulation and Anaphylaxis" Current Issues in Molecular Biology 47, no. 11: 963. https://doi.org/10.3390/cimb47110963
APA StyleZheng, D., Zhang, H., Mao, C., Liang, J., & Li, X. (2025). Zingerone Targets LKB1/AMPK to Block FcεRI-Dependent Mast Cell Degranulation and Anaphylaxis. Current Issues in Molecular Biology, 47(11), 963. https://doi.org/10.3390/cimb47110963






