Mitochondrial Collapse Responsible for Chagasic and Post-Ischemic Heart Failure Is Reversed by Cell Therapy Under Different Transcriptomic Topologies
Abstract
1. Introduction
2. Materials and Methods
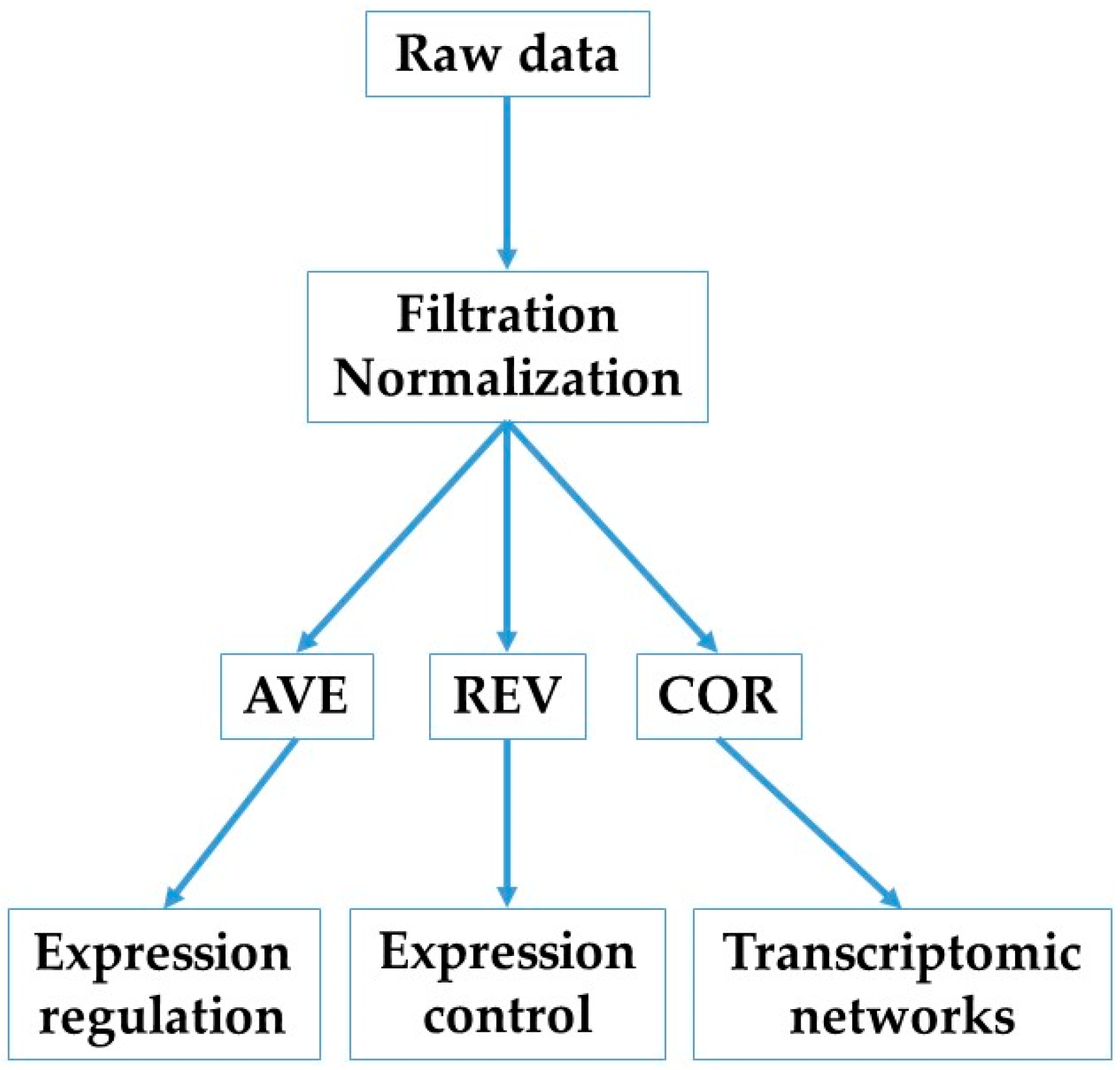
2.1. Experimental Data
2.2. Primary Independent Characteristics of Individual Genes
- (1)
- synergistically expressed, i.e., their expression levels oscillate in phase across biological replicates.
- (2)
- antagonistically expressed, i.e., their expression levels oscillate in antiphase across biological replicates.
- (3)
- independently expressed genes, i.e., the expression of one gene has no influence on the expression of the other gene.
2.3. Transcriptomic Changes in Individual Genes and Functional Pathways
3. Results
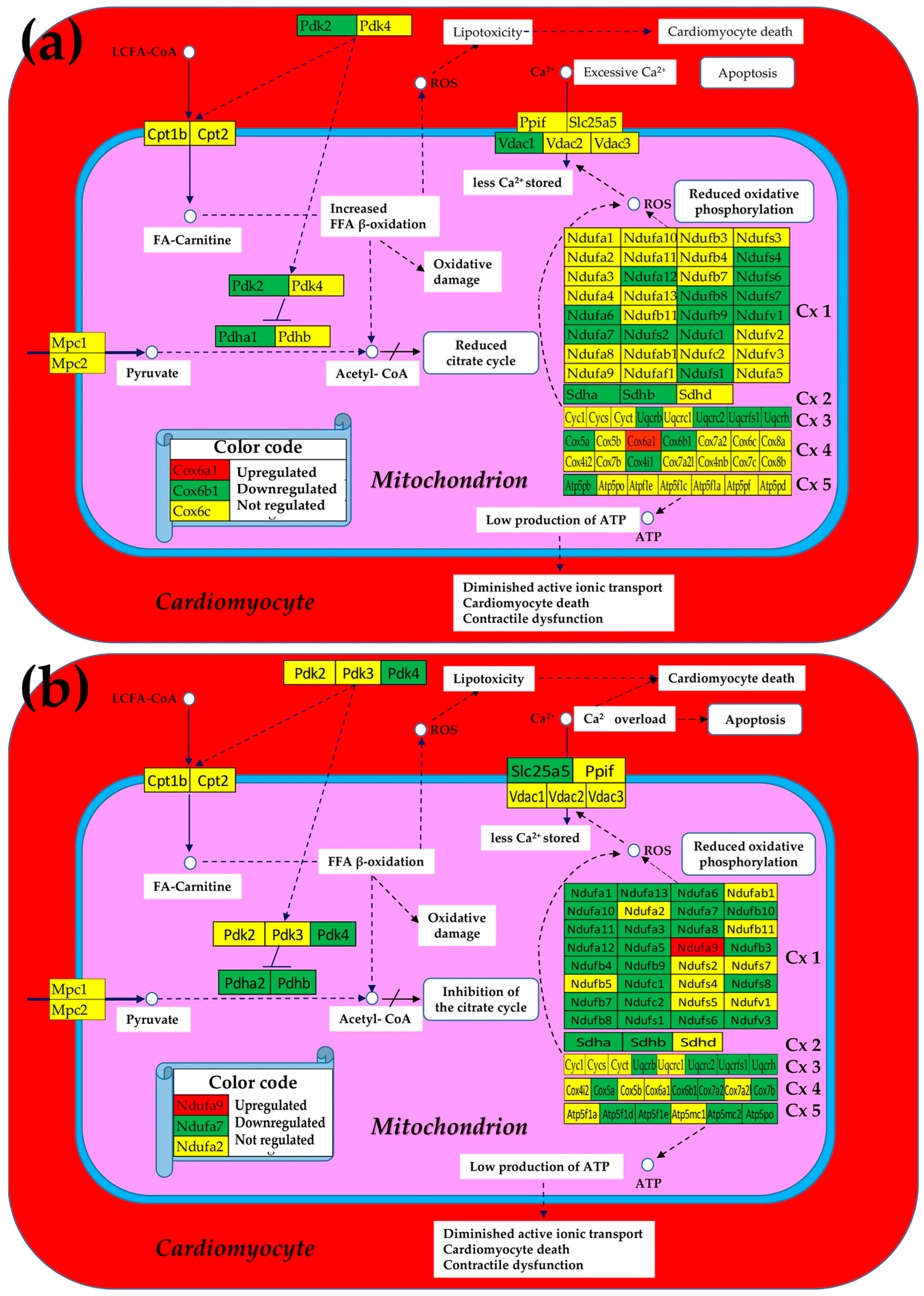
3.1. Both Chagasic Disease and Post-Ischemic Heart Failure Are Characterized by Substantial Downregulation of Mitochondrial Genes
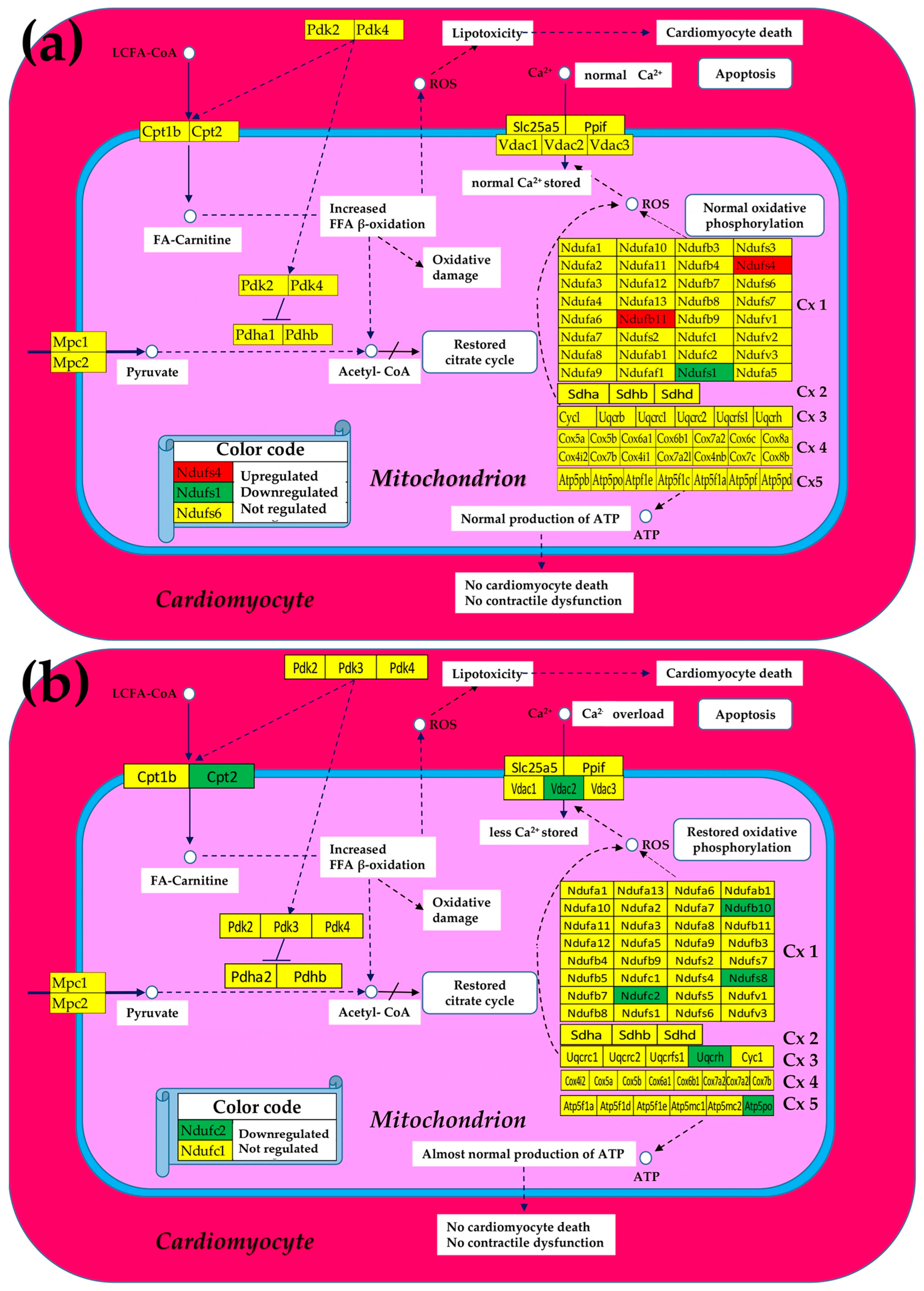
3.2. Cell Treatment Restores the Normal Expression of Most Mitochondrial Genes Altered in Both Chagasic Disease and Post-Ischemic Heart Failure
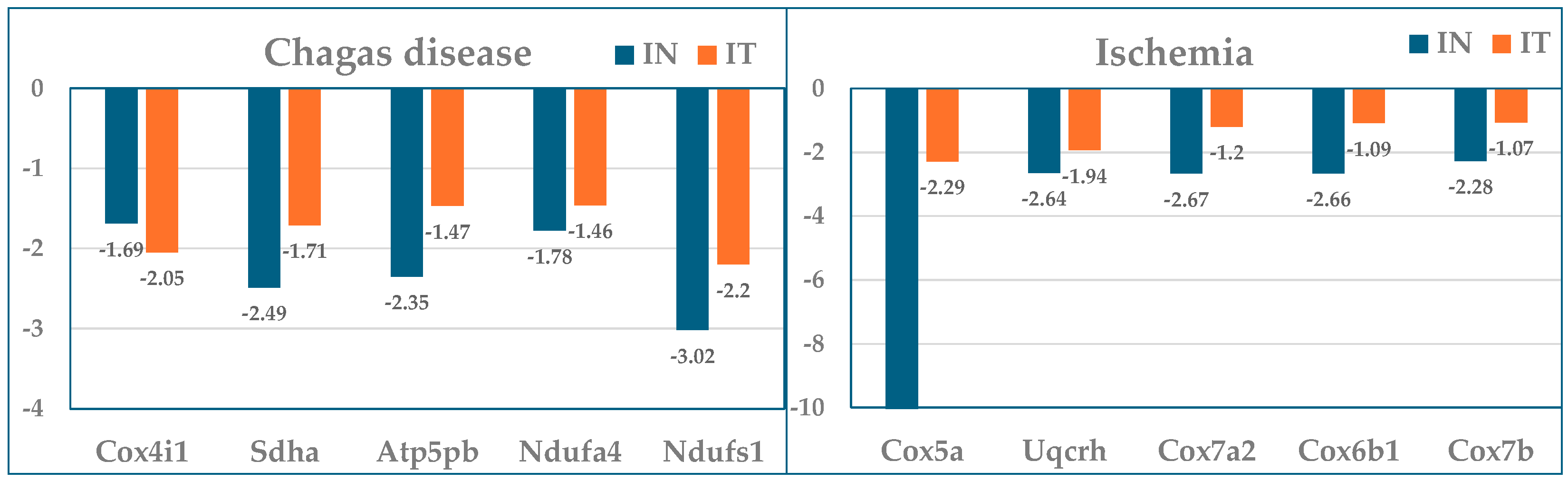
3.3. The Largest Mitochondrial Gene Contributors to the Transcriptomic Alterations in Both Chagasic and Post-Ischemic Mice
3.4. The Most and the Least Controlled Mitochondrial Genes in CCC and IHF Mice
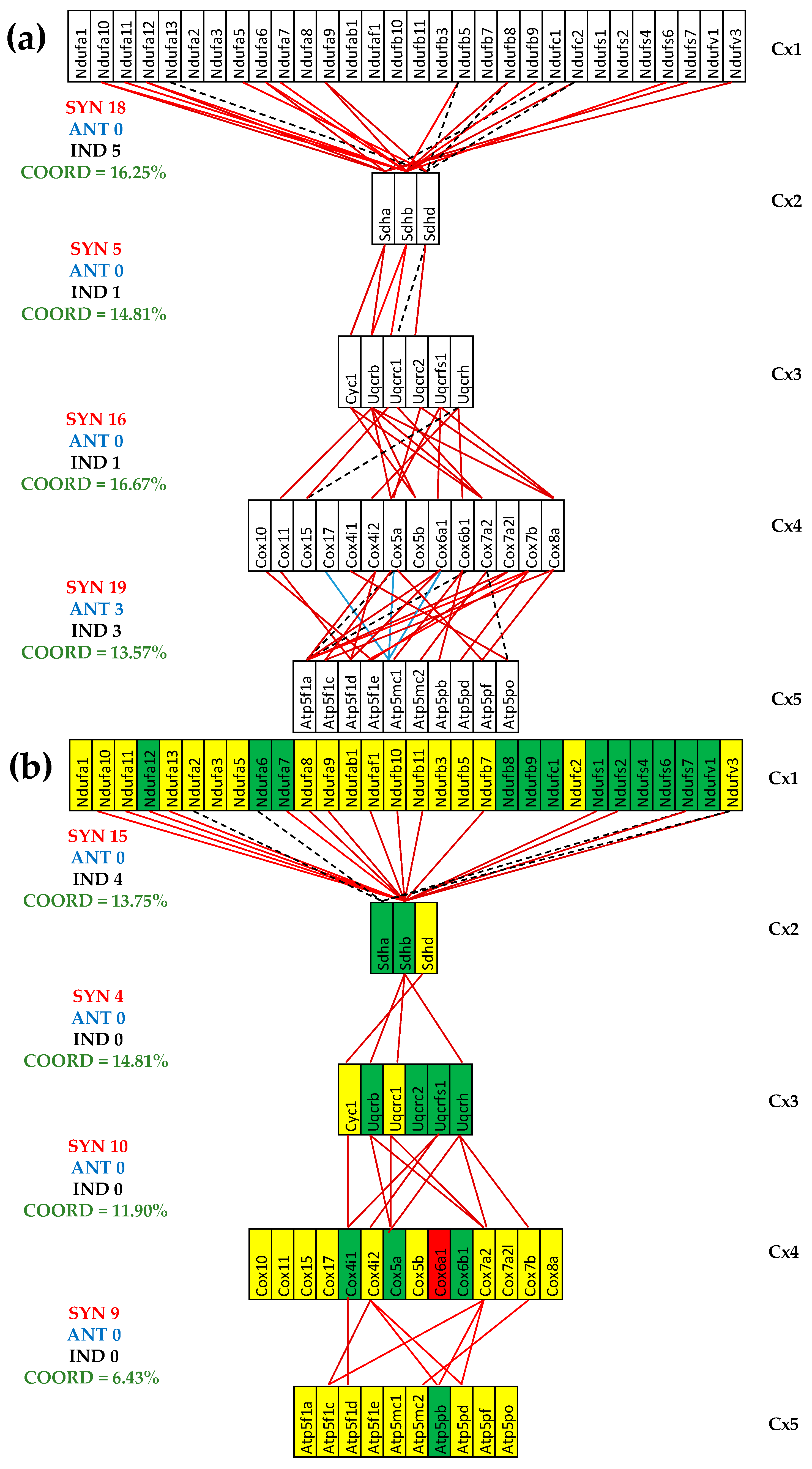
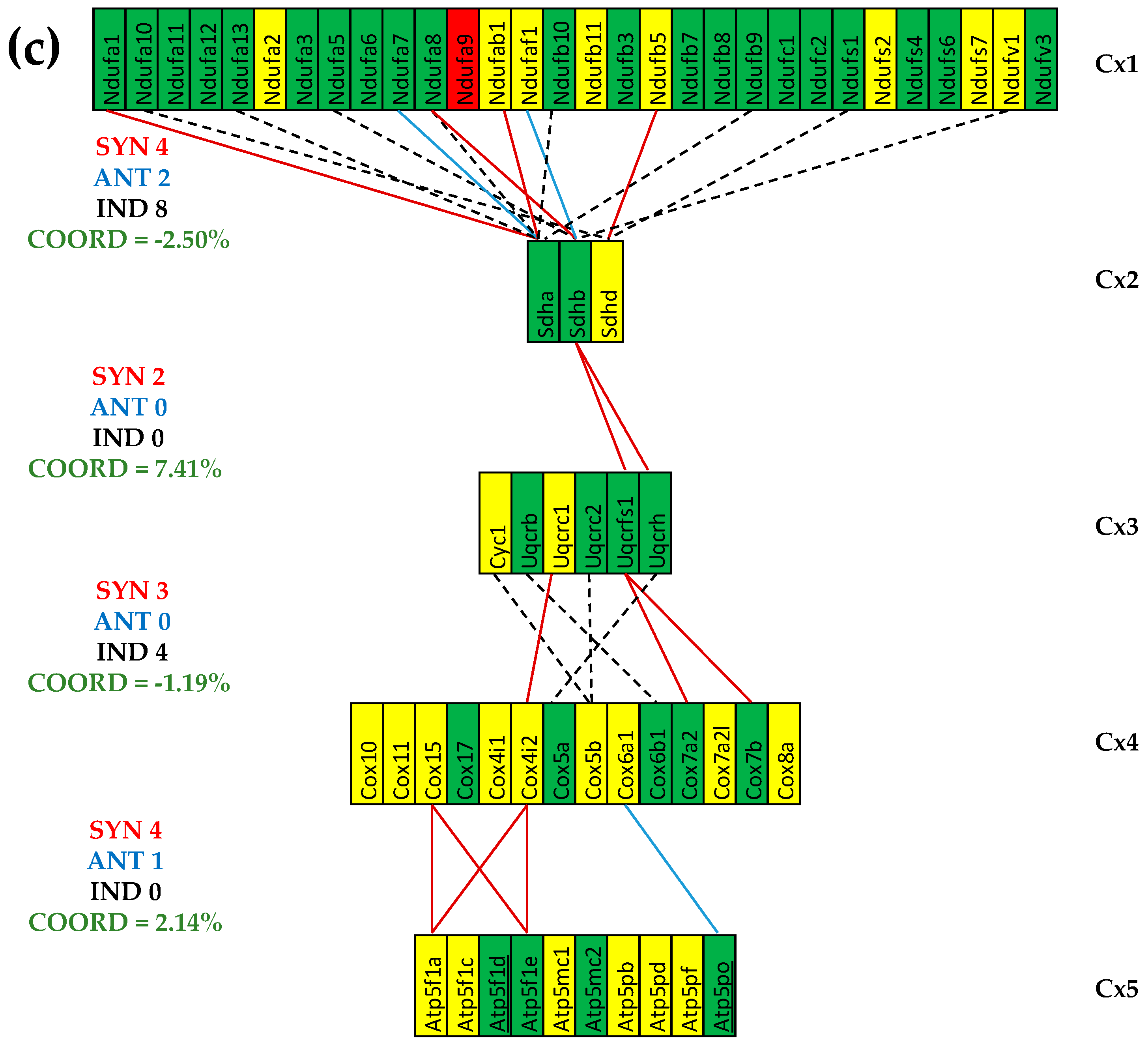
3.5. Both CCC and IHF Alter the Transcriptomic Networks of the Mitochondrial Genes by Partially Decoupling the Oxidative Phosphorylation Complexes
3.6. Stem Cell Treatment Benefits for the Transcriptomic Coupling of the Oxidative Phosphorylation Complexes
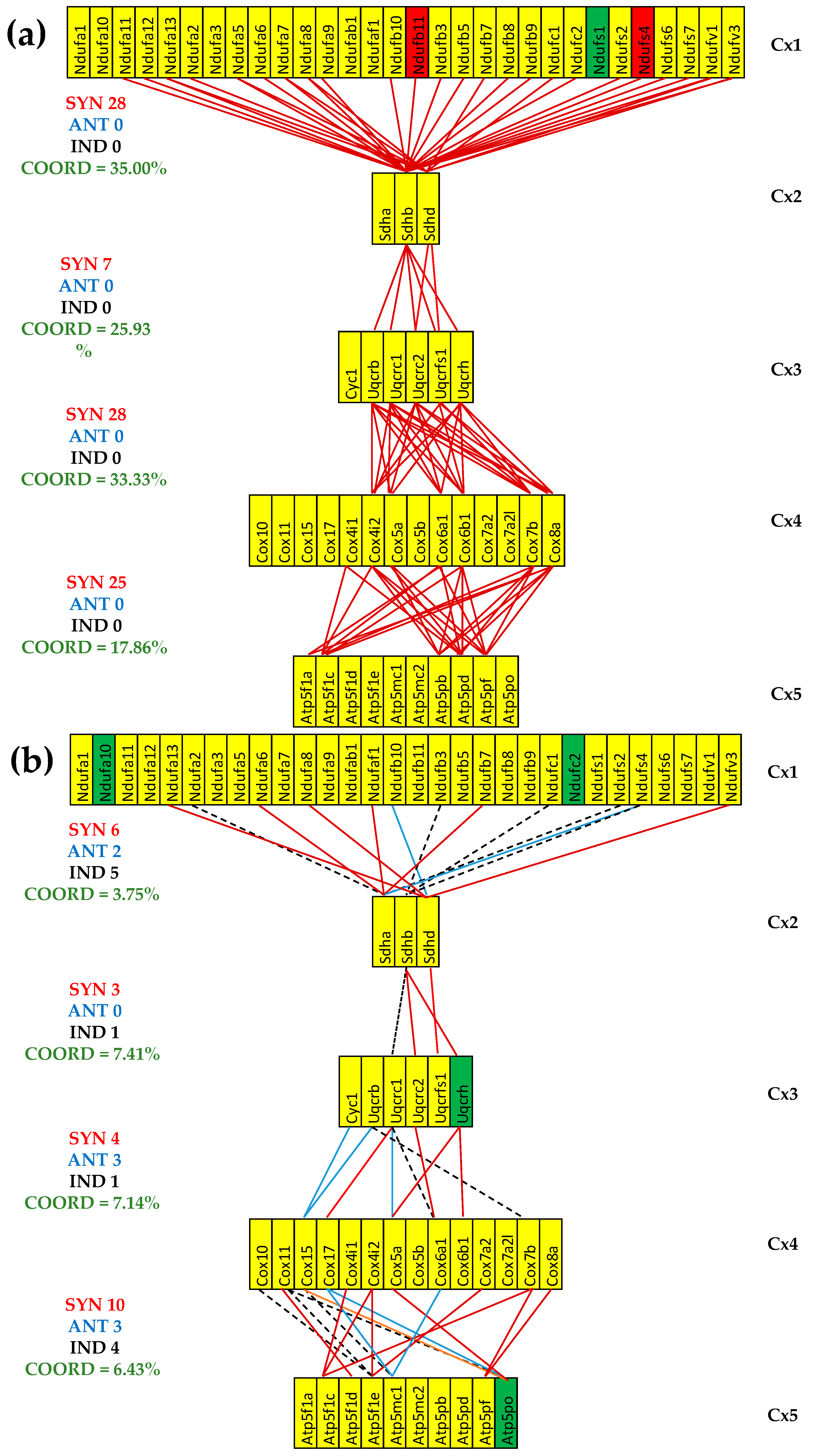
3.7. Both CCC and IHF Alter the Hierarchy of Mitochondrial Genes
4. Discussion
- (i)
- Gene hierarchy is altered in cardiomyopathy, as revealed by the differences between the top five genes in healthy mice compared to the sets of five in the other heart conditions.
- (ii)
- Each type of cardiomyopathy induces distinct alteration of the gene hierarchy (see GCH differences between CCC and IHF for the most prominent genes in healthy mice).
- (iii)
- Restoration of the normal expression level is not accompanied by the reinstatement of the genes in their right hierarchy, indicated by the non-unit GCH-FC. For instance, Uqcrh, found by us as downregulated in both untreated CCC and IHF mice, was reported as upregulated in patients with hypertrophic cardiomyopathy [106]. Cell treatment restored the normal expression in the CCC but not in the IHF mice. However, the restored expression level is not followed by the full restoration of its GCH after the treatment. Instead, its RCS became 6.67× stronger than in the normal condition (even stronger than in the untreated CCC, where it was 4.66×).
- (iv)
- Cell treatment has different effects on the two types of heart afflictions. For instance, the somehow low GCH of Cox6b1 healthy mice that was considerably raised in both untreated cardiomyopathies (GCH-FC(IN-CCC) = 4.81, GCH-FC(IN-IHF) = 5.53) is raised even more in treated CCC (GCH-FC) = 7.34) but downgraded in treated IHF (GCH-FC = −2.10).
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinton, A., Jr.; Claypool, S.M.; Neikirk, K.; Senoo, N.; Wanjalla, C.N.; Kirabo, A.; Williams, C.R. Mitochondrial Structure and Function in Human Heart Failure. Circ. Res. 2024, 135, 372–396. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W., 2nd; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1alpha/PI3K/Akt signaling. J. Cell Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef]
- Koka, S.; Aluri, H.S.; Xi, L.; Lesnefsky, E.J.; Kukreja, R.C. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: Potential role of NO/SIRT1/PGC-1alpha signaling. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1558–H1568. [Google Scholar] [CrossRef]
- Nunes, J.P.S.; Roda, V.M.P.; Andrieux, P.; Kalil, J.; Chevillard, C.; Cunha-Neto, E. Inflammation and mitochondria in the pathogenesis of chronic Chagas disease cardiomyopathy. Exp. Biol. Med. 2023, 248, 2062–2071. [Google Scholar] [CrossRef]
- Zhu, S.G.; Kukreja, R.C.; Das, A.; Chen, Q.; Lesnefsky, E.J.; Xi, L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J. Am. Coll. Cardiol. 2011, 57, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Schaub, G.A. Trypanosoma cruzi/Triatomine Interactions—A Review. Pathogens 2025, 14, 392. [Google Scholar] [CrossRef]
- Ouali, R.; Bousbata, S. Rhodnius prolixus (kissing bug). Trends Parasitol. 2025, 41, 1064–1065. [Google Scholar] [CrossRef]
- Mukherjee, S.; Belbin, T.J.; Spray, D.C.; Iacobas, D.A.; Weiss, L.M.; Kitsis, R.N.; Wittner, M.; Jelicks, L.A.; Scherer, P.E.; Ding, A.; et al. Microarray analysis of changes in gene expression in a murine model of chronic chagasic cardiomyopathy. Parasitol. Res. 2003, 91, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Tanowitz, H.B.; Garg, N.J. Pathogenesis of Chronic Chagas Disease: Macrophages, Mitochondria, and Oxidative Stress. Curr. Clin. Microbiol. Rep. 2018, 5, 45–54. [Google Scholar] [CrossRef]
- Hernandez, S.; Srikanth, K.K.; Bommireddi, A.; Leong, T.K.; Miller, D.A.; Ambrosy, A.P.; Zaroff, J. Chagas Disease in Northern California: Observed Prevalence, Clinical Characteristics, and Outcomes Within an Integrated Health Care Delivery System. Perm. J. 2025, 29, 40–48. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.C.; da Silva, M.T.S.; Bezerra Dos Santos, L.; Abdala, M.G.G.; Lopes, A.B.O.; de Oliveira, G.A.; Guedes-da-Silva, F.H.; Rigoni, T.D.S.; Damasceno, F.S.; Barros-Neto, J.A.; et al. Epidemiological assessment of the first year of mandatory notification of chronic Chagas disease in Alagoas, Northeast Brazil. Acta Trop. 2025, 270, 107805. [Google Scholar] [CrossRef]
- Beatty, N.L.; Hamer, G.L.; Moreno-Peniche, B.; Mayes, B.; Hamer, S.A. Chagas Disease, an Endemic Disease in the United States. Emerg. Infect. Dis. 2025, 31, 1691–1697. [Google Scholar] [CrossRef]
- Bunkofske, M.E.; Sanchez-Valdez, F.J.; Tarleton, R.L. The importance of persistence and dormancy in Trypanosoma cruzi infection and Chagas disease. Curr. Opin. Microbiol. 2025, 86, 102615. [Google Scholar] [CrossRef]
- Telleria, J.; Costales, J.A. An Overview of Trypanosoma cruzi Biology Through the Lens of Proteomics: A Review. Pathogens 2025, 14, 337. [Google Scholar] [CrossRef]
- Garg, N.; Popov, V.L.; Papaconstantinou, J. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: Implications in chagasic myocarditis development. Biochim. Biophys. Acta 2003, 1638, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Báez, A.; Lo Presti, M.S.; Rivarola, H.W.; Mentesana, G.G.; Pons, P.; Fretes, R.; Paglini-Oliva, P. Mitochondrial involvement in chronic chagasic cardiomyopathy. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Coll, H. Chagas disease: Host responses, parasite evasion and vaccine advances. Trans. R. Soc. Trop. Med. Hyg. 2025, traf109. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.J.D.N.; da Silva, P.S.; Saraiva, R.M.; Sangenis, L.H.C.; de Holanda, M.T.; Sperandio da Silva, G.M.; Mendes, F.S.N.S.; Xavier, I.G.G.; Costa, H.S.; Gonçalves, T.R.; et al. Food insecurity is associated with decreased quality of life in patients with chronic Chagas disease. PLoS ONE 2025, 20, e0328466. [Google Scholar] [CrossRef]
- Echeverría, L.E.; Serrano-García, A.Y.; Rojas, L.Z.; Silva-Sieger, F.; Navarro, M.; Aguilera, L.; Gómez-Ochoa, S.A.; Morillo, C.A. Beyond cardiac embolism and cryptogenic stroke: Unveiling the mechanisms of cerebrovascular events in Chagas disease. Lancet Reg. Health Am. 2025, 50, 101203. [Google Scholar] [CrossRef]
- Souza-Silva, T.G.; Figueiredo, A.; Morais, K.L.P.; Apostólico, J.; Pantaleao, A.; Mutarelli, A.; Araújo, S.S.; Nunes, M.D.C.P.; Gollob, K.J.; Dutra, W.O. Single-cell targeted transcriptomics reveals subset-specific immune signatures differentiating asymptomatic and cardiac patients with chronic Chagas disease. J. Infect. Dis. 2025, jiaf269. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; So, J.; Castro-Sesquen, Y.E.; DeToy, K.; Gutierrez Guarnizo, S.A.; Jahanbakhsh, F.; Malaga Machaca, E.; Miranda-Schaeubinger, M.; Chakravarti, I.; Cooper, V.; et al. Immunologic changes in the peripheral blood transcriptome of individuals with early-stage chronic Chagas cardiomyopathy: A cross-sectional study. Lancet Reg. Health Am. 2025, 45, 101090. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Soliman-Aboumarie, H. Review of Current Management of Myocardial Infarction. J. Clin. Med. 2025, 14, 6241. [Google Scholar] [CrossRef]
- Mueller, C.; White, H.D.; Lopez-Ayala, P.; de Silva, R.; Kaski, J.C. Great debate: The universal definition of myocardial infarction is flawed and should be put to rest. Eur. Heart J. 2025, ehaf641. [Google Scholar] [CrossRef]
- Taggart, C.; Ferry, A.V.; Chapman, A.R.; Schulberg, S.D.; Bularga, A.; Wereski, R.; Boeddinghaus, J.; Kimenai, D.M.; Lowry, M.T.H.; Chew, D.P.; et al. The assessment and management of patients with type 2 myocardial infarction: An international Delphi study. Eur. Heart J. Qual. Care Clin. Outcomes 2025, qcaf069. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Z.; Li, Y.; Zhang, H.; Guo, H.; Chen, G.; Wei, P.; Lin, F.; Zhao, G. Mitochondrial dysfunction as a central hub linking Na+/Ca2+ homeostasis and inflammation in ischemic arrhythmias: Therapeutic implications. Front. Cardiovasc. Med. 2025, 12, 1506501. [Google Scholar] [CrossRef]
- Meco, M.; Giustiniano, E.; Nisi, F.; Zulli, P.; Agosteo, E. MAPK, PI3K/Akt Pathways, and GSK-3β Activity in Severe Acute Heart Failure in Intensive Care Patients: An Updated Review. J. Cardiovasc. Dev. Dis. 2025, 12, 266. [Google Scholar] [CrossRef]
- Yang, Q.; Ji, H.; Modarresi Chahardehi, A. JAK/STAT pathway in myocardial infarction: Crossroads of immune signaling and cardiac remodeling. Mol. Immunol. 2025, 186, 206–217. [Google Scholar] [CrossRef]
- Deng, G.; Yang, Y.; Qing, O.; Linhui, J.; Haotao, S.; Liu, C.; Li, G.; Nasser, M.I. Chrysin Attenuates Myocardial Cell Apoptosis in Mice. Cardiovasc. Toxicol. 2025, 25, 1791–1806. [Google Scholar] [CrossRef]
- Lachtermacher, S.; Esporcatte, B.L.; Montalvao, F.; Costa, P.C.; Rodrigues, D.C.; Belem, L.; Rabischoffisky, A.; Faria Neto, H.C.; Vasconcellos, R.; Iacobas, S.; et al. Cardiac gene expression and systemic cytokine profile are complementary in a murine model of post-ischemic heart failure. Braz. J. Med. Biol. Res. 2010, 43, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Lachtermacher, S.; Esporcatte, B.L.; da Silva de Azevedo Fortes, F.; Rocha, N.N.; Montalvao, F.; Costa, P.C.; Belem, L.; Rabischoffisky, A.; Faria Neto, H.C.; Vasconcellos, R.; et al. Functional and transcriptomic recovery of infarcted mouse myocardium treated with bone marrow mononuclear cells. Stem Cell Rev. Rep. 2012, 8, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Yang, F.; Iacobas, D.A.; Xi, L. Mental disorders after myocardial infarction: Potential mediator role for chemokines in heart-brain interaction? J. Geriatr. Cardiol. 2024, 21, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.; de Lima, R.S.; Rocha, L.L.; Vasconcelos, J.F.; Rogatto, S.R.; dos Santos, R.R.; Iacobas, S.; Goldenberg, R.C.; Iacobas, D.A.; Tanowitz, H.B.; et al. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J. Infect. Dis. 2010, 202, 416–426. [Google Scholar] [CrossRef]
- Goldenberg, R.C.; Iacobas, D.A.; Iacobas, S.; Rocha, L.L.; da Silva de Azevedo Fortes, F.; Vairo, L.; Nagajyothi, F.; Campos de Carvalho, A.C.; Tanowitz, H.B.; Spray, D.C. Transcriptomic alterations in Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2009, 11, 1140–1149. [Google Scholar] [CrossRef]
- Adesse, D.; Goldenberg, R.C.; Fortes, F.S.; Jasmin; Iacobas, D.A.; Iacobas, S.; Campos de Carvalho, A.C.; de Narareth Meirelles, M.; Huang, H.; Soares, M.B.; et al. Gap junctions and chagas disease. Adv. Parasitol. 2011, 76, 63–81. [Google Scholar] [CrossRef]
- Xinxin, Z.; Pan, H.; Qiao, L. Research progress of connexin 43 in cardiovascular diseases. Front. Cardiovasc. Med. 2025, 12, 1650548. [Google Scholar] [CrossRef]
- Caetano-da-Silva, J.E.; Gonçalves-Santos, E.; Domingues, E.L.B.C.; Caldas, I.S.; Lima, G.D.A.; Diniz, L.F.; Gonçalves, R.V.; Novaes, R.D. The mitochondrial uncoupler 2,4-dinitrophenol modulates inflammatory and oxidative responses in Trypanosoma cruzi-induced acute myocarditis in mice. Cardiovasc. Pathol. 2024, 72, 107653. [Google Scholar] [CrossRef]
- Nisimura, L.M.; Coelho, L.L.; de Melo, T.G.; Vieira, P.C.; Victorino, P.H.; Garzoni, L.R.; Spray, D.C.; Iacobas, D.A.; Iacobas, S.; Tanowitz, H.B.; et al. Trypanosoma cruzi Promotes Transcriptomic Remodeling of the JAK/STAT Signaling and Cell Cycle Pathways in Myoblasts. Front. Cell Infect. Microbiol. 2020, 10, 255. [Google Scholar] [CrossRef]
- Narimani, S.; Rahbarghazi, R.; Salehipourmehr, H.; Taghavi Narmi, M.; Lotfimehr, H.; Mehdipour, R. Therapeutic Potential of Endothelial Progenitor Cells in Angiogenesis and Cardiac Regeneration: A Systematic Review and Meta-Analysis of Rodent Models. Adv Pharm Bull. 2025, 15, 268–283. [Google Scholar] [CrossRef]
- Soares, M.B.; Lima, R.S.; Souza, B.S.; Vasconcelos, J.F.; Rocha, L.L.; Dos Santos, R.R.; Iacobas, S.; Goldenberg, R.C.; Lisanti, M.P.; Iacobas, D.A.; et al. Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle 2011, 10, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Tanowitz, H.B.; Campos de Carvalho, A.; Spray, D.C. Functional genomic fabrics are remodeled in a mouse model of Chagasic cardiomyopathy and restored following cell therapy. Microbes Infect. 2018, 20, 185–195. [Google Scholar] [CrossRef]
- Oxidative Phosphorylation. Available online: https://www.kegg.jp/pathway/mmu00190 (accessed on 1 September 2025).
- Diabetic Cardiomyopathy. Available online: https://www.kegg.jp/pathway/mmu05415 (accessed on 1 September 2025).
- Genomic Data: Gene Expression Changes Associated with Myocarditis and Fibrosis in Hearts of Mice with Chronic Chagasic Cardiomyopathy. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17363 (accessed on 1 September 2025).
- Genomic Data: Cardiac Gene Expression and Systemic Cytokine Profile Are Complementary in a Murine Model of Post Ischemic Heart Failure. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE18703 (accessed on 1 September 2025).
- Genomic Data: Therapy with Bone Marrow Cells Recovers Gene Expression Alterations in Hearts of Mice with Chronic Chagasic Cardiomyopathy. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE24088 (accessed on 1 September 2025).
- Genomic Data: Functional and Transcriptomic Recovery of Infarcted Mouse Myocardium Treated with Bone Marrow Mononuclear Cells. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29769 (accessed on 1 September 2025).
- Adesse, D.; Iacobas, D.A.; Iacobas, S.; Garzoni, L.R.; Meirelles, M.d.N.; Tanowitz, H.B.; Spray, D.C. Transcriptomic signatures of alterations in a myoblast cell line infected with four distinct strains of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2010, 82, 846–854. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Lee, P.R.; Cohen, J.E.; Fields, R.D. Coordinated Activity of Transcriptional Networks Responding to the Pattern of Action Potential Firing in Neurons. Genes 2019, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A. Biomarkers, Master Regulators and Genomic Fabric Remodeling in a Case of Papillary Thyroid Carcinoma. Genes 2020, 11, 1030. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Xi, L. Theory and Applications of the (Cardio) Genomic Fabric Approach to Post-Ischemic and Hypoxia-Induced Heart Failure. J. Pers. Med. 2022, 12, 1246. [Google Scholar] [CrossRef]
- Parker, A.M.; Lees, J.G.; Murray, A.J.; Velagic, A.; Lim, S.Y.; De Blasio, M.J.; Ritchie, R.H. Precision Medicine: Therapeutically Targeting Mitochondrial Alterations in Heart Failure. JACC Basic Transl. Sci. 2025, 10, 101345. [Google Scholar] [CrossRef]
- Mongelli, A.; Mengozzi, A.; Geiger, M.; Gorica, E.; Mohammed, S.A.; Paneni, F.; Ruschitzka, F.; Costantino, S. Mitochondrial epigenetics in aging and cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1204483. [Google Scholar] [CrossRef]
- Pietrangelo, D.; Lopa, C.; Litterio, M.; Cotugno, M.; Rubattu, S.; Lombardi, A. Metabolic Disturbances Involved in Cardiovascular Diseases: The Role of Mitochondrial Dysfunction, Altered Bioenergetics and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 6791. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Ostadal, P.; Tappia, P.S. Involvement of Oxidative Stress in Mitochondrial Abnormalities During the Development of Heart Disease. Biomedicines 2025, 13, 1338. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Blandino, A.; Scherer, D.; Zulantay, I.; Apt, W.; Varela, N.M.; Llancaqueo, M.; Garcia, L.; Ortiz, L.; Nicastri, E.; et al. Small-RNA sequencing identifies serum microRNAs associated with abnormal electrocardiography findings in patients with Chagas disease. J. Infect. 2025, 91, 106613. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, H. Diagnosis and treatment of post-acute myocardial infarction ventricular aneurysm: A review. Medicine 2025, 8, e43696. [Google Scholar] [CrossRef] [PubMed]
- Formosa, L.E.; Muellner-Wong, L.; Reljic, B.; Sharpe, A.J.; Jackson, T.D.; Beilharz, T.H.; Stojanovski, D.; Lazarou, M.; Stroud, D.A.; Ryan, M.T. Dissecting the Roles of Mitochondrial Complex I Intermediate Assembly Complex Factors in the Biogenesis of Complex I. Cell Rep. 2020, 31, 107541. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, H.; Luo, L.; Wu, W. Nicotinamide Adenine Dinucleotide Supplementation to Alleviate Heart Failure: A Mitochondrial Dysfunction Perspective. Nutrients 2025, 17, 1855. [Google Scholar] [CrossRef] [PubMed]
- Bozdemir, N.; Cakir, C.; Topcu, U.; Uysal, F. A Comprehensive Review of Mitochondrial Complex I During Mammalian Oocyte Maturation. Genesis 2025, 63, e70017. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, Y.; Chen, R.; Gong, Y.; Zhang, M.; Guan, R.; Rotstein, O.D.; Liu, X.; Wen, X.Y. ndufa7 plays a critical role in cardiac hypertrophy. J. Cell Mol. Med. 2020, 24, 13151–13162. [Google Scholar] [CrossRef]
- Wyckelsma, V.L.; Levinger, I.; McKenna, M.J.; Formosa, L.E.; Ryan, M.T.; Petersen, A.C.; Anderson, M.J.; Murphy, R.M. Preservation of skeletal muscle mitochondrial content in older adults: Relationship between mitochondria, fibre type and high-intensity exercise training. J. Physiol. 2017, 595, 3345–3359. [Google Scholar] [CrossRef]
- Meng, C.; Jin, X.; Xia, L.; Shen, S.M.; Wang, X.L.; Cai, J.; Chen, G.Q.; Wang, L.S.; Fang, N.Y. Alterations of mitochondrial enzymes contribute to cardiac hypertrophy before hypertension development in spontaneously hypertensive rats. J. Proteome Res. 2009, 8, 2463–2475. [Google Scholar] [CrossRef]
- Huang, L.; Jin, X.; Xia, L.; Wang, X.; Yu, Y.; Liu, C.; Shao, D.; Fang, N.; Meng, C. Characterization of mitochondrial NADH dehydrogenase 1alpha subcomplex 10 variants in cardiac muscles from normal Wistar rats and spontaneously hypertensive rats: Implications in the pathogenesis of hypertension. Mol. Med. Rep. 2016, 13, 961–966. [Google Scholar] [CrossRef]
- Iverson, T.M.; Singh, P.K.; Cecchini, G. An evolving view of complex II-noncanonical complexes, megacomplexes, respiration, signaling, and beyond. J. Biol. Chem. 2023, 299, 104761. [Google Scholar] [CrossRef]
- Lin, S.; Fasham, J.; Al-Hijawi, F.; Qutob, N.; Gunning, A.; Leslie, J.S.; McGavin, L.; Ubeyratna, N.; Baker, W.; Zeid, R.; et al. Consolidating biallelic SDHD variants as a cause of mitochondrial complex II deficiency. Eur. J. Hum. Genet. 2021, 29, 1570–1576. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Qiao, L.; Yu, R.; Ren, H.; Zhao, A.; Sun, Y.; Wang, A.; Li, B.; Wang, X.; et al. Exploring the biological basis for the identification of different syndromes in ischemic heart failure based on joint multi-omics analysis. Front. Pharmacol. 2025, 16, 1641422. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, N.; Schenkl, C.; Komlodi, T.; da Silva-Buttkus, P.; Heyne, E.; Rohde, J.; Amarie, O.V.; Rathkolb, B.; Gnaiger, E.; Doenst, T.; et al. Knockout of the Complex III subunit Uqcrh causes bioenergetic impairment and cardiac contractile dysfunction. Mamm. Genome 2023, 34, 229–243. [Google Scholar] [CrossRef]
- Čunátová, K.; Fernández-Vizarra, E. Pathological variants in nuclear genes causing mitochondrial complex III deficiency: An update. J. Inherit. Metab. Dis. 2024, 47, 1278–1291. [Google Scholar] [CrossRef]
- Sinkler, C.A.; Kalpage, H.; Shay, J.; Lee, I.; Malek, M.H.; Grossman, L.I.; Huttemann, M. Tissue- and Condition-Specific Isoforms of Mammalian Cytochrome c Oxidase Subunits: From Function to Human Disease. Oxidative Med. Cell Longev. 2017, 2017, 1534056. [Google Scholar] [CrossRef]
- Wen, J.J.; Garg, N. Oxidative modification of mitochondrial respiratory complexes in response to the stress of Trypanosoma cruzi infection. Free Radic. Biol. Med. 2004, 37, 2072–2081. [Google Scholar] [CrossRef]
- Pandey, R.; Velasquez, S.; Durrani, S.; Jiang, M.; Neiman, M.; Crocker, J.S.; Benoit, J.B.; Rubinstein, J.; Paul, A.; Ahmed, R.P. MicroRNA-1825 induces proliferation of adult cardiomyocytes and promotes cardiac regeneration post-ischemic injury. Am. J. Transl. Res. 2017, 9, 3120–3137. [Google Scholar] [PubMed]
- Elbatarny, M.; Lu, Y.T.; Hu, M.; Coles, J.; Mital, S.; Ross-White, A.; Honjo, O.; Barron, D.J.; Gramolini, A.O. Systems biology approaches investigating mitochondrial dysfunction in cyanotic heart disease: A systematic review. EBioMedicine 2025, 118, 105839. [Google Scholar] [CrossRef]
- Qiu, J.; Gu, Y. Analysis of the prognostic value of mitochondria-related genes in patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2024, 24, 408. [Google Scholar] [CrossRef] [PubMed]
- Hejzlarova, K.; Mracek, T.; Vrbacky, M.; Kaplanova, V.; Karbanova, V.; Nuskova, H.; Pecina, P.; Houstek, J. Nuclear genetic defects of mitochondrial ATP synthase. Physiol. Res. 2014, 63, S57–S71. [Google Scholar] [CrossRef]
- Mayr, J.A.; Havlickova, V.; Zimmermann, F.; Magler, I.; Kaplanova, V.; Jesina, P.; Pecinova, A.; Nuskova, H.; Koch, J.; Sperl, W.; et al. Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 epsilon subunit. Hum. Mol. Genet. 2010, 19, 3430–3439. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Hasek, L.Y.; Harris, K.L.; Berman, A.G.; Damen, F.W.; Goergen, C.J.; Ellis, J.M. Loss of cardiac carnitine palmitoyltransferase 2 results in rapamycin-resistant, acetylation-independent hypertrophy. J. Biol. Chem. 2017, 292, 18443–18456. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Escamilla, I.; Benedicto, C.; Pérez-Carrillo, L.; Delgado-Arija, M.; González-Torrent, I.; Vilchez, R.; Martínez-Dolz, L.; Portolés, M.; Tarazón, E.; Roselló-Lletí, E. Alterations in Mitochondrial Oxidative Phosphorylation System: Relationship of Complex V and Cardiac Dysfunction in Human Heart Failure. Antioxidants 2024, 13, 285. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, A.; Cheng, H.; Zheng, Y.; Li, B. PCBP2 alleviates myocardial infarction by inhibiting cardiomyocyte ferroptosis via the NDUFS1/NRF2 pathway. Mol. Immunol. 2025, 186, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Li, L.; Ma, L.; Song, C.; Li, W.; Zhang, Y.; Cheng, W.; Chen, Y.; Yang, Y.; Wang, Q.; et al. Activation of RXRα exerts cardioprotection through transcriptional upregulation of Ndufs4 in heart failure. Sci. Bull. 2024, 69, 1202–1207. [Google Scholar] [CrossRef]
- Guo, P.; Hu, S.; Liu, X.; He, M.; Li, J.; Ma, T.; Huang, M.; Fang, Q.; Wang, Y. CAV3 alleviates diabetic cardiomyopathy via inhibiting NDUFA10-mediated mitochondrial dysfunction. J. Transl. Med. 2024, 22, 390. [Google Scholar] [CrossRef] [PubMed]
- Friederich, M.W.; Erdogan, A.J.; Coughlin, C.R., 2nd; Elos, M.T.; Jiang, H.; O’Rourke, C.P.; Lovell, M.A.; Wartchow, E.; Gowan, K.; Chatfield, K.C.; et al. Mutations in the accessory subunit NDUFB10 result in isolated complex I deficiency and illustrate the critical role of intermembrane space import for complex I holoenzyme assembly. Hum. Mol. Genet. 2017, 26, 702–716. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wan, J.; Zhang, P.; Pei, F. COX6B1 relieves hypoxia/reoxygenation injury of neonatal rat cardiomyocytes by regulating mitochondrial function. Biotechnol. Lett. 2019, 41, 59–68. [Google Scholar] [CrossRef]
- Nogueira, S.S.; Souza, M.A.; Santos, E.C.; Caldas, I.S.; Gonçalves, R.V.; Novaes, R.D. Oxidative stress, cardiomyocytes premature senescence and contractile dysfunction in in vitro and in vivo experimental models of Chagas disease. Acta Trop. 2023, 244, 106950. [Google Scholar] [CrossRef]
- Gomez, J.; Coll, M.; Guarise, C.; Cifuente, D.; Masone, D.; Tello, P.F.; Piñeyro, M.D.; Robello, C.; Reta, G.; Sosa, M.Á.; et al. New insights into the pro-oxidant mechanism of dehydroleucodine on Trypanosoma cruzi. Sci. Rep. 2024, 14, 18875. [Google Scholar] [CrossRef]
- Santos-Miranda, A.; Joviano-Santos, J.V.; Ribeiro, G.A.; Botelho, A.F.M.; Rocha, P.; Vieira, L.Q.; Cruz, J.S.; Roman-Campos, D. Reactive oxygen species and nitric oxide imbalances lead to in vivo and in vitro arrhythmogenic phenotype in acute phase of experimental Chagas disease. PLoS Pathog. 2020, 16, e1008379, Erratum in PLoS Pathog. 2020, 16, e1009049. [Google Scholar] [CrossRef]
- Paillard, M.; Abdellatif, M.; Andreadou, I.; Bär, C.; Bertrand, L.; Brundel, B.J.J.; Chiva-Blanch, G.; Davidson, S.M.; Dawson, D.; Di Lisa, F.; et al. Mitochondrial targets in ischaemic heart disease and heart failure, and their potential for a more efficient clinical translation. A scientific statement of the ESC Working Group on Cellular Biology of the Heart and the ESC Working Group on Myocardial Function. Eur. J. Heart Fail. 2025, 27, 1720–1736. [Google Scholar] [CrossRef]
- Kohlhaas, M.; Nickel, A.G.; Maack, C. Mitochondrial energetics and calcium coupling in the heart. J. Physiol. 2017, 595, 3753–3763. [Google Scholar] [CrossRef]
- Frade, A.F.; Guerin, H.; Nunes, J.P.S.; Silva, L.; Roda, V.M.P.; Madeira, R.P.; Brochet, P.; Andrieux, P.; Kalil, J.; Chevillard, C.; et al. Cardiac and Digestive Forms of Chagas Disease: An Update on Pathogenesis, Genetics, and Therapeutic Targets. Mediat. Inflamm. 2025, 2025, 8862004. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.H.; Yao, B.C.; Chen, Q.L.; Jiang, N.; Wang, L.Q.; Guo, Z.G. NDUFB11 and NDUFS3 play a role in atherosclerosis and chronic stress. Aging 2023, 15, 8026–8043. [Google Scholar] [CrossRef]
- Nagajyothi, J.F.; Weiss, L.M. Advances in understanding the role of adipose tissue and mitochondrial oxidative stress in Trypanosoma cruzi infection. F1000Research 2019, 8, 1152. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.K. OXPHOS mediators in acute myeloid leukemia patients: Prognostic biomarkers and therapeutic targets for personalized medicine. World J. Surg. Oncol. 2024, 22, 298. [Google Scholar] [CrossRef] [PubMed]
- Elkenani, M.; Barallobre-Barreiro, J.; Schnelle, M.; Mohamed, B.A.; Beuthner, B.E.; Jacob, C.F.; Paul, N.B.; Yin, X.; Theofilatos, K.; Fischer, A.; et al. Cellular and extracellular proteomic profiling of paradoxical low-flow low-gradient aortic stenosis myocardium. Front. Cardiovasc. Med. 2024, 11, 1398114. [Google Scholar] [CrossRef]
- Chen, F.; Bai, J.; Zhong, S.; Zhang, R.; Zhang, X.; Xu, Y.; Zhao, M.; Zhao, C.; Zhou, Z. Molecular Signatures of Mitochondrial Complexes Involved in Alzheimer’s Disease via Oxidative Phosphorylation and Retrograde Endocannabinoid Signaling Pathways. Oxidative Med. Cell Longev. 2022, 2022, 9565545. [Google Scholar] [CrossRef]
- Alvim, J.M.; Venturini, G.; Oliveira, T.G.M.; Seidman, J.G.; Seidman, C.E.; Krieger, J.E.; Pereira, A.C. mTOR signaling inhibition decreases lysosome migration and impairs the success of Trypanosoma cruzi infection and replication in cardiomyocytes. Acta Trop. 2023, 240, 106845. [Google Scholar] [CrossRef] [PubMed]
- Nàger, M.; Calvoli, M.; Larsen, K.B.; Birgisdottir, A.B. The multifaceted role of autophagy and mitophagy in cardiovascular health and disease. Autophagy Rep. 2025, 4, 2572511. [Google Scholar] [CrossRef]
- Piquereau, J.; Veksler, V.; Novotova, M.; Ventura-Clapier, R. Energetic Interactions Between Subcellular Organelles in Striated Muscles. Front. Cell Dev. Biol. 2020, 8, 581045. [Google Scholar] [CrossRef]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef]
- Social Science Statistics. Available online: https://www.socscistatistics.com/pvalues/pearsondistribution.aspx (accessed on 1 September 2025).
- Ahmad, B.; Dumbuya, J.S.; Li, W.; Tang, J.X.; Chen, X.; Lu, J. Evaluation of GFM1 mutations pathogenicity through in silico tools, RNA sequencing and mitophagy pahtway in GFM1 knockout cells. Int. J. Biol. Macromol. 2025, 304 Pt 2, 140970, Erratum in Int. J. Biol. Macromol. 2025, 318 Pt 1, 145547. [Google Scholar] [CrossRef] [PubMed]
- Lemeshko, V.V. Mechanism of Na+ ions contribution to the generation and maintenance of a high inner membrane potential in mitochondria. Biochim. Biophys. Acta Bioenerg. 2025, 1867, 149571. [Google Scholar] [CrossRef] [PubMed]
- Fedor, J.G.; Jones, A.J.Y.; Di Luca, A.; Kaila, V.R.I.; Hirst, J. Correlating kinetic and structural data on ubiquinone binding and reduction by respiratory complex I. Proc. Natl. Acad. Sci. USA 2017, 114, 12737–12742. [Google Scholar] [CrossRef]
- Galemou Yoga, E.; Haapanen, O.; Wittig, I.; Siegmund, K.; Sharma, V.; Zickermann, V. Mutations in a conserved loop in the PSST subunit of respiratory complex I affect ubiquinone binding and dynamics. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 573–581. [Google Scholar] [CrossRef]
- Röpke, M.; Saura, P.; Riepl, D.; Pöverlein, M.C.; Kaila, V.R.I. Functional Water Wires Catalyze Long-Range Proton Pumping in the Mammalian Respiratory Complex I. J. Am. Chem. Soc. 2020, 142, 21758–21766. [Google Scholar] [CrossRef]
- Chen, P.; Yawar, W.; Farooqui, A.R.; Ali, S.; Lathiya, N.; Ghous, Z.; Sultan, R.; Alhomrani, M.; Alghamdi, S.A.; Almalki, A.A.; et al. Transcriptomics data integration and analysis to uncover hallmark genes in hypertrophic cardiomyopathy. Am. J. Transl. Res. 2024, 16, 637–653. [Google Scholar] [CrossRef]
- Iacobas, S.; Ede, N.; Iacobas, D.A. The Gene Master Regulators (GMR) Approach Provides Legitimate Targets for Personalized, Time-Sensitive Cancer Gene Therapy. Genes 2019, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.; Lima, R.S.; Rocha, L.L.; Takyia, C.M.; Pontes-de-Carvalho, L.; de Carvalho, A.C.; Ribeiro-dos-Santos, R. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. Am. J. Pathol. 2004, 164, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Jasmin; Jelicks, L.A.; Tanowitz, H.B.; Peters, V.M.; Mendez-Otero, R.; de Carvalho, A.C.C.; Spray, D.C. Molecular imaging, biodistribution and efficacy of mesenchymal bone marrow cell therapy in a mouse model of Chagas disease. Microbes Infect. 2014, 16, 923–935. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, H.; Liu, H.; Xu, M.; Runyan, R.B.; Eisenberg, C.A.; Markwald, R.R.; Borg, T.K.; Gao, B.Z. Mesenchymal stem cell-cardiomyocyte interactions under defined contact modes on laser-patterned biochips. PLoS ONE 2013, 8, e56554. [Google Scholar] [CrossRef]
- He, L.; Nguyen, N.B.; Ardehali, R.; Zhou, B. Heart Regeneration by Endogenous Stem Cells and Cardiomyocyte Proliferation: Controversy, Fallacy, and Progress. Circulation 2020, 142, 275–291. [Google Scholar] [CrossRef]
- Mello, D.B.; Mesquita, F.C.P.; Silva dos Santos, D.; Asensi, K.D.; Dias, M.L.; Campos de Carvalho, A.C.; Goldenberg, R.C.d.S.; Kasai-Brunswick, T.H. Mesenchymal Stromal Cell-Based Products: Challenges and Clinical Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 6063. [Google Scholar] [CrossRef]
- Carvalho, A.B.; Kasai-Brunswick, T.H.; Campos de Carvalho, A.C. Advanced cell and gene therapies in cardiology. EBioMedicine 2024, 103, 105125. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Obiomon, E.A.; Iacobas, S. Genomic Fabrics of the Excretory System’s Functional Pathways Remodeled in Clear Cell Renal Cell Carcinoma. Curr. Issues Mol. Biol. 2023, 45, 9471–9499. [Google Scholar] [CrossRef] [PubMed]

| The Largest Contributors to the Mitochondrial Transcriptome Alteration in CCC Mice | ||||||||
| Gene | Description | AVE | X-IN | p | |WIR| | X-IT | p | |WIR| |
| Cox4i1 | Cytochrome c oxidase subunit IV isoform 1 | 165 | −1.69 | 0.02 | 111 | −2.05 | 0.45 | 95 |
| Sdha | Succinate dehydrogenase complex, subunit A, flavoprotein | 61 | −2.49 | 0.00 | 91 | −1.71 | 0.16 | 36 |
| Atp5pb | ATP synthase peripheral stalk-membrane subunit b | 62 | −2.35 | 0.00 | 83 | −1.47 | 0.27 | 21 |
| Ndufa4 | Mlrq-like protein | 105 | −1.78 | 0.09 | 76 | −1.46 | 0.27 | 35 |
| Ndufs1 | NADH dehydrogenase (ubiquinone) Fe-S protein 1 | 34 | −3.02 | 0.01 | 68 | −2.20 | 0.05 | 39 |
| Average mitochondrial |WIR| | 54 | 49 | ||||||
| The largest overall contributors to the entire transcriptome alteration in CCC mice | ||||||||
| Pln | Phospholamban | 148 | −2.64 | 0.09 | 222 | −2.65 | 0.31 | 167 |
| Overall average |WIR| | 2.24 | 1.15 | ||||||
| The Largest Contributors to the Mitochondrial Transcriptome Alteration in IHF Mice | ||||||||
| Gene | Description | AVE | X-IN | p | |WIR| | X-IT | p | |WIR| |
| Cox5a | Cytochrome c oxidase, subunit Va | 49 | −59.69 | 0.09 | 2603 | −2.29 | 0.28 | 45 |
| Uqcrh | Ubiquinol-cytochrome c reductase hinge protein | 59 | −2.64 | 0.02 | 94 | −1.94 | 0.03 | 54 |
| Cox7a2 | Cytochrome c oxidase, subunit VIIa 2 | 49 | −2.67 | 0.00 | 81 | −1.20 | 0.16 | 8 |
| Cox6b1 | Cytochrome c oxidase, subunit VIb polypeptide 1 | 49 | −2.66 | 0.00 | 80 | −1.09 | 0.48 | 2 |
| Cox7b | Cytochrome c oxidase subunit VIIb | 64 | −2.28 | 0.03 | 80 | −1.07 | 0.69 | 1 |
| Average mitochondrial |WIR| | 69 | 12 | ||||||
| The largest overall contributors to the entire transcriptome alteration in IHF mice | ||||||||
| Cox5a | Cytochrome c oxidase, subunit Va | 49 | −59.69 | 0.09 | 2603 | −2.29 | 0.28 | 45 |
| Overall average |WIR| | 1.63 | 0.70 | ||||||
| The Most and the Least Controlled Mito Genes in CCC Mice | RCS-FC | |||||
| Gene | Description | CN | IN | IT | IN | IT |
| Ndufa10 | NADH:ubiquinone oxidoreductase subunit A10 | 3.86 | −1.17 | −0.64 | −32.80 | −22.70 |
| Cox7b | Cytochrome c oxidase subunit VIIb | 2.59 | −0.42 | −1.59 | −8.05 | −18.12 |
| Ndufb10 | NADH:ubiquinone oxidoreductase subunit B10 | 2.15 | −0.95 | −0.68 | −8.56 | −7.13 |
| Vdac2 | Voltage-dependent anion channel 2 | 1.06 | 2.59 | −0.63 | 2.89 | −3.23 |
| Mpc2 | Mitochondrial pyruvate carrier 2 | 0.32 | 1.54 | 0.68 | 2.34 | 1.28 |
| Cox4nb | COX4 neighbor | 0.81 | 1.28 | 0.98 | 1.38 | 1.12 |
| Ndufb5 | NADH:ubiquinone oxidoreductase subunit B5 | 0.43 | −0.53 | 1.66 | −1.95 | 2.33 |
| Ndufb11 | NADH:ubiquinone oxidoreductase subunit B11 | 0.99 | 0.54 | 1.62 | −1.36 | 1.55 |
| Ndufaf4 | NADH:ubiquinone oxidoreductase subunit A4 | 0.29 | −0.92 | 1.37 | −2.32 | 2.11 |
| Sdhd | Succinate dehydrogenase complex, subunit D, integral membrane protein | −1.87 | −0.32 | −0.30 | 2.92 | 2.96 |
| Cox5a | Cytochrome c oxidase, subunit Va | −1.58 | 1.17 | −2.53 | 6.69 | −1.94 |
| Ndufa9 | NADH:ubiquinone oxidoreductase subunit A9 | −1.26 | −2.64 | −0.32 | −2.60 | 1.93 |
| Cpt2 | Carnitine palmitoyltransferase 2 | 0.53 | −1.67 | 0.21 | −4.60 | −1.25 |
| Pdk2 | Pyruvate dehydrogenase kinase, isoenzyme 2 | 0.60 | −1.43 | −0.67 | −4.09 | −2.41 |
| Ppif | Peptidylprolyl isomerase F (cyclophilin F) | 0.57 | −1.41 | −0.56 | −3.94 | −2.18 |
| Cox7b | Cytochrome c oxidase subunit VIIb | 2.59 | −0.42 | −1.59 | −8.05 | −18.12 |
| Atp5pf | ATP synthase peripheral stalk subunit F6 | 1.52 | −0.81 | −1.58 | −5.01 | −8.53 |
| Cox4i1 | Cytochrome c oxidase subunit IV isoform 1 | 0.66 | −0.20 | −1.56 | −1.81 | −4.65 |
| The Most and the Least Controlled Mito Genes in IHF Mice | RCS-FC | |||||
| Cox6b1 | Cytochrome c oxidase, subunit VIb polypeptide 1 | 1.36 | 4.38 | 0.05 | 8.13 | −2.48 |
| Atp5f1e | ATP synthase F1 subunit epsilon | −0.79 | 2.68 | 0.68 | 11.07 | 2.77 |
| Atp5mc2 | ATP synthase membrane subunit c locus 2 | 0.52 | 1.75 | −0.37 | 2.35 | −1.86 |
| Ndufb11 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 11 | 0.74 | 0.87 | 2.38 | 1.10 | 3.11 |
| Ndufv1 | NADH dehydrogenase (ubiquinone) flavoprotein 1 | −1.14 | −0.97 | 1.79 | 1.12 | 7.58 |
| Cpt1b | Carnitine palmitoyltransferase 1b, muscle | 0.16 | −1.42 | 1.64 | −2.99 | 2.79 |
| Ndufa9 | NADH:ubiquinone oxidoreductase subunit A9 | −1.26 | −2.64 | −0.32 | −2.60 | 1.93 |
| Cyc1 | Cytochrome c-1 | −1.05 | −1.67 | 0.93 | −1.54 | 3.95 |
| Ndufa11 | NADH:ubiquinone oxidoreductase subunit A11 | −0.36 | −1.58 | −0.29 | −2.34 | 1.04 |
| Cox5a | Cytochrome c oxidase, subunit Va | −1.58 | 1.17 | −2.53 | 6.69 | −1.94 |
| Mpc1 | Mitochondrial pyruvate carrier 1 | −1.05 | −0.92 | −1.71 | 1.10 | −1.58 |
| Ndufc2 | NADH:ubiquinone oxidoreductase subunit C2 | 0.38 | −0.30 | −1.52 | −1.61 | −3.75 |
| The most controlled genes in the entire transcriptome in CN and IHF mice | RCS-FC | |||||
| Tmem186 | Transmembrane protein 186 | 5.19 | −0.10 | 0.70 | −39.13 | −22.50 |
| Cd164 | CD164 antigen | 0.09 | 5.28 | 0.57 | 36.56 | 1.40 |
| Atp13a2 | ATPase type 13A2 | 0.66 | −0.03 | 4.55 | −1.61 | 14.82 |
| The least controlled genes in the entire transcriptome in CN and IHF mice | RCS-FC | |||||
| Gmcl1 | Germ cell-less homolog 1 (Drosophila) | −2.40 | −0.33 | −2.50 | 4.19 | −1.07 |
| Idh3g | Isocitrate dehydrogenase 3 (NAD+), gamma | −1.10 | −2.83 | 0.80 | −3.32 | 3.72 |
| Tsc22d4 | TSC22 domain family, member 4 | 0.98 | 0.25 | −2.91 | −1.67 | −14.86 |
| Most Prominent Mito Genes in CCC Mice | GCH | GCH-FC | |||
| GENE | Description | IN | IT | IN | IT |
| Cox4i2 | cytochrome c oxidase subunit 4I2 | 13.20 | 17.02 | 8.74 | 11.27 |
| Ndufb7 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 7 | 11.73 | 17.82 | 14.09 | 21.42 |
| Cox6b1 | Cytochrome c oxidase, subunit VIb polypeptide 1 | 11.48 | 17.52 | 4.81 | 7.34 |
| Uqcrh | Ubiquinol-cytochrome c reductase hinge protein | 11.21 | 16.07 | 4.66 | 6.67 |
| Ndufs4 | NADH dehydrogenase (ubiquinone) Fe-S protein 4 | 11.00 | 12.23 | 4.66 | 5.18 |
| Ndufa7 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 7 | 7.48 | 19.80 | 1.42 | 3.77 |
| Ndufc1 | NADH:ubiquinone oxidoreductase subunit C1 | 4.60 | 19.71 | −1.18 | 3.63 |
| Ndufa2 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2 | 6.54 | 19.70 | 7.64 | 23.01 |
| Ndufa10 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 10 | 9.54 | 19.57 | 1.71 | 3.52 |
| Uqcrb | Ubiquinol-cytochrome c reductase binding protein | 9.95 | 19.55 | 2.44 | 4.80 |
| Most Prominent Mito Genes in IHF Mice | GCH | GCH-FC | |||
| Cox6b1 | Cytochrome c oxidase, subunit VIb polypeptide 1 | 24.16 | 2.08 | 5.53 | −2.10 |
| Atp5f1e | ATP synthase F1 subunit epsilon | 9.01 | 2.00 | 3.70 | −1.22 |
| Atp5mc2 | ATP synthase membrane subunit c locus 2 | 5.13 | 1.12 | 1.88 | −2.44 |
| Ndufs5 | NADH dehydrogenase (ubiquinone) Fe-S protein 5 | 4.22 | 1.40 | 1.13 | −2.65 |
| Ndufa1 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 1 | 4.21 | 3.60 | 1.38 | 1.18 |
| Ndufb11 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 11 | 3.01 | 6.39 | −1.90 | 1.12 |
| Ndufs4 | NADH dehydrogenase (ubiquinone) Fe-S protein 4 | 0.80 | 5.92 | −9.72 | −1.31 |
| Ndufv1 | NADH dehydrogenase (ubiquinone) flavoprotein 1 | 0.72 | 4.48 | −3.68 | 1.68 |
| Cyc1 | Cytochrome c-1 | 0.52 | 4.26 | −4.69 | 1.73 |
| Ndufa1 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 1 | 4.21 | 3.60 | 1.38 | 1.18 |
| Most Prominent Mito Genes in Healthy Mice | GCH | CCC | IHF | ||
| Ndufb10 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex, 10 | 13.07 | 7.21 | 2.26 | |
| Ndufa10 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 10 | 9.79 | 9.54 | 1.01 | |
| Uqcrb | Ubiquinol-cytochrome c reductase binding protein | 9.69 | 9.95 | 2.91 | |
| Ndufaf1 | NADH:ubiquinone oxidoreductase complex assembly factor 1 | 8.14 | 8.31 | 1.67 | |
| Ndufs4 | NADH dehydrogenase (ubiquinone) Fe-S protein 4 | 7.76 | 11.00 | 0.80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobas, D.A.; Manzoor, S.; Daniels, D.; Iacobas, S.; Xi, L. Mitochondrial Collapse Responsible for Chagasic and Post-Ischemic Heart Failure Is Reversed by Cell Therapy Under Different Transcriptomic Topologies. Curr. Issues Mol. Biol. 2025, 47, 940. https://doi.org/10.3390/cimb47110940
Iacobas DA, Manzoor S, Daniels D, Iacobas S, Xi L. Mitochondrial Collapse Responsible for Chagasic and Post-Ischemic Heart Failure Is Reversed by Cell Therapy Under Different Transcriptomic Topologies. Current Issues in Molecular Biology. 2025; 47(11):940. https://doi.org/10.3390/cimb47110940
Chicago/Turabian StyleIacobas, Dumitru A., Shavaiz Manzoor, Dennis Daniels, Sanda Iacobas, and Lei Xi. 2025. "Mitochondrial Collapse Responsible for Chagasic and Post-Ischemic Heart Failure Is Reversed by Cell Therapy Under Different Transcriptomic Topologies" Current Issues in Molecular Biology 47, no. 11: 940. https://doi.org/10.3390/cimb47110940
APA StyleIacobas, D. A., Manzoor, S., Daniels, D., Iacobas, S., & Xi, L. (2025). Mitochondrial Collapse Responsible for Chagasic and Post-Ischemic Heart Failure Is Reversed by Cell Therapy Under Different Transcriptomic Topologies. Current Issues in Molecular Biology, 47(11), 940. https://doi.org/10.3390/cimb47110940







