Abstract
Oral squamous cell carcinoma (OSCC) remains one of the most prevalent and aggressive malignancies worldwide, with late diagnosis contributing to poor survival rates. Recent evidence suggests that periodontitis may act as a co-factor in development of OSCC through persistent inflammation, microbial dysbiosis, and subsequent tissue remodeling. Identifying molecular signatures that link periodontitis with early oral cancerization is therefore of paramount importance for risk assessment, prevention, and timely intervention. This narrative review aims to provide an integrative overview of the current knowledge on molecular, genetic, and epigenetic biomarkers associated with oral cancer risk in patients with periodontitis. Specifically, periodontal pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum promote oral cancerization by modulating molecular, genetic, and epigenetic pathways, including p53, Cyclin D1, Ki-67, p16INK4A, DNA methylation, histone modifications, and microRNA regulation. Therefore, this review provides a discussion about the role of inflammatory mediators, oxidative stress-related molecules, microbial-derived products, genetic markers and epigenetic mechanisms as early molecular signals of malignant transformation. The study of these salivary biomarkers (salivaomics) has emerged as a promising non-invasive diagnostic tool, although variability in sampling, biomarker stability, and confounding factors such as coexisting periodontal disease remain significant limitations. By synthesizing the available evidence, this review summarizes recent evidence linking periodontitis to oral cancerization, highlights potential salivary, proteomic, and inflammatory biomarkers, and considers the role of periodontal therapy in improving inflammatory profiles and modulating tumor-related biomarkers. Finally, it explores future perspectives, including the integration of Artificial Intelligence (AI) to enhance biomarker-based diagnosis and risk stratification in OSCC patients.
1. Introduction
Periodontitis is a chronic, multifactorial inflammatory disease mediated by the host immune response to bacterial colonization of the periodontium and related dysbiosis, which, if not preventively treated, can lead to periodontal tissue destruction and tooth loss [1]. Periodontitis is the 6th most prevalent disease worldwide, with moderate forms affecting approximately 50% of the global population [2]. However, persistent inflammation, coupled with the release of tissue degradation products and nutrients, fosters the overgrowth of highly pathogenic bacterial species and leads to clinical signs of periodontal attachment loss [3]. During periodontitis, the bacteria present in the periodontal pocket can also spread out in the bloodstream, resulting in bacteremia and the onset or exacerbation of preexisting inflammatory conditions [2]. This, together with the establishment of a systemic pro-inflammatory state, may underlie the association between periodontitis and various systemic diseases—a topic extensively debated in the literature, particularly over the last few decades [2,4]. Among principal systemic implications are cardiovascular, cerebrovascular, neurological, metabolic, and oncologic disorders [5,6,7,8,9]. Although the precise mechanisms driving carcinogenesis remain incompletely understood, numerous studies have supported a link between periodontitis and several cancer types, including head and neck cancers as well as colorectal, breast, lung and prostate cancers [6,10,11].
Among head and neck squamous cell carcinoma (HNSCC), oral squamous cell carcinoma (OSCC) accounts for approximately 90% of head and neck malignancies and is the sixth most common cancer worldwide, associated with high morbidity, mortality, and recurrence rates [12]. Since 1990, the incidence and the prevalence of oral and lip cancer have increased significantly (by +161.8% and +142.18%, respectively), and projections for 2050 reflect a further rise, with a substantial global impact [13].
Oral hygiene and the composition of the oral microbiota also play a critical role in the risk of developing OSCC. Indeed, patients with OSCC have been shown to exhibit poorer oral hygiene and more advanced periodontitis compared to healthy individuals [14]. Ecological changes affecting the oral microbiome during periodontitis are responsible for the onset of a localized inflammatory state in the head and neck region, particularly within the oral cavity. Nevertheless, the potential for a reverse causal relationship between the two conditions must be considered. Oral squamous cell carcinoma (OSCC), through the induction of pain during toothbrushing, may adversely affect oral hygiene practices. This impairment may facilitate biofilm accumulation, initiate an inflammatory response, and ultimately promote the onset of periodontitis [15].
Although numerous studies have demonstrated an association between periodontitis and oral cancer, it is important to note that such evidence does not establish a definitive causal relationship between the two conditions. One of the main reasons why a clear causal link between periodontitis and oral cancer cannot be asserted is the substantial difference in their respective prevalence rates [16].
The pathogenesis of cancer, as well as the malignant transformation of oral potentially malignant disorders (OPMDs), is strongly associated with the persistence of a chronic inflammatory state, as first demonstrated by Virchow in 1863 through the identification of a lymphoreticular infiltrate within neoplastic tissues [17,18,19,20]. Indeed, the literature supports that prolonged inflammation accounts for approximately 15–20% of malignant tumors [21]. Inflammatory cells and cytokines contribute to the establishment of a favorable tumor microenvironment (TME), which plays a crucial role in cell survival, growth, and proliferation, as well as in the onset of genetic and epigenetic alterations [20]. The mechanisms by which periodontitis and oral bacteria contribute to OSCC development are diverse and include the disruption of epithelial barrier integrity through the infiltration of periodontal pathogens, the induction of persistent inflammation, modulation of signaling pathways, promotion of invasion and metastasis, and alteration of host immune responses [22,23,24]. In this context, periodontopathogenic bacteria exert their effects through a dual mechanism: indirectly, by inducing and perpetuating the immune response; and directly, through specific virulence factors and bacterial metabolic products. The recognition of pathogen-associated molecular patterns (PAMPs) by Toll-like receptors (TLRs) triggers the release of a cascade of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) and the recruitment of neutrophils, leading to the initiation of the immune response, followed by complement activation and adaptive immunity [25,26]. The chronicity of inflammation is sustained by the persistence of risk factors, such as dysbiosis and immune dysregulation, which lead to cellular proliferation, inhibition of apoptosis, and DNA damage—features commonly implicated in the pathogenesis of various non-communicable diseases, including OSCC [22,27].
Moreover, individual periodontal pathogens are characterized by distinct pathogenic factors that modulate apoptotic pathways, DNA repair mechanisms, and immune evasion. The most prevalent microorganisms in OSCC, Porphyromonas gingivalis and Fusobacterium nucleatum, exhibit high pathogenic potential and contribute to tumorigenesis through molecular mechanisms involved in cancer initiation, progression, and tumor dissemination [12]. Recent studies have shown increasing interest in tissue-resident microorganisms and their role in modulating inflammatory cytokines and factors involved in immune evasion [24].
Indeed, P. gingivalis and F. nucleatum exhibit multiple virulence determinants, including lipopolysaccharide (LPS) for immune recognition, toxins (gingipains) mediating tissue damage, and fimbriae and adhesins (FadA) facilitating adhesion and colonization [6,17,28,29]. Oral dysbiosis thus represents a substantial cofactor in cancer development, acting synergistically with other well-established risk factors such as tobacco and alcohol consumption [26,30,31]. Furthermore, periodontal pathogens induce significant alterations in molecular and epigenetic biomarkers implicated in carcinogenesis [29].
The diagnosis of OSCC is often delayed and this condition is mostly detected at its final stages (III or IV), which decreases the survival rate and leads to a noticeable decline in quality of life [32,33,34]. According to Carreras-Torres et al., the 5-year survival rate for OSCC has remained around 50% over the past decades [35]. A systematic review by Grafton Clarke et al. reported that survival is markedly higher in early-stage disease (>80%) but falls below 30% in advanced cases. Diagnostic delay is often attributable to two levels: the patient level and the healthcare professional level [36].
Although tissue biopsy remains the gold standard for diagnosing OSCC, there has been a significant transition from histopathological to molecular diagnostic approaches [37]. This shift is driven both by enhanced understanding of the molecular mechanisms underlying carcinogenesis and by the advent of advanced analytical technologies [38]. In recent years, emerging biomarkers have been identified for the stratification of malignant transformation risk, including alterations in the oral bacteriome and other molecular signatures within the TME [30]. Identifying OSCC biomarkers can help prevent oral cancer [37]. In fact, they play a central role in early diagnosis, which is often key to effectively treating the disease and intervening at a stage when it is more responsive to treatment [39].
Despite advances in the study and identification of novel salivary biomarkers for non-invasive OSCC diagnosis, their clinical application still faces considerable limitations. Moreover, only a few studies have investigated biomarkers common to both periodontitis and cancer. The main challenges of salivaomics include variability in salivary composition, the sensitivity and specificity of individual biomarkers, technical requirements and high costs, as well as the presence of confounding factors [40,41,42]. Importantly, periodontitis itself is regarded as a confounding factor in OSCC biomarker research, since the levels of certain markers may be affected by its presence [43]. This underscores the urgency of conducting broader studies that account for concomitant inflammatory conditions and periodontal status.
In this context, the present review aims to illustrate and discuss recent evidence regarding molecular, genetic, and epigenetic biomarkers as early risk factors for oral carcinogenesis in patients with periodontitis.
2. Periodontal Inflammation and Risk Biomarkers for Oral Cancerization
Assessing the risk of oral cancerization in periodontitis patients typically involves the use of molecular and genetic biomarkers, epigenetic modifications, and emerging novel biomarkers [44,45]. Table 1 summarizes changes induced by periodontal bacteria on the main targets.

Table 1.
Changes induced by periodontal bacteria.
Table 1.
Changes induced by periodontal bacteria.
| Periodontal Bacteria | Target | Activity Changes | Effects on Tumorigenesis |
|---|---|---|---|
| P. gingivalis | P53 | Reduced ↓ [46,47] | Increased proliferation of malignant cells, cellular senescence and DNA damage [48] |
| P. gingivalis/F. nucleatum | Cyclin D1 | Increased ↑ [47,49,50] | Increased cell proliferation [51] |
| Periodontal bacteria | Ki-67 | Increased ↑ [28,52,53] | Increased cell proliferation [54] |
| F. nucleatum | p16INK4A | Increased ↑ [55] | Increased cell proliferation and cellular senescence [56,57] |
| F. nucleatum/P. gingivalis | Histone H2A phosphorylation | Increased ↑ [6,58] | Increased cell proliferation [58] |
| P. gingivalis | Promoter hypermethylation | Increased ↑ [47] | Increased cell proliferation [59] |
| P. gingivalis | DNA hypomethylation | Increased ↑ [47,60] | Increased cell proliferation [47,60] |
| P. gingivalis | miR-125a | Reduced ↓ [61] | Increased cell proliferation [61] |
| P. gingivalis | miR-200a | Reduced ↓ [61] | Increased cell proliferation [61] |
| P. gingivalis | miR-21 | Increased ↑ [47] | Increased cell proliferation |
| Periodontal bacteria | hsa-miR-224 | Increased ↑ [62] | Increased cell proliferation [62] |
| Periodontal bacteria | hsa-miR-210 | Increased ↑ [62] | Increased cell proliferation [62] |
| Periodontal bacteria | hsa-miR-31 | Increased ↑ [62] | Increased cell proliferation [62] |
| Periodontal bacteria | hsa-miR-497 | Reduced ↓ [62] | Increased cell proliferation [62] |
| Periodontal bacteria | hsa-miR-29c | Reduced ↓ [62] | Increased cell proliferation [62] |
| Periodontal bacteria | hsa-miR-486 | Reduced ↓ [62] | Increased cell proliferation [62] |
| Periodontal bacteria | miR-19b-3b | Increased ↑ [58] | Increased cell proliferation [26] |
| Periodontal bacteria | miR-181b-2-3p | Controversial ↑ [63] | Increased cell proliferation [63] |
| Periodontal bacteria | miR-495-3p | Controversial ↑ [64] | Increased cell proliferation [64] |
| P. gingivalis | MMP-1 | Increased ↑ [65] | Extracellular matrix degradation, tumor cell invasion, and metastasis [65] |
| P. gingivalis | MMP-3 | Increased ↑ [65] | Increased cell proliferation, tumor cell invasion and metastasis [65] |
| Periodontal bacteria | MMP-8 | Increased ↑ [65] | Controversial role [65] |
| Periodontal bacteria | MMP-12 | Increased ↑ [58] | Increased cell proliferation, tumor cell invasion [65] |
| F. nucleatum | MMP-13 | Increased ↑ [58] | Increased cell proliferation, tumor cell invasion and metastasis [65] |
| Periodontal bacteria | NETs | Increased ↑ [58] | Extracellular matrix degradation, tumor cell adhesion, invasion, and metastasis [66] |
| Periodontal bacteria | IL-1β and IL-8 | Increased ↑ [58] | Increased cell proliferation and angiogenesis [19,67] |
| P. gingivalis | IL-6 | Increased ↑ [19,67] | Increased cell proliferation and angiogenesis [19,67] |
2.1. Molecular Biomarkers
Periodontitis and oral preneoplastic and neoplastic disorders can share several molecular biomarkers, including proteins and mediators implicated in the proliferation of cancer cells. Alterations in the levels of these biomarkers have been observed in patients with periodontitis and OSCC [46,47].
The p53 protein is a transcription factor encoded by the tumor-suppressor gene TP53, which plays a crucial role in genome defense [12]. It involves aging phenomena and senescence, cellular differentiation, cell cycle regulation, apoptosis, and DNA repair (Figure 1). Mutations in the encoding gene (due to deletion or demethylation) are implicated in the development of various malignancies, including OSCC [29,46]. Chattopadhyay et al. reported the presence of TP53 mutations in exon 4, at codon 63, in the saliva of patients with OSCC, as detected through circulating tumor DNA (ctDNA) analysis [59]. Mutations affecting TP53 have been linked to OSCC, particularly in the early stages of tumor development [68]. Specifically, a study by Jagadeesan et al. found that approximately 80% of oral carcinomas exhibit a TP53 mutation, and 10% of dysplasia cases too [12]. According to Radaic et al., TP53 mutation represents one of the most frequent alterations in oral carcinoma, with a frequency of 75–85% in HPV-negative HNSCC cases [44,69]. Furthermore, the study by Starska-Kowarska, comparing salivary samples from healthy individuals and OSCC patients, confirmed the high prevalence of TP53 mutations (93.3%) in cancer cases compared to controls [68]. P53 regulates the expression of p16INK4a, CDKN2A (cyclin-dependent kinase inhibitor 2A), and p52, and its inactivation prevents cell cycle arrest in the G1 phase, thereby allowing the proliferation of potentially malignant cells. [48]. Additionally, it may impair the ability of cells to repair damaged DNA, predisposing them to further mutations. Studies show how some periodontal pathogens affect p53 activity, resulting in an increased predisposition to carcinogenesis and the malignant transformation of (OPMDs). For example, P. gingivalis modulates cell cycle regulation by reducing both the levels and activity of p53 in gingival epithelial cells, an effect induced by the activation of the PI3K/Akt pathway [46,47] (Table 1). Furthermore, LPS from P. gingivalis induces senescence in human gingival fibroblasts (HGFs) by increasing p53 concentration [70,71]. The results suggest a potential discrepancy in the effects exerted by P. gingivalis on gingival epithelial cells and fibroblasts. Nevertheless, these observations are consistent with recent evidence indicating that P. gingivalis can employ distinct strategies to enhance host cell survival, while simultaneously modulating multiple anti-apoptotic signaling pathways [72]. Previous studies have demonstrated that the ability of P. gingivalis to elicit either pro- or anti-apoptotic responses is tightly dependent on the specific host cell type and/or the presence of particular bacterial components, such as cysteine proteinases or LPS [72]. Abnormalities in p53 within fibroblasts have been associated with increased tumor aggressiveness, metastasis, and poor response to therapy [73]. Several studies further suggest that p53 may represent a key factor linking periodontal inflammation to carcinogenesis. Indeed, p53 is involved in the antioxidant response, and its levels are influenced by hypoxia and the presence of reactive oxygen species (ROS) [71]. F. nucleatum has also been implicated in regulating cell survival and proliferation mechanisms through the Ku70/p53 pathway [47]. In addition, F. nucleatum induces DNA damage and downregulation of the TP53 gene, thus predisposing cells to proliferation [21].
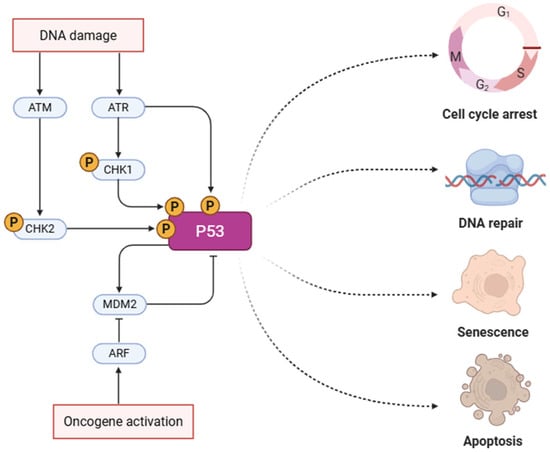
Figure 1.
P53 pathway in oncogenesis. This pathway highlights the activation of p53 in response to DNA damage or oncogene activation. DNA damage activates ATM/ATR kinases, which phosphorylate CHK1/CHK2 and subsequently p53. Stabilized p53 directs the cell toward one of several outcomes: cell cycle arrest, DNA repair, senescence, or apoptosis, depending on the context and damage severity [74]. Created in https://BioRender.com, accessed on 24 May 2025.
Cyclin D1 is a cell cycle regulatory protein that facilitates the G1-to-S phase transition by binding to CDK4 and CDK6, implicating it in uncontrolled cell proliferation in various tumors [51]. Elevated levels of Cyclin D1 are also associated with a poor prognosis, making it a potential prognostic factor for OSCC when used in conjunction with other biomarkers. Its overexpression has been associated with OSCC and is induced through signaling pathways mediated by NF-kB, STAT3, and TLR2 [28]. Several studies have reported a link between periodontal pathogens and Cyclin D1 levels. The review by Lafuente Ibáñez Mendoza et al. demonstrated that in murine OSCC models, P. gingivalis infection leads to an increase in Cyclin D1 [28]. P. gingivalis infection induces inflammatory cytokines (e.g., IL-6), activating NF-kB and STAT3, which in turn upregulate Cyclin D1 [28,75,76].
Similarly, P. gingivalis can raise Cyclin D1 levels through a negative feedback mechanism on the miR-21/PDCD4/AP-1 pathway [47] (Table 1). F. nucleatum infection has been associated with elevated Cyclin D1 activity in OSCC, accelerating cell cycle progression and contributing to increased cell proliferation [49,50] (Table 1). Moreover, the adhesin FadA of F. nucleatum promotes colorectal cancer oncogenesis through the expression of annexin 1 (ANXA1) and Cyclin D1 [77,78].
Ki-67 is a nuclear protein involved in the active phases of the cell cycle and cellular proliferation and is expressed in all proliferating cells [54]. Its overexpression has been associated with inflammatory states and increased invasiveness in various cancers, including but not limited to OSCC (Table 1). Several studies have recognized its role as both a diagnostic and prognostic biomarker. Under physiological conditions, Ki-67-positive cells are confined to the basal layer of the epithelium; however, in potentially malignant and malignant lesions, its expression extends above the basal layer and is significantly increased [76]. Furthermore, Ki-67 may serve as a predictive marker for treatment response. When assessed in combination with p16INK4A, it has proven to be a more reliable and valid biomarker compared to p16 alone. In particular, OSCC tumors characterized by low Ki-67 expression and high p16 levels (p16+/Mib−) demonstrated superior overall survival rates (83% at 5 years) and improved disease-free survival compared to other subgroups, such as p16+/Mib+ tumors (25% at 5 years) [49]. However, according to Jagadeesan et al., when evaluated in isolation, Ki-67 exhibits a weaker correlation with OSCC compared to other biomarkers [12]. Salivary Ki-67 has been detected at high concentrations in inflammatory conditions and has been positively correlated with the severity of periodontitis [76]. The association between periodontal inflammation and Ki-67 levels may be explained by the presence of inflammatory mediators and signaling factors that contribute to the establishment of a pro-tumorigenic microenvironment in response to periodontal pathogens. These factors—including platelet-derived growth factor (PDGF), nitric oxide (NO), transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)—can induce Ki-67 overexpression, thereby predisposing tissues to the onset and progression of OMPDs and OSCC [76]. Periodontal pathogens can also modulate Ki-67 levels, thereby promoting tumorigenesis. Indeed, studies have shown that in murine models, T. denticola enhances tumor progression via the TGF-β signaling pathway and increased Ki-67 expression [52]. It has also been hypothesized that the upregulation of Ki-67 may be closely linked to P. gingivalis-induced NF-kB activation in oral epithelial cells exposed to periodontal pathogens [28] (Table 1). Another study on murine models of periodontitis demonstrated a correlation between elevated Ki-67 proliferation index and OSCC development, with significantly higher levels in advanced stages, associated with increased tumor burden, enhanced cellular proliferation, and decreased survival rates [53] (Table 1).
The p16INK4A protein is a key cell cycle regulator that inhibits the transition from G1 to S phase by suppressing cyclin-dependent kinases CDK4 and CDK6 [76]. Its overexpression is associated with uncontrolled cellular proliferation and the development of multiple cancers, including OSCC. P16 is one of the principal biomarkers of cellular senescence (Figure 2). Cellular senescence represents a manifestation of cellular aging and is characterized by metabolic and structural alterations, resistance to apoptosis, and permanent cell cycle arrest. The persistence of senescent cells may influence neighboring cells by promoting chronic inflammatory processes [1]. Moreover, p16-rich cells have been implicated in the development of various chronic diseases. Recent evidence has demonstrated that the periodontal microenvironment can induce premature senescence through inflammation and oxidative stress, thereby predisposing individuals to the onset of disease even at a young age [1]. Exposure to periodontal pathogens—such as Fusobacterium nucleatum—has been shown to induce senescence-like features in gingival keratinocytes, accompanied by increased p16 expression. Epithelial cell senescence reduces their intrinsic repair capacity, thereby compromising periodontal homeostasis and increasing susceptibility to tissue damage [55]. In cases of chronic and persistent injury, these cells tend to accumulate and exert pro-tumorigenic effects due to the acquisition of the so-called senescence-associated secretory phenotype (SASP), which is characterized by altered gene expression and the release of inflammatory mediators. The SASP, within the context of carcinogenesis, promotes the development of epithelial tumors including OSCC [79]. By regulating proteins known as cyclin-dependent kinase inhibitors, including p16INK4a and p14ARF, senescence leads to an irreversible arrest of the cell cycle. According to Albuquerque-Souza et al., also F. Nucleatum induces cellular senescence through the upregulation of p16INK4a. This mechanism highlights the carcinogenic potential of this microorganism. Furthermore, it plays a fundamental role in HPV-related cervical and oropharyngeal cancers, in conjunction with the underexpression of retinoblastoma protein (pRb) [55] (Table 1). Immunohistochemical analyses of OSCC have demonstrated an association between P16 and P53 expression with more aggressive lesions, lymphovascular invasion, and poor prognosis [73]. In HPV infection cases, P16 and pRb levels may serve as prognostic factors; however, conclusive studies on this subject are still lacking [48,80]. The role of p16 as a biomarker in OSCC remains controversial due to inconsistent findings. Loss of p16 expression has been reported in early stages of OSCC development, whereas its positivity does not appear to correlate reliably with histopathological diagnosis [76]. Although p16 alone has demonstrated minimal prognostic relevance in OSCC, a study by Richter et al. suggests that, when used in combination with Ki-67, it may serve as a potential predictor of survival and malignant transformation [54].
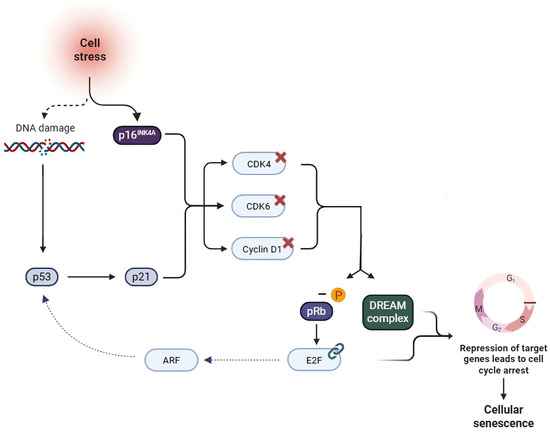
Figure 2.
p16INK4A/pRB and p53/p21 pathways in cellular senescence. Cellular stress, genomic damage, telomere shortening, and inflammatory cytokines contribute to the activation of the p16INK4A/pRB and p53/p21 pathways, and the DREAM complex (a multiprotein complex composed of DP, Rb-like, E2F, and MuvB) [56]. The upregulation of p16 leads to the inhibition of CDK4–6 and Cyclin D1, resulting in the dephosphorylation of pRB. In its active form, pRB binds to the transcription factor E2F, which, together with the DREAM complex, represses the transcription of target genes leading to cell cycle arrest [56]. Concurrently, p53 promotes the expression of dephosphorylated pRB through the activation of p21, further contributing to the establishment of cellular senescence. Moreover, a functional link between the two pathways has been identified, mediated by the transcription factor E2F, which activates the tumor suppressor gene ARF, thereby inhibiting MDM2 and stabilizing p53 [57].Created in https://BioRender.com, accessed on 29 October 2025.
2.2. Epigenetic Changes and Genetic Biomarkers
Epigenetics refers to the study of reversible and heritable changes in gene expression not encoded within the DNA sequence. Epigenetic mechanisms include DNA methylation, histone modifications, and microRNAs [81,82]. Such modifications influence gene expression by inducing activation or silencing, with DNA methylation and histone modifications playing key roles in oncogenesis and potentially representing a point of convergence between periodontitis and OSCC development [51]. Indeed, oral bacteria such as P. gingivalis and F. nucleatum have been shown to trigger epigenetic alterations in gingival epithelial cells [81]. For example, both species have been associated with up to 125-fold increases in OSCC cell proliferation through histone H2A phosphorylation, leading to the downregulation of p53 and Ku70 pathways [6] (Table 1).
Promoter hypermethylation, which induces gene silencing, represents an early event in OSCC development. The inactivation of tumor suppressor genes such as CDKN2A, hMLH1, and hMSH2 promotes tumor progression [51]. Identifying hypermethylated genes involved in proliferation, adhesion, DNA repair, and angiogenesis may provide a valuable tool for early OSCC diagnosis [59]. Several studies have reported frequent hypermethylation events in the 9p21 chromosomal region, encompassing the p14ARF, p15INK4b, and p16INK4a gene clusters [68]. Loss of CDKN2A function and its encoded protein, p16, has been observed in 80% of OSCC cases [83]. Conversely, hypomethylation can activate oncogenes, increasing cancer risk [51]. For example, P. gingivalis, through its virulence factors (particularly LPS), induces an increase in IL-6 that drives epigenetic changes—including promoter hypermethylation and DNA hypomethylation—thereby facilitating cellular replication [47,60] (Table 1). A bioinformatics analysis by Li et al. revealed overlapping methylation patterns between periodontitis and OSCC. Specifically, altered methylation profiles (not limited to hypermethylation) were identified in genes such as MPPED1, PROC, TUBA4B, PLD6, RSPH4A, RSPH9, and CSPG4 in both conditions [84]. Similarly, a review by Lavu et al. reported comparable proportions of hypermethylation in E-cadherin and COX-2 in patients with periodontitis and breast cancer, suggesting a potential correlation [81].
Histone modifications, including acetylation and methylation, influence chromatin remodeling and transcription regulation. Acetylation facilitates transcription, whereas deacetylation suppresses it. Other modifications, such as methylation, phosphorylation, and ubiquitination, differentially affect chromatin architecture and gene expression [51]. F. nucleatum and P. gingivalis have been shown to downregulate the p53 and Ku70 signaling pathways through histone H2A phosphorylation, thereby promoting increased cell proliferation in OSCC cells [58] (Table 1).
Ribosomal microRNAs (miRNAs) found in saliva are small non-coding RNAs (20–22 nucleotides) involved in gene regulation. These molecules influence cell differentiation, proliferation, apoptosis, and migration, with expression patterns akin to oncogenes and tumor suppressors [12]. Based on their activity, miRNAs are classified as oncogenic (also known as oncomirs), tumor suppressors, prometastatic (metastamiRs), and metastasis suppressors [58].
Notably, miR-125a and miR-200a, which are downregulated in periodontitis as a result of P. gingivalis exposure, are also found to be downregulated in OSCC patients, suggesting a potential tumor-suppressive role [61] (Table 1 and Table 2). In addition, another study reports the high accuracy of miRNA-136, miRNA-27B, and miR-27b in saliva for the diagnosis of OSCC [85].
Salivary rinse or oral brushing can detect methylated genes linked to OSCC with high sensitivity (≥75%) and specificity (≥90%) [47]. P. gingivalis can indeed promote the proliferation of tumor cells in OSCC through the modulation of miRNA expression, particularly increasing miR-21, which deregulates the miR-21/PDCD4/AP-1 pathway [47] (Table 1).
A study by Chen et al. demonstrated a significant association among periodontitis, OSCC, and three miRNAs: hsa-miR-19b-3p, hsa-miR-181b-2-3p, and hsa-miR-495-3p. Hsa-miR-19b-3p is associated with overall survival in oncology patients and is found in high concentrations in those with periodontitis [26]. Similarly, hsa-miR-181b-2-3p, an oncogenic miRNA, is upregulated in OSCC patients. miR-181b-2-3p has been identified as a reliable marker for lymph node metastasis, together with three other miRNAs (miR-21-5p, miR-107, miR-1247-3p) [63]. However, there are no definitive studies yet regarding changes in miR-181b expression levels in relation to disease progression. In fact, miR-181b appears to increase progressively with the severity of epithelial dysplasia, subsequently decreasing in OSCC samples. Therefore, miR-181b may act during the early stages of epithelial dysplasia development and later undergo suppression following malignant transformation. Conversely, in leukoplakia samples with low-grade dysplasia, miR-181b appears to be downregulated. Overall, current evidence highlights a substantial variability in the mechanisms of action of the different miR-181b isoforms, depending on the tumor microenvironment context. Further studies are needed to better elucidate their precise role in oral carcinogenesis [86]. Although hsa-miR-495-3p exhibits anti-oncogenic activity in various cancers, in the case of OSCC it appears to serve as a reliable prognostic risk factor, with elevated levels indicative of a poorer prognosis [26] (Table 1 and Table 2). The role of mir-495 remains rather controversial, as the available evidence presents highly conflicting data regarding its expression in OSCC samples. According to a study by Lv et al., miR-495 is downregulated in OSCC and, in vitro, limits OSCC cell proliferation and invasion through the repression of its target Notch1. Similarly, in gastric cancer, it appears to exert a tumor-suppressive effect [64]. The findings of You et al. support these results, demonstrating that miR-495 inhibits EMT, proliferation, migration, and invasion of cancer stem cells in OSCC by regulating the TGF-β signaling pathway [87].
A network analysis by Li et al. identified 18 co-expressed miRNAs in both OSCC and periodontitis, implicated in immune, inflammatory, and carcinogenic responses. Among them, three were co-upregulated (hsa-miR-224, hsa-miR-210, hsa-miR-31) and three co-downregulated (hsa-miR-497, hsa-miR-29c, hsa-miR-486). Notably, hsa-miR-224 and hsa-miR-31 emerged as reliable biomarkers for OSCC [62] (Table 2).
Periodontal pathogens and their carcinogenic metabolites are responsible for the genetic and epigenetic alterations underlying tumorigenesis [84]. Oxidative stress, a consequence of chronic periodontal inflammation, promotes the release of ROS, such as superoxide radicals and hydrogen peroxide, as well as reactive nitrogen intermediates (RNI), including nitric oxide and peroxynitrite [22,23,88]. These free radicals modify and damage DNA, thereby predisposing to tumorigenesis [22]. DNA damage—including base-pair mismatches, replication errors, oxidative deamination, and hydrolysis—can impair normal mechanisms of cell cycle regulation, DNA repair, and apoptosis, and may result from oxidative stress [89]. The most frequent mutation detected in OSCC samples involves the TP53 gene (93.3%). Mutation of the p53 protein represents an early biomarker of oral cancer [68].
Mutations in tumor suppressor genes and chromosomal abnormalities contribute to increased cancer susceptibility [83]. Identifying these genetic alterations aids in patient risk stratification [90]. These genetic aberrations include mutations, loss of heterozygosity (LOH) and alterations in DNA repair mechanisms [45].
The relationship between tumor suppressor gene (TSG) mutations and the levels of genetic and epigenetic biomarkers has not yet been fully elucidated in the literature. Mutations in TSGs directly affect cellular proliferative activity and, consequently, the levels of proliferative biomarkers [91]. For instance, mutation of the CDKN2A gene directly impacts the p16INK4A/pRB pathway through the activation of the transcription factor E2F, resulting in increased cellular proliferation [56]. Furthermore, an inverse causal relationship also exists between epigenetic alterations and TSG mutations. Indeed, aberrant hypermethylation and consequent silencing of genes involved in DNA repair mechanisms lead to high genomic instability, which in turn contributes to the accumulation of additional mutations and epigenetic modifications [92].
LOH refers to the loss of an allele at a chromosomal locus, resulting in gene function loss [74]. A high incidence of loss of heterozygosity (LOH) has also been observed in salivary and tissue samples of OSCC patients at chromosomal regions 9p, 3p, and 17p [68].
In OSCC, LOH frequently affects the CDKN2A gene on chromosome 9p, encoding the p16 protein [56]. LOH in OSCC also impacts chromosome 3p, which harbors genes involved in cellular proliferation, DNA synthesis, and cellular adhesion. Chromosome 3p alterations are associated with survival and recurrence risk [83]. This condition can arise from various mechanisms, including coding region mutations, promoter methylation, chromosomal rearrangements, and DNA repair defects [83]. Interestingly, LOH tends to occur more frequently at specific loci, associated with risk factors such as tobacco and alcohol consumption [68]. Future research may investigate the role of periodontal pathogens as potential risk factors for LOH.
2.3. Emerging Biomarkers
Recent research has explored novel biomarkers, including salivary and proteomic epigenetic mediators, which offer potential for high-sensitivity and high-specificity biomarker for disease detection [12,93].
Liquid biopsy, which involves the analysis of biological fluids, and salivaomics, the study of salivary omics markers, represent innovative, non-invasive techniques for the early detection of neoplasms. Salivaomics encompasses multiple domains, including genomics, transcriptomics, proteomics, epigenomics, metabolomics, metagenomics, and microbiomics. Saliva collection and analysis represent a promising and cost-effective strategy for OSCC diagnosis [68,94].
Proteins secreted by TME cells provide valuable insights into tumor progression [95]. Saliva contains a diverse range of proteins that have been extensively studied as potential biomarkers [44]. TME proteomics has identified proteins involved in metabolic pathways (e.g., glucose metabolism), extracellular matrix remodeling, and hypoxia [51]. These biomarkers can be employed not only for assessing disease progression but also for evaluating associated risk factors [44].
Notably, elevated concentrations of metalloproteinases (MMPs) and neutrophil elastase, characteristic of periodontitis, have been linked to OSCC and its recurrence [96].
MMPs are implicated in carcinogenesis, angiogenesis, and metastasis. Chen et al.’s study revealed elevated levels of MMP-12 and MMP-13 in OSCC patients, demonstrating that these enzymes may serve as diagnostic and prognostic biomarkers [26]. Furthermore, the expression of MMP-13 is closely linked to the activation of oncogenic-related pathways (NF-κB or MAPK/p38 pathways) by F. nucleatum [58] (Table 1). Periodontal inflammation mediated by neutrophils induces the secretion of elastase, one of the protein components of NETs [26] (Table 1). Several studies have demonstrated the role of neutrophil elastase in extracellular matrix degradation, tumor cell adhesion, invasion, and metastasis in OSCC [66] (Table 2). For instance, a study by Monea et al. demonstrated the upregulation of MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-12, and MMP-13 in samples from oral cancer patients, with MMP-1, MMP-3, and MMP-9 identified as promising diagnostic biomarkers [65]. Moreover, a study by Jansson et al. demonstrated the upregulation of MMP-1, MMP-2, MMP-3 in gingival crevicular fluid from periodontitis patients [97] (Table 1 and Table 2). Notably, MMP-8, a key mediator of tissue destruction in periodontitis, is a shared biomarker between periodontitis and OSCC [65] (Table 2).
Cytokines and inflammatory mediators also represent promising biomarkers in oral cancer and are closely associated with periodontitis. IL-6 and IL-8, NF-kB-dependent cytokines, are central messengers involved in inflammatory responses, cell proliferation, and angiogenesis, and serve as sensitive early biomarkers for OSCC [19,67].
IL-1β and IL-8, key mediators of periodontal inflammation, are also involved in OSCC. Specifically, IL-1β plays a critical role in both acute and chronic inflammatory responses and promotes cell proliferation. Its levels are elevated in patients with periodontitis and OSCC, suggesting its potential use as a biomarker. IL-8 (CXCL8) is essential for neutrophil and granulocyte chemotaxis and contributes to disease progression and metastasis through the activation of the NF-κB signaling pathway [26] (Table 2).

Table 2.
Differential expression patterns of biomarkers in both periodontitis and OSCC.
Table 2.
Differential expression patterns of biomarkers in both periodontitis and OSCC.
| Target | Expression Pattern in Periodontitis | Expression Pattern in OSCC |
|---|---|---|
| miR-125a | Reduced ↓ | Reduced ↓ [61] |
| miR-200a | Reduced ↓ | Reduced ↓ [61] |
| miR-19b-3p | Increased ↑ | Increased ↑ [26] |
| miR-181b-2-3p | Reduced ↓ | Controversial [26,63] |
| miR-495-3p | Reduced ↓ | Controversial [86] |
| hsa-miR-224 | Increased ↑ | Increased ↑ [62] |
| hsa-miR-210 | Increased ↑ | Increased ↑ [62] |
| hsa-miR-31 | Increased ↑ | Increased ↑ [62] |
| hsa-miR-497 | Reduced ↓ | Reduced ↓ [62] |
| hsa-miR-29c | Reduced ↓ | Reduced ↓ [62] |
| hsa-miR-486 | Reduced ↓ | Reduced ↓ [62] |
| MMP-1, MMP-2, MMP-3 | Increased ↑ [97] | Increased ↑ [65] |
| MMP-12 | Increased ↑ | Increased ↑ [58] |
| MMP-13 | Increased ↑ | Increased ↑ [58] |
| MMP-8 | Increased ↑ | Increased ↑ [65] |
| NETs | Increased ↑ | Increased ↑ [96] |
| IL-1β | Increased ↑ | Increased ↑ [58] |
| IL-6 | Increased ↑ | Increased ↑ [19,67] |
| IL-8 | Increased ↑ | Increased ↑ [58] |
2.4. The Role of Periodontal Therapy in Biomarker Levels
Periodontal therapy significantly reduces both local and systemic inflammation, improving inflammatory markers and modulating tumor-related biomarkers. Furthermore, non-surgical periodontal therapy aims to restore a balanced condition from both an immunological and microbiological perspective; focusing on microbiome-driven immunological pathways represents a promising therapeutic strategy for the management of chronic periodontitis and OSCC [15,98,99].
Mechanical instrumentation and plaque removal lead to a decrease in IL-6 and IL-8 levels, while the oral microbiome benefits from a decrease in periodontal pathogens [100]. While in gingival crevicular fluid (GCF), a reduction in local levels of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-17, and IL-23, as well as other mediators including MMP-8 and VEGF, is observed [101,102]. VEGF plays a crucial role in OSCC, as it is involved in both angiogenesis and tumor progression [46].
Moreover, periodontal treatment contributes to the downregulation of TNF-α, MMP, and pro-inflammatory cytokines and it significantly influences neutrophil ROS production, thereby reducing NETs formation and the consequent oxidative stress and tissue damage [98,100,103].
Recent studies have highlighted the influence of non-surgical periodontal therapy on miRNA levels, particularly miR-21, which regulates key signaling pathways involved in tumor progression [58,85]. Indeed, if the expression of specific miRNAs (e.g., miR-21, miR-146a, and miR-155) is strongly correlated with IL-1β or TNF-α levels in periodontitis, the elimination of such pro-inflammatory stimuli following periodontal therapy could lead to a significant reduction in their expression [104].
P. gingivalis modulates the expression of miRNA-205-5p, which is known for its immunomodulatory properties, in gingival epithelial cells. As there is a reduction in bacterial load of P. gingivalis after mechanical debridement, the level of miRNA-205-5p expression also tends to normalize, thereby contributing to the restoration of balance in the inflammatory response [29,105].
Current evidence does not provide direct proof of the role of periodontal therapy in the suppression of OSCC. Periodontal therapy represents a crucial tool in the primary prevention of OSCC by targeting oral dysbiosis and both local and systemic low-grade chronic inflammation [28]. At present, oral cancer treatment relies on conventional approaches such as surgical excision, radiotherapy, and chemotherapy, as well as innovative strategies including immunotherapy and molecular targeting [90,106]. Considering the beneficial effects of periodontal management on inflammatory mediators and biomarkers, scaling and root planing may influence disease progression and reduce tumor aggressiveness.
Recent studies have demonstrated that inflammatory mediators play a critical role in the progression of OSCC, indeed persistent exposure to inflammatory signals promote chemoresistance and enhance the aggressiveness of tumor cells. In particular, IL-6 has been implicated in conferring resistance to paclitaxel. These findings suggest that periodontitis-associated pathogens and inflammatory mediators are key contributors to the development of chemoresistance in OSCC [107].
In light of these considerations, an approach that integrates periodontal therapy into the management of patients with periodontitis appears to be a prudent and potentially effective strategy for preventing the onset and progression of OSCC [46,108].
3. Current Limitations
The research of biomarkers has a pivotal role for an early diagnosis, which is often the key in effectively treating a disease and it can help to intervene at a stage when the disease is more responsive to treatment [48]. For the study of tumor, its possible to use markers in serum, tissue, and other body fluids. However, the investigation of blood biochemical have some disadvantages, in fact blood sampling is an invasive procedure and has a potential risk of disease transmission through needle stick injuries. For this reason, saliva is used for non-invasive diagnostic medium [109]. The use of salivary samples for biomarker research nonetheless presents certain limitations: the methods of sampling and of nucleic acid extraction and analytical procedures can distort the results too, like stimulated and unstimulated saliva, the time of collection, pH, and flow rate, oral rinse, oral swab, scraping, cytobrush, and biopsy [30,68]. Moreover, the concentration and stability of biomarkers in salivary samples are influenced by several unhealthy lifestyle behaviors, such as alcohol consumption, smoking, as well as by systemic diseases, pharmacological treatments, radiotherapy, the presence of enzymes and the condition of the gums and teeth [68]. Finally, to establish saliva as a reliable medium for clinical diagnostics, it is necessary to standardize the saliva collection method, processing, analysis, and reporting of liquid biopsy results [94].
Among the limitations is the lack of long-term longitudinal studies too. Indeed, in the absence of such studies, it is difficult to definitively establish a strong correlation between periodontitis and cancer, control for confounding factors, and assess the benefits of periodontal therapy [15].
4. Future Directions
Since biomarker assessment and the role of periodontal therapy, alongside traditional risk predictors of cancerization, enables optimal patient care and more precise risk stratification, clinically detected factors and biomarkers may be incorporated into artificial intelligence (AI) algorithms to assist clinicians in diagnosis and risk stratification [93,110].
One of specialized algorithms such as machine learning (ML) can identify patterns and relationships within datasets, while deep learning (DL) employs complex multilayer neural networks capable of analyzing intricate data structures. These technologies have various applications: ML can aid in the identification and validation of biomarkers, support decision-making systems based on patient data, and generate risk scores for malignant transformation [93,111]. Conversely, DL is particularly useful in analyzing histopathological images, distinguishing between benign and malignant lesions, and assessing tumor size and depth of invasion [93]. The integration of AI in clinical practice could enhance the identification and management of oral cancer patients by enabling the development of personalized treatment plans based on individual risk factors [44].
Moreover, recent studies have identified biomarkers in saliva, blood, buccal swabs, and other body fluids, along with promising artificial intelligence (AI) strategies, as potential tools for the early detection of oral cancer [111]. Recent studies have also demonstrated that AI can serve as a non-invasive and cost-effective tool to understand the role of the microbiome in OSCC, thereby improving diagnosis, monitoring progression, and treatment. However, the clinical application of AI requires standardized protocols, diverse patient cohorts, and validation through large-scale longitudinal studies [112].
Starting from the use of salivary samples, the presence of dental diseases such as periodontitis and oral cancer could potentially be diagnosed through metabolomics, which focuses on the qualitative and quantitative analysis of endogenous low-molecular-weight metabolites [68]. Therefore, the application of salivary metabolomics to simultaneously assess a range of metabolites could prove useful for identifying biomarkers the diagnosis and monitoring of OSCC [94].
Finally, the identification of bacterial species capable of promoting carcinogenesis may serve as a valuable diagnostic tool OSCC. Metagenomic analyses have revealed significant differences in the oral microbiota between patients with precancerous lesions or oral cancer and healthy patients. Various technologies have been employed to study the oral microbiota, including culture-based methods, microscopy, DNA microarrays, PCR, 16S rRNA gene sequencing, and high-throughput sequencing. Recent studies have associated the presence of specific bacteria—such as Prevotella, Streptococcus, Salmonella, Fusobacterium nucleatum, and Porphyromonas gingivalis—with OSCC. Therefore, the analysis of shifts in the composition and activity of oral bacterial communities represents a promising biomarker for the progression of neoplastic lesions in the oral epithelium and for the early diagnosis of OSCC [68].
5. Conclusions
This review highlights the current evidence on the status update, emphasizing the common pattern and pathways between chronic periodontal inflammation and oral carcinogenesis. It also highlighted the crucial role of certain molecular mediators, as well as genetic and epigenetic biomarker alterations induced by periodontal pathogens. In this regard, potential clinical patient stratification based on P. gingivalis and F. nucleatum concentrations in gingival tissues could aid in tumor initiation and progression, modulating pathways related to cell proliferation, apoptosis, DNA repair, and immune evasion.
Furthermore, current evidence suggests that salivary biomarkers, particularly those related to proteomics, hold promising opportunities for the early and non-invasive diagnosis of OSCC. In this regard, periodontal therapy, driven by reducing the expression of key periodontal pathogenic bacteria and the related immune response—a pathway that has been shown to be shared between preneoplastic lesions and OSCC, both in controlling local inflammation and modulating tumor-related biomarkers—has been shown to currently represent one of the main strategies to be applied to reduce various environmental cancer risk factors in patients with periodontitis. Looking ahead, integrating biomarker assessment with artificial intelligence-based diagnostic tools could support and enhance more accurate risk stratification and personalized patient management. Overall, enhancing our understanding of the shared mechanisms between periodontitis and OSCC will be crucial for developing preventive strategies, improving early diagnosis, and ultimately improving patient survival outcomes.
However, potentially effective mechanisms in vitro or on limited samples still need to be better evaluated in larger study populations, as saliva composition has been shown to vary significantly from one sample to another. Therefore, the current methodologies developed in recent years will require further studies to validate their large-scale standardization and better validate their clinical applicability.
Author Contributions
Conceptualization, G.I.; methodology, A.P.; validation, R.C. and M.M. (Marco Mascitti); formal analysis, R.C. and M.M. (Marco Mascitti); investigation, G.M.M., M.M. (Morena Munzone) and G.I.; data curation, G.M.M., M.M. (Morena Munzone) and A.P.; writing—original draft preparation, G.M.M. and M.M. (Morena Munzone); writing—review and editing, G.M.M., M.M. (Morena Munzone) and A.P.; visualization, R.C. and M.M. (Marco Mascitti); supervision, G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OSCC | Oral squamous cell carcinoma |
| OPMDs | Oral potentially malignant disorders |
| HNSCC | Head and neck squamous cell carcinoma |
| TME | Tumor microenvironment |
| PAMPs | Pathogen-associated molecular patterns |
| TLRs | Toll-like receptors |
| CtDNA | Circulating tumor DNA |
| HGFs | Human gingival fibroblasts |
| ROS | Reactive oxygen species |
| PDGF | Platelet-derived growth factor |
| NO | Nitric oxide |
| TGF-β | Transforming growth factor-beta |
| HGF | Hepatocyte growth factor |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| pRb | Retinoblastoma protein |
| MiRNA | microRNA |
| TSG | Tumor suppressor gene |
| LOH | Loss of heterozygosity |
| MMP | Metalloproteinase |
| NETs | Neutrophil extracellular traps |
| AI | Artificial intelligence |
| ML | Machine learning |
| DL | Deep learning |
References
- Rattanaprukskul, K.; Xia, X.J.; Jiang, M.; Albuquerque-Souza, E.; Bandyopadhyay, D.; Sahingur, S.E. Molecular Signatures of Senescence in Periodontitis: Clinical Insights. J. Dent. Res. 2024, 103, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Albahri, J.; Allison, H.; Whitehead, K.; Muhamadali, H. The role of salivary metabolomics in chronic periodontitis: Bridging oral and systemic diseases. Metabolomics 2025, 21, 24. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, K.; Liu, T.; Chen, Y.; Li, R.; Dong, J.; Xing, L. Causal network between periodontitis and systemic inflammation: Triangulating evidence from Mendelian randomization and sequencing datasets. J. Periodontol. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Polizzi, A.; Nibali, L.; Tartaglia, G.M.; Isola, G. Impact of nonsurgical periodontal treatment on arterial stiffness outcomes related to endothelial dysfunction: A systematic review and meta-analysis. J. Periodontol. 2025, 96, 330–345. [Google Scholar] [CrossRef]
- Baima, G.; Minoli, M.; Michaud, D.S.; Aimetti, M.; Sanz, M.; Loos, B.G.; Romandini, M. Periodontitis and risk of cancer: Mechanistic evidence. Periodontol. 2000 2024, 96, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Marruganti, C.; Suvan, J.E.; D’Aiuto, F. Periodontitis and metabolic diseases (diabetes and obesity): Tackling multimorbidity. Periodontol. 2000, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Jungbauer, G.; Stähli, A.; Zhu, X.; Auber Alberi, L.; Sculean, A.; Eick, S. Periodontal microorganisms and Alzheimer disease—A causative relationship? Periodontol. 2000 2022, 89, 59–82. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Serra, S.; Boato, M.; Sculean, A. Relationship between periodontitis and systemic diseases: A bibliometric and visual study. Periodontol. 2000, 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Bonilla, M.; Peñalver, I.; Mesa-López, M.J.; Mesa, F. Association Between Periodontitis and Cancer: A Perspective Review of Mechanisms and Clinical Evidence. J. Clin. Med. 2025, 14, 6334. [Google Scholar] [CrossRef]
- Camañes-Gonzalvo, S.; Montiel-Company, J.M.; Lobo-de-Mena, M.; Safont-Aguilera, M.J.; Fernández-Diaz, A.; López-Roldán, A.; Paredes-Gallardo, V.; Bellot-Arcís, C. Relationship between oral microbiota and colorectal cancer: A systematic review. J. Periodontal Res. 2024, 59, 1071–1082. [Google Scholar] [CrossRef]
- Jagadeesan, D.; Sathasivam, K.V.; Fuloria, N.K.; Balakrishnan, V.; Khor, G.H.; Ravichandran, M.; Solyappan, M.; Fuloria, S.; Gupta, G.; Ahlawat, A.; et al. Comprehensive insights into oral squamous cell carcinoma: Diagnosis, pathogenesis, and therapeutic advances. Pathol.-Res. Pract. 2024, 261, 155489. [Google Scholar] [CrossRef]
- Alves-Costa, S.; Romandini, M.; Nascimento, G.G. Lip and Oral Cancer, Caries and Other Oral Conditions: Estimates From the 2021 Global Burden of Disease Study and Projections up to 2050. J. Periodontal Res. 2025, 60, 544–558. [Google Scholar] [CrossRef]
- Unlu, O.; Demirci, M.; Paksoy, T.; Eden, A.B.; Tansuker, H.D.; Dalmizrak, A.; Aktan, C.; Senel, F.; Sunter, A.V.; Yigit, O.; et al. Oral microbial dysbiosis in patients with oral cavity cancers. Clin. Oral Investig. 2024, 28, 377. [Google Scholar] [CrossRef]
- Pigossi, S.C.; Oliveira, J.A.; de Medeiros, M.C.; Soares, L.F.F.; D’Silva, N.J. Demystifying the link between periodontitis and oral cancer: A systematic review integrating clinical, pre-clinical, and in vitro data. Cancer Metastasis Rev. 2025, 44, 67. [Google Scholar] [CrossRef]
- Mehrnia, N.; Sonis, S. Periodontitis and Oral Cancer Risk. Dent. Clin. N. Am. 2025, 69, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Munzone, M.; Marmo, G.M.; Polizzi, A.; Marya, A.; Blasi, A.; Isola, G. Periodontal inflammation as a negative stimulus for oral cancerization: The hidden role of periodontitis in oral cancerization. Oncologie 2025, 27, 659–672. [Google Scholar] [CrossRef]
- Polizzi, A.; Tartaglia, G.M.; Santonocito, S.; Alibrandi, A.; Verzì, A.E.; Isola, G. Impact of Topical Fluocinonide on Oral Lichen Planus Evolution: Randomized Controlled Clinical Trial. Oral Dis. 2025, 31, 510–521. [Google Scholar] [CrossRef]
- Rani, N.A.J.; Vardhan, B.G.H.; Srinivasan, S.; Gopal, S.K. Evaluation of Salivary Interleukin-6 in Patients with Oral Squamous Cell Carcinoma, Oral Potentially Malignant Disorders, Chronic Periodontitis and in Healthy Controls—A Cross-Sectional Comparative Study. Ann. Maxillofac. Surg. 2023, 13, 70–75. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Geng, F.; Zhang, Y.; Lu, Z.; Zhang, S.; Pan, Y. Fusobacterium nucleatum Caused DNA Damage and Promoted Cell Proliferation by the Ku70/p53 Pathway in Oral Cancer Cells. DNA Cell Biol. 2020, 39, 144–151. [Google Scholar] [CrossRef]
- Farhad, S.Z.; Karbalaeihasanesfahani, A.; Dadgar, E.; Nasiri, K.; Esfahaniani, M.; Nabi Afjadi, M. The role of periodontitis in cancer development, with a focus on oral cancers. Mol. Biol. Rep. 2024, 51, 814. [Google Scholar] [CrossRef]
- Li, R.; Hou, M.; Yu, L.; Luo, W.; Liu, R.; Wang, H. Association between periodontal disease and oral squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2023, 61, 394–402. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, X.; Tang, Q.; Zhang, J.; Bai, J.; Jing, F.; Gao, L.; Zhang, H.; Li, T. Differences in the landscape of colonized microorganisms in different oral potentially malignant disorders and squamous cell carcinoma: A multi-group comparative study. BMC Microbiol. 2024, 24, 318. [Google Scholar] [CrossRef]
- Dopico, J.; Botelho, J.; Ouro, A.; Domínguez, C.; Machado, V.; Aramburu-Nuñez, M.; Custodia, A.; Blanco, T.; Vázquez-Reza, M.; Romaus-Sanjurjo, D.; et al. Association between periodontitis and peripheral markers of innate immunity activation and inflammation. J. Periodontol. 2023, 94, 11–19. [Google Scholar] [CrossRef]
- Chen, X.; Lei, H.; Cheng, Y.; Fang, S.; Sun, W.; Zhang, X.; Jin, Z. CXCL8, MMP12, and MMP13 are common biomarkers of periodontitis and oral squamous cell carcinoma. Oral Dis. 2024, 30, 390–407. [Google Scholar] [CrossRef]
- Chew, R.J.J.; Tan, K.S.; Chen, T.; Al-Hebshi, N.N.; Goh, C.E. Quantifying periodontitis-associated oral dysbiosis in tongue and saliva microbiomes—An integrated data analysis. J. Periodontol. 2025, 96, 55–66. [Google Scholar] [CrossRef]
- Lafuente Ibáñez de Mendoza, I.; Maritxalar Mendia, X.; García de la Fuente, A.M.; Quindós Andrés, G.; Aguirre Urizar, J.M. Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: A systematic review. J. Periodontal Res. 2020, 55, 13–22. [Google Scholar] [CrossRef]
- Ciani, L.; Libonati, A.; Dri, M.; Pomella, S.; Campanella, V.; Barillari, G. About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview. Int. J. Mol. Sci. 2024, 25, 5083. [Google Scholar] [CrossRef]
- Pignatelli, P.; Curia, M.C.; Tenore, G.; Bondi, D.; Piattelli, A.; Romeo, U. Oral bacteriome and oral potentially malignant disorders: A systematic review of the associations. Arch. Oral Biol. 2024, 160, 105891. [Google Scholar] [CrossRef]
- Smędra, A.; Berent, J. The Influence of the Oral Microbiome on Oral Cancer: A Literature Review and a New Approach. Biomolecules 2023, 13, 815. [Google Scholar] [CrossRef]
- Keinänen, A.; Uittamo, J.; Snäll, J. Do we recognize oral cancer? Primary professional delay in diagnosis of oral squamous cell carcinoma. Clin. Oral Investig. 2024, 28, 131. [Google Scholar] [CrossRef]
- Antonoglou, G.N.; Romandini, M.; Meurman, J.H.; Surakka, M.; Janket, S.J.; Sanz, M. Periodontitis and edentulism as risk indicators for mortality: Results from a prospective cohort study with 20 years of follow-up. J. Periodontal Res. 2023, 58, 12–21. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Salvucci, A.; Togni, L.; Mascitti, M.; Sartini, D.; Salvolini, E.; Santarelli, A.; Lo Muzio, L.; Emanuelli, M. Paraoxonase-2 expression in oral squamous cell carcinoma. Hum. Cell 2023, 36, 1211–1213. [Google Scholar] [CrossRef]
- Carreras-Torras, C.; Gay-Escoda, C. Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e305–e315. [Google Scholar] [CrossRef]
- Grafton-Clarke, C.; Chen, K.W.; Wilcock, J. Diagnosis and referral delays in primary care for oral squamous cell cancer: A systematic review. Br. J. Gen. Pract. 2019, 69, e112–e126. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Sheikh, N. Oral Potentially Malignant Disorders: A Comprehensive Review of Diagnostic Approaches and Management Strategies. J. Bahria Univ. Med. Dent. Coll. 2024, 14, 83–89. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, H.; Mou, Q.; Wong, P.Y.; Lan, L.; Ng, C.W.K.; Lei, P.; Cheung, M.K.; Wang, D.; Wong, E.W.Y.; et al. Integrative analysis reveals associations between oral microbiota dysbiosis and host genetic and epigenetic aberrations in oral cavity squamous cell carcinoma. NPJ Biofilms Microbiomes 2024, 10, 39. [Google Scholar] [CrossRef]
- You, J.R.; Chen, Y.T.; Hsieh, C.Y.; Chen, S.Y.; Lin, T.Y.; Shih, J.S.; Chen, G.T.; Feng, S.W.; Peng, T.Y.; Wu, C.Y.; et al. Exploring Possible Diagnostic Precancerous Biomarkers for Oral Submucous Fibrosis: A Narrative Review. Cancers 2023, 15, 4812. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Hu, C.C.; Wang, S.G.; Gao, Z.; Qing, M.F.; Pan, S.; Liu, Y.Y.; Li, F. Emerging salivary biomarkers for early detection of oral squamous cell carcinoma. World J. Clin. Oncol. 2025, 16, 103803. [Google Scholar] [CrossRef]
- Safari, Z.; Firouzi, A.; Rezaeikalantari, N.; Mohammadi, S.; Ranjbar, N.; Shahpori, H.; Khaleghi, P.; Bagherianlemraski, M.; Zandi, S.; Rafieyan, S. The salivary exosomal microRNA as a potential biomarker in patients with periodontitis and oral cancers. Chem. Biol. Drug Des. 2023, 101, 1204–1215. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Jordan, L.; Chen, H.S.; Kang, D.; Oxford, L.; Plemons, J.; Parks, H.; Rees, T. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J. Periodontal Res. 2017, 52, 428–437. [Google Scholar] [CrossRef]
- Radaic, A.; Kamarajan, P.; Cho, A.; Wang, S.; Hung, G.-C.; Najarzadegan, F.; Wong, D.T.; Ton-That, H.; Wang, C.-Y.; Kapila, Y.L. Biological biomarkers of oral cancer. Periodontol. 2000 2024, 96, 250–280. [Google Scholar] [CrossRef] [PubMed]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef]
- Karmakar, S.; Modak, B.; Solomon, M.C. Exploring the causal relationship between chronic periodontitis and oral cancer: An insight. Oral Oncol. Rep. 2024, 11, 100468. [Google Scholar] [CrossRef]
- Zhou, Y.; Meyle, J.; Groeger, S. Periodontal pathogens and cancer development. Periodontol. 2000 2024, 96, 112–149. [Google Scholar] [CrossRef]
- Pekarek, L.; Garrido-Gil, M.J.; Sánchez-Cendra, A.; Cassinello, J.; Pekarek, T.; Fraile-Martinez, O.; García-Montero, C.; Lopez-Gonzalez, L.; Rios-Parra, A.; Álvarez-Mon, M.; et al. Emerging histological and serological biomarkers in oral squamous cell carcinoma: Applications in diagnosis, prognosis evaluation and personalized therapeutics (Review). Oncol. Rep. 2023, 50, 213. [Google Scholar] [CrossRef]
- Richter, M.; Doll, C.; Mrosk, F.; Hofmann, E.; Koerdt, S.; Heiland, M.; Neumann, K.; Beck, M.; Dommerich, S.; Jöhrens, K.; et al. The combined assessment of p16(INK4a) and Mib/Ki-67 in oral squamous cell carcinoma. Front. Oncol. 2024, 14, 1493281. [Google Scholar] [CrossRef]
- Acharya, S.; Hegde, U.; Acharya, A.B.; Nitin, P. Dysbiosis linking periodontal disease and oral squamous cell carcinoma-A brief narrative review. Heliyon 2024, 10, e32259. [Google Scholar] [CrossRef]
- Akbari, E.; Epstein, J.B.; Samim, F. Unveiling the Hidden Links: Periodontal Disease, Fusobacterium Nucleatum, and Cancers. Curr. Oncol. Rep. 2024, 26, 1388–1397. [Google Scholar] [CrossRef]
- Peng, R.T.; Sun, Y.; Zhou, X.D.; Liu, S.Y.; Han, Q.; Cheng, L.; Peng, X. Treponema denticola Promotes OSCC Development via the TGF-β Signaling Pathway. J. Dent. Res. 2022, 101, 704–713. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Shen, X.; Lyu, J.; Yan, C.; Tang, B.; Ma, W.; Xie, H.; Zhao, L.; Cheng, L.; et al. Oral Microbiota from Periodontitis Promote Oral Squamous Cell Carcinoma Development via γδ T Cell Activation. mSystems 2022, 7, e0046922. [Google Scholar] [CrossRef]
- de Villalaín, L.; Álvarez-Teijeiro, S.; Rodríguez-Santamarta, T.; Fernández Del Valle, Á.; Allonca, E.; Rodrigo, J.P.; de Vicente, J.C.; García-Pedrero, J.M. Emerging Role of Decoy Receptor-2 as a Cancer Risk Predictor in Oral Potentially Malignant Disorders. Int. J. Mol. Sci. 2023, 24, 14382. [Google Scholar] [CrossRef]
- Albuquerque-Souza, E.; Shelling, B.; Jiang, M.; Xia, X.J.; Rattanaprukskul, K.; Sahingur, S.E. Fusobacterium nucleatum triggers senescence phenotype in gingival epithelial cells. Mol. Oral Microbiol. 2024, 39, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kandhaya-Pillai, R.; Miro-Mur, F.; Alijotas-Reig, J.; Tchkonia, T.; Schwartz, S.; Kirkland, J.L.; Oshima, J. Key elements of cellular senescence involve transcriptional repression of mitotic and DNA repair genes through the p53-p16/RB-E2F-DREAM complex. Aging 2023, 15, 4012–4034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nakajima, R.; Shirasawa, M.; Fikriyanti, M.; Zhao, L.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Expanding Roles of the E2F-RB-p53 Pathway in Tumor Suppression. Biology 2023, 12, 1511. [Google Scholar] [CrossRef]
- Khijmatgar, S.; Yong, J.; Rübsamen, N.; Lorusso, F.; Rai, P.; Cenzato, N.; Gaffuri, F.; Del Fabbro, M.; Tartaglia, G.M. Salivary biomarkers for early detection of oral squamous cell carcinoma (OSCC) and head/neck squamous cell carcinoma (HNSCC): A systematic review and network meta-analysis. Jpn. Dent. Sci. Rev. 2024, 60, 32–39. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Panda, M. Recent trends of saliva omics biomarkers for the diagnosis and treatment of oral cancer. J. Oral Biosci. 2019, 61, 84–94. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Hsu, C.-C.; Chen, Y.-W.; Fu, E. Hypomethylation of the interleukin-6 promoter in gingival tissue of patients with periodontitis. J. Periodontol. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Salta, S.; Lopez, R.; Henrique, R.; Suarez-Cunqueiro, M.; Jeronimo, C. DNA methylation markers for oral cancer detection in non- and minimally invasive samples: A systematic review. Clin. Epigenetics 2024, 16, 105. [Google Scholar] [CrossRef]
- Li, Z.; Fu, R.; Wen, X.; Zhang, L. Network analysis reveals miRNA crosstalk between periodontitis and oral squamous cell carcinoma. BMC Oral Health 2023, 23, 19. [Google Scholar] [CrossRef]
- Liu, K.; Zhu, S.; Brooks, D.; Bowlby, R.; Durham, J.; Ma, Y.; Moore, R.; Mungall, A.; Jones, S.; Poh, C. Tumor microRNA profile and prognostic value for lymph node metastasis in oral squamous cell carcinoma patients. Oncotarget 2020, 11, 2204–2215. [Google Scholar] [CrossRef]
- Lv, L.; Wang, Q.; Yang, Y.; Ji, H. MicroRNA-495 targets Notch1 to prohibit cell proliferation and invasion in oral squamous cell carcinoma. Mol. Med. Rep. 2019, 19, 693–702. [Google Scholar] [CrossRef]
- Monea, M.; Pop, A.M. The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review. Curr. Issues Mol. Biol. 2022, 44, 6306–6322. [Google Scholar] [CrossRef]
- Zhai, R.; Gong, Z.; Wang, M.; Ni, Z.; Zhang, J.; Wang, M.; Zhang, Y.; Zeng, F.; Gu, Z.; Chen, X.; et al. Neutrophil extracellular traps promote invasion and metastasis via NLRP3-mediated oral squamous cell carcinoma pyroptosis inhibition. Cell Death Discov. 2024, 10, 214. [Google Scholar] [CrossRef]
- Dikova, V.; Jantus-Lewintre, E.; Bagan, J. Potential Non-Invasive Biomarkers for Early Diagnosis of Oral Squamous Cell Carcinoma. J. Clin. Med. 2021, 10, 1658. [Google Scholar] [CrossRef]
- Starska-Kowarska, K. Salivaomic Biomarkers—An Innovative Approach to the Diagnosis, Treatment, and Prognosis of Oral Cancer. Biology 2025, 14, 852. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Shi, J.; Hao, X.Y.; Tong, Y.; Qian, W.B.; Sun, Y. SIRT6 alleviates senescence induced by Porphyromonas gingivalis in human gingival fibroblasts. Mol. Biol. Rep. 2024, 51, 976. [Google Scholar] [CrossRef]
- Memmert, S.; Gölz, L.; Pütz, P.; Jäger, A.; Deschner, J.; Appel, T.; Baumgarten, G.; Rath-Deschner, B.; Frede, S.; Götz, W. Regulation of p53 under hypoxic and inflammatory conditions in periodontium. Clin. Oral Investig. 2016, 20, 1781–1789. [Google Scholar] [CrossRef]
- Yilmaz, Ö. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology 2008, 154, 2897–2903. [Google Scholar] [CrossRef]
- Amano, Y.; Hasegawa, M.; Kihara, A.; Matsubara, D.; Fukushima, N.; Nishino, H.; Mori, Y.; Inamura, K.; Niki, T. Clinicopathological and prognostic significance of stromal p16 and p53 expression in oral squamous cell carcinoma. Ann. Diagn. Pathol. 2025, 75, 152439. [Google Scholar] [CrossRef] [PubMed]
- Mesgari, H.; Esmaelian, S.; Nasiri, K.; Ghasemzadeh, S.; Doroudgar, P.; Payandeh, Z. Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review. Cancers 2023, 15, 5600. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Hua, R.; Wang, Y.; Li, Y.; Zhang, H. Promising dawn in tumor microenvironment therapy: Engineering oral bacteria. Int. J. Oral Sci. 2024, 16, 24. [Google Scholar] [CrossRef]
- Ali Ahmed, M.A.; Shetty, S.; Rahman, B.; Gopalakrishnan, A.R.K.; Ismail, A.A.; Acharya, A.B. Evaluation of salivary Ki-67 in health and periodontitis. BMC Oral Health 2025, 25, 366. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Baik, J.E.; Lagana, S.M.; Han, R.P.; Raab, W.J.; Sahoo, D.; Dalerba, P.; Wang, T.C.; Han, Y.W. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/β-catenin modulator Annexin A1. EMBO Rep. 2019, 20, e47638. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhou, H.; Liu, Q.; Zhang, X.; Hu, F. Periodontitis Exacerbates Colorectal Cancer by Altering Gut Microbiota-Derived Metabolomics in Mice. J. Periodontal Res. 2025; Epub ahead of print. [Google Scholar] [CrossRef]
- Niklander, S.E.; Aránguiz, P.; Faunes, F.; Martínez-Flores, R. Aging and oral squamous cell carcinoma development: The role of cellular senescence. Front. Oral Health 2023, 4, 1285276. [Google Scholar] [CrossRef]
- López-Ansio, M.; Ramos-García, P.; González-Moles, M.Á. Predictive Value of the Loss of pRb Expression in the Malignant Transformation Risk of Oral Potentially Malignant Disorders: A Systematic Review and Meta-Analysis. Cancers 2025, 17, 329. [Google Scholar] [CrossRef]
- Lavu, V.; Venkatesan, V.; Rao, S.R. The epigenetic paradigm in periodontitis pathogenesis. J. Indian Soc. Periodontol. 2015, 19, 142–149. [Google Scholar] [CrossRef]
- Liaw, A.; Liu, C.; Ivanovski, S.; Han, P. The Relevance of DNA Methylation and Histone Modification in Periodontitis: A Scoping Review. Cells 2022, 11, 3211. [Google Scholar] [CrossRef]
- Arslan Bozdag, L.; Inan, S.; Elif Gultekin, S. Microsatellite Instability and Loss of Heterozygosity as Prognostic Markers in Oral Squamous Cell Carcinoma: Molecular Mechanisms, Detection Techniques, and Therapeutic Strategies. Genes Chromosomes Cancer 2024, 63, e70002. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Zhou, Y.; Acharya, A.; Savkovic, V.; Xu, C.; Wu, N.; Deng, Y.; Hu, X.; Li, H.; et al. Shared genetic and epigenetic mechanisms between chronic periodontitis and oral squamous cell carcinoma. Oral Oncol. 2018, 86, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Lu, Y.; Liu, T.; Shang, H.; Lu, H.; Dong, B.; Xu, Y. Genetic and therapeutic for oral lichen planus and diabetes mellitus: A comprehensive study. BMC Oral Health 2024, 24, 1226. [Google Scholar] [CrossRef] [PubMed]
- Aghiorghiesei, A.I.; Nutu, A.; Mehterov, N.; Kontos, C.K.; Vladimirov, B.; Buduru, R.; Braicu, C.; Berindan-Neagoe, I. Roles of miR-181 Family Members in OSCC: Implications for Therapy and Diagnostics. Cancer Med. 2025, 14, e71266. [Google Scholar] [CrossRef]
- You, X.; Zhou, Z.; Chen, W.; Wei, X.; Zhou, H.; Luo, W. MicroRNA-495 confers inhibitory effects on cancer stem cells in oral squamous cell carcinoma through the HOXC6-mediated TGF-β signaling pathway. Stem Cell Res. Ther. 2020, 11, 117. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and its Analogues in Oral Squamous Cell Carcinoma: State-of-the-art and Therapeutic Potential. Anticancer. Agents Med. Chem. 2025, 25, 313–329. [Google Scholar] [CrossRef]
- Belloni, A.; Campagna, R.; Notarstefano, V.; Pozzi, V.; Orilisi, G.; Pompei, V.; Togni, L.; Mascitti, M.; Sartini, D.; Giorgini, E.; et al. Deepening Cisplatin sensitivity on Oral Squamous cell Carcinoma cell lines after PON2 knockdown: A FTIRM investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 330, 125726. [Google Scholar] [CrossRef]
- Pomella, S.; Melaiu, O.; Cifaldi, L.; Bei, R.; Gargari, M.; Campanella, V.; Barillari, G. Biomarkers Identification in the Microenvironment of Oral Squamous Cell Carcinoma: A Systematic Review of Proteomic Studies. Int. J. Mol. Sci. 2024, 25, 8929. [Google Scholar] [CrossRef]
- Girod, S.C.; Pfeiffer, P.; Ries, J.; Pape, H.D. Proliferative activity and loss of function of tumour suppressor genes as ‘biomarkers’ in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 1998, 36, 252–260. [Google Scholar] [CrossRef]
- Nizar Jawad, Z. Epigenetic and genetic events of oral squamous cell carcinoma: Perspective on DNA methylation, silencing of tumor suppressor gene, and activating oncogenes. Cell. Mol. Biol. 2025, 71, 96–104. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, A.; Srinivasan, K. A Current Review of Machine Learning and Deep Learning Models in Oral Cancer Diagnosis: Recent Technologies, Open Challenges, and Future Research Directions. Diagnostics 2023, 13, 1353. [Google Scholar] [CrossRef]
- Rigotti, P.; Polizzi, A.; Quinzi, V.; Blasi, A.; Lombardi, T.; Lo Muzio, E.; Isola, G. Cell-Free DNA as a Prognostic Biomarker in Oral Carcinogenesis and Oral Squamous Cell Carcinoma: A Translational Perspective. Cancers 2025, 17, 2366. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, J.; He, W.; Ren, J. Neutrophil extracellular traps in cancer. MedComm 2024, 5, e647. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, J.J. Expression Levels of miR-181 Family Members in Oral Biofluids as Biomarkers for Periodontitis Severity. Tohoku J. Exp. Med. 2024, 264, 121–130. [Google Scholar] [CrossRef]
- Jansson, L.; Lundmark, A.; Modin, C.; Gustafsson, A.; Yucel-Lindberg, T. Levels of matrix metalloproteinase-1 (MMP-1), MMP-2, MMP-3, osteopontin, pentraxin-3, and thymic stromal lymphopoietin in crevicular fluid samples from peri-implantitis, periodontitis, and healthy sites. J. Periodontal Res. 2025, 60, 473–483. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, W.; Lu, Y.; Li, H.; Yang, Y.; Geng, F.; Liu, J.; Lin, L.; Pan, Y.; Li, C. Association between periodontitis and inflammatory comorbidities: The common role of innate immune cells, underlying mechanisms and therapeutic targets. Int. Immunopharmacol. 2024, 128, 111558. [Google Scholar] [CrossRef]
- Isola, G.; Pesce, P.; Polizzi, A.; Lo Giudice, A.; Cicciù, M.; Scannapieco, F.A. Effects of minimally invasive non-surgical therapy on C-reactive protein, lipoprotein-associated phospholipase A2, and clinical outcomes in periodontitis patients: A 1-year randomized, controlled clinical trial. J. Periodontol. 2024, 95, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Teles, F.; Martin, L.; Patel, M.; Hu, W.; Bittinger, K.; Kallan, M.J.; Chandrasekaran, G.; Cucchiara, A.J.; Giannobile, W.V.; Stephens, D.; et al. Gingival Crevicular Fluid Biomarkers During Periodontitis Progression and After Periodontal Treatment. J. Clin. Periodontol. 2025, 52, 40–55. [Google Scholar] [CrossRef]
- Guzeldemir-Akcakanat, E.; Sunnetci-Akkoyunlu, D.; Balta-Uysal, V.M.; Özer, T.; Işik, E.B.; Cine, N. Differentially expressed miRNAs associated with generalized aggressive periodontitis. Clin. Oral Investig. 2023, 28, 7. [Google Scholar] [CrossRef]
- Magdum, D.B.; Kulkarni, N.A.; Kavle, P.G.; Paraye, S.; Pohankar, P.S.; Giram, A.V. Salivary Neutrophil-to-Lymphocyte Ratio as a Prognostic Predictor of Oral Premalignant and Malignant Disorders: A Prospective Study. Cureus 2024, 16, e56273. [Google Scholar] [CrossRef]
- Walther, K.A.; Gröger, S.; Vogler, J.A.H.; Wöstmann, B.; Meyle, J. Inflammation indices in association with periodontitis and cancer. Periodontol. 2000 2024, 96, 281–315. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Pesce, P.; Kocher, T. Effect of quadrantwise versus full-mouth subgingival instrumentation on clinical and microbiological parameters in periodontitis patients: A randomized clinical trial. J. Periodontal Res. 2024, 59, 647–656. [Google Scholar] [CrossRef]
- Nocini, R.; Vianini, M.; Girolami, I.; Calabrese, L.; Scarpa, A.; Martini, M.; Morbini, P.; Marletta, S.; Brunelli, M.; Molteni, G.; et al. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin. Exp. Dent. Res. 2022, 8, 690–698. [Google Scholar] [CrossRef]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer—current concepts of the etiopathogenesis. Oncol. Rev. 2020, 14, 465. [Google Scholar] [CrossRef]
- Li, T.-J.; Hao, Y.-h.; Tang, Y.-l.; Liang, X.-h. Periodontal Pathogens: A Crucial Link Between Periodontal Diseases and Oral Cancer. Front. Microbiol. 2022, 13, 919633. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Xiao, T.; Wang, B.; Wang, L.; Liu, G.; Wang, R.; Xie, C.; Tang, Z. Mechanisms and markers of malignant transformation of oral submucous fibrosis. Heliyon 2024, 10, e23314. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Li, Z.T.; Zhou, G. Developmental synergism in the management of oral potentially malignant disorders. Photodiagnosis Photodyn. Ther. 2023, 42, 103563. [Google Scholar] [CrossRef]
- Viet, C.T.; Zhang, M.; Dharmaraj, N.; Li, G.Y.; Pearson, A.T.; Manon, V.A.; Grandhi, A.; Xu, K.; Aouizerat, B.E.; Young, S. Artificial Intelligence Applications in Oral Cancer and Oral Dysplasia. Tissue Eng. Part. A 2024, 30, 640–651. [Google Scholar] [CrossRef]
- Soghli, N.; Khormali, A.; Mahboubi, D.; Peng, A.; Miguez, P.A. Recent advancements in artificial intelligence-powered cancer prediction from oral microbiome. Periodontol. 2000, 2025; Epub ahead of print. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).