Novel Variants in Sperm Mitochondrial Cytochrome C Oxidase II (MT-CO2) Gene Associated with Asthenozoospermia in Jordan
Abstract
1. Introduction
2. Materials and Methods
2.1. Semen Samples Collection and Analysis
2.2. Sperm mtDNA Extraction
2.3. Polymerase Chain Reaction (PCR)
2.4. Sequencing
2.5. Data and Statistical Analysis
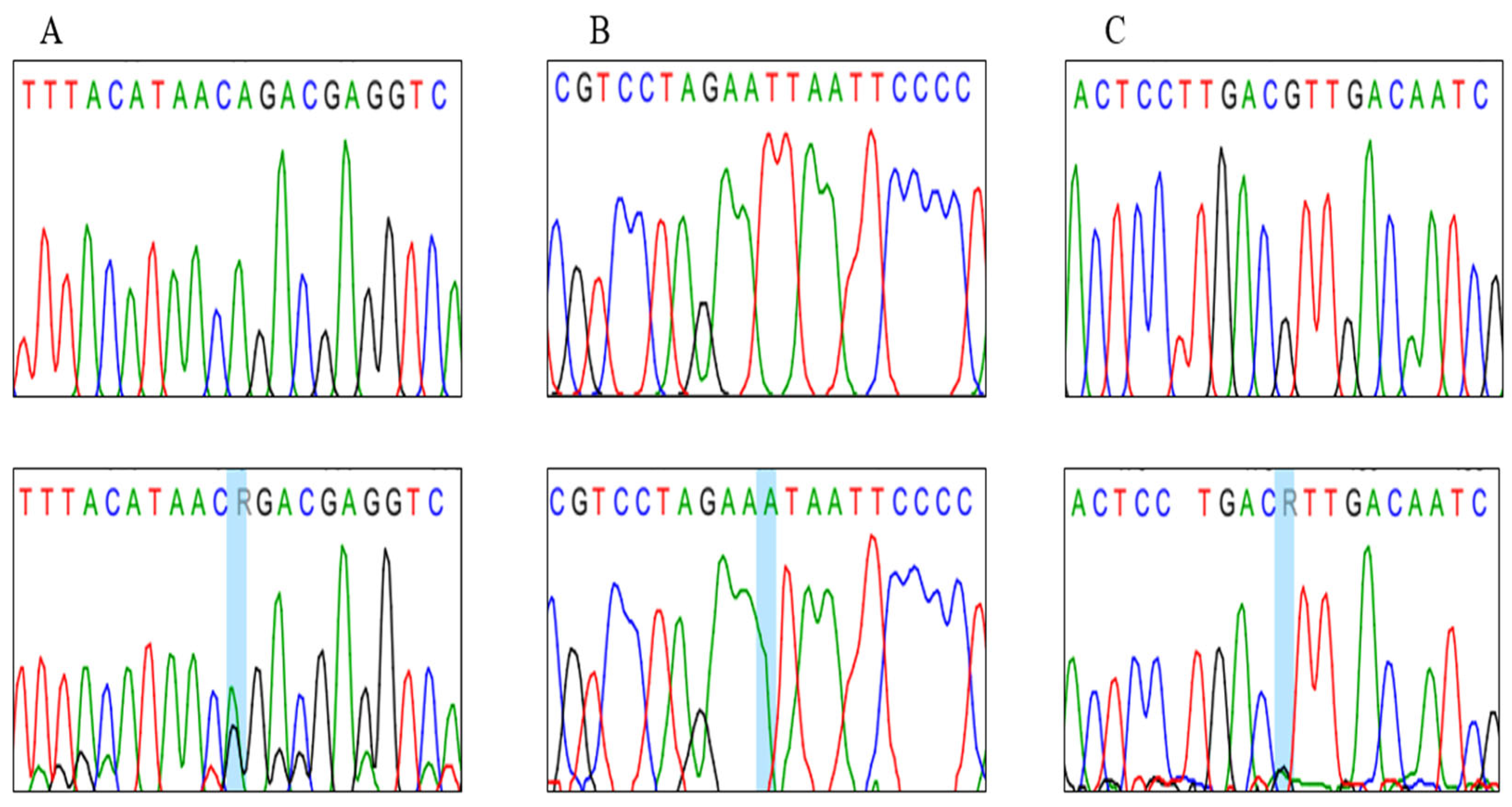
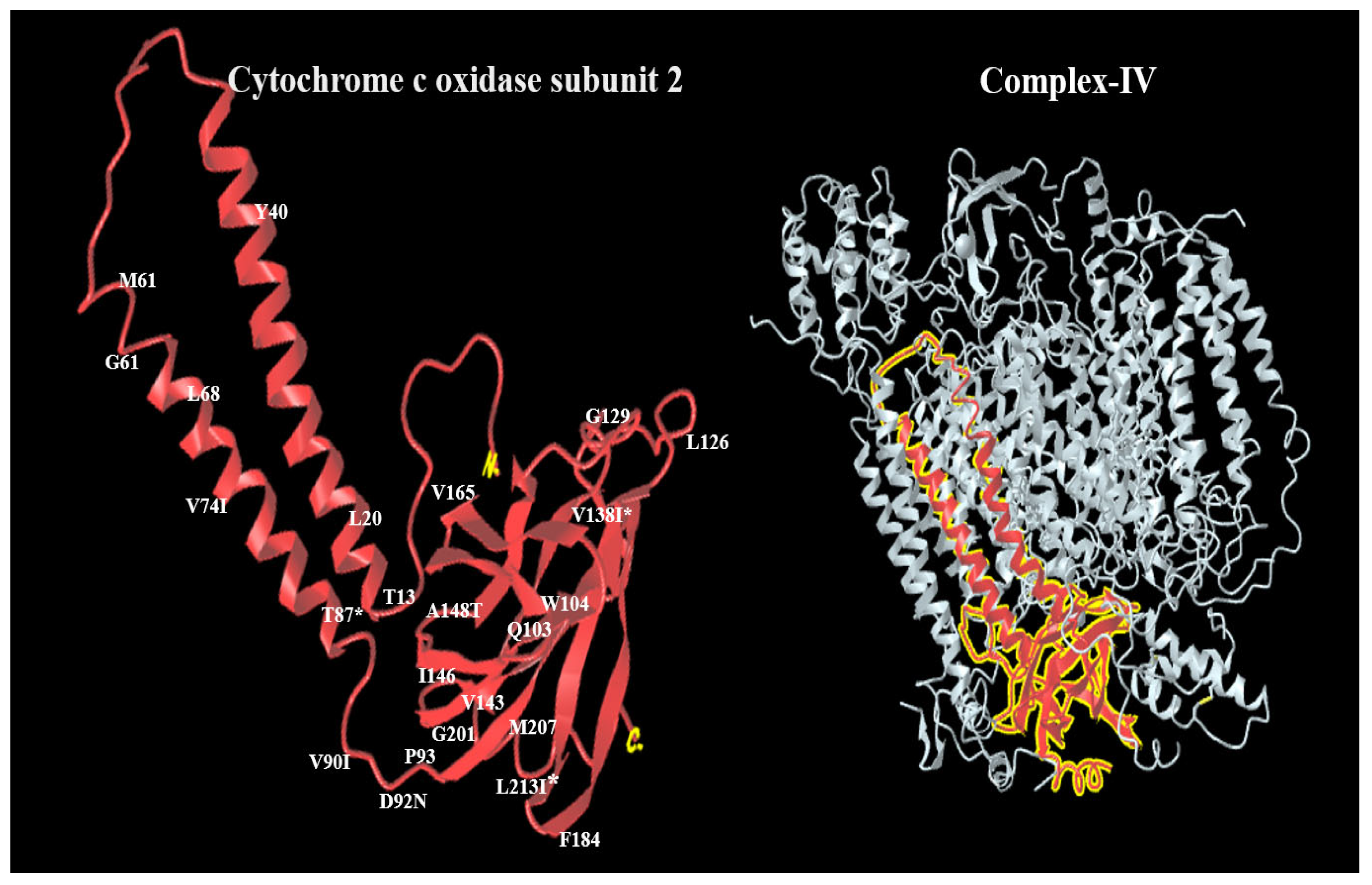
3. Results
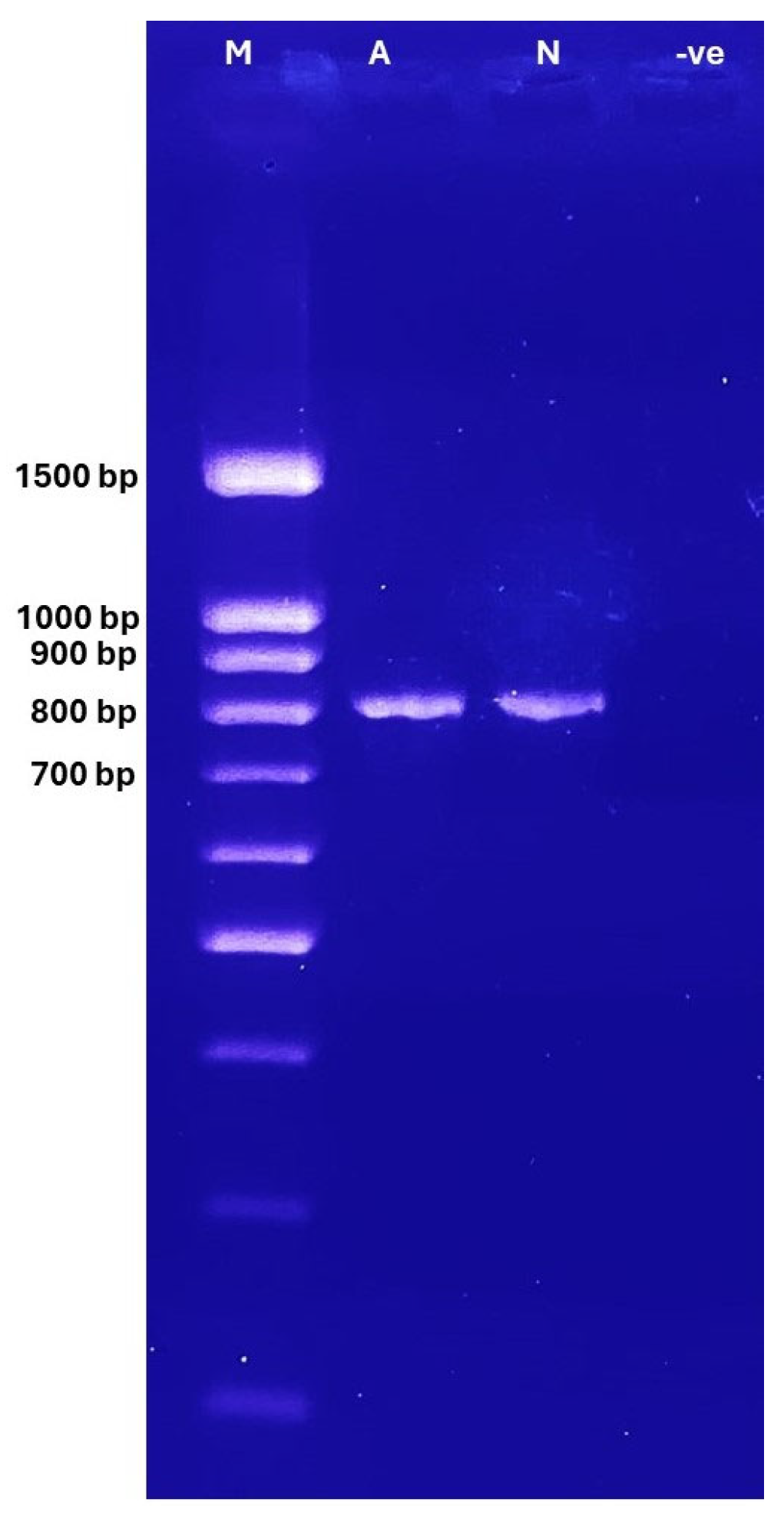
3.1. Gel Electrophoresis
3.2. Genotypes Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mansour, H.A.E.-H. Infertility diagnosis and management. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 81. [Google Scholar] [CrossRef]
- Brugo-Olmedo, S.; Chillik, C.; Kopelman, S. Definition and causes of infertility. Reprod. Biomed. Online 2001, 2, 173–185. [Google Scholar] [CrossRef]
- Thwaites, A.; Hall, J.; Barrett, G.; Stephenson, J. Contraception after in vitro fertilisation (IVF): A qualitative study of the views of women who have had spontaneous pregnancies after successful IVF. Reprod. Health 2022, 19, 40. [Google Scholar] [CrossRef]
- Withers, M. Infertility Among Women in Low- and Middle-Income Countries. In Handbook of Global Health; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar]
- Eisenberg, M.L.; Esteves, S.C.; Lamb, D.J.; Hotaling, J.M.; Giwercman, A.; Hwang, K.; Cheng, Y.-S. Male infertility. Nat. Rev. Dis. Primers 2023, 9, 49. [Google Scholar] [CrossRef]
- Leslie, S.; Soon-Sutton, T.; Khan, M.A. Male Infertility; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Selvam, M.K.P.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Mughal, I. Role of Human Sperm Mitochondrial DNA in Infertility. Ph.D. Thesis, Quaid-i-Azam University, Islamabad, Pakistan, 2015. [Google Scholar]
- Boguenet, M.; Bouet, P.-E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef]
- Chianese, R.; Pierantoni, R. Mitochondrial reactive oxygen species (ROS) production alters sperm quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S. Semen analysis and sperm function tests: How much to test? Indian J. Urol. IJU J. Urol. Soc. India 2011, 27, 41. [Google Scholar] [CrossRef]
- Dias, T.R.; Cho, C.-L.; Agarwal, A. Sperm assessment: Traditional approaches and their indicative value. In In Vitro Fertilization: A Textbook of Current and Emerging Methods and Devices; Springer International Publishing: Cham, Switzerland, 2019; pp. 249–263. [Google Scholar]
- Barbăroșie, C.; Agarwal, A.; Henkel, R. Diagnostic value of advanced semen analysis in evaluation of male infertility. Andrologia 2021, 53, e13625. [Google Scholar] [CrossRef]
- Al Zoubi, M.S.; Al-Batayneh, K.; Alsmadi, M.; Rashed, M.; Al-Trad, B.; Al Khateeb, W.; Aljabali, A.; Otoum, O.; Al-Talib, M.; Batiha, O. 4977-bp human mitochondrial DNA deletion is associated with asthenozoospermic infertility in Jordan. Andrologia 2020, 52, e13379. [Google Scholar] [CrossRef]
- Al Zoubi, M.S.; Al-Talafha, A.M.; Al Sharu, E.; Al-Trad, B.; Alzu’bi, A.; AbuAlarjah, M.I.; Shehab, Q.; Alsmadi, M.; Al-Batayneh, K.M. Correlation of Sperm Mitochondrial DNA 7345 Bp and 7599 Bp Deletions with Asthenozoospermia in Jordanian Population. J. Reprod. Infertil. 2021, 22, 165. [Google Scholar]
- Al Zoubi, M.S.; Bataineh, H.; Rashed, M.; Al-Trad, B.; Aljabali, A.A.; Al-Zoubi, R.M.; Al Hamad, M.; Issam AbuAlArjah, M.; Batiha, O.; Al-Batayneh, K.M. CAG repeats in the androgen receptor gene is associated with oligozoospermia and teratozoospermia in infertile men in Jordan. Andrologia 2020, 52, e13728. [Google Scholar] [CrossRef]
- Saleh Jaweesh, M.; Hammadeh, M.E.; Dahadhah, F.W.; Al Zoubi, M.S.; Amor, H. Association between the single nucleotide variants of the mitochondrial cytochrome B gene (MT-CYB) and the male infertility. Mol. Biol. Rep. 2022, 49, 3609–3616. [Google Scholar] [CrossRef]
- Dahadhah, F.W.; Jaweesh, M.S.; Al Zoubi, M.S.; Alarjah, M.I.A.; Hammadeh, M.E.; Amor, H. Mitochondrial nicotinamide adenine dinucleotide hydride dehydrogenase (NADH) subunit 4 (MTND4) polymorphisms and their association with male infertility. J. Assist. Reprod. Genet. 2021, 38, 2021–2029. [Google Scholar] [CrossRef]
- Rajender, S.; Rahul, P.; Mahdi, A.A. Mitochondria, spermatogenesis and male infertility. Mitochondrion 2010, 10, 419–428. [Google Scholar] [CrossRef]
- Cowan, K.J. The mitochondria: Powerhouse of the cell. In Functional Metabolism: Regulation and Adaptation; John Wiley and Sons: Hoboken, NJ, USA, 2004; pp. 211–241. [Google Scholar]
- Amor, H.; Hammadeh, M.E. A systematic review of the impact of mitochondrial variations on male infertility. Genes 2022, 13, 1182. [Google Scholar] [CrossRef] [PubMed]
- Saleh Jaweesh, M. The Association Between Mitochondrial CYB, CO3, ATP6 and ATP8 Polymorphisms and Male Infertility. Ph.D. Thesis, University of Saarland, Saarbrücken, Germany, 2023. [Google Scholar]
- Benkhalifa, M.; Ferreira, Y.J.; Chahine, H.; Louanjli, N.; Miron, P.; Merviel, P.; Copin, H. Mitochondria: Participation to infertility as source of energy and cause of senescence. Int. J. Biochem. Cell Biol. 2014, 55, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–178. [Google Scholar]
- Kadenbach, B.; Hüttemann, M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015, 24, 64–76. [Google Scholar] [CrossRef]
- Metodiev, M.D.; Thompson, K.; Alston, C.L.; Morris, A.A.; He, L.; Assouline, Z.; Rio, M.; Bahi-Buisson, N.; Pyle, A.; Griffin, H. Recessive mutations in TRMT10C cause defects in mitochondrial RNA processing and multiple respiratory chain deficiencies. Am. J. Hum. Genet. 2016, 98, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Siwar, B.-G.; Myriam, G.; Afif, B.M.; Emna, M.-R.; Nozha, C.; Afifa, S.; Faiza, F.; Leila, A.-K. Two novel mutations in COII and tRNAHis mitochondrial genes in asthenozoospermic infertiles men. Biochem. Biophys. Res. Commun. 2014, 450, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Joshi, M.B.; Reddy, A.G.; Rasalkar, A.A.; Singh, L. Sperm mitochondrial mutations as a cause of low sperm motility. J. Androl. 2003, 24, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, S.A.; Rajkovic, A. Genetics of human female infertility. Biol. Reprod. 2019, 101, 549–566. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza-Tesarik, R. Mitochondria in human fertility and infertility. Int. J. Mol. Sci. 2023, 24, 8950. [Google Scholar] [CrossRef]
- Brischigliaro, M.; Zeviani, M. Cytochrome c oxidase deficiency. Biochim. Biophys. Acta (BBA)-Bioenerg. 2021, 1862, 148335. [Google Scholar] [CrossRef]
- Kanungo, S.; Morton, J.; Neelakantan, M.; Ching, K.; Saeedian, J.; Goldstein, A. Mitochondrial disorders. Ann. Transl. Med. 2018, 6, 475. [Google Scholar] [CrossRef] [PubMed]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility—An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Darehbagh, R.R.; Khalafi, B.; Allahveisi, A.; Habiby, M. Effects of the mitochondrial genome on germ cell fertility: A review of the literature. Int. J. Fertil. Steril. 2022, 16, 70. [Google Scholar]
- Zierz, C.M.; Baty, K.; Blakely, E.L.; Hopton, S.; Falkous, G.; Schaefer, A.M.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Ng, Y.S.; Taylor, R.W. A novel pathogenic variant in MT-CO2 causes an isolated mitochondrial complex IV deficiency and late-onset cerebellar ataxia. J. Clin. Med. 2019, 8, 789. [Google Scholar] [CrossRef]
- Ayekoue, J.E.H.; N’zi, K.G.S.; Ako, A.A.B.; N’guessan, M.-F.; Yayé, Y.G.; Coulibaly, F.A.; Djaman, A.J. Polymorphism of mitochondrial DNA genes involved in asthenozoospermia in infertile patients of Côte d’Ivoire. Reprod. Dev. Med. 2023, 7, 38–43. [Google Scholar] [CrossRef]
- Saleh Jaweesh, M.; Hammadeh, M.E.; Dahadhah, F.W.; Al Smadi, M.A.; Al Zoubi, M.S.; Alarjah, M.I.A.; Amor, H. A lack of a definite correlation between male sub-fertility and single nucleotide polymorphisms in sperm mitochondrial genes MT-CO3, MT-ATP6 and MT-ATP8. Mol. Biol. Rep. 2022, 49, 10229–10238. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.-H.; Huang, X.-H.; Geng, X.-J.; Li, Q.; Zhang, Y.; Dou, Q. Correlation between sperm mitochondrial ND5 and ND6 gene variations and total fertilisation failure. Arch. Med. Sci. 2020, 16, 692–698. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequences (5′ → 3′) | Cycling Conditions | Product Size (bp) | |
|---|---|---|---|---|
| Forward | 5-CATGGCCTCCATGACTTTTT-3 | Initial denaturation 94 °C 5 min Denaturation 94 °C 40 s Annealing 62 °C 40 s Extension 72 °C 45 s Final extension 72 °C 5 min | 35 cycles | 820 |
| Reverse | 5-GTTAGCTTTACAGTGGGCTC-3 |
| Parameter | Age (Mean) | Motility (Mean) | p-Value t-Test |
|---|---|---|---|
| Asthenozoospermic (N = 119) | 34.3 | 12.58 | 0.001 |
| Normozoospermic (N = 77) | 32.66 | 62.10 |
| Position | Codon Change | Amino Acid Change | Homoplasmy/Heteroplasmy/ | No. of Individuals | Polyphen2 Prediction |
|---|---|---|---|---|---|
| m.7846 A>G | ACA>ACG | T87T | Heteroplasmy (A, G) | 5 Asthenozoospermic 2 Normozoospermic | - |
| m.8222 T>A | TTA>TAA | L213I | Homoplasmy (A, A) | 1 Asthenozoospermic 1 Normozoospermic | Possibly damaging |
| m.7997 G>A | GTT>ATT | V138I | Homoplasmy (G, G) Heteroplasmy (G, A) | 3 Asthenozoospermic 1 Normozoospermic | Probably damaging |
| rs ID | Contig Position | Codon Change | Amino Acid Change | Type of Variant | Homoplasmy/Heteroplasmy/ | Frequency of Mutation Asthenozoospermic (N) (%) | Frequency of Mutation Normozoospermic (N) (%) | p Value (Chi-Square Test). |
|---|---|---|---|---|---|---|---|---|
| rs28358879 | 7624T>A | ACT > ACA | Thr13 | Synonymous | Homoplasmy (A, A) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs1556423316 | 7645T>C | CTT > CTC | Leu20 | Synonymous | Homoplasmy (C, C) Heteroplasmy (T, C) | 7/109 (6.42%) 3/109 (2.75%) | 14/58 (24.14%) 5/58 (8.62%) | 0.0072 |
| rs1556423330 | 7705T>C | TAT > TAC | Tyr40 | Synonymous | Homoplasmy (C, C) | 3/116 (2.59%) | 0/77 (0%) | 0.1603 |
| rs41534044 | 7768A>G | ATA > ATG | Met61 | Synonymous | Homoplasmy (G, G) | 3/116 (2.59%) | 0/77 (0%) | 0.1603 |
| rs368038563 | 7771A>G | GAA > GAG | Glu62 | Synonymous | Homoplasmy (G, G) | 2/117 (1.71%) | 0/77 (0%) | 0.2529 |
| rs386829014 | 7789G>A | CTG > CTA | Leu68 | Synonymous | Homoplasmy (A, A) | 2/117 (1.71%) | 1/76 (1.31%) | 0.8315 |
| rs879119797 | 7805G>A | GTC > ATC | Val74Ile | Missense | Homoplasmy (A, A) | 3/116 (2.59%) | 2/75 (1.33%) | 0.9736 |
| rs386420037 | 7853G>A | GTC > ATC | Val90Ile | Missense | Homoplasmy (A, A) | 2/117 (1.71%) | 1/76 (1.31%) | 0.8315 |
| rs373105186 | 7859G>A | GAT > AAT | Asp92Asn | Missense | Homoplasmy (A, A) | 1/118 (0.85%) | 1/76 (1.31%) | 0.7552 |
| rs879034269 | 7864C>T | CCC > CCT | Pro93 | Synonymous | Homoplasmy (C, C) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs386829020 | 7894A>G | CAA> CAG | Gln103 | Synonymous | Heteroplasmy (A, G) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs1603221196 | 7897G>A | TGG> TGA | Trp104 | Synonymous | Homoplasmy (A, A) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs373420717 | 7961T>C | TTA > CTA | Leu126 | Synonymous | Homoplasmy (C, C) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs879023568 | 7963A>G | TTA > TTG | Leu126 | Synonymous | Homoplasmy (G, G) | 2/117 (1.71%) | 0/77 (0%) | 0.2529 |
| rs878954138 | 7972A>G | GAA> GAG | Glu129 | Synonymous | Heteroplasmy (A, G) | 0/119 (0%) | 1/76 (1.31%) | 0.2126 |
| rs879223416 | 8014A>G | GTA > GTT | Val143 | Synonymous | Homoplasmy (G, G) | 1/118 (0.85%) | 4/73 (5.48%) | 0.0590 |
| rs879039143 | 8023T>C | ATT > ATC | Ile146 | Synonymous | Homoplasmy (C, C) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs1116904 | 8027G>A | GCC> ACC | Ala148Thr | Missense | Homoplasmy (A, A) Heteroplasmy (G, A) | 0/118 (0%) 1/118 (0.85%) | 1/76 (1.31%) 0/76 (0%) | 0.7552 |
| rs371628304 | 8080C>T | GTC > GTA | Val165 | Synonymous | Homoplasmy (T, T) | 1/118 (0.85%) | 1/76 (1.31%) | 0.7552 |
| rs879043235 | 8137C>T | TTC > TTT | Phe184 | Synonymous | Homoplasmy (T, T) | 1/118 (0.85%) | 0/77 (0%) | 0.4200 |
| rs28651339 | 8188A>G | GGA > GGG | Gly201 | Synonymous | Homoplasmy (G, G) Heteroplasmy (A, G) | 1/118 (0.85%) 0/118 (0%) | 0/76 (0%) 1/76 (1.31%) | 0.3340 |
| rs28358883 | 8206G>A | ATG > ATA | Met207 | Synonymous | Homoplasmy (A, A) Heteroplasmy (G, A) | 4/114 (3.51%) 1/114 (0.88%) | 1/76 (1.31%) 0/76 (0%) | 0.4852 |
| rs386829020 | 7894 A>G | CAA> CAG | Gln103 | Synonymous | Heteroplasmy (A, G) | 1/118 (0.85%) | 0/77 (0%) | 0.4253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Zoubi, M.S.; AlQuraan, R.N.; Al-Smadi, A.; AlSmadi, M.A.; AbuAlArja, M.; Alkaraki, A.K.; Al-Trad, B.; Al-Zoubi, R.M.; Al-Batayneh, K. Novel Variants in Sperm Mitochondrial Cytochrome C Oxidase II (MT-CO2) Gene Associated with Asthenozoospermia in Jordan. Curr. Issues Mol. Biol. 2025, 47, 901. https://doi.org/10.3390/cimb47110901
Al Zoubi MS, AlQuraan RN, Al-Smadi A, AlSmadi MA, AbuAlArja M, Alkaraki AK, Al-Trad B, Al-Zoubi RM, Al-Batayneh K. Novel Variants in Sperm Mitochondrial Cytochrome C Oxidase II (MT-CO2) Gene Associated with Asthenozoospermia in Jordan. Current Issues in Molecular Biology. 2025; 47(11):901. https://doi.org/10.3390/cimb47110901
Chicago/Turabian StyleAl Zoubi, Mazhar Salim, Razan N. AlQuraan, Asmaa Al-Smadi, Mohammad A. AlSmadi, Manal AbuAlArja, Almuthanna K. Alkaraki, Bahaa Al-Trad, Raed M. Al-Zoubi, and Khalid Al-Batayneh. 2025. "Novel Variants in Sperm Mitochondrial Cytochrome C Oxidase II (MT-CO2) Gene Associated with Asthenozoospermia in Jordan" Current Issues in Molecular Biology 47, no. 11: 901. https://doi.org/10.3390/cimb47110901
APA StyleAl Zoubi, M. S., AlQuraan, R. N., Al-Smadi, A., AlSmadi, M. A., AbuAlArja, M., Alkaraki, A. K., Al-Trad, B., Al-Zoubi, R. M., & Al-Batayneh, K. (2025). Novel Variants in Sperm Mitochondrial Cytochrome C Oxidase II (MT-CO2) Gene Associated with Asthenozoospermia in Jordan. Current Issues in Molecular Biology, 47(11), 901. https://doi.org/10.3390/cimb47110901








