Long-Term Liver-Targeted AAV8 Gene Therapy for Mucopolysaccharidosis IVA
Abstract
1. Introduction
2. Materials and Methods
2.1. Cassette Design and Expression of AAV Vector Production
2.2. Animal Experimentation
2.3. GALNS Enzyme Activity Assay
2.4. Glycosaminoglycans Assay
2.5. Glycosaminoglycan Extraction from Tissues
2.6. Detection of Plasma Anti-GALNS IgG Antibodies
2.7. Quantification of AAV Genome Copies
2.8. Assessment of Bone Pathology
2.9. Micro-Computed Tomography Analysis of Femur
2.10. Data Analysis
3. Results
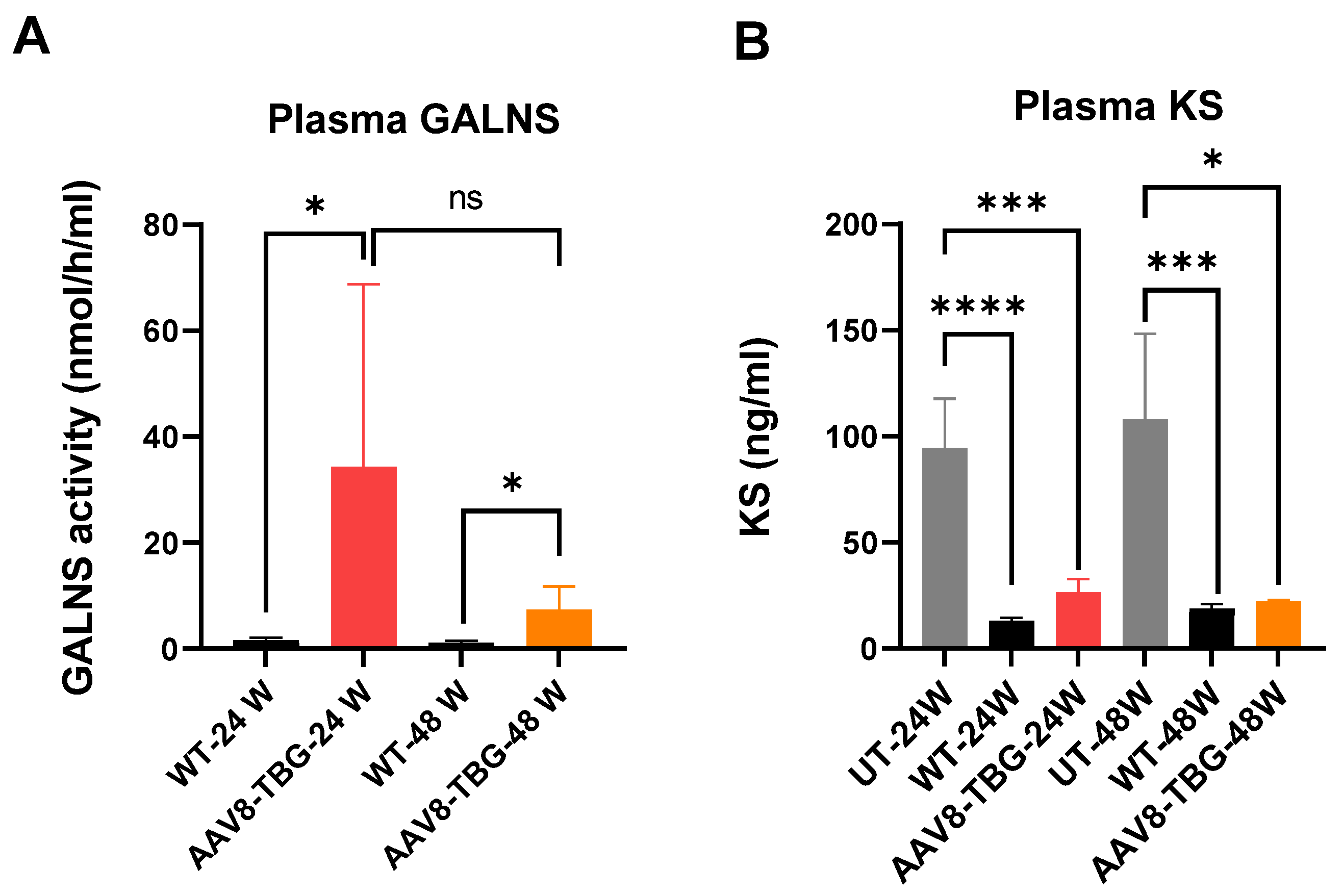
3.1. Enzyme Activity and KS Level in Plasma
3.1.1. GALNS Enzyme Activity in Plasma
3.1.2. Keratan Sulfate Levels in Plasma
3.2. GALNS Enzyme Activity in Tissues
3.2.1. GALNS Enzyme Activity in Liver
3.2.2. GALNS Enzyme Activity in Muscle
3.2.3. GALNS Enzyme Activity in Bone
3.2.4. GALNS Enzyme Activity in the Heart
3.2.5. GALNS Enzyme Activity in Spleen
3.2.6. GALNS Enzyme Activity in the Trachea
3.3. KS Levels in Tissues
3.3.1. KS Level in the Liver
3.3.2. KS Level in Muscle
3.3.3. KS Level in Bone (Humerus)
3.4. Biodistribution of AAV Genome
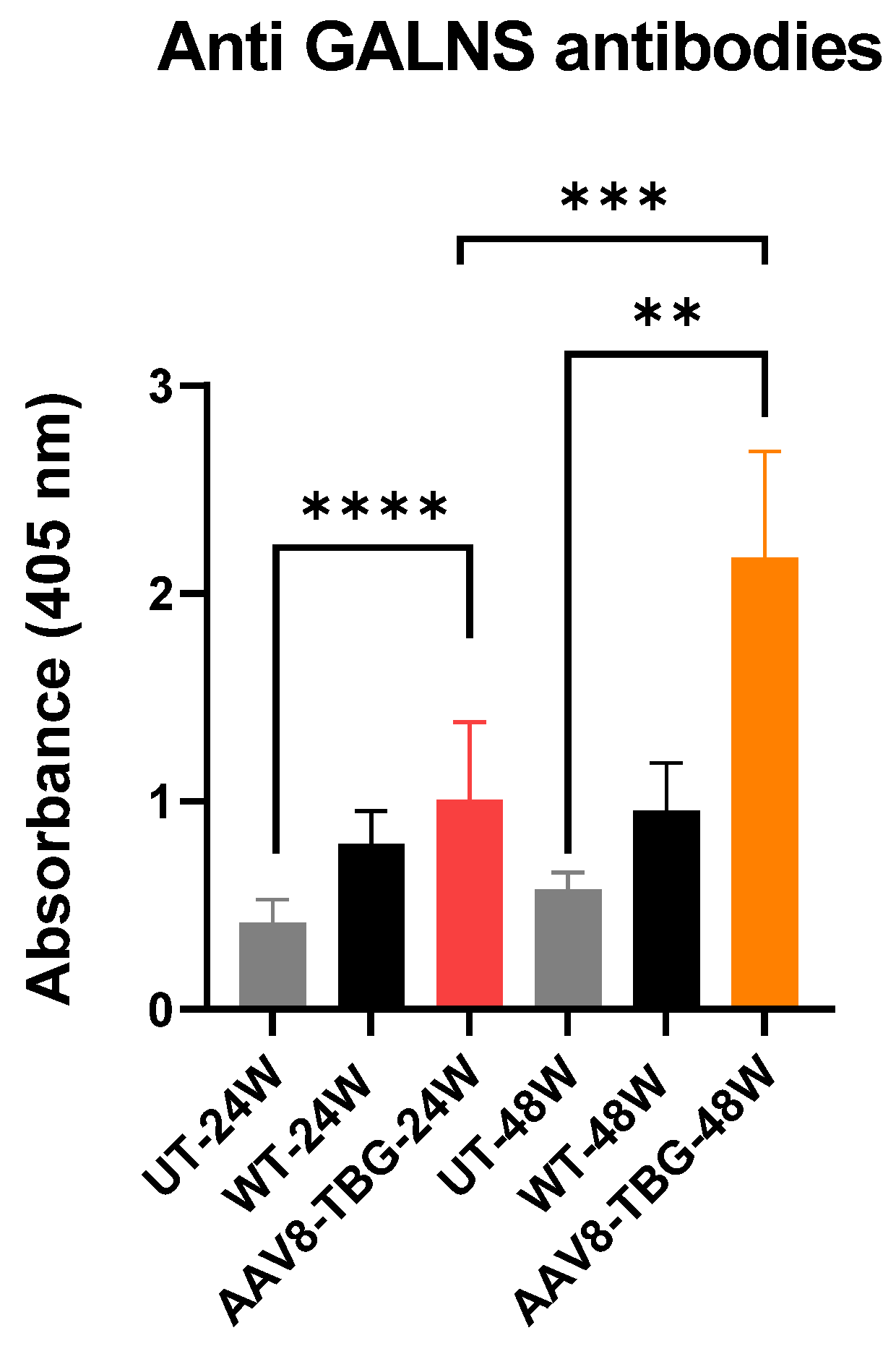
3.5. Humoral Response Against GALNS
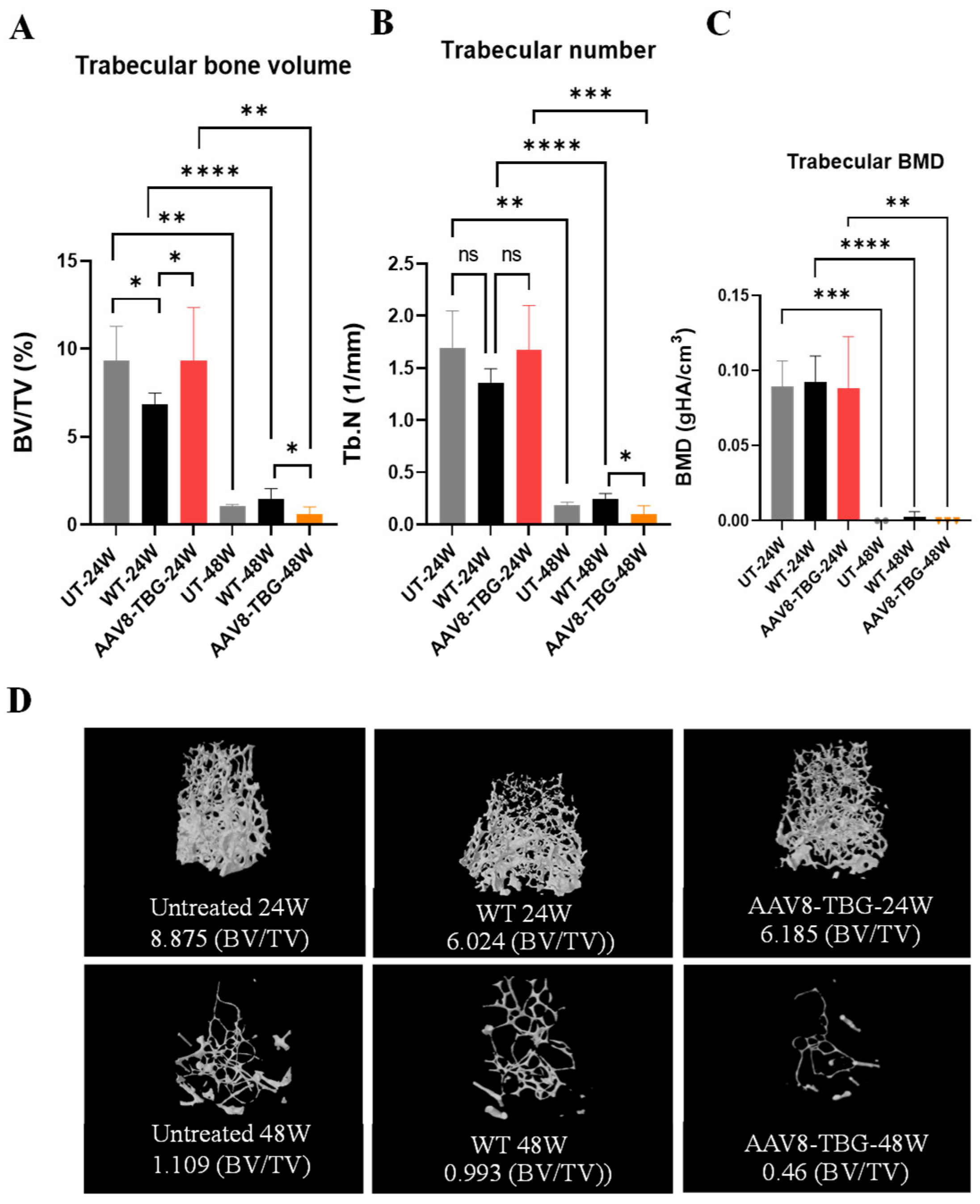
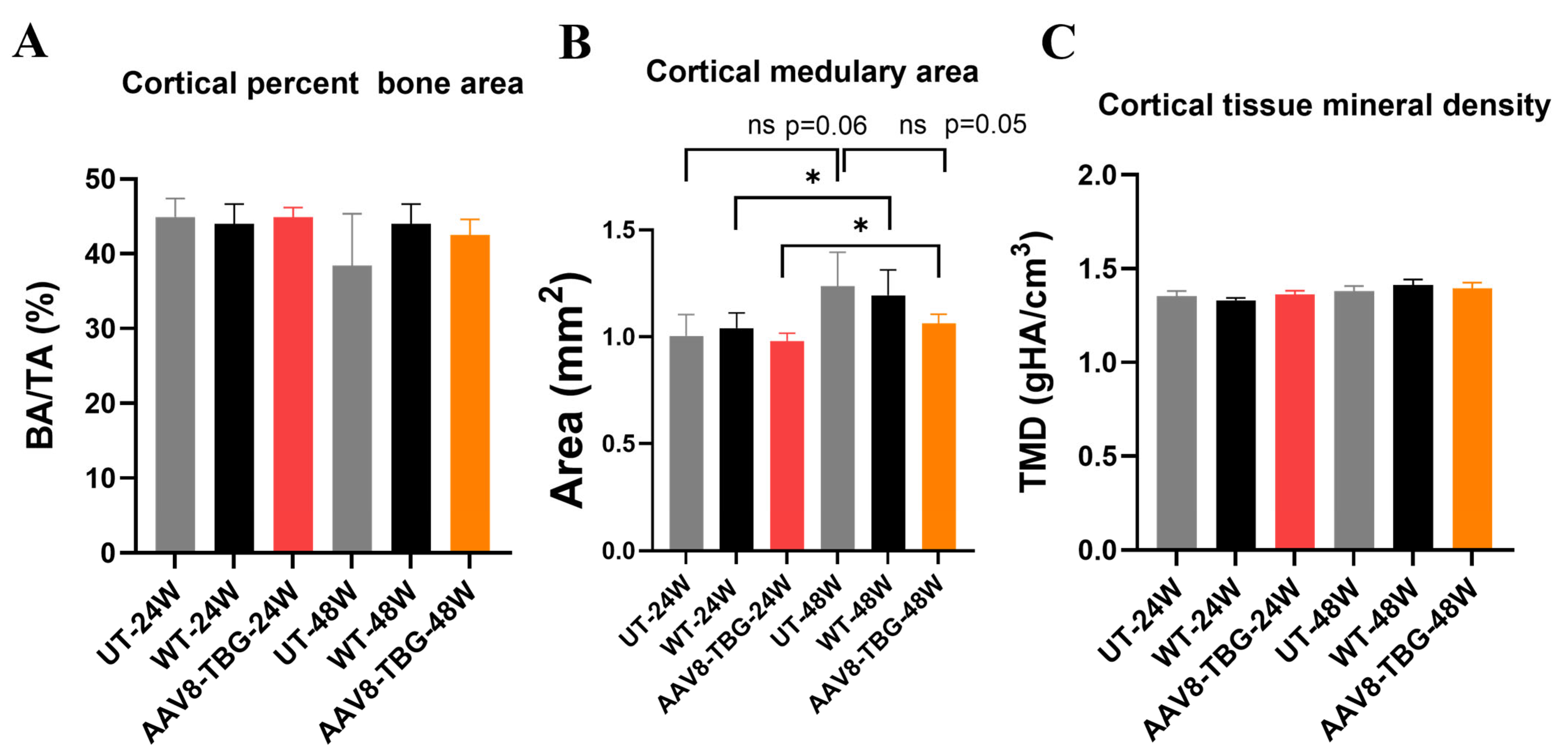
3.6. Micro-Computed Tomography Analysis of the Femur
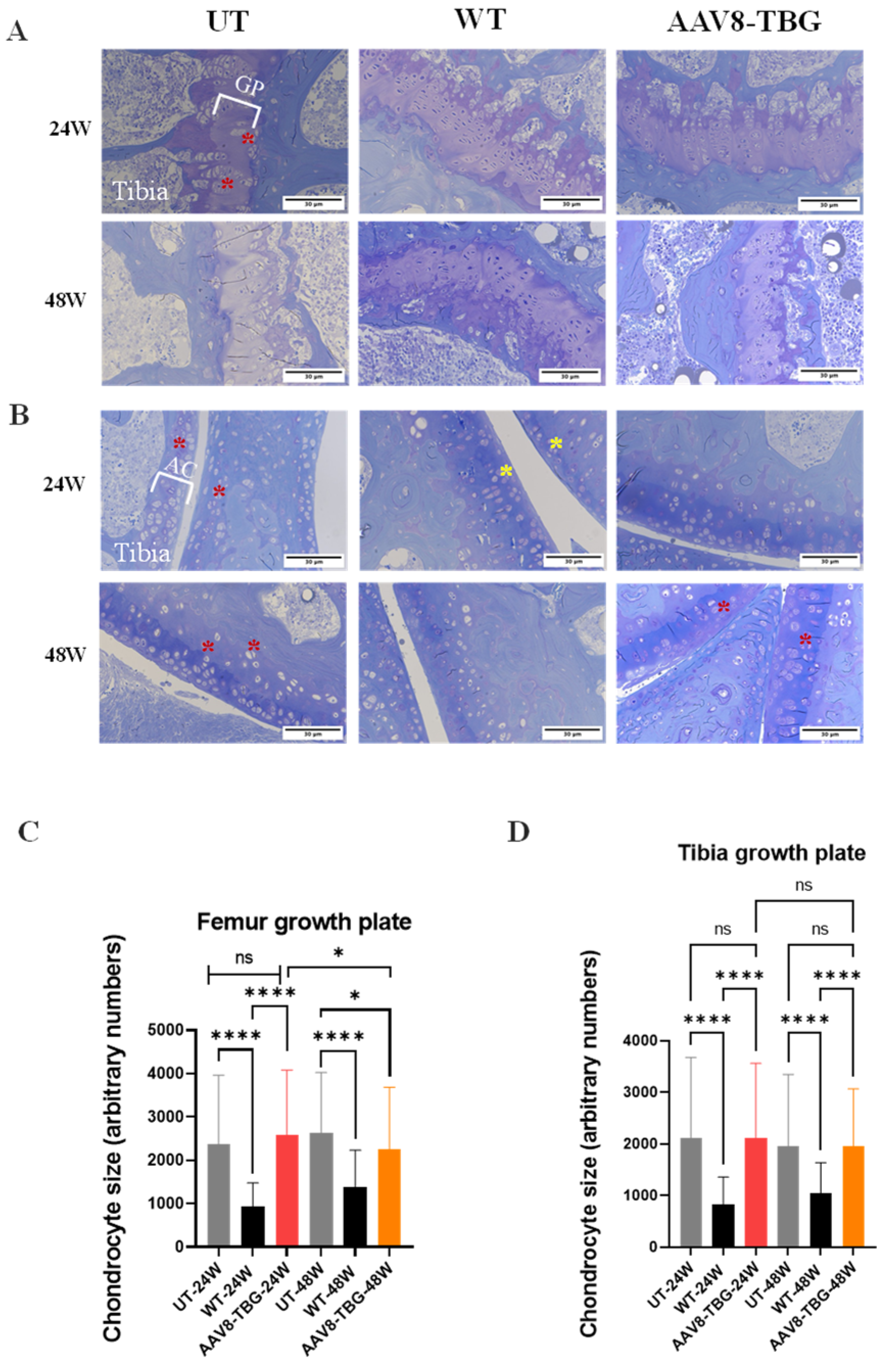
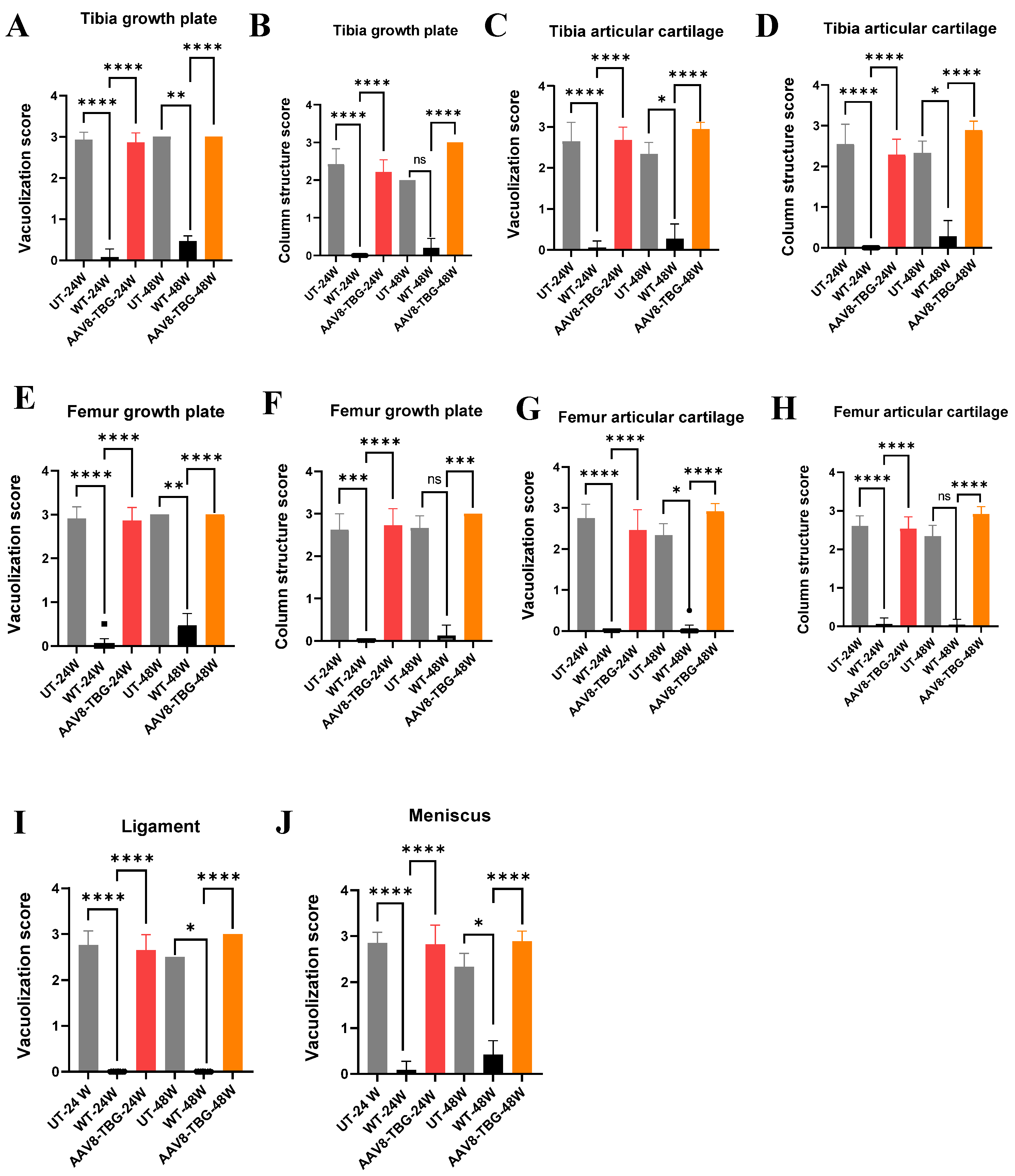
3.7. Bone and Cartilage Pathology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matalon, R.; Arbogast, B.; Justice, P.; Brandt, I.K.; Dorfman, A. Morquio’s syndrome: Deficiency of a chondroitin sulfate N-acetylhexosamine sulfate sulfatase. Biochem. Biophys. Res. Commun. 1974, 61, 759–765. [Google Scholar] [CrossRef]
- Di Ferrante, N.; Ginsberg, L.C.; Donnelly, P.V.; Di Ferrante, D.T.; Caskey, C.T. Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses. Science 1978, 199, 79–81. [Google Scholar] [CrossRef]
- Dorfman, A.; Arbogast, B.; Matalon, R. The enzymic defects in Morquio and Maroteaux-Lamy syndrome. Adv. Exp. Med. Biol. 1976, 68, 261–276. [Google Scholar]
- Singh, J.; Di Ferrante, N.; Niebes, P.; Tavella, D. N-acetylgalactosamine-6-sulfate sulfatase in man. Absence of the enzyme in Morquio disease. J. Clin. Investig. 1976, 57, 1036–1040. [Google Scholar] [CrossRef]
- Orii, T.; Kiman, T.; Sukegawa, K.; Kanemura, T.; Hattori, S.; Taga, T.; Hirota, K. Late onset Nacetylgalactosamine-6-sulfate sulfatase deficiency in two brothers. Connect. Tissue 1981, 13, 169–175. [Google Scholar]
- Neufeld, E.F.; Muenzer, J. The Metabolic and Molecular Bases of Inherited Disease. In The Mucopolysaccharidoses; Scriver, C., Ed.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Tomatsu, S.; Montano, A.M.; Oikawa, H.; Smith, M.; Barrera, L.; Chinen, Y.; Thacker, M.M.; Mackenzie, W.G.; Suzuki, Y.; Orii, T. Mucopolysaccharidosis type IVA (Morquio A disease): Clinical review and current treatment. Curr. Pharm. Biotechnol. 2011, 12, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Montaño, A.M.; Oikawa, H.; Giugliani, R.; Harmatz, P.; Smith, M.; Suzuki, Y.; Orii, T. Impairment of Body Growth in Mucopolysaccharidoses. Handb. Growth Growth Monit. Health Dis. 2012, 1, 2091–2117. [Google Scholar]
- Yasuda, E.; Fushimi, K.; Suzuki, Y.; Shimizu, K.; Takami, T.; Zustin, J.; Patel, P.; Ruhnke, K.; Shimada, T.; Boyce, B.; et al. Pathogenesis of Morquio A syndrome: An autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013, 109, 301–311. [Google Scholar] [CrossRef]
- Sawamoto, K.; Fushimi, K.; Suzuki, Y.; Shimizu, K.; Takami, T.; Zustin, J.; Patel, P.; Ruhnke, K.; Shimada, T.; Boyce, B. Mucopolysaccharidosis IVA: Diagnosis, Treatment, and Management. Int. J. Mol. Sci. 2020, 21, 1517. [Google Scholar] [CrossRef]
- Melbouci, M.; Mason, R.W.; Suzuki, Y.; Fukao, T.; Orii, T.; Tomatsu, S. Growth impairment in mucopolysaccharidoses. Mol. Genet. Metab. 2018, 124, 1–10. [Google Scholar] [CrossRef]
- Montano, A.M.; Tomatsu, S.; Gottesman, G.S.; Smith, M.; Orii, T. International Morquio A Registry: Clinical manifestation and natural course of Morquio A disease. J. Inherit. Metab. Dis. 2007, 30, 165–174. [Google Scholar] [CrossRef]
- Lavery, C.; Hendriksz, C. Mortality in patients with morquio syndrome a. JIMD Rep. 2015, 15, 59–66. [Google Scholar]
- Pizarro, C.; Davies, R.R.; Spurrier, E.A.; Theroux, M.; Averill, L.W.; Tomatsu, S.; Harmatz, P. Surgical reconstruction for severe tracheal obstruction in Morquio A syndrome. Ann. Thorac. Surg. 2016; in press. [Google Scholar]
- Tomatsu, S.; Averill, L.W.; Sawamoto, K.; Mackenzie, W.G.; Bober, M.B.; Pizarro, C.; Goff, C.J.; Xie, L.; Orii, T.; Theroux, M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol. Genet. Metab. 2016, 117, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Akyol, M.U.; Alden, T.D.; Amartino, H.; Ashworth, J.; Belani, K.; Berger, K.I.; Borgo, A.; Braunlin, E.; Eto, Y.; Gold, J.I.; et al. Recommendations for the management of MPS IVA: Systematic evidence- and consensus-based guidance. Orphanet J. Rare Dis. 2019, 14, 137. [Google Scholar] [CrossRef]
- Hughes, D.; Giugliani, R.; Guffon, N.; Jones, S.A.; Mengel, K.E.; Parini, R.; Matousek, R.; Hawley, S.M.; Quartel, A. Clinical outcomes in a subpopulation of adults with Morquio A syndrome: Results from a long-term extension study of elosulfase alfa. Orphanet J. Rare Dis. 2017, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luan, Z.; Jiang, H.; Fang, J.; Qin, M.; Lee, V.; Chen, J. Allogeneic Hematopoietic Stem Cell Transplantation in Thirty-Four Pediatric Cases of Mucopolysaccharidosis-A Ten-Year Report from the China Children Transplant Group. Biol. Blood Marrow Transpl. 2016, 22, 2104–2108. [Google Scholar] [CrossRef]
- Yabe, H.; Tanaka, A.; Chinen, Y.; Kato, S.; Sawamoto, K.; Yasuda, E.; Shintaku, H.; Suzuki, Y.; Orii, T.; Tomatsu, S. Hematopoietic stem cell transplantation for Morquio A syndrome. Mol. Genet. Metab. 2016, 117, 84–94. [Google Scholar] [CrossRef]
- Qi, Y.; Musson, D.G.; Schweighardt, B.; Tompkins, T.; Jesaitis, L.; Shaywitz, A.J.; Yang, K.; O’Neill, C.A. Pharmacokinetic and pharmacodynamic evaluation of elosulfase alfa, an enzyme replacement therapy in patients with Morquio A syndrome. Clin. Pharmacokinet. 2014, 53, 1137–1147. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montano, A.M.; Ohashi, A.; Gutierrez, M.A.; Oikawa, H.; Oguma, T.; Dung, V.C.; Nishioka, T.; Orii, T.; Sly, W.S. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2008, 17, 815–824. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montano, A.M.; Oikawa, H.; Dung, V.C.; Hashimoto, A.; Oguma, T.; Gutierrez, M.L.; Takahashi, T.; Shimada, T.; Orii, T.; et al. Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): Effect and limitations. Expert Opin. Orphan Drugs 2015, 3, 1279–1290. [Google Scholar] [CrossRef]
- Sawamoto, K.; Suzuki, Y.; Mackenzie, W.G.; Theroux, M.C.; Pizarro, C.; Yabe, H.; Orii, K.E.; Mason, R.W.; Orii, T.; Tomatsu, S. Current therapies for Morquio A syndrome and their clinical outcomes, Expert Opinion on Orphan Drugs. In Proceedings of the 14th International Symposium of Mucopolysaccharidoses and Related Diseases, Bonn, Germany, 14–17 July 2016. [Google Scholar]
- Tomatsu, S.; Sawamoto, K.; Almeciga-Diaz, C.J.; Shimada, T.; Bober, M.B.; Chinen, Y.; Yabe, H.; Montano, A.M.; Giugliani, R.; Kubaski, F.; et al. Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome. Drug Des. Devel. Ther. 2015, 9, 1937–1953. [Google Scholar] [CrossRef]
- Do Cao, J.; Wiedemann, A.; Quinaux, T.; Battaglia-Hsu, S.F.; Mainard, L.; Froissart, R.; Bonnemains, C.; Ragot, S.; Leheup, B.; Journeau, P.; et al. 30 months follow-up of an early enzyme replacement therapy in a severe Morquio A patient: About one case. Mol. Genet. Metab. Rep. 2016, 9, 42–45. [Google Scholar] [CrossRef]
- Doherty, C.; Stapleton, M.; Piechnik, M.; Mason, R.W.; Mackenzie, W.G.; Yamaguchi, S.; Kobayashi, H.; Suzuki, Y.; Tomatsu, S. Effect of enzyme replacement therapy on the growth of patients with Morquio A. J. Hum. Genet. 2019, 64, 625–635. [Google Scholar] [CrossRef]
- Tomatsu, S.; Montano, A.M.; Dung, V.C.; Ohashi, A.; Oikawa, H.; Oguma, T.; Orii, T.; Barrera, L.; Sly, W.S. Enhancement of drug delivery: Enzyme-replacement therapy for murine Morquio A syndrome. Mol. Ther. 2010, 18, 1094–1102. [Google Scholar] [CrossRef]
- Chinen, Y.; Higa, T.; Tomatsu, S.; Suzuki, Y.; Orii, T.; Hyakuna, N. Long-term therapeutic efficacy of allogenic bone marrow transplantation in a patient with mucopolysaccharidosis IVA. Mol. Genet. Metab. Rep. 2014, 1, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Khan, S.; Stapleton, M.; Wang, J.; Chen, J.; Wynn, R.; Yabe, H.; Chinen, Y.; Boelens, J.J.; Mason, R.W.; et al. Hematopoietic Stem Cell Transplantation for Mucopolysaccharidoses: Past, Present, and Future. Biol. Blood Marrow Transpl. 2019, 25, e226–e246. [Google Scholar] [CrossRef] [PubMed]
- Vellodi, A.; Young, E.; Cooper, A.; Lidchi, V.; Winchester, B.; Wraith, J.E. Long-term follow-up following bone marrow transplantation for Hunter disease. J. Inherit. Metab. Dis. 1999, 22, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Almeciga-Diaz, C.J.; Hidalgo, O.A.; Olarte-Avellaneda, S.; Rodriguez-Lopez, A.; Guzman, E.; Garzon, R.; Pimentel-Vera, L.N.; Puentes-Tellez, M.A.; Rojas-Rodriguez, A.F.; Gorshkov, K.; et al. Identification of Ezetimibe and Pranlukast as Pharmacological Chaperones for the Treatment of the Rare Disease Mucopolysaccharidosis Type IVA. J. Med. Chem. 2019, 62, 6175–6189. [Google Scholar] [CrossRef]
- Sawamoto, K.; Tomatsu, S. Development of Substrate Degradation Enzyme Therapy for Mucopolysaccharidosis IVA Murine Model. Int. J. Mol. Sci. 2019, 20, 4139. [Google Scholar] [CrossRef]
- Leal, A.F.; Cifuentes, J.; Torres, C.E.; Suarez, D.; Quezada, V.; Gomez, S.C.; Cruz, J.C.; Reyes, L.H.; Espejo-Mojica, A.J.; Almeciga-Diaz, C.J. Delivery and assessment of a CRISPR/nCas9-based genome editing system on in vitro models of mucopolysaccharidoses IVA assisted by magnetite-based nanoparticles. Sci. Rep. 2022, 12, 15045. [Google Scholar] [CrossRef]
- Celik, B.; Rintz, E.; Sansanwal, N.; Khan, S.; Bigger, B.; Tomatsu, S. Lentiviral Vector-Mediated Ex Vivo Hematopoietic Stem Cell Gene Therapy for Mucopolysaccharidosis IVA Murine Model. Hum. Gene Ther. 2024, 35, 917–937. [Google Scholar] [CrossRef]
- Sawamoto, K.; Karumuthil-Melethil, S.; Khan, S.; Stapleton, M.; Bruder, J.T.; Danos, O.; Tomatsu, S. Liver-Targeted AAV8 Gene Therapy Ameliorates Skeletal and Cardiovascular Pathology in a Mucopolysaccharidosis IVA Murine Model. Mol. Ther. Methods Clin. Dev. 2020, 18, 50–61. [Google Scholar] [CrossRef]
- Khan, S.A.; Alvarez, J.V.; Nidhi, F.N.U.; Benincore-Florez, E.; Tomatsu, S. Evaluation of AAV vectors with tissue-specific or ubiquitous promoters in a mouse model of mucopolysaccharidosis type IVA. Mol. Ther. Methods Clin. Dev. 2025, 33, 101447. [Google Scholar] [CrossRef]
- Buning, H.; Perabo, L.; Coutelle, O.; Quadt-Humme, S.; Hallek, M. Recent developments in adeno-associated virus vector technology. J. Gene. Med. 2008, 10, 717–733. [Google Scholar] [CrossRef]
- Rabinowitz, J.E.; Samulski, R. Building a better vector: The manipulation of AAV virions. Virology 2000, 278, 301–308. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef]
- Paneda, A.; Vanrell, L.; Mauleon, I.; Crettaz, J.S.; Berraondo, P.; Timmermans, E.J.; Beattie, S.G.; Twisk, J.; van Deventer, S.; Prieto, J.; et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum. Gene Ther. 2009, 20, 908–917. [Google Scholar] [CrossRef]
- Nam, H.J.; Lane, M.D.; Padron, E.; Gurda, B.; McKenna, R.; Kohlbrenner, E.; Aslanidi, G.; Byrne, B.; Muzyczka, N.; Zolotukhin, S.; et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J. Virol. 2007, 81, 12260–12271. [Google Scholar] [CrossRef] [PubMed]
- Cabanes-Creus, M.; Navarro, R.G.; Zhu, E.; Baltazar, G.; Liao, S.H.Y.; Drouyer, M.; Amaya, A.K.; Scott, S.; Nguyen, L.H.; Westhaus, A.; et al. Novel human liver-tropic AAV variants define transferable domains that markedly enhance the human tropism of AAV7 and AAV8. Mol. Ther. Methods Clin. Dev. 2022, 24, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Fuess, S.; Storm, T.A.; Muramatsu, S.; Nara, Y.; Kay, M.A. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J. Virol. 2005, 79, 214–224. [Google Scholar] [CrossRef]
- Thomas, C.E.; Storm, T.A.; Huang, Z.; Kay, M.A. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J. Virol. 2004, 78, 3110–3122. [Google Scholar] [CrossRef]
- Nam, H.J.; Gurda, B.L.; McKenna, R.; Potter, M.; Byrne, B.; Salganik, M.; Muzyczka, N.; Agbandje-McKenna, M. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J. Virol. 2011, 85, 11791–11799. [Google Scholar] [CrossRef]
- Monahan, P.E.; Lothrop, C.D.; Sun, J.; Hirsch, M.L.; Kafri, T.; Kantor, B.; Sarkar, R.; Tillson, D.M.; Elia, J.R.; Samulski, R.J. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: A strategy for broad clinical application. Mol. Ther. 2010, 18, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Couto, L.B.; Patarroyo-White, S.; Liu, T.; Nagy, D.; Vargas, J.A.; Zhou, S.; Scallan, C.D.; Sommer, J.; Vijay, S.; et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006, 108, 3321–3328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, T.; Qiao, C.; Zhou, L.; Wang, B.; Zhang, J.; Chen, C.; Li, J.; Xiao, X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005, 23, 321–328. [Google Scholar] [CrossRef]
- Rossi, A.; Romano, R.; Fecarotta, S.; Dell’Anno, M.; Pecorella, V.; Passeggio, R.; Zancan, S.; Parenti, G.; Santamaria, F.; Borgia, F.; et al. Multi-year enzyme expression in patients with mucopolysaccharidosis type VI after liver-directed gene therapy. Med 2024, 6, 100544. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.A. The mucopolysaccharidoses: A success of molecular medicine. Expert Rev. Mol. Med. 2008, 10, e1, Erratum in Expert Rev. Mol. Med. 2009, 11, e21. [Google Scholar] [CrossRef]
- Tomatsu, S.; Orii, K.O.; Vogler, C.; Nakayama, J.; Levy, B.; Grubb, J.H.; Gutierrez, M.A.; Shim, S.; Yamaguchi, S.; Nishioka, T.; et al. Mouse model of N-acetylgalactosamine-6-sulfate sulfatase deficiency (Galns-/-) produced by targeted disruption of the gene defective in Morquio A disease. Hum. Mol. Genet. 2003, 12, 3349–3358. [Google Scholar] [CrossRef]
- Oguma, T.; Tomatsu, S.; Montano, A.M.; Okazaki, O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 2007, 368, 79–86. [Google Scholar] [CrossRef]
- Oguma, T.; Toyoda, H.; Toida, T.; Imanari, T. Analytical method for keratan sulfates by high-performance liquid chromatography/turbo-ionspray tandem mass spectrometry. Anal. Biochem. 2001, 290, 68–73. [Google Scholar] [CrossRef]
- Oguma, T.; Tomatsu, S.; Okazaki, O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed. Chromatogr. 2007, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Yoshida, K.; Shibata, Y.; Kimata, K. Tetrasulfated disaccharide unit in heparan sulfate: Enzymatic formation and tissue distribution. J. Biol. Chem. 2008, 283, 31237–31245. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, S.; Orii, K.O.; Vogler, C.; Grubb, J.H.; Snella, E.M.; Gutierrez, M.; Dieter, T.; Holden, C.C.; Sukegawa, K.; Orii, T.; et al. Production of MPS VII mouse (Gustm(hE540A x mE536A)Sly) doubly tolerant to human and mouse beta-glucuronidase. Hum. Mol. Genet. 2003, 12, 961–973. [Google Scholar] [CrossRef]
- Tomatsu, S.; Gutierrez, M.; Nishioka, T.; Yamada, M.; Yamada, M.; Tosaka, Y.; Grubb, J.H.; Montano, A.M.; Vieira, M.B.; Trandafirescu, G.G.; et al. Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum. Mol. Genet. 2005, 14, 3321–3335. [Google Scholar] [CrossRef]
- Santi, L.; De Ponti, G.; Dina, G.; Pievani, A.; Corsi, A.; Riminucci, M.; Khan, S.; Sawamoto, K.; Antolini, L.; Gregori, S.; et al. Neonatal combination therapy improves some of the clinical manifestations in the Mucopolysaccharidosis type I murine model. Mol. Genet. Metab. 2020, 130, 197–208. [Google Scholar] [CrossRef]
- Chen, H.H.; LaFave, M.C.; Varshney, G.K.; Trivedi, N.S.; Carrillo-Carrasco, N.; Senac, J.S.; Wu, W.; Hoffmann, V.; Elkahloun, A.G.; Burgess, S.M. Enzyme replacement therapy for mucopolysaccharidoses; past, present, and future. J. Hum. Genet. 2019, 64, 1153–1171. [Google Scholar] [CrossRef]
- Sands, M.S. AAV-mediated liver-directed gene therapy. Methods Mol. Biol. 2011, 807, 141–157. [Google Scholar]
- Greig, J.A.; Martins, K.M.; Breton, C.; Lamontagne, R.J.; Zhu, Y.; He, Z.; White, J.; Zhu, J.X.; Chichester, J.A.; Zheng, Q.; et al. Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration. Nat. Biotechnol. 2024, 42, 1232–1242. [Google Scholar] [CrossRef]
- McIntosh, J.; Lenting, P.J.; Rosales, C.; Lee, D.; Rabbanian, S.; Raj, D.; Patel, N.; Tuddenham, E.G.; Christophe, O.D.; McVey, J.H.; et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 2013, 121, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Tuddenham, E.G.; Rangarajan, S.; Rosales, C.; McIntosh, J.; Linch, D.C.; Chowdary, P.; Riddell, A.; Pie, A.J.; Harrington, C.; et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011, 365, 2357–2365. [Google Scholar] [CrossRef]
- Piechnik, M.; Amendum, P.C.; Sawamoto, K.; Stapleton, M.; Khan, S.; Fnu, N.; Alvarez, V.; Pachon, A.M.H.; Danos, O.; Bruder, J.T.; et al. Sex Difference Leads to Differential Gene Expression Patterns and Therapeutic Efficacy in Mucopolysaccharidosis IVA Murine Model Receiving AAV8 Gene Therapy. Int. J. Mol. Sci. 2022, 23, 12693. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef]
- Piechnik, M.; Sawamoto, K.; Ohnishi, H.; Kawamoto, N.; Ago, Y.; Tomatsu, S. Evading the AAV Immune Response in Mucopolysaccharidoses. Int. J. Mol. Sci. 2020, 21, 3433. [Google Scholar] [CrossRef]
- Keeler, G.D.; Markusic, D.M.; Hoffman, B.E. Liver induced transgene tolerance with AAV vectors. Cell. Immunol. 2019, 342, 103728. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Reiss, U.; Tuddenham, E.; Chowdary, P.; McIntosh, J.; Riddell, A.; Pie, J.; Mahlangu, J.N.; Recht, M.; Shen, Y.M.; et al. Adeno-Associated Mediated Gene Transfer for Hemophilia B: 8 Year Follow Up and Impact of Removing” Empty Viral Particles” on Safety and Efficacy of Gene Transfer; American Society of Hematology: Washington, DC, USA, 2018. [Google Scholar]
- Ertl, H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022, 13, 975803. [Google Scholar] [CrossRef]
- Greig, J.A.; Limberis, M.P.; Bell, P.; Chen, S.J.; Calcedo, R.; Rader, D.J.; Wilson, J.M. Non-Clinical Study Examining AAV8.TBG.hLDLR Vector-Associated Toxicity in Chow-Fed Wild-Type and LDLR(+/−) Rhesus Macaques. Hum. Gene Ther. Clin. Dev. 2017, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.J.; LaFave, M.C.; Varshney, G.K.; Trivedi, N.S.; Carrillo-Carrasco, N.; Senac, J.S.; Wu, W.; Hoffmann, V.; Elkahloun, A.G.; Burgess, S.M.; et al. Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J. Clin. Investig. 2015, 125, 870–880. [Google Scholar] [CrossRef] [PubMed]
- George, L.A.; Ragni, M.V.; Rasko, J.E.J.; Raffini, L.J.; Samelson-Jones, B.J.; Ozelo, M.; Hazbon, M.; Runowski, A.R.; Wellman, J.A.; Wachtel, K.; et al. Long-Term Follow-Up of the First in Human Intravascular Delivery of AAV for Gene Transfer: AAV2-hFIX16 for Severe Hemophilia B. Mol. Ther. 2020, 28, 2073–2082. [Google Scholar] [CrossRef]
- Kiourtis, C.; Wilczynska, A.; Nixon, C.; Clark, W.; May, S.; Bird, T.G. Specificity and off-target effects of AAV8-TBG viral vectors for the manipulation of hepatocellular gene expression in mice. Biol. Open 2021, 10, bio058678. [Google Scholar] [CrossRef]
- Wang, L.; Calcedo, R.; Nichols, T.C.; Bellinger, D.A.; Dillow, A.; Verma, I.M.; Wilson, J.M. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood 2005, 105, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Domenger, C.; Grimm, D. Next-generation AAV vectors-do not judge a virus (only) by its cover. Hum. Mol. Genet. 2019, 28, R3–R14. [Google Scholar] [CrossRef] [PubMed]
- Ozelo, M.C.; Mahlangu, J.; Pasi, K.J.; Giermasz, A.; Leavitt, A.D.; Laffan, M.; Symington, E.; Quon, D.V.; Wang, J.D.; Peerlinck, K.; et al. Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A. N. Engl. J. Med. 2022, 386, 1013–1025. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Thorrez, L.; Acosta-Sanchez, A.; Petrus, I.; Wang, L.; Ma, L.D.E.; Waele, L.; Iwasaki, Y.; Gillijns, V.; Wilson, J.M.; et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 2007, 5, 16–24. [Google Scholar] [CrossRef]
- Cantore, A.; Naldini, L. WFH State-of-the-art paper 2020: In vivo lentiviral vector gene therapy for haemophilia. Haemophilia 2021, 27 (Suppl. 3), 122–125. [Google Scholar] [CrossRef]
- Batty, P.; Lillicrap, D. Adeno-associated viral vector integration: Implications for long-term efficacy and safety. J. Thromb. Haemost. 2024, 22, 2945–2960. [Google Scholar] [CrossRef]
- Nathwani, A.C. Gene therapy for hemophilia. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 569–578. [Google Scholar] [CrossRef]
- Arabi, F.; Mansouri, V.; Ahmadbeigi, N. Gene therapy clinical trials, where do we go? An. overview. Biomed. Pharmacother. 2022, 153, 113324. [Google Scholar] [CrossRef]
- Tessitore, A.; Faella, A.; O’Malley, T.; Cotugno, G.; Doria, M.; Kunieda, T.; Matarese, G.; Haskins, M.; Auricchio, A. Biochemical, pathological, and skeletal improvement of mucopolysaccharidosis VI after gene transfer to liver but not to muscle. Mol. Ther. 2008, 16, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ferla, R.; O’Malley, T.; Calcedo, R.; O’Donnell, P.; Wang, P.; Cotugno, G.; Claudiani, P.; Wilson, J.M.; Haskins, M.; Auricchio, A. Gene therapy for mucopolysaccharidosis type VI is effective in cats without pre-existing immunity to AAV8. Hum. Gene Ther. 2013, 24, 163–169. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.R.; Bigger, B.W. Delivering gene therapy for mucopolysaccharide diseases. Front. Mol. Biosci. 2022, 9, 965089. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Xie, J.; Wang, D.; Kim, J.M.; Tai, P.W.L.; Gravallese, E.; Gao, G.; Shim, J.H. Bone-targeting AAV-mediated silencing of Schnurri-3 prevents bone loss in osteoporosis. Nat. Commun. 2019, 10, 2958. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, H.; Zhou, C.; Yu, H.; Yao, S.; Fu, F.; Seeley, R.; Ji, X.; Yang, Y.; Chen, P.; et al. Comparative intra-articular gene transfer of seven adeno-associated virus serotypes reveals that AAV2 mediates the most efficient transduction to mouse arthritic chondrocytes. PLoS ONE 2020, 15, e0243359. [Google Scholar] [CrossRef]
- Brooks, P.J.; Miller, T.M.; Revah, F.; Suh, J.; Garrison, B.R.; Starke, L.C.; MacLachlan, T.K.; Neilan, E.G.; Raychaudhuri, G.; Kassim, S.H.; et al. The Bespoke Gene Therapy Consortium: Facilitating development of AAV gene therapies for rare diseases. Nat. Rev. Drug Discov. 2024, 23, 157–158. [Google Scholar] [CrossRef]
- Allison, B.; Kimberly, K.; Stuart, M.W.; Shunji, T. Accelerating Medicines Partnership Bespoke Gene Therapy Consortium for Rare Disorders. Jpn. J. Clin. Med. 2024, 82, 769–777. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.A.; Benincore-Florez, E.; Nidhi, F.; Álvarez, J.V.; Holder, D.A.; Tomatsu, S. Long-Term Liver-Targeted AAV8 Gene Therapy for Mucopolysaccharidosis IVA. Curr. Issues Mol. Biol. 2025, 47, 900. https://doi.org/10.3390/cimb47110900
Khan SA, Benincore-Florez E, Nidhi F, Álvarez JV, Holder DA, Tomatsu S. Long-Term Liver-Targeted AAV8 Gene Therapy for Mucopolysaccharidosis IVA. Current Issues in Molecular Biology. 2025; 47(11):900. https://doi.org/10.3390/cimb47110900
Chicago/Turabian StyleKhan, Shaukat A., Eliana Benincore-Florez, FNU Nidhi, Jose Victor Álvarez, Dione A. Holder, and Shunji Tomatsu. 2025. "Long-Term Liver-Targeted AAV8 Gene Therapy for Mucopolysaccharidosis IVA" Current Issues in Molecular Biology 47, no. 11: 900. https://doi.org/10.3390/cimb47110900
APA StyleKhan, S. A., Benincore-Florez, E., Nidhi, F., Álvarez, J. V., Holder, D. A., & Tomatsu, S. (2025). Long-Term Liver-Targeted AAV8 Gene Therapy for Mucopolysaccharidosis IVA. Current Issues in Molecular Biology, 47(11), 900. https://doi.org/10.3390/cimb47110900






