Abstract
Epidemiological studies have provided evidence to show that the consumption of coffee and green tea has beneficial effects against cancer. Chlorogenic acid (CGA) in coffee and epigallocatechin-3-O-gallate (EGCG) in tea are involved in these effects. Research also suggests that the anticancer effects of coffee and tea may vary depending on the type of cancer, although the reasons for this remain unclear. As bioactive food factors, CGA and EGCG can contribute to epigenetic modification to exert their anticancer activity. One of the anticancer mechanisms is the one associated with reactive oxygen species (ROS). CGA and EGCG possess activities that initiate anticancer pathways by down-regulating ROS and NF-κB, and up-regulating AMPK. CGA and EGCG can regulate non-coding RNAs, including cancer-associated microRNAs. This review provides updated information on how CGA and EGCG exhibit anticancer activity via ROS-dependent anticancer pathways by regulating microRNA expression.
1. Introduction
Coffee and tea, made from Camellia sinensis (tea plant), are the most consumed beverages worldwide [1]. Intake of these beverages is believed to have beneficial effects in various diseases, including cancer. Chlorogenic acid (CGA) in coffee and epigallocatechin-3-O-gallate (EGCG) in tea (Figure 1) have been shown to contribute to these effects, and we have discussed these aspects [2,3,4].
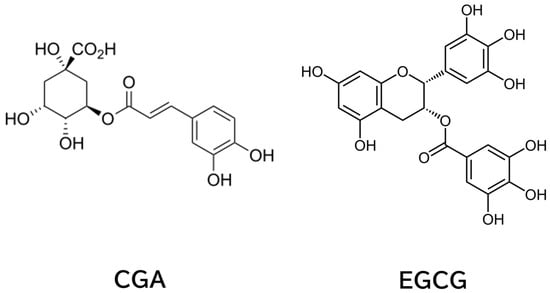
Figure 1.
Chemical Structures of CGA and EGCG.
This paper provides updated information, including observational epidemiology of coffee and tea consumption, regulation of microRNA (miR) by CGA and EGCG, and roles of miR in reactive oxygen species (ROS)-mediated anticancer pathways.
2. Observational Epidemiology of Coffee and Tea Consumption
This section is divided by subheadings. It will provide a concise and precise description of the experimental results, their interpretation, and the experimental conclusions that can be drawn.
2.1. Human Studies on Consumption of Coffee and Tea
Several epidemiological studies have shown the anticancer effects of coffee. A meta-analysis reported in 2020 for observational epidemiological studies on coffee consumption for 26 different cancers involving 364,749 cancer cases provided evidence to show that coffee consumption is inversely associated with cancer risk of endometrium, liver, oral cavity, pharyngeal, and skin cancers [3,5]. In addition, this meta-analysis suggested a reduced risk for mouth, pharynx, larynx, and skin cancers. It may also be inversely associated with the risk of breast, colon, colorectal, esophageal, and nonmelanoma skin cancers.
However, the same analysis showed that higher coffee intake was associated with an increased risk of bladder cancer, childhood acute lymphocytic leukemia, and possibly lung cancer. The additional results of epidemiological studies on coffee consumption reported before 2020 are available in a previous review [3].
There have been many observational epidemiological studies on tea consumption as well. For example, a survey in 2013 for prospective cohort and case–controlled studies revealed that green tea consumption showed risk-reducing effects across a total of 39 cancer sites, including breast, colon, esophagus, kidney/bladder, lung, ovary, pancreas, prostate, and stomach cancers, whereas 46 cases showed no risk reduction [6]. In the case of black tea, 28 and 92 cases showed risk reduction and no risk reduction, respectively, for these cancers [6]. These findings suggest that green and black teas have preventive effects in some types of cancer.
Additional information is available based on literature published before 2020 in our previous review, which also contains comparisons between coffee and tea [3].
Here, updated information is presented in Table 1. This adds evidence to show the favorable effects of green tea consumption on some types of cancer, such as breast, colon, lung, and blood cancers, and coffee consumption on liver, endometrial, and skin cancers. Coffee consumption may be related to the higher risk of bladder cancer. In contrast, tea consumption is related to an increased risk of skin cancer, while daily coffee consumption is a protective factor [7]. Tea consumption was reported to be associated insignificantly with an increased risk for thyroid cancer [8]. Thus, green tea and coffee are likely to have some differences in site-specific anticancer effects.

Table 1.
Anticancer effects in an observational epidemiology study of coffee and tea *.
When creating a correlation plot using country-level coffee consumption and age-standardized cancer incidence based on the data of the FAO/European Coffee Report 2023–2024 (https://www.ecf-coffee.org/european-coffee-report-2023-2024/ accessed on 22 October 2025) and age-standardized cancer incidence data (excluding non-melanoma skin cancer) from WHO/IARC GLOBOCAN 2022 (https://www.wcrf.org/preventing-cancer/cancer-statistics/global-cancer-data-by-country/#global-cancer-incidence-both-sexes accessed on 22 October 2025), the results revealed a weak positive association between per capita coffee consumption and total cancer incidence, indicating that coffee intake alone does not explain global variation in cancer occurrence (Figure 2). However, a meta-analysis of 59 cohort studies reported that higher coffee intake was associated with a reduced overall cancer risk (relative risk (RR) = 0.87, 95% confidence interval (CI) = 0.82–0.92), and specifically lower risks of liver, endometrial, and prostate cancers, whereas an increased risk was observed for lung cancer (RR = 2.18, CI: 1.26–3.78) [106]. A meta-analysis review [82] concluded that coffee consumption shows highly suggestive inverse associations with liver and endometrial cancer risks, suggesting potential protective effects against certain cancer types rather than overall carcinogenicity.
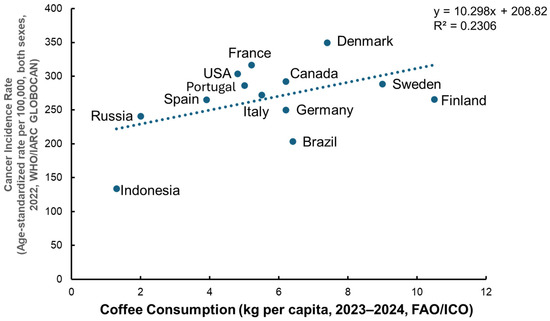
Figure 2.
Relationship between coffee consumption and age-standardized total cancer incidence. A correlation analysis was conducted using country-level data on coffee consumption (kg per capita, 2023–2024) from the FAO/International Coffee Organization (ICO) and age-standardized total cancer incidence (per 100,000 population, both sexes, excluding non-melanoma skin cancer, 2022) from WHO/IARC GLOBOCAN. The analysis revealed a weak positive association between per capita coffee consumption and total cancer incidence (R2 = 0.23), indicating that coffee intake alone does not explain global variations in cancer occurrence. Data sources: FAO/European Coffee Report 2023–2024 (https://www.ecf-coffee.org/european-coffee-report-2023-2024/, accessed on 22 October 2025); WHO/IARC GLOBOCAN 2022 (https://www.wcrf.org/preventing-cancer/cancer-statistics/global-cancer-data-by-country/#global-cancer-incidence-both-sexes, accessed on 22 October 2025).
Some studies have found conflicting results regarding the effects of coffee and tea consumption. These discrepancies may stem from differences in confounding factors such as the extraction method, degree of roasting, coffee species, serving temperature, method of quantifying consumption, beverage temperature, acrylamide contents, alcohol consumption, cigarette smoking status and healthcare accessibility, and differences in genetic and environmental factors such as sex, race, and age, lifestyle, genetic polymorphisms, and intestinal microbiota [107,108]. It is expected that future studies will clarify the reason for the inconsistent results and may provide clues to establish the anticancer effects of these beverage intakes.
A recent systematic review and meta-analysis by Zhang et al. [109] found that green tea/EGCG consumption reduced cancer risk with statistical significance compared to controls. These authors noted risk reduction in prostate cancer (RR = 0.43, CI = 0.22–0.83), oral cancer (RR = 0.44, CI = 0.01–0.87), gallbladder cancer (RR = 0.72, CI = 0.51–0.94), and hematological cancers (RR = 0.72, CI = 0.49–0.95), suggesting that green tea or EGCG intake may prevent some types of cancer.
2.2. Human Clinical Intervention Studies on Consumption of Coffee and Tea
Human intervention studies on coffee/CGA consumption are scarce. Kang et al. [110] conducted a phase I trial for CGA in injectable form in patients with recurrent high-grade glioma. The results indicated that CGA has a favorable safety profile and provides certain clinical benefits to patients with high-grade glioma relapsing following standard therapies. This grade of CGA would promote studies to test the clinical application of CGA for various diseases, including cancer.
A recent systematic review and dose–response meta-analysis of randomized controlled trials (RCTs) by Samavat et al. [111] found that consumption of green coffee bean extract significantly decreased systolic blood pressure (SBP) (weighted mean difference (WMD) = −2.95 mmHg; CI = −4.27 to −1.62; p < 0.001) and diastolic blood pressure (DBP) (WMD = −2.15 mmHg; CI = −2.59 to −1.72; p < 0.001). The anticancer effect of coffee consumption awaits verification by future RCTs.
A comprehensive review on CGA by Gupta et al. [112] also pointed out that only a few clinical reports have demonstrated the effectiveness of CGA as a therapeutic agent.
As for tea, habitual tea consumption is generally safe (e.g., 704 mg/day in beverage), but higher doses of EGCG (≥800 mg/day) may cause liver toxicity [113]. Filippini et al. [97] evaluated the results of 11 RCTs on green tea supplementation. In most cases, evidence for the beneficial effect of green tea extract on cancer was insufficient. Adverse effects of green tea extract intake were also reported, suggesting that future RCT studies are required to confirm green tea’s effects [97].
Polyphenon® E is a standardized catechin preparation of green tea extract and is proposed as a topical treatment of genital warts. Its efficacy has been demonstrated in several clinical studies [114]. Genital warts are caused by human papillomaviruses (HPVs), suggesting their possible application to HPV-associated cancers such as cervical cancer and lymphocytic leukemia [114]. A clinical trial showed that the treatment with Polyphenon® E ointment or capsules or both of 51 patients with HPV-infected cervical lesions resulted in an overall 69% response rate as compared with that of 10% in the untreated groups [115]. A review article by Norman et al. [116] reported nine clinical studies with a National Clinical Trial number. Although Sinicrope et al. [117] reported that Polyphenon® E did not significantly reduce the number of rectal aberrant crypt foci, further clinical intervention studies may provide clear evidence of the anticancer effects of green tea.
3. Regulatory Effects of Coffee/CGA and Tea/EGCG on miRs
Throughout this section, we specify the concentrations of CGA and EGCG used in each cited study (e.g., 25 µM and 50 µM CGA for miR-20a suppression, 10–60 µM EGCG for miR-483-3p downregulation) so that readers can evaluate dose–response relationships.
As bioactive food factors, CGA and EGCG can contribute to epigenetic modification and exert their anticancer activity. For example, hypermethylation or hypomethylation of DNA is closely related to tumorigenesis [118]. Lee and Zhu [119] demonstrated that CGA inhibits the growth of cultured MCF-7 cells through the inhibition of DNA methyltransferase (DNMT), and Pal et al. [120] showed that EGCG reduces the proliferation of HeLa cells and expression of DNMT-1. As shown below, inhibition of DNA methyltransferase would affect the biogenesis of miRs.
EGCG is known to decrease histone deacetylase activity, leading to the increased acetylation levels of histone H3 and H4 [121]. Combining the two findings that ionizing radiation triggers histone modification, such as acetylation of histone H3 and histone H4, leading to upregulation of miR-34a, and that EGCG can upregulate miR-34a, suggests that EGCG-induced histone modifications contribute to the upregulation of miR-34a.
These are the examples to explain how CGA and EGCG can modulate miR expression. EGCG’s ability to bind DNA and RNA [122] represents another possible mechanism. These proposed mechanisms are based on limited in vitro evidence; thus, additional experimental and clinical studies are required to determine whether CGA and EGCG modulate miRNA expression through DNMT inhibition, histone modifications, direct nucleic acid binding, or other yet to be identified pathways.
miRs, which are small single-stranded molecules (ca. 20 to 25 nucleotides), play a role in epigenetic modification involved in tumorigenesis [4,123]. Various dietary polyphenols, including CGA and EGCG, have been shown to regulate miRs as exemplified above and to exert beneficial effects in diseases such as cancer. Several examples have been described in previous papers [4]. Here we provide updated information in Table 2.

Table 2.
Regulatory effects of CGA and EGCG on miRs.
CGA and EGCG have been shown to upregulate miR-200c. Davalos et al. [164] found that all cell lines with hypermethylation at CpG islands have significant loss of mature and pri-miRNA expression for the miR-200 family as compared with the CpG unmethylated cell lines derived from the same tumor type, indicating that DNA methylation modulates expression of miR.
Chang et al. [165] have demonstrated that p53 directly induces the expression of miR-34a by promoting transcriptional activity. Since upregulation of p53 by CGA and EGCG is well known [4], these dietary factors are expected to upregulate miR-34a.
Oncogenic miR-17 family miR-20a, miR-93, and miR-106b bind to p21 mRNA and suppress its expression, leading to cancer development [128]. Several studies have demonstrated downregulation of these miRs by CGA and EGCG [4]. Figure 3 illustrates how these miRs downregulate p21 expression.
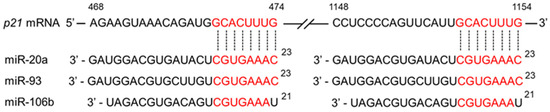
Figure 3.
Schematic presentation of the downregulation of p21 expression by miRs. The binding of three miR-17 family miRs (miR-20a, miR-93, miR-106b) to p21 mRNA reduces p21 protein expression. CGA and EGCG may contribute to anticancer effects by suppressing the expression of these miRs, thereby increasing p21 levels.
Table 2 provides an update on studies to show the regulatory effects of CGA and EGCG on miRs. There are some differences in these regulations. This may be due to cancer-specific differences or differences between cell subtypes of the same cancer origin. For example, miR-125b was upregulated in cervical carcinoma SiHa cells, but downregulated in CA33 cells and HeLa cells upon treatment with EGCG [139].
Several studies on EGCG have provided the results of microarray and next-generation sequencing (NGS) [132,135,136,161] (Table 3). Although some of these data have been evaluated by quantitative reverse transcription-polymerase chain reaction (qRT-PCR), the majority remain unconfirmed. Table 3 shows similar results to those based on qRT-PCR, as exemplified by EGCG’s upregulation of miR-34a and let-7a, but there are some conflicting findings. Yamada et al. [163] demonstrated that, in addition to let-7b, both let-7a and let-7e were upregulated in a real-time PCR analysis. Although differences may be due to those in cell types used, caution is warranted regarding data based solely on microarray/NGS analysis.

Table 3.
Microarray/NGS analysis for upregulation or downregulation by EGCG/Polyphenon-60 in different cancer cells.
Thus, NGS platforms provide a large number of findings, but these may not be biologically robust and may lack reproducibility. Therefore, validation of these findings would be necessary. It should be pointed out that similar research on CGA is very scarce, although the reason for this is unclear.
4. miR Targets in ROS-Associated Anticancer Pathways
CGA and EGCG are well known to possess both antioxidant and pro-oxidant properties, as discussed previously [2]. However, since the pro-oxidant properties of CGA and EGCG require higher concentrations (e.g., 500 μM EGCG) [166], their radical scavenging activity is thought to primarily contribute to their anticancer activity. Accordingly, the present paper is concerned with the pathways in which CGA and EGCG act as radical scavengers (see Figure 4).
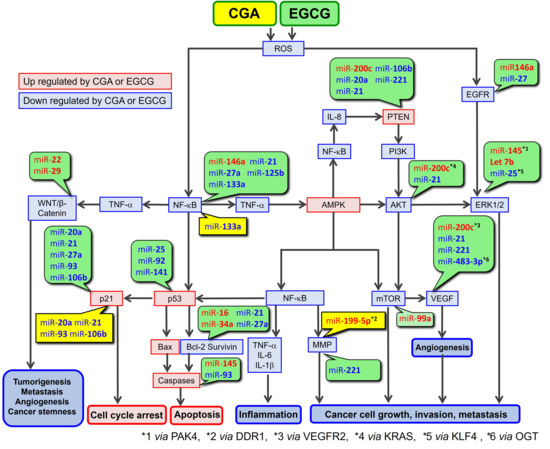
Figure 4.
ROS-mediated anticancer pathways involving CGA and EGCG, and microRNA regulation of the components of this pathway. CGA’s and EGCG’s effects on miRs are presented in yellow and green boxes, respectively.
Table 4 and Table 5 summarize how miRs regulate targets in ROS-mediated anticancer pathways. As indicated in Table 2, some miRs undergo cell type-dependent regulation (e.g., upregulation and downregulation) by CGA and EGCG. Table 4 and Table 5 list only mRNAs relevant to the anticancer activity of CGA and/or EGCG along this pathway.

Table 4.
miRs upregulated by EGCG or CGA and their regulatory effects on proposed molecular targets.

Table 5.
miRs downregulated by EGCG, Polyphenon-60, or CGA and their regulatory effects on proposed molecular targets.
5. Involvement of miRs in the Anticancer Pathway Associated with ROS-Scavenging Activities of CGA and EGCG
Table 6 provides updated information that was given previously [2]. It shows a continuing recognition that CGA and EGCG have the activities to down-regulate ROS and nuclear factor-κB (NF-κB), and to up-regulate AMP-activated protein kinase (AMPK) in various biological activities. Based on these data and our previous discussions [2,3,108], Figure 4 depicts a proposed ROS-mediated anticancer pathway in which CGA and EGCG can be involved. Figure 4 also includes information on how miRs, based on the data in Table 4 and Table 5, regulate the components of this pathway.

Table 6.
Regulatory effects of CGA and EGCG on ROS, AMPK, and NF-κB.
Table 6.
Regulatory effects of CGA and EGCG on ROS, AMPK, and NF-κB.
| Polyphenols | AMPK Up Stimulation/ Upregulation | ROS Down Suppression/ Downregulation | NF-κB Down Suppression/ Downregulation |
|---|---|---|---|
| CGA | Ping et al. [196] Silva et al. [197] Saadatagah et al. [198] | Wójciak et al. [199] Huimei Chen et al. [200] Sharma et al. [201] | Komeili-Movahhed et al. [202] Negm et al. [203] Lin et al. [204] |
| EGCG | Peng et al. [205] Tian et al. [206] Wang et al. [207] | Yuan et al. [208] Khan et al. [209] Haoxiang Chen et al. [210] | X. Li et al. [211] Z.-D. Li et al. [212] Zhang et al. [213] |
AKT, AKT serine/threonine kinase 1; Bax, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma 2; DDR1, discoidin domain receptor 1; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; OGT, O-GlcNAc transferase; KLF4, Kruppel-like factor 4; KRAS, KRAS proto-oncogene; IL, interleukin; MMP, matrix metalloproteinase; mTOR, mammalian target of rapamycin; PAK4, p21-activated kinase 4; PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin homolog deleted on chromosome 10; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; Wnt, wingless-related integration site.
6. Perspectives
In a recent study investigating the effects of a nutritional supplement on human obesity, Joshua et al. [214] found that consumption of a supplement containing green coffee bean and green tea extracts resulted in significantly reduced plasma levels of miR-34a and miR-122. A similar experimental approach would reveal the efficacy of CGA and EGCG.
In an animal experiment, Kang et al. [158] showed that oral administration of EGCG at a concentration equivalent to daily achievable dosages of tea drinkers suppressed miR483-3p-induced metastasis of hepatocellular carcinoma cells. EGCG induced hypermethylation of the miR483-3p promoter region via epigenetic mechanisms, thereby downregulating miR483-3p expression in these cells. These authors suggested that regular tea consumption can suppress metastasis through downregulation of miR-483-3p, which upregulates vimentin expression and downregulates E-cadherin expression [158], since these events are associated with cancer invasiveness and metastasis [215].
As one of the possible mechanisms, Figure 5 illustrates how exogenous EGCG can suppress metastasis via O-GlcNAc transferase (OGT), which is involved as a target of miR-483-3p [195].
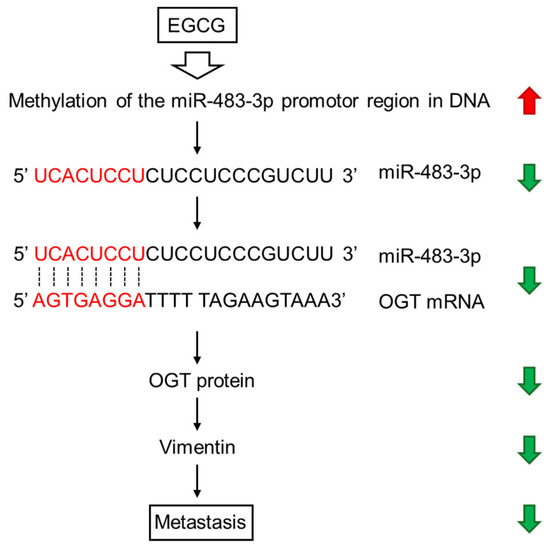
Figure 5.
EGCG-induced DNA methylation exerts anticancer effects through downregulation of miR-483-3p, OGT, and vimentin.
One intriguing emerging research area involves analyzing miRs in foods that may exert functional effects after ingestion. Zhang et al. [216] found that plant food-derived miRNAs are present in human and animal sera, and demonstrated that one of these exogenous mature plant miRs is functional. Huang et al. [217] found, based on an NGS analysis, the presence of various miRs as new components of matcha, one of the green tea products. Thus, it would be interesting to examine whether or not food-derived miRs can have beneficial effects in human diseases, including cancer.
A recent comprehensive review by Fujimura et al. [218] pointed out that a variety of biomolecules, such as citrus polyphenols and sulfur-containing food factors, can potentiate EGCG sensing by 67-kD laminin receptor (67LR). Similar synergistic effects may be expected for other polyphenols, including CGA. It would be interesting to examine whether or not such a combination induces any changes in miRs.
7. Conclusions
Human observational epidemiological studies have yielded only limited evidence for the reduced cancer risk by consumption of coffee and tea, although CGA and EGCG, major bioactive constituents of these beverages, have been shown to have beneficial effects against various types of cancer. Although a global correlation plot shows a trend of increased risk of total cancer cases with coffee consumption, several epidemiological studies have suggested inverse associations in some specific cancer types. Future studies, including human clinical intervention studies, would be needed to confirm the cancer-preventive effect of these food-derived bioactive factors.
Furthermore, coffee and tea contain a wide range of bioactive constituents besides CGA and EGCG—such as caffeine, diterpenes, trigonelline, melanoidins, and acrylamide—that may individually or synergistically influence cancer risk. Therefore, attributing the observed anticancer effects solely to CGA or EGCG is an oversimplification; direct comparisons using preparations with and without these compounds or 100% purified CGA/EGCG are necessary to clarify their specific contributions. These inconsistencies may also reflect heterogeneity across study populations (e.g., age, sex, smoking prevalence, and genetic background) and differences in the concentrations of bioactive constituents present in various coffee and tea preparations. Variation in dose or exposure level of CGA/EGCG could result in divergent biological effects. Future studies should consider these demographic and compositional variables when interpreting risk estimates.
CGA and EGCG share similar properties in that they can scavenge ROS, which triggers the anticancer pathway leading to cell cycle arrest, apoptosis, and the prevention of inflammation and metastasis. These polyphenols can modulate the expression/activity of multiple components of this pathway by increasing the expression of tumor-suppressing miRs and decreasing the expression of oncogenic miRs in general. Therefore, the effects of these miRs may additively or synergistically enhance the anticancer effects of CGA and EGCG.
EGCG appears to have a specific feature in that it can act via 67LR in anticancer effects and induce miR-let-7b [163]. Although no data are available for CGA, this unique mechanism of action may be one potential explanation for the differences between coffee and tea consumption in cancer-specific effects observed in epidemiological studies. Further studies would clarify the reason for the observed differences in the anticancer effects of the consumption of coffee/CGA and tea/EGCG. In addition, most evidence described in this review remains in vitro or indirect, and future human studies would be needed before firm conclusions can be drawn.
Author Contributions
M.I., Corresponding author, Project design, Outline of the whole manuscript, Literature search, Text preparation of Abstract, Section 6 and Section 7; S.H., Literature search, Text preparation of Section 2, Preparation of Table 1, Drawing of Figure 3; T.O., Literature search, Text preparation of Section 3, Preparation of Table 2, Table 4, and Table 5, Drawing of Figure 2 and Figure 4; N.M. Corresponding author, Outline of the whole manuscript, Literature search, Text preparation of Section 4 and References, Preparation of Table 3 and Table 6; R.F., Literature search, Text preparation of Section 5, Drawing of Figure 5; Y.N., Text preparation of Section 1, Literature search, Drawing of Figure 1. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Grant-in-Aid for Scientific Research (JSPS KAKENHI Grant Number JP24K08772 to S.H.) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CGA | Chlorogenic acid |
| CI | 95% confidence interval |
| DNMT | DNA methyltransferase |
| EGCG | Epigallocatechin-3-O-gallate |
| HPVs | Human papillomaviruses |
| miR | MicroRNA |
| NGS | Next-generation sequencing |
| OGT | O-GlcNAc transferase |
| ROS | Reactive oxygen species |
| RR | Relative risk |
| RCT | Randomized controlled trial |
| 67LR | 67 kDa laminin receptor |
References
- Treskes, R.W.; Clausen, J.; Marott, J.L.; Jensen, G.B.; Holtermann, A.; Gyntelberg, F.; Jensen, M.T. Use of sugar in coffee and tea and long-term risk of mortality in older adult Danish men: 32 years of follow-up from a prospective cohort study. PLoS ONE 2023, 18, e0292882. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Ohishi, T.; Nakamura, Y.; Fukutomi, R.; Miyoshi, N. Anti-Cancer Effects of Dietary Polyphenols via ROS-Mediated Pathway with Their Modulation of MicroRNAs. Molecules 2022, 27, 3816. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Oishi, Y.; Isemura, M.; Miyoshi, N. Contribution of Non-Coding RNAs to Anticancer Effects of Dietary Polyphenols: Chlorogenic Acid, Curcumin, Epigallocatechin-3-Gallate, Genistein, Quercetin and Resveratrol. Antioxidants 2022, 11, 2352. [Google Scholar] [CrossRef]
- Zhao, L.-G.; Li, Z.-Y.; Feng, G.-S.; Ji, X.-W.; Tan, Y.-T.; Li, H.-L.; Gunter, M.J.; Xiang, Y.-B. Coffee drinking and cancer risk: An umbrella review of meta-analyses of observational studies. BMC Cancer 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Hong, J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Ferhatosmanoğlu, A.; Selcuk, L.B.; Arıca, D.A.; Ersöz, Ş.; Yaylı, S. Frequency of skin cancer and evaluation of risk factors: A hospital-based study from Turkey. J. Cosmet. Dermatol. 2022, 21, 6920–6927. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Cristaldi, A.; Okatyeva, V.; Bianco, S.L.; Conti, G.O.; Zuccarello, P.; Copat, C.; Caltabiano, R.; Cannizzaro, M.; Ferrante, M. Dietary habits and thyroid cancer risk: A hospital-based case-control study in Sicily (South Italy). Food Chem. Toxicol. 2020, 146, 111778. [Google Scholar] [CrossRef]
- Yu, E.Y.W.; Dai, Y.; Wesselius, A.; van Osch, F.; Brinkman, M.; van den Brandt, P.; Grant, E.J.; White, E.; Weiderpass, E.; Gunter, M.; et al. Coffee consumption and risk of bladder cancer: A pooled analysis of 501,604 participants from 12 cohort studies in the BLadder Cancer Epidemiology and Nutritional Determinants (BLEND) international study. Eur. J. Epidemiol. 2020, 35, 523–535. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Zhao, L.; Mucci, L.A.; Giovannucci, E.L. Decaffeinated coffee consumption and risk of total and site-specific cancer. Ann. Oncol. 2025, 36, 819–831. [Google Scholar] [CrossRef]
- Hashemian, M.; Sinha, R.; Murphy, G.; Weinstein, S.J.; Liao, L.M.; Freedman, N.D.; Abnet, C.C.; Albanes, D.; Loftfield, E. Coffee and tea drinking and risk of cancer of the urinary tract in male smokers. Ann. Epidemiol. 2019, 34, 33–39. [Google Scholar] [CrossRef]
- Zhao, L.-G.; Li, Z.-Y.; Feng, G.-S.; Ji, X.-W.; Tan, Y.-T.; Li, H.-L.; Gunter, M.J.; Xiang, Y.-B. Tea Drinking and Risk of Cancer Incidence: A Meta-Analysis of Prospective Cohort Studies and Evidence Evaluation. Adv. Nutr. 2021, 12, 402–412. [Google Scholar] [CrossRef]
- Al-Zalabani, A.H.; Wesselius, A.; Yu, E.Y.-W.; van den Brandt, P.; Grant, E.J.; White, E.; Skeie, G.; Liedberg, F.; Weiderpass, E.; Zeegers, M.P. Tea consumption and risk of bladder cancer in the Bladder Cancer Epidemiology and Nutritional Determinants (BLEND) Study: Pooled analysis of 12 international cohort studies. Clin. Nutr. 2022, 41, 1122–1130. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Albers, R.; Chen, Y.-T.; Steineck, G.; Kellen, E.; Johnson, K.C.; Lu, C.-M.; Pohlabeln, H.; La Vecchia, C.; Porru, S.; et al. The Association between Tea Consumption and Bladder Cancer Risk Based on the Bladder Cancer Epidemiology and Nutritional Determinants (BLEND) International Consortium. Nutr. Cancer 2025, 77, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Greenop, K.R.; Petridou, E.; Bailey, H.D.; Orsi, L.; Kang, A.Y.; Baka, M.; Bonaventure, A.; Kourti, M.; Metayer, C.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: A pooled analysis from the childhood Leukemia International Consortium. Cancer Causes Control 2018, 29, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Dessypris, N.; Clavel, J.; Metayer, C.; Erdmann, F.; Orsi, L.; Kang, A.Y.; Schüz, J.; Bonaventure, A.; Greenop, K.R.; et al. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: A Childhood Leukemia International Consortium (CLIC) study. Cancer Epidemiol. 2019, 62, 101581. [Google Scholar] [CrossRef] [PubMed]
- Msallem, E.; Pacquement, H.; Olivier, L.; Brugières, L.; Parker, J.L.; Garnier, N.; Lambilliotte, A.; Faure, L.; Clavel, J.; Bonaventure, A. Association Between Perinatal Factors and Childhood Lymphoma-A Pooled Analysis of the ESCALE and ESTELLE Studies (SFCE). Pediatr. Blood Cancer 2025, 72, e31439. [Google Scholar] [CrossRef]
- Flores-García, M.K.; Flores-Collado, G.; Mérida-Ortega, Á.; Ugalde-Resano, R.; González-Rocha, A.; Denova-Gutiérrez, E.; Muñoz-Aguirre, P.; Zapata-Tarrés, M.; López-Carrillo, L. Maternal and infant diet play a role in acute leukemia development: An expanded systematic review and meta-analysis. Clin. Nutr. ESPEN 2025, 66, 515–522. [Google Scholar] [CrossRef]
- Torres-Duarte, K.; Rodríguez, L.M.C.; Mora-Becerra, C.; Moreno-Chaparro, J.; Gaitán-Duarte, H. Association Between Maternal Diet During Pregnancy and the Risk of Childhood Acute Lymphoblastic Leukemia. An Overview. Cancer Rep. 2025, 8, e70231. [Google Scholar] [CrossRef]
- Pranata, R.; Feraldho, A.; Lim, M.A.; Henrina, J.; Vania, R.; Golden, N.; July, J. Coffee and tea consumption and the risk of glioma: A systematic review and dose-response meta-analysis. Br. J. Nutr. 2022, 127, 78–86. [Google Scholar] [CrossRef]
- Malmir, H.; Shayanfar, M.; Mohammad-Shirazi, M.; Tabibi, H.; Sharifi, G.; Esmaillzadeh, A. Tea and coffee consumption in relation to glioma: A case-control study. Eur. J. Nutr. 2019, 58, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mirtavoos-Mahyari, H.; Salehipour, P.; Parohan, M.; Sadeghi, A.; Coffee, E.O. Black Tea and Green Tea Consumption on the Risk of Non-Hodgkin’s Lymphoma: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Nutr. Cancer 2019, 71, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Cote, D.J.; Bever, A.M.; Wilson, K.M.; Smith, T.R.; Smith-Warner, S.A.; Stampfer, M.J. A prospective study of tea and coffee intake and risk of glioma. Int. J. Cancer 2020, 146, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Onyije, F.M.; Dolatkhah, R.; Olsson, A.; Bouaoun, L.; Deltour, I.; Erdmann, F.; Bonaventure, A.; Scheurer, M.E.; Clavel, J.; Schüz, J. Risk factors for childhood brain tumours: A systematic review and meta-analysis of observational studies from 1976 to 2022. Cancer Epidemiol. 2024, 88, 102510. [Google Scholar] [CrossRef]
- Hu, Z.; Ye, J.; Shi, S.; Luo, C.; Wang, T.; Liu, Y.; Ye, J.; Sun, X.; Ke, Y.; Hou, C. Maternal smoking, consumption of alcohol, and caffeinated beverages during pregnancy and the risk of childhood brain tumors: A meta-analysis of observational studies. BMC Public Health 2024, 24, 1238. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Jin, Y.; Guo, J. Association between tea and coffee consumption and brain cancer risk: An updated meta-analysis. World J. Surg. Oncol. 2019, 17, 51. [Google Scholar] [CrossRef]
- Creed, J.H.; Smith-Warner, S.A.; Gerke, T.A.; Egan, K.M. A prospective study of coffee and tea consumption and the risk of glioma in the UK Biobank. Eur. J. Cancer 2020, 129, 123–131. [Google Scholar] [CrossRef]
- Wang, Z.; Arthur, R.; Shadyab, A.H.; Saquib, N.; Johnson, K.C.; Snetselaar, L.G.; Mu, L.; Chen, Z.; Luo, J. Association of tea-drinking habits with the risk of non-Hodgkin lymphoma: A prospective cohort study among postmenopausal women. Br. J. Nutr. 2023, 129, 1543–1551. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Yang, Y.; Xie, J.; Liu, M.; Zhang, Y.; Zhang, Y.; Zhao, Q. Does coffee, tea and caffeine consumption reduce the risk of incident breast cancer? A systematic review and network meta-analysis. Public Health Nutr. 2021, 24, 6377–6389. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoo, D.M.; Min, C.; Choi, H.G. Association between Coffee Consumption/Physical Exercise and Gastric, Hepatic, Colon, Breast, Uterine Cervix, Lung, Thyroid, Prostate, and Bladder Cancer. Nutrients 2021, 13, 3927. [Google Scholar] [CrossRef]
- Do, T.M.; Nguyen, Q.H.N.; Le, N.H.D.; Nguyen, H.D.; Phung, A.H.T.; Tran, T.S.; Nguyen, T.V.; Ho-Pham, L.T. Association between dietary factors and breast cancer risk: A matched case-control study in Vietnam. BMC Cancer 2024, 24, 1224. [Google Scholar] [CrossRef] [PubMed]
- Schmit, S.L.; Nwogu, O.; Matejcic, M.; DeRenzis, A.; Lipworth, L.; Blot, W.J.; Raskin, L. Coffee consumption and cancer risk in African Americans from the Southern Community Cohort Study. Sci. Rep. 2020, 10, 17907. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, B.; Lam, T.H.; Cheng, K.K.; Zhang, W.; Xu, L. The mediating roles of anthropo-metabolic biomarkers on the association between beverage consumption and breast cancer risk. Nutr. J. 2025, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Abalsamo, A.; Acito, M.; Villarini, M.; Moretti, M.; Realdon, S. Green Tea Consumption and Risk of Breast Cancer and Recurrence-A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1886. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liao, Y.-H.; Lin, Y.; Liu, Q.; Xie, X.-M.; Tang, L.-Y.; Ren, Z.-F. Effects of tea consumption and the interactions with lipids on breast cancer survival. Breast Cancer Res. Treat. 2019, 176, 679–686. [Google Scholar] [CrossRef]
- van Die, M.D.; Bone, K.M.; Visvanathan, K.; Kyrø, C.; Aune, D.; Ee, C.; Paller, C.J. Phytonutrients and outcomes following breast cancer: A systematic review and meta-analysis of observational studies. JNCI Cancer Spectr. 2024, 8, pkad104. [Google Scholar] [CrossRef]
- Lamchabbek, N.; Elattabi, C.; Bour, A.; Chimera, B.; Boutayeb, S.; Belyamani, L.; Faure, E.; Huybrechts, I.; Khalis, M. Associations Between Dietary Factors and Breast Cancer Risk: A Systematic Review of Evidence from the MENA Region. Nutrients 2025, 17, 394. [Google Scholar] [CrossRef]
- Romelli, M.; Gnagnarella, P.; Gaeta, A.; Serrano, D.; Ermini, I.; Cavalcabo’, N.D.B.; Saieva, C.; Iadevaia, S.; Gandini, S.; Caini, S. Coffee and tea intake and survival of cancer patients: A systematic review and meta-analysis. Cancer Causes Control 2025. [Google Scholar] [CrossRef]
- Shin, S.; Fu, J.; Shin, W.-K.; Huang, D.; Min, S.; Kang, D. Association of food groups and dietary pattern with breast cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 282–297. [Google Scholar] [CrossRef]
- Nordestgaard, A.T. Causal relationship from coffee consumption to diseases and mortality: A review of observational and Mendelian randomization studies including cardiometabolic diseases, cancer, gallstones and other diseases. Eur. J. Nutr. 2022, 61, 573–587. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Hung, H.-Y.; You, J.-F.; Chiang, J.-M.; Chin, C.-C. Common habitual behaviors and synchronous colorectal cancer risk: A retrospective case-control study. Int. J. Color. Dis. 2019, 34, 1421–1430. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhao, R.; Xia, L.; Cui, Y.-P.; Rao, Z.-Y.; Zhou, Y.; Wu, X.-T. Dose-response meta-analysis of coffee consumption and risk of colorectal adenoma. Eur. J. Clin. Nutr. 2020, 74, 297–306. [Google Scholar] [CrossRef]
- Mackintosh, C.; Yuan, C.; Ou, F.-S.; Zhang, S.; Niedzwiecki, D.; Chang, I.-W.; O’Neil, B.H.; Mullen, B.C.; Lenz, H.-J.; Blanke, C.D.; et al. Association of Coffee Intake With Survival in Patients With Advanced or Metastatic Colorectal Cancer. JAMA Oncol. 2020, 6, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chinnathambi, S.; Kumar, M.; Pandian, G.N. Food Intake and Colorectal Cancer. Nutr. Cancer 2023, 75, 1710–1742. [Google Scholar] [CrossRef] [PubMed]
- Oyelere, A.M.; Kok, D.E.; Bos, D.; Gunter, M.J.; Ferrari, P.; Keski-Rahkonen, P.; de Wilt, J.H.W.; van Halteren, H.K.; Kouwenhoven, E.A.; van Duijnhoven, F.J.B.; et al. Coffee consumption is associated with a reduced risk of colorectal cancer recurrence and all-cause mortality. Int. J. Cancer 2024, 154, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Lehoczki, A.; Laukkanen, J.A. Coffee consumption, cancer, and healthy aging: Epidemiological evidence and underlying mechanisms. GeroScience 2025, 47, 1517–1555. [Google Scholar] [CrossRef]
- Oyelere, A.M.; Verstraete, F.F.; Kok, D.E.; Bos, D.; Gunter, M.J.; de Wilt, J.H.W.; Keski-Rahkonen, P.; van Duijnhoven, F.J.B.; Kampman, E. Coffee consumption and mortality in colorectal cancer patients: Does the co-existence of cardiometabolic disease matter? Clin. Nutr. ESPEN 2025, 67, 62–70. [Google Scholar] [CrossRef]
- Rosato, V.; Guercio, V.; Bosetti, C.; Gracia-Lavedan, E.; Villanueva, C.M.; Polesel, J.; Toffoluti, F.; Moreno, V.; Martin, V.; Aragonés, N.; et al. Coffee consumption and colorectal cancer risk: A multicentre case-control study from Italy and Spain. Eur. J. Cancer Prev. 2021, 30, 204–210. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Murphy, N.; Key, T.J. Diet and colorectal cancer in UK Biobank: A prospective study. Int. J. Epidemiol. 2020, 49, 246–258. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Yan, G.; Xu, B.; Sun, M.; Feng, M. Causal relationships between coffee intake, apolipoprotein B and gastric, colorectal, and esophageal cancers: Univariable and multivariable Mendelian randomization. Eur. J. Nutr. 2024, 63, 469–483. [Google Scholar] [CrossRef]
- Wada, K.; Oba, S.; Tsuji, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Hori, A.; Tanabashi, S.; Matsushita, S.; et al. Green tea intake and colorectal cancer risk in Japan: The Takayama study. Jpn. J. Clin. Oncol. 2019, 49, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Quang, L.N.; Hien, N.Q.; Quang, N.T.; Chung, N.T. Active Lifestyle Patterns Reduce the Risk of Colorectal Cancer in the North of Vietnam: A Hospital-Based Case-Control Study. Cancer Control 2019, 26, 1073274819864666. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; He, X.; Zheng, H.; Deng, D.; He, F.; Li, R.; Ni, X.; Li, S.; Xu, F. Association between green tea intake and digestive system cancer risk in European and East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2024, 63, 1103–1111. [Google Scholar] [CrossRef]
- Gao, Y.; Zhai, P.; Jiang, F.; Zhou, F.; Wang, X. Association between coffee drinking and endometrial cancer risk: A meta-analysis. J. Obstet. Gynaecol. Res. 2022, 48, 774–795. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Cai, J.; Dong, Y.; Chen, H.; Bo, Z.; Zhao, X.; Xia, M.; Han, M. A multi-omic approach reveals utility of CD45 expression in prognosis and novel target discovery. Front. Genet. 2022, 13, 928328. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Du, M.; Gunter, M.J.; Setiawan, V.W.; Schouten, L.J.; Shu, X.-O.; Wentzensen, N.; Bertrand, K.A.; Cook, L.S.; Friedenreich, C.M.; et al. Epidemiology of Endometrial Cancer Consortium (E2C2), Coffee consumption and risk of endometrial cancer: A pooled analysis of individual participant data in the Epidemiology of Endometrial Cancer Consortium (E2C2). Am. J. Clin. Nutr. 2022, 116, 1219–1228. [Google Scholar] [CrossRef]
- Ong, J.-S.; Law, M.H.; An, J.; Han, X.; Gharahkhani, P.; Whiteman, D.C.; Neale, R.E.; MacGregor, S. Association between coffee consumption and overall risk of being diagnosed with or dying from cancer among >300,000 UK Biobank participants in a large-scale Mendelian randomization study. Int. J. Epidemiol. 2019, 48, 1447–1456. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J.; Lin, K.; Lv, Y.; Wang, H.; Lin, J. Tea Consumption and the Risk of Endometrial Cancer: An Updated Meta-Analysis. Nutr. Cancer 2021, 73, 1849–1855. [Google Scholar] [CrossRef]
- Masukume, G.; Mmbaga, B.T.; Dzamalala, C.P.; Mlombe, Y.B.; Finch, P.; Nyakunga-Maro, G.; Mremi, A.; Middleton, D.R.S.; Narh, C.T.; Chasimpha, S.J.D.; et al. A very-hot food and beverage thermal exposure index and esophageal cancer risk in Malawi and Tanzania: Findings from the ESCCAPE case-control studies. Br. J. Cancer 2022, 127, 1106–1115. [Google Scholar] [CrossRef]
- Carter, P.; Yuan, S.; Kar, S.; Vithayathil, M.; Mason, A.M.; Burgess, S.; Larsson, S.C. Coffee consumption and cancer risk: A Mendelian randomisation study. Clin. Nutr. 2022, 41, 2113–2123. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Ramirez, Y.; O’Connell, C.; de Gonzalez, A.B.; Dawsey, S.M.; Abnet, C.C.; Freedman, N.D.; Loftfield, E. Hot beverage intake and oesophageal cancer in the UK Biobank: Prospective cohort study. Br. J. Cancer 2025, 132, 652–659. [Google Scholar] [CrossRef]
- Kaimila, B.; Mulima, G.; Kajombo, C.; Salima, A.; Nietschke, P.; Pritchett, N.; Chen, Y.; Murphy, G.; Dawsey, S.M.; Gopal, S.; et al. Tobacco and other risk factors for esophageal squamous cell carcinoma in Lilongwe Malawi: Results from the Lilongwe esophageal cancer case: Control study. PLOS Glob. Public Health 2022, 2, e0000135. [Google Scholar] [CrossRef] [PubMed]
- Eser, S.; Özgür, S.; Shayan, N.A.; Abdianwall, M.H. Risk Factors Related to Esophageal Cancer, a Case-Control Study in Herat Province of Afghanistan. Arch. Iran. Med. 2022, 25, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jia, G.; Zhou, X.; Yang, Z. Diet and Esophageal Cancer Risk: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. Adv. Nutr. 2022, 13, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Martimianaki, G.; Bertuccio, P.; Alicandro, G.; Pelucchi, C.; Bravi, F.; Carioli, G.; Bonzi, R.; Rabkin, C.S.; Liao, L.M.; Sinha, R.; et al. Coffee consumption and gastric cancer: A pooled analysis from the Stomach cancer Pooling Project consortium. Eur. J. Cancer Prev. 2022, 31, 117–127. [Google Scholar] [CrossRef]
- Liu, M.; Song, S.-S.; Park, S. High Polygenic Risk Scores Positively Associated with Gastric Cancer Risk Interact with Coffee and Polyphenol Intake and Smoking Status in Korean Adults. Nutrients 2024, 16, 3263. [Google Scholar] [CrossRef]
- Kim, J.H.; Jun, S.; Kim, J. Dietary intake and cancer incidence in Korean adults: A systematic review and meta-analysis of observational studies. Epidemiol. Health 2023, 45, e2023102. [Google Scholar] [CrossRef]
- Poorolajal, J.; Moradi, L.; Mohammadi, Y.; Cheraghi, Z.; Gohari-Ensaf, F. Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiol. Health 2020, 42, e2020004. [Google Scholar] [CrossRef]
- Pelucchi, C.; La Vecchia, C.; Bonzi, R.; Negri, E.; Corso, G.; Boccia, S.; Boffetta, P.; Camargo, M.C.; Curado, M.P.; Lunet, N.; et al. StoP Project Working Group, The global gastric cancer consortium: An update from the Stomach cancer Pooling (StoP) project. Eur. J. Cancer Prev. 2024, 33, 433–437. [Google Scholar] [CrossRef]
- Sasazuki, S.; Tamakoshi, A.; Matsuo, K.; Ito, H.; Wakai, K.; Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Inoue, M.; et al. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan, Green tea consumption and gastric cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2012, 42, 335–346. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Zhou, L.; Li, G.; Yi, D.; Zhang, Y.; Wu, Y.; Liu, X.; Wu, X.; Song, Q.; et al. Association between green tea intake and risk of gastric cancer: A systematic review and dose-response meta-analysis of observational studies. Public Health Nutr. 2017, 20, 3183–3192. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; et al. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan, Coffee, green tea and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2019, 49, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Bhurwal, A.; Rattan, P.; Yoshitake, S.; Pioppo, L.; Reja, D.; Dellatore, P.; Rustgi, V. Inverse Association of Coffee with Liver Cancer Development: An Updated Systematic Review and Meta-analysis. J. Gastrointestin. Liver Dis. 2020, 29, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J.; Volterrani, D. Coffee Consumption and Cancer Risk: An Assessment of the Health Implications Based on Recent Knowledge. Med. Princ. Pract. 2021, 30, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Markozannes, G.; Kanellopoulou, A.; Critselis, E.; Alhardan, S.; Karafousia, V.; Kasimis, J.C.; Katsaraki, C.; Papadopoulou, A.; Zografou, M.; et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat. Commun. 2021, 12, 4579. [Google Scholar] [CrossRef]
- Cai, X.; Li, X.; Liang, C.; Zhang, M.; Dong, Z.; Yu, W. The effect of metabolism-related lifestyle and clinical risk factors on digestive system cancers in East Asian populations: A two-sample Mendelian randomization analysis. Sci. Rep. 2024, 14, 9474. [Google Scholar] [CrossRef]
- Chen, J.-G.; Zhang, Y.-H.; Lu, J.-H.; Kensler, T.W. Liver Cancer Etiology: Old Issues and New Perspectives. Curr. Oncol. Rep. 2024, 26, 1452–1468. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Tan, Y.-T.; Liu, D.-K.; Gao, L.-F.; Li, H.-L.; Xiang, Y.-B. Cumulative consumption of tea is associated with lower risk of liver cancer: Updated results from the Shanghai Women’s Health Study. Int. J. Cancer 2023, 152, 1115–1123. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Christopher, C.N.; Tabung, F.K.; Bao, W.; Garcia, D.O.; Shadyab, A.H.; Saquib, N.; Neuhouser, M.L.; Tinker, L.F.; et al. Association of dietary insulinemic and inflammatory potential with risk of liver cancer and chronic liver disease mortality in postmenopausal women: A prospective cohort study. Am. J. Clin. Nutr. 2023, 118, 530–537. [Google Scholar] [CrossRef]
- Seow, W.J.; Koh, W.-P.; Jin, A.; Wang, R.; Yuan, J.-M. Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population. Eur. J. Nutr. 2019, 59, 3083–3091. [Google Scholar] [CrossRef]
- Bunjaku, J.; Lama, A.; Pesanayi, T.; Shatri, J.; Chamberlin, M.; Hoxha, I. Lung Cancer and Lifestyle Factors: Umbrella Review. Hematol. Oncol. Clin. N. Am. 2024, 38, 171–184. [Google Scholar] [CrossRef]
- Jabbari, M.; Salari-Moghaddam, A.; Bagheri, A.; Larijani, B.; Esmaillzadeh, A. A systematic review and dose-response meta-analysis of prospective cohort studies on coffee consumption and risk of lung cancer. Sci. Rep. 2024, 14, 14991. [Google Scholar] [CrossRef]
- Jin, S.; Je, Y. Coffee Consumption and Risk of Lung Cancer: A Meta-Analysis of Prospective Cohort Studies. Nutr. Cancer 2024, 76, 552–562. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lai, C.-Y.; Lin, I.-H.; Tsai, C.-H.; Tsai, S.-M.; Lam, K.-L.; Wang, J.-Y.; Chen, C.-C.; Wong, R.-H. Joint Effects of Cigarette Smoking and Green Tea Consumption with miR-29b and DNMT3B mRNA Expression in the Development of Lung Cancer. Genes 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, Z.; Liu, H.; An, L.; Yang, T.; Zhang, B.; Liu, G.; Sun, D. Associations of lifestyle factors with oral cancer risk: An umbrella review. J. Stomatol. Oral. Maxillofac. Surg. 2025, 126, 102234. [Google Scholar] [CrossRef] [PubMed]
- Neetha, M.C.; Panchaksharappa, M.G.; Pattabhiramasastry, S.; Shivaprasad, N.V.; Venkatesh, U.G. Chemopreventive Synergism between Green Tea Extract and Curcumin in Patients with Potentially Malignant Oral Disorders: A Double-blind, Randomized Preliminary Study. J. Contemp. Dent. Pract. 2020, 21, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.L.; Jeong, G.H.; Yang, J.W.; Lee, K.H.; Kronbichler, A.; van der Vliet, H.J.; Grosso, G.; Galvano, F.; Aune, D.; Kim, J.Y.; et al. Tea Consumption and Risk of Cancer: An Umbrella Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2020, 11, 1437–1452. [Google Scholar] [CrossRef]
- Shafiei, F.; Salari-Moghaddam, A.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Coffee and caffeine intake and risk of ovarian cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2019, 29, 579–584. [Google Scholar] [CrossRef]
- Huang, C.; Bu, H.; Wang, Y.; Chu, R.; Zhao, W.; Liu, Y.; Wu, H.; Yao, S. Association between coffee and tea consumption and ovarian cancer incidence: A prospective analysis in the PLCO dataset. Int. J. Cancer 2024, 155, 1033–1044. [Google Scholar] [CrossRef]
- Nagle, C.M.; Ibiebele, T.I.; Bandera, E.V.; Cramer, D.; Doherty, J.A.; Giles, G.G.; Goodman, M.T.; Hanley, G.E.; Harris, H.R.; Jensen, A.; et al. Pre-diagnosis tea and coffee consumption and survival after a diagnosis of ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2024, 131, 1043–1049. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, K.; Zhong, J.; Tang, S.; Xu, S.; Lu, W.; Wu, Y.; Xia, D. Association between Different Types of Tea Consumption and Risk of Gynecologic Cancer: A Meta-Analysis of Cohort Studies. Nutrients 2023, 15, 403. [Google Scholar] [CrossRef]
- Gersekowski, K.; DeFazio, A.; Friedlander, M.; Obermair, A.; Webb, P.M. Green tea consumption, primary treatment outcome and survival after a diagnosis of ovarian cancer. J. Epidemiol. Community Health 2025, 79, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Gregg, J.R.; Kim, J.; Logothetis, C.; Hanash, S.; Zhang, X.; Manyam, G.; Muir, K.; Group, U.K.P.S.C.; Giles, G.G.; Stanford, J.L.; et al. Coffee Intake, Caffeine Metabolism Genotype, and Survival Among Men with Prostate Cancer. Eur. Urol. Oncol. 2023, 6, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Papadimitriou, N.; Lagiou, P.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; Murphy, N.; Gunter, M.; Freisling, H.; Tzoulaki, I.; et al. Coffee and tea consumption and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2019, 144, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Gkiouras, K.; Papageorgiou, S.Τ.; Myrogiannis, I.; Mykoniatis, I.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. Dietary Factors and Supplements Influencing Prostate Specific-Antigen (PSA) Concentrations in Men with Prostate Cancer and Increased Cancer Risk: An Evidence Analysis Review Based on Randomized Controlled Trials. Nutrients 2020, 12, 2985. [Google Scholar] [CrossRef]
- Perletti, G.; Magri, V.; Vral, A.; Stamatiou, K.; Trinchieri, A. Green tea catechins for chemoprevention of prostate cancer in patients with histologically-proven HG-PIN or ASAP. Concise review and meta-analysis. Arch. Ital. Urol. Androl. 2019, 91, 153–156. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 3, CD005004. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Wang, Y.; Xu, Y. Effect of dietary antioxidants on the risk of prostate cancer. Systematic review and network meta-analysis. Nutr. Hosp. 2023, 40, 657–667. [Google Scholar] [CrossRef]
- Rhee, J.; Lim, R.K.; Purdue, M.P. Coffee consumption and risk of renal cancer: A meta-analysis of cohort evidence. Cancer Causes Control 2022, 33, 101–108. [Google Scholar] [CrossRef]
- Chen, Y.; Abe, S.K.; Inoue, M.; Yamaji, T.; Iwasaki, M.; Nomura, S.; Hashizume, M.; Tsugane, S.; Sawada, N.; Group, J.P.C.S. Green tea and coffee consumption and risk of kidney cancer in Japanese adults. Sci. Rep. 2022, 12, 20274. [Google Scholar] [CrossRef]
- Oh, C.C.; Jin, A.; Yuan, J.-M.; Koh, W.-P. Coffee, tea, caffeine, and risk of nonmelanoma skin cancer in a Chinese population: The Singapore Chinese Health Study. J. Am. Acad. Dermatol. 2019, 81, 395–402. [Google Scholar] [CrossRef]
- Paiva, M.; Yumeen, S.; Kahn, B.J.; Nan, H.; Cho, E.; Saliba, E.; Qureshi, A. Coffee, Citrus, and Alcohol: A Review of What We Drink and How it May Affect our Risk for Skin Cancer. Yale J. Biol. Med. 2023, 96, 205–210. [Google Scholar] [CrossRef]
- Shao, C.C.; Luo, D.; Pang, G.D.; Xiao, J.; Yang, X.R.; Zhang, Y.; Jia, H.Y. A dose-response meta-analysis of coffee consumption and thyroid cancer occurrence. Int. J. Food Sci. Nutr. 2020, 71, 176–185. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Alghamdi, M.A.; Cayssials, V.; Franceschi, S.; Almquist, M.; Hennings, J.; Sandström, M.; Tsilidis, K.K.; Weiderpass, E.; Boutron-Ruault, M.-C.; et al. Coffee and tea drinking in relation to the risk of differentiated thyroid carcinoma: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2019, 58, 3303–3312. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, P.; Liu, H.; Li, T.; Wang, H.; Jiang, D.; Liu, L.; Ye, H. Coffee and Risk of Pancreatic Cancer: Insights from Two-Sample and Multivariable Mendelian Randomization Analyses. Nutrients 2024, 16, 3723. [Google Scholar] [CrossRef]
- Yu, X.; Bao, Z.; Zou, J.; Dong, J. Coffee consumption and risk of cancers: A meta-analysis of cohort studies. BMC Cancer 2011, 11, 96. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-Cancer Effects of Green Tea by Either Anti- or Pro- Oxidative Mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Q.; Hu, J.; Zhang, F.; Yu, Y.; Ma, L. Green Tea and Epigallocatechin Gallate (EGCG) for Cancer Prevention: A Systematic Review and Meta-Analysis. Am. J. Chin. Med. 2025, 53, 1755–1784. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Li, S.; Kang, X.; Deng, J.; Yang, H.; Chen, F.; Jiang, J.; Zhang, J.; Li, W. Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up. Cancer Biol. Med. 2023, 20, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Samavat, S.; Ashtary-Larky, D.; Naeini, F.; Nazarian, B.; Kashkooli, S.; Clark, C.C.T.; Bagheri, R.; Asbaghi, O.; Babaali, M.; Goudarzi, M.A.; et al. The effects of green coffee bean extract on blood pressure and heart rate: A systematic review and dose-response meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2024, 18, 103120. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, N.; Tanabe, H.; Suzuki, T.; Saeki, K.; Hara, Y. Applications of a Standardized Green Tea Catechin Preparation for Viral Warts and Human Papilloma Virus-Related and Unrelated Cancers. Molecules 2020, 25, 2588. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.-S.; Yoo, J.; Huh, S.-W.; Kim, C.-K.; Lee, J.-M.; Namkoong, S.-E.; Bae, S.-M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef]
- Noman, A.M.; Sultan, M.T.; Mazhar, A.; Baig, I.; Javaid, J.; Hussain, M.; Imran, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Mujtaba, A.; et al. Anticancer Molecular Mechanisms of Epigallocatechin Gallate: An Updated Review on Clinical Trials. Food Sci. Nutr. 2025, 13, e70735. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Viggiano, T.R.; Buttar, N.S.; Song, L.M.W.K.; Schroeder, K.W.; Kraichely, R.E.; Larson, M.V.; Sedlack, R.E.; Kisiel, J.B.; Gostout, C.J.; et al. Randomized Phase II Trial of Polyphenon E versus Placebo in Patients at High Risk of Recurrent Colonic Neoplasia. Cancer Prev. Res. 2021, 14, 573–580. [Google Scholar] [CrossRef]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Dévoz, P.P.; Antunes, L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020, 43, e20190347. [Google Scholar] [CrossRef]
- Lee, W.J.; Zhu, B.T. Inhibition of DNA methylation by caffeic acid and chlorogenic acid, two common catechol-containing coffee polyphenols. Carcinogenesis 2006, 27, 269–277. [Google Scholar] [CrossRef]
- Pal, D.; Sur, S.; Roy, R.; Mandal, S.; Panda, C.K. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J. Cell. Physiol. 2018, 234, 825–836. [Google Scholar] [CrossRef]
- Nandakumar, V.; Vaid, M.; Katiyar, S.K. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 2011, 32, 537–544. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Sei, Y.; Yamaguchi, K.; Suganuma, M.; Fujiki, H. DNA and RNA as new binding targets of green tea catechins. J. Biol. Chem. 2006, 281, 17446–17456. [Google Scholar] [CrossRef]
- Sakamoto, N.; Honma, R.; Sekino, Y.; Goto, K.; Sentani, K.; Ishikawa, A.; Oue, N.; Yasui, W. Non-coding RNAs are promising targets for stem cell-based cancer therapy. Non-Coding RNA Res. 2017, 2, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bai, M.; Sun, Z.; Yao, N.; Zhang, A.; Guo, S.; Asemi, Z. Epigallocatechin-3-gallate and cancer: Focus on the role of microRNAs. Cancer Cell Int. 2023, 23, 241. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of p53-targeting microRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Al-Maghout, T.; Bissinger, R.; Zeng, N.; Pelzl, L.; Salker, M.S.; Cheng, A.; Singh, Y.; Lang, F. Epigallocatechin-3-gallate (EGCG) up-regulates miR-15b expression thus attenuating store operated calcium entry (SOCE) into murine CD4+ T cells and human leukaemic T cell lymphoblasts. Oncotarget 2017, 8, 89500–89514. [Google Scholar] [CrossRef][Green Version]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Mirzaaghaei, S.; Foroughmand, A.M.; Saki, G.; Shafiei, M. Combination of Epigallocatechin-3-gallate and Silibinin: A Novel Approach for Targeting Both Tumor and Endothelial Cells. ACS Omega 2019, 4, 8421–8430. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Xue, J.; Zhou, X.; Luo, L.; Ma, Q.; Chen, Y.-F.; Zhang, J.; Zhang, S.-L.; Zhao, L. Antischistosomiasis Liver Fibrosis Effects of Chlorogenic Acid through IL-13/miR-21/Smad7 Signaling Interactions In Vivo and In Vitro. Antimicrob. Agents Chemother. 2017, 61, e01347-16. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Asim, M.; Hafeez, B.B.; Adhami, V.M.; Tarapore, R.S.; Mukhtar, H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J. 2011, 25, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Fix, L.N.; Shah, M.; Efferth, T.; Farwell, M.A.; Zhang, B. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60. Cancer Genom. Proteom. 2010, 7, 261–277. [Google Scholar]
- El Gizawy, H.A.; Boshra, S.A.; Mostafa, A.; Mahmoud, S.H.; Ismail, M.I.; Alsfouk, A.A.; Taher, A.T.; Al-Karmalawy, A.A. Pimenta dioica (L.) Merr. Bioactive Constituents Exert Anti-SARS-CoV-2 and Anti-Inflammatory Activities: Molecular Docking and Dynamics, In Vitro, and In Vivo Studies. Molecules 2021, 26, 5844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2016, 37, 4373–4382. [Google Scholar] [CrossRef]
- Banerjee, S.; Mandal, A.K.A. Role of epigallocatechin-3- gallate in the regulation of known and novel microRNAs in breast carcinoma cells. Front. Genet. 2022, 13, 995046. [Google Scholar] [CrossRef]
- Li, B.-B.; Huang, G.-L.; Li, H.-H.; Kong, X.; He, Z.-W. Epigallocatechin-3-gallate Modulates MicroRNA Expression Profiles in Human Nasopharyngeal Carcinoma CNE2 Cells. Chin. Med. J. 2017, 130, 93–99. [Google Scholar] [CrossRef]
- Dharshini, L.C.P.; Mandal, A.K.A. Regulation of gene expression by modulating microRNAs through Epigallocatechin-3-gallate in cancer. Mol. Biol. Rep. 2024, 51, 230. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Liu, M.; Yan, Q.; Zhao, W.; Yang, P.; Gao, Q.; Wei, J.; Zhao, W.; Ma, L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp. Ther. Med. 2018, 17, 1742–1748. [Google Scholar] [CrossRef]
- Nakayama, T.; Funakoshi-Tago, M.; Tamura, H. Coffee reduces KRAS expression in Caco-2 human colon carcinoma cells via regulation of miRNAs. Oncol. Lett. 2017, 14, 1109–1114. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, D.; Wan, X.; Bai, Y.; Yuan, C.; Wang, T.; Yuan, D.; Zhang, C.; Liu, C. Chlorogenic Acid Suppresses miR-155 and Ameliorates Ulcerative Colitis through the NF-κB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2020, 64, e2000452. [Google Scholar] [CrossRef]
- Luque-Badillo, A.C.; Hernandez-Tapia, G.; Ramirez-Castillo, D.A.; Espinoza-Serrano, D.; Cortes-Limon, A.M.; Cortes-Gallardo, J.P.; Jacobo-Velázquez, D.A.; Martinez-Fierro, M.L.; Rios-Ibarra, C.P. Gold nanoparticles enhance microRNA 31 detection in colon cancer cells after inhibition with chlorogenic acid. Oncol. Lett. 2021, 22, 742. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Suárez, M.; Torres, J.L.; Salvadó, M.J.; Arola, L.; Arola-Arnal, A. Resveratrol and EGCG bind directly and distinctively to miR-33a and miR-122 and modulate divergently their levels in hepatic cells. Nucleic Acids Res. 2014, 42, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, Y.; Yu, J.; Chang, X. Hawthorn polyphenols reduce high glucose-induced inflammation and apoptosis in ARPE-19 cells by regulating miR-34a/SIRT1 to reduce acetylation. J. Food Biochem. 2021, 45, e13623. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, X.; Cao, N.; Chen, C.; Yi, J.; Hao, L.; Ji, Y.; Liu, X.; Lu, J. EGCG enhances cancer cells sensitivity under 60Coγ radiation based on miR-34a/Sirt1/p53. Food Chem. Toxicol. 2019, 133, 110807. [Google Scholar] [CrossRef]
- Mostafa, S.M.; Gamal-Eldeen, A.M.; El Maksoud, N.A.; Fahmi, A.A. Epigallocatechin gallate-capped gold nanoparticles enhanced the tumor suppressors let-7a and miR-34a in hepatocellular carcinoma cells. An. Acad. Bras. Cienc. 2020, 92, e20200574. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ai, W.; Banik, N.L.; Ray, S.K. Overexpression of miR-7-1 increases efficacy of green tea polyphenols for induction of apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-DZ cells. Neurochem. Res. 2013, 38, 420–432. [Google Scholar] [CrossRef]
- Zhou, D.-H.; Wang, X.; Feng, Q. EGCG enhances the efficacy of cisplatin by downregulating hsa-miR-98-5p in NSCLC A549 cells. Nutr. Cancer 2014, 66, 636–644. [Google Scholar] [CrossRef]
- Khedr, N.F.; Zahran, E.S.; Ebeid, A.M.; Melek, S.T.; Werida, R.H. Effect of green coffee on miR-133a, miR-155 and inflammatory biomarkers in obese individuals. Diabetol. Metab. Syndr. 2024, 16, 256. [Google Scholar] [CrossRef]
- Suetsugu, F.; Tadokoro, T.; Fujita, K.; Fujihara, S.; Sasaki, K.; Omayu, E.; Nakatani, K.; Koyama, Y.; Kozuka, K.; Matsui, T.; et al. Antitumor Effects of Epigallocatechin-3-Gallate on Colorectal Cancer: An In Vitro and In Vivo Study. Anticancer Res. 2025, 45, 2937–2947. [Google Scholar] [CrossRef]
- Zhou, N.; Yuan, Y.; Lin, H.; Wang, J.; Lin, H.; Ashktorab, H.; Smoot, D.; Jin, Z.; Zhuang, S.; Qin, Y. Epigallocatechin Gallate Induces miR-192/215 Suppression of EGR1 in Gastric Cancer. Anticancer Res. 2025, 45, 1935–1951. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ping, Z.; Xuemei, G.; Hongjuan, M.; Yi, H.; Xiaoli, L.; Zhongxiang, Z. Chlorogenic acid regulates the proliferation and migration of high-grade serous ovarian cancer cells through modulating the miR199a5p/DDR1 axis. Acta Biochim. Pol. 2022, 69, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, V.; Mandal, A.K.A. Next-Generation Sequencing Reveals the Role of Epigallocatechin-3-Gallate in Regulating Putative Novel and Known microRNAs Which Target the MAPK Pathway in Non-Small-Cell Lung Cancer A549 Cells. Molecules 2019, 24, 368. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.A.; Jordan, H.R.; Tollefsbol, T.O. Effects of SAHA and EGCG on Growth Potentiation of Triple-Negative Breast Cancer Cells. Cancers 2018, 11, 23. [Google Scholar] [CrossRef]
- Arffa, M.L.; Zapf, M.A.; Kothari, A.N.; Chang, V.; Gupta, G.N.; Ding, X.; Al-Gayyar, M.M.; Syn, W.; Elsherbiny, N.M.; Kuo, P.C.; et al. Epigallocatechin-3-Gallate Upregulates miR-221 to Inhibit Osteopontin-Dependent Hepatic Fibrosis. PLoS ONE 2016, 11, e0167435. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wang, H.-H.; Chen, T.-H.; Chiang, M.-C.; Hung, P.-H.; Chen, Y.-J. Involvement of MicroRNA-296 in the Inhibitory Effect of Epigallocatechin Gallate against the Migratory Properties of Anoikis-Resistant Nasopharyngeal Carcinoma Cells. Cancers 2020, 12, 973. [Google Scholar] [CrossRef]
- Kang, Q.; Tong, Y.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.-W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021, 12, 3381–3392. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, C.; Chen, L.; Chen, A.; Wu, X.; Zhou, M.; Haq, I.U.; Mariyam, Z.; Feng, Q. EGCG inhibits CSC-like properties through targeting miR-485/CD44 axis in A549-cisplatin resistant cells. Mol. Carcinog. 2018, 57, 1835–1844. [Google Scholar] [CrossRef]
- Shaalan, Y.M.; Handoussa, H.; Youness, R.A.; Assal, R.A.; El-Khatib, A.H.; Linscheid, M.W.; El Tayebi, H.M.; Abdelaziz, A.I. Destabilizing the interplay between miR-1275 and IGF2BPs by Tamarix articulata and quercetin in hepatocellular carcinoma. Nat. Prod. Res. 2018, 32, 2217–2220. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Chen, Y.-J.; Chang, W.-A.; Li, W.-M.; Ke, H.-L.; Wu, W.-J.; Kuo, P.-L. Effects of Epigallocatechin Gallate (EGCG) on Urinary Bladder Urothelial Carcinoma-Next-Generation Sequencing and Bioinformatics Approaches. Medicina 2019, 55, 768. [Google Scholar] [CrossRef]
- Sasaki, K.; Fujita, K.; Fujihara, S.; Iwama, H.; Kitaoka, A.; Suetsugu, F.; Mimura, S.; Tani, J.; Morishita, A.; Masaki, T.; et al. The Polyphenol (-)-Epigallocatechin-3-gallate (EGCG) Inhibits the Proliferation of Gastric Cancer Cells and Alters microRNA Signatures. Anticancer Res. 2025, 45, 2925–2936. [Google Scholar] [CrossRef]
- Yamada, S.; Tsukamoto, S.; Huang, Y.; Makio, A.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci. Rep. 2016, 6, 19225. [Google Scholar] [CrossRef] [PubMed]
- Davalos, V.; Moutinho, C.; Villanueva, A.; Boque, R.; Silva, P.; Carneiro, F.; Esteller, M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 2012, 31, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Khiewkamrop, P.; Phunsomboon, P.; Richert, L.; Pekthong, D.; Srisawang, P. Epistructured catechins, EGCG and EC facilitate apoptosis induction through targeting de novo lipogenesis pathway in HepG2 cells. Cancer Cell Int. 2018, 18, 46. [Google Scholar] [CrossRef]
- Weiss, J. The health system in Africa: Poor medicine and large problems. Dtsch. Med. Wochenschr. 2009, 134, p42. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.; Sun, Y.; Liu, P. Quercetin suppresses the tumorigenesis of oral squamous cell carcinoma by regulating microRNA-22/WNT1/β-catenin axis. J. Pharmacol. Sci. 2019, 140, 128–136. [Google Scholar] [CrossRef]
- Tan, M.; Wu, J.; Cai, Y. Suppression of Wnt signaling by the miR-29 family is mediated by demethylation of WIF-1 in non-small-cell lung cancer. Biochem. Biophys. Res. Commun. 2013, 438, 673–679. [Google Scholar] [CrossRef]
- Yao, S.; Gao, M.; Wang, Z.; Wang, W.; Zhan, L.; Wei, B. Upregulation of MicroRNA-34a Sensitizes Ovarian Cancer Cells to Resveratrol by Targeting Bcl-2. Yonsei Med. J. 2021, 62, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-F.; Fu, J.-Y.; Han, L.; Gao, G.-B.; Zhang, W.-X.; Yu, S.-M.; Li, N.; Li, Y.-J.; Lu, Y.-F.; Ding, X.-F.; et al. The Antipsychotic Drug Aripiprazole Suppresses Colorectal Cancer by Targeting LAMP2a to Induce RNH1/miR-99a/mTOR-Mediated Autophagy and Apoptosis. Adv. Sci. 2024, 11, e2409498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gong, J.; Ding, C.; Chen, G. Quercetin induces the apoptosis of human ovarian carcinoma cells by upregulating the expression of microRNA-145. Mol. Med. Rep. 2015, 12, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Yang, Z.; Du, H.; Wu, Z.; Gong, J.; Yan, J.; Zheng, Q. MiR-145 regulates PAK4 via the MAPK pathway and exhibits an antitumor effect in human colon cells. Biochem. Biophys. Res. Commun. 2012, 427, 444–449. [Google Scholar] [CrossRef]
- Ravindran, F.; Koroth, J.; Manjunath, M.; Narayan, S.; Choudhary, B. Curcumin derivative ST09 modulates the miR-199a-5p/DDR1 axis and regulates proliferation and migration in ovarian cancer cells. Sci. Rep. 2021, 11, 23025. [Google Scholar] [CrossRef]
- Soubani, O.; Ali, A.S.; Logna, F.; Ali, S.; Philip, P.A.; Sarkar, F.H. Re-expression of miR-200 by novel approaches regulates the expression of PTEN and MT1-MMP in pancreatic cancer. Carcinogenesis 2012, 33, 1563–1571. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, S.; Wu, H.; Zhang, L.; Dai, X.; Hu, J.; Xue, J.; Liu, T.; Liang, Y.; Wu, G. MiR-200c increases the radiosensitivity of non-small-cell lung cancer cell line A549 by targeting VEGF-VEGFR2 pathway. PLoS ONE 2013, 8, e78344. [Google Scholar] [CrossRef]
- Ding, X.; Zhong, T.; Jiang, L.; Huang, J.; Xia, Y.; Hu, R. miR-25 enhances cell migration and invasion in non-small-cell lung cancer cells via ERK signaling pathway by inhibiting KLF4. Mol. Med. Rep. 2018, 17, 7005–7016. [Google Scholar] [CrossRef]
- Hameiri-Grossman, M.; Porat-Klein, A.; Yaniv, I.; Ash, S.; Cohen, I.J.; Kodman, Y.; Haklai, R.; Elad-Sfadia, G.; Kloog, Y.; Chepurko, E.; et al. The association between let-7, RAS and HIF-1α in Ewing Sarcoma tumor growth. Oncotarget 2015, 6, 33834–33848. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, W.; Zhang, W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin. Transl. Oncol. 2014, 16, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.S.; Shahryari, V.; Deng, G.; Thamminana, S.; Saini, S.; Majid, S.; Chang, I.; Hirata, H.; Ueno, K.; Yamamura, S.; et al. Up-regulation of microRNA-21 correlates with lower kidney cancer survival. PLoS ONE 2012, 7, e31060. [Google Scholar] [CrossRef]
- Liu, P.; Liang, H.; Xia, Q.; Li, P.; Kong, H.; Lei, P.; Wang, S.; Tu, Z. Resveratrol induces apoptosis of pancreatic cancers cells by inhibiting miR-21 regulation of BCL-2 expression. Clin. Transl. Oncol. 2013, 15, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, Z.; Li, A.; Jenkins, G.; Yang, X.; Hu, J.; Guo, W. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol. Cell. Biochem. 2013, 382, 137–143. [Google Scholar] [CrossRef]
- Shi, D.-L.; Shi, G.-R.; Xie, J.; Du, X.-Z.; Yang, H. MicroRNA-27a Inhibits Cell Migration and Invasion of Fibroblast-Like Synoviocytes by Targeting Follistatin-Like Protein 1 in Rheumatoid Arthritis. Mol. Cells 2016, 39, 611–618. [Google Scholar] [CrossRef]
- Gandhy, S.U.; Kim, K.; Larsen, L.; Rosengren, R.J.; Safe, S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer 2012, 12, 564. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Khandkar, M.; Banik, N.L.; Ray, S.K. Alterations in expression of specific microRNAs by combination of 4-HPR and EGCG inhibited growth of human malignant neuroblastoma cells. Brain Res. 2012, 1454, 1–13. [Google Scholar] [CrossRef]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef]
- Song, M.; Zhou, W.; Fan, J.; Jia, C.; Xiong, W.; Wei, H.; Tao, S. Diarrheal microbiota-derived extracellular vesicles drive intestinal homeostasis dysfunction via miR-125b/NF-κB-mediated macrophage polarization. Gut Microbes 2025, 17, 2541036. [Google Scholar] [CrossRef]
- Zhou, J.; Lei, Y.; Chen, J.; Zhou, X. Potential ameliorative effects of epigallocatechin-3-gallate against testosterone-induced benign prostatic hyperplasia and fibrosis in rats. Int. Immunopharmacol. 2018, 64, 162–169. [Google Scholar] [CrossRef]
- Wang, S.-S.; Feng, L.; Hu, B.-G.; Lu, Y.-F.; Wang, W.-M.; Guo, W.; Suen, C.-W.; Jiao, B.-H.; Pang, J.-X.; Fu, W.-M.; et al. miR-133a Promotes TRAIL Resistance in Glioblastoma via Suppressing Death Receptor 5 and Activating NF-κB Signaling. Mol. Ther. Nucleic Acids 2017, 8, 482–492. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy Isoflavone Genistein-Mediated Downregulation of miR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Allegri, L.; Rosignolo, F.; Mio, C.; Filetti, S.; Baldan, F.; Damante, G. Effects of nutraceuticals on anaplastic thyroid cancer cells. J. Cancer Res. Clin. Oncol. 2018, 144, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Dubaybo, H.; Ali, S.; Goncalves, P.; Kollepara, S.L.; Sethi, S.; Philip, P.A.; Li, Y. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27(kip1), p57(kip2), and PUMA. Am. J. Cancer Res. 2013, 3, 465–477. [Google Scholar]
- Zhang, S.; Tang, D.; Zang, W.; Yin, G.; Dai, J.; Sun, Y.U.; Yang, Z.; Hoffman, R.M.; Guo, X. Synergistic Inhibitory Effect of Traditional Chinese Medicine Astragaloside IV and Curcumin on Tumor Growth and Angiogenesis in an Orthotopic Nude-Mouse Model of Human Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 465–473. [Google Scholar] [CrossRef]
- Kim, O.-H.; Jeon, T.J.; Kang, H.; Chang, E.S.; Hong, S.A.; Kim, M.K.; Lee, H.J. hsa-mir-483-3p modulates delayed breast cancer recurrence. Sci. Rep. 2025, 15, 693. [Google Scholar] [CrossRef]
- Ping, P.; Yang, T.; Ning, C.; Zhao, Q.; Zhao, Y.; Yang, T.; Gao, Z.; Fu, S. Chlorogenic acid attenuates cardiac hypertrophy via up-regulating Sphingosine-1-phosphate receptor1 to inhibit endoplasmic reticulum stress. ESC Hear. Fail. 2024, 11, 1580–1593. [Google Scholar] [CrossRef]
- Silva, M.A.; Izidoro, M.; Bonifácio, B.S.; Schenkman, S. Untargeted Metabolomics of Epimastigote Forms of Trypanosoma cruzi. Bio-Protocol. 2025, 15, e5368. [Google Scholar] [CrossRef]
- Saadatagah, S.; Naderian, M.; Larouche, M.; Gaudet, D.; Kullo, I.J.; Ballantyne, C.M. Epidemiology and longitudinal course of chylomicronemia: Insights from NHANES and a large health care system. J. Clin. Lipidol. 2025, 19, 432–441. [Google Scholar] [CrossRef]
- Wójciak, M.; Paduch, R.; Drozdowski, P.; Wójciak, W.; Żuk, M.; Płachno, B.J.; Sowa, I. Antioxidant and Anti-Inflammatory Effects of Nettle Polyphenolic Extract: Impact on Human Colon Cells and Cytotoxicity Against Colorectal Adenocarcinoma. Molecules 2024, 29, 5000. [Google Scholar] [CrossRef]
- Chen, H.; Shi, J.; Tang, Y.; Chen, X.; Wang, Z.; Liu, Q.; Wu, K.; Yao, X. Exploring the effect of chlorogenic acid on oxidative stress and autophagy in dry eye mice via the AMPK/ULK1 pathway. Eur. J. Pharmacol. 2025, 991, 177311. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, P.; Islam, A.; Bhardwaj, M.; Kumar, V.; Prakash, H. The neuroprotective role of chlorogenic acid and Fisetin in differentiated neuronal cell line-SHSY5Y against amyloid-β-induced neurotoxicity. Toxicol. Vitr. 2025, 109, 106110. [Google Scholar] [CrossRef] [PubMed]
- Komeili-Movahhed, T.; Heidari, F.; Moslehi, A. Chlorogenic acid alleviated testicular inflammation and apoptosis in tunicamycin induced endoplasmic reticulum stress. Physiol. Int. 2023, 110, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Negm, A.; El-Neanaey, A.A.; Khadr, A.E.S.; Kamel, M.A.E.N.; Ismail, A.E.-H.A.; El Sayed, I.E.T.; Darwish, W.S.; Eldaim, M.A.A.; Okaz, R.S.; Bahr, M.H.; et al. Chlorogenic Acid Ameliorates CCl4-induced Liver Fibrosis by Modulating the PI3K/AKT/mTOR Autophagy Pathway. Anticancer Agents Med. Chem. 2025, 25, 913–920. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Ding, X.; Xu, H.; Xiong, C.; Tang, M.; Peng, Y. Chlorogenic acid mitigates DHEA-induced oxidative stress in granulosa cells and alleviates ferroptosis via the NF-κB signaling pathway in PCOS. Eur. J. Pharmacol. 2025, 1002, 177870. [Google Scholar] [CrossRef]
- Peng, Y.; Qi, Z.; Xu, Y.; Yang, X.; Cui, Y.; Sun, Q. AMPK and metabolic disorders: The opposite roles of dietary bioactive components and food contaminants. Food Chem. 2024, 437, 137784. [Google Scholar] [CrossRef]
- Tian, C.; Feng, Y.; Chen, T.; Zhang, Z.; He, X.; Jiang, L.; Liu, M. EGCG Restores Keratinocyte Autophagy to Promote Diabetic Wound Healing through the AMPK/ULK1 Pathway. Front. Biosci. 2023, 28, 324. [Google Scholar] [CrossRef]
- Wang, H.; An, Y.; Rajput, S.A.; Qi, D. Resveratrol and (-)-Epigallocatechin-3-gallate Regulate Lipid Metabolism by Activating the AMPK Pathway in Hepatocytes. Biology 2024, 13, 368. [Google Scholar] [CrossRef]
- Yuan, M.; Hu, L.; Zhu, C.; Li, Q.; Tie, H.; Ruan, H.; Wu, T.; Zhang, H.; Xu, L. Comparison and Assessment of Anti-Inflammatory and Antioxidant Capacity Between EGCG and Phosphatidylcholine-Encapsulated EGCG. J. Cosmet. Dermatol. 2025, 24, e16628. [Google Scholar] [CrossRef]
- Khan, I.M.; Gul, H.; Khan, S.; Nassar, N.; Khalid, A.; Swelum, A.A.; Wang, Z. Green tea polyphenol epigallocatechin-3-gallate mediates an antioxidant response via Nrf2 pathway in heat-stressed poultry: A review. Poult. Sci. 2025, 104, 105071. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Q.; Li, M.; Ren, M.; Liu, S.; An, G.; Ren, Y.; Liu, P.; Du, L.; Sun, X.; et al. Polyphenol-based self-assembled nanoparticles treating uveitis by inflammation-oxidative stress suppression. Mater. Today Bio. 2025, 33, 102052. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Han, G.; Yang, Y.; Wang, S.; Lv, X.; Gao, M. S100A4/NF-κB axis mediates the anticancer effect of epigallocatechin-3-gallate in platinum-resistant ovarian cancer. IScience 2024, 27, 108885. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-D.; Liu, F.; Zeng, Y.; Liu, Y.; Luo, W.; Yuan, F.; Li, S.; Li, Q.; Chen, J.; Fujita, M.; et al. EGCG suppresses PD-1 expression of T cells via inhibiting NF-κB phosphorylation and nuclear translocation. Int. Immunopharmacol. 2024, 133, 112069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Zhang, T.; Zi, M.; Wang, S.; Zhang, Q. Green tea epigallocatechin gallate attenuate metabolic dysfunction-associated steatotic liver disease by regulation of pyroptosis. Lipids Health Dis. 2025, 24, 180. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Mastrolonardo, A.J.; Xhuti, D.; Di Carlo, A.; Manta, K.; Fuda, M.R.; Tarnopolsky, M.A. Novel Multi-Ingredient Supplement Facilitates Weight Loss and Improves Body Composition in Overweight and Obese Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 3693. [Google Scholar] [CrossRef]
- Srinivasan, D.; Balakrishnan, R.; Chauhan, A.; Kumar, J.; Girija, D.M.; Shrestha, R.; Shrestha, R.; Subbarayan, R. Epithelial-Mesenchymal Transition in Cancer: Insights Into Therapeutic Targets and Clinical Implications. Medcomm 2025, 6, e70333. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Morikawa-Ichinose, T.; Lee, S.-U.; Tatsumi, Y.; Ichitani, M.; Kumazoe, M.; Tachibana, H.; Fujimura, Y. Comprehensive microRNA analysis toward exploring a new functional component in Matcha green tea. Food Chem. Mol. Sci. 2025, 10, 100265. [Google Scholar] [CrossRef]
- Fujimura, Y.; Kumazoe, M.; Tachibana, H. 67-kDa Laminin Receptor-Mediated Cellular Sensing System of Green Tea Polyphenol EGCG and Functional Food Pairing. Molecules 2022, 27, 5130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).