Establishment of Genetic Transformation System of Non-Embryogenic Callus in Rosa rugosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Media
2.2. Vector Construction
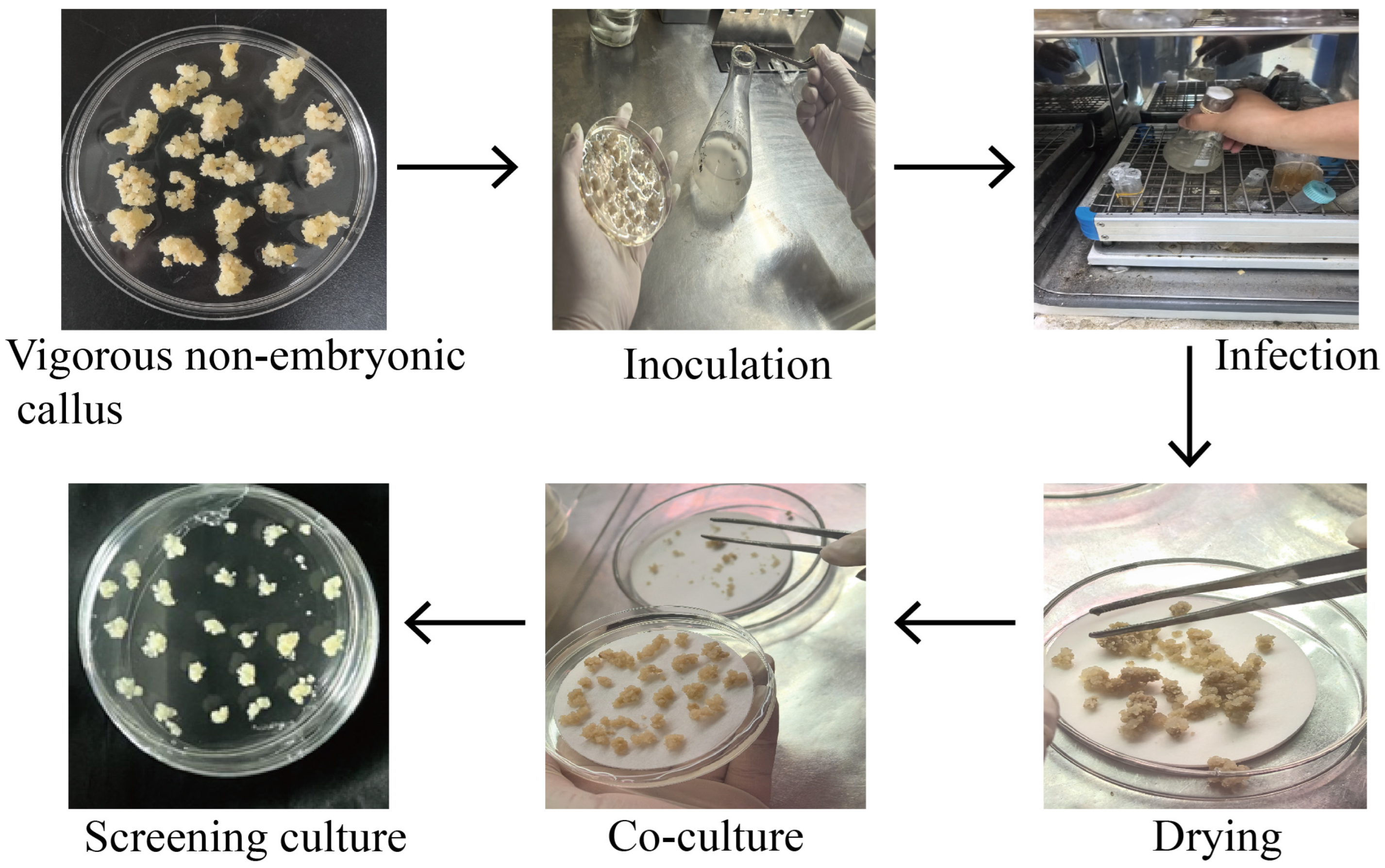
2.3. Transformation Procedure
2.4. Carotenoid Detection
2.5. GFP Fluorescence Observation
2.6. PCR Identification of Positive Calli
2.7. Statistical Analysis
3. Results
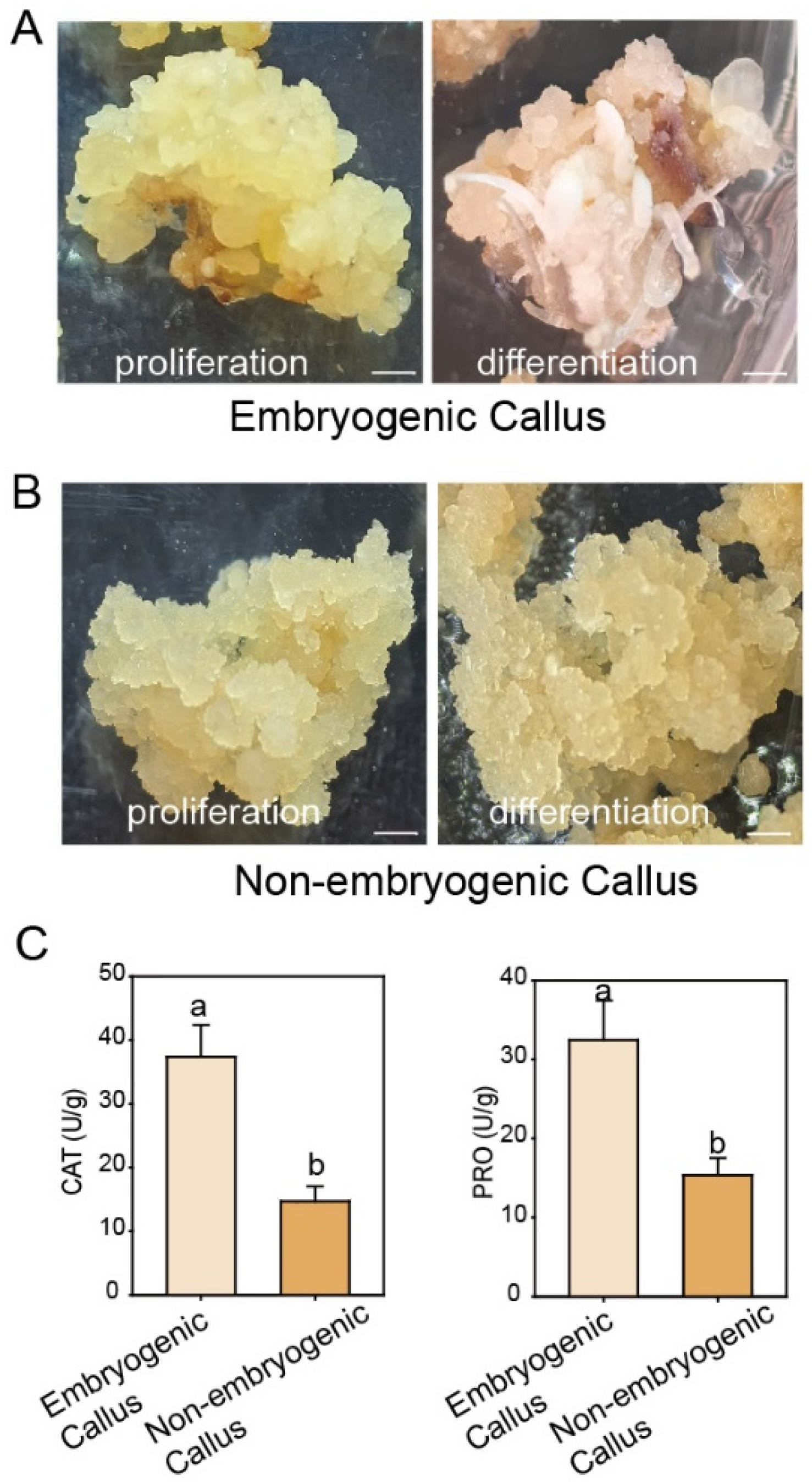
3.1. Comparison of Embryogenic and Non-Embryogenic Callus
3.2. Antibiotic Concentration Screening
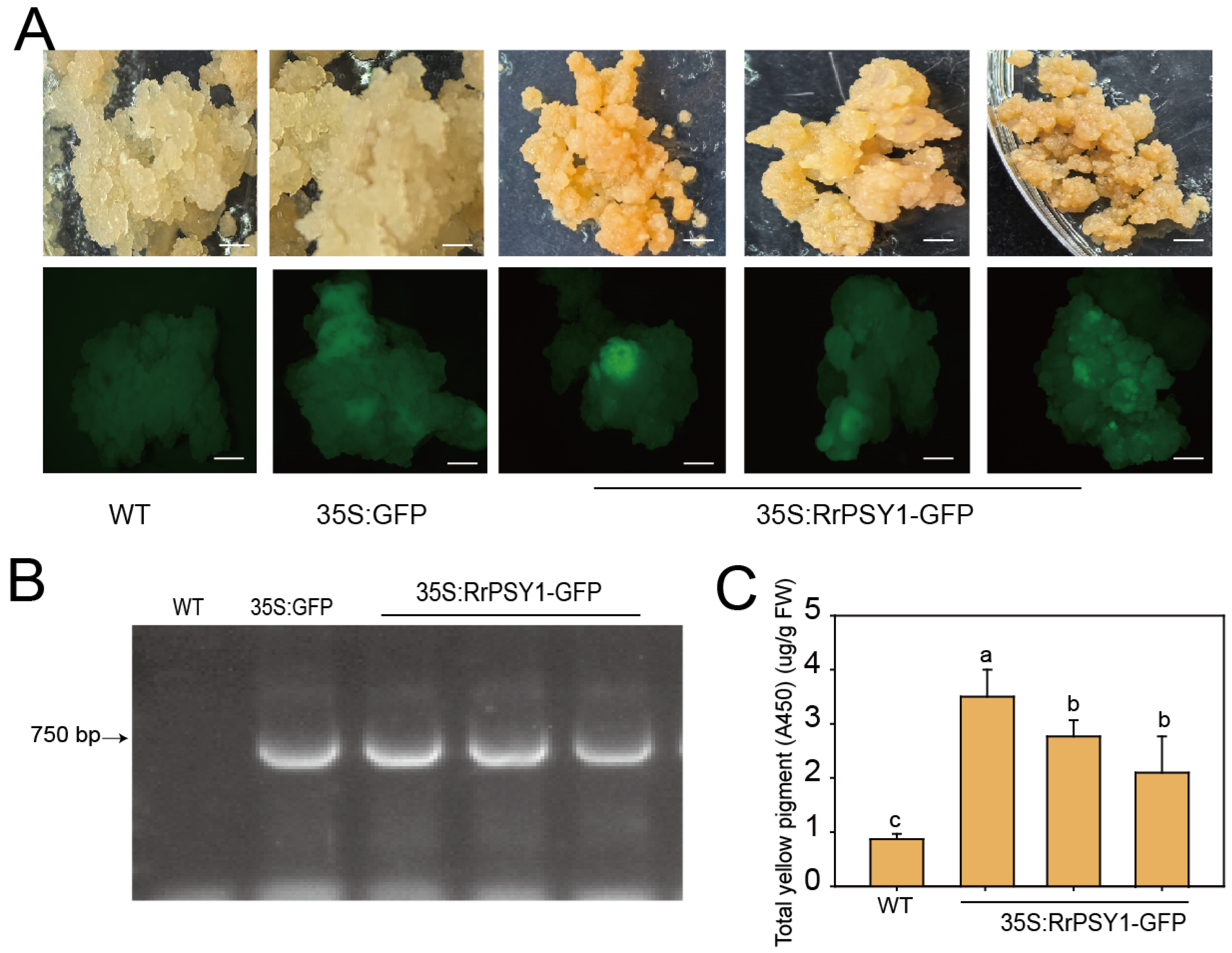
3.3. Genetic Transformation of Callus with RrPSY1 Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.Y.; Zhang, X.L. Regulation of somatic embryogenesis in higher plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Marsoni, M.; Bracale, M.; Espen, L.; Prinsi, B.; Negri, A.S.; Vannini, C. Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Rep. 2008, 27, 347–356. [Google Scholar] [CrossRef]
- Caeiro, A.; Jarak, I.; Correia, S.; Canhoto, J.; Carvalho, R. Primary Metabolite Screening Shows Significant Differences between Embryogenic and Non-Embryogenic Callus of Tamarillo (Solanum betaceum Cav.). Plants 2023, 12, 2869. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Canhoto, J.M. Somatic Embryogenesis of Tamarillo (Solanum betaceum Cav.). In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Jain, S., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 85, pp. 171–179. ISBN 978-3-319-79086-2. [Google Scholar]
- Lopes, M.L.; Ferreira, M.R.; Carloto, J.M.; Cruz, G.S.; Canhoto, J.M. Somatic Embryogenesis Induction in Tamarillo (Cyphomandra betacea). In Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Newton, R.J., Eds.; Springer: Dordrecht, The Netherland, 2000; Volume 67, pp. 433–455. ISBN 978-94-017-3030-3. [Google Scholar]
- Gautier, F.; Label, P.; Eliášová, K.; Leplé, J.-C.; Motyka, V.; Boizot, N.; Vondráková, Z.; Malbeck, J.; Trávníčková, A.; Metté, C.L.; et al. Cytological, Biochemical and Molecular Events of the Embryogenic State in Douglas-fir (Pseudotsuga menziesii [Mirb.]). Front. Plant Sci. 2019, 10, 118. [Google Scholar] [CrossRef]
- Zhao, F.X.; Chen, S.W.; Perl, A.; Dai, R.; Xu, H.; Ma, H. The Establishment of an Agrobacterium-Mediated Transformation Platform for the Non-Embryogenic Calli of Vitis vinifera L. Agric. Sci. 2011, 10, 686–694. [Google Scholar] [CrossRef]
- Wann, S.R.; Johnson, M.A.; Noland, T.L.; Carlson, J. Biochemical differences between embryogenic and nonembryogenic callus of Picea abies (L.) Karst. Plant Cell Rep. 1987, 6, 39–42. [Google Scholar] [CrossRef]
- Swedlund, B.; Locy, R.D. Sorbitol as the Primary Carbon Source for the Growth of Embryogenic Callus of Maize. Plant Physiol. 1993, 103, 1339–1346. [Google Scholar] [CrossRef]
- Niedz, R.P.; Evens, T.J. Regulating NH4+, NO3+, and K+ concentration and proportions improves in vitro tissue growth. Vitr. Cell. Dev. Biol.-Anim. 2008, 44, S72. [Google Scholar]
- Hesami, M.; Pepe, M.; de Ronne, M.; Yoosefzadeh-Najafabadi, M.; Adamek, K.; Torkamaneh, D.; Jones, A.M.P. Transcriptomic Profiling of Embryogenic and Non-Embryogenic Callus Provides New Insight into the Nature of Recalcitrance in Cannabis. Int. J. Mol. Sci. 2023, 24, 14625. [Google Scholar] [CrossRef]
- Dal Santo, S.; De Paoli, E.; Pagliarani, C.; Amato, A.; Celii, M.; Boccacci, P.; Zenoni, S.; Gambino, G.; Perrone, I. Stress responses and epigenomic instability mark the loss of somatic embryogenesis competence in grapevine. Plant Physiol. 2022, 188, 490–508. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Bao, W.; Sui, M.; Bai, Y. Transcriptome Analysis of Embryogenic and Non-Embryogenic Callus of Picea Mongolica. Curr. Issues Mol. Biol. 2023, 45, 5232–5247. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, P.F.; Li, R.; Hyden, B.; An, X.; Jing, R.; Zhao, X.; Zhang, Y.; Qiao, H.; Han, Y.; et al. Cellular and metabolic characteristics of peach anther-derived callus. Sci. Hortic. 2023, 311, 111796. [Google Scholar] [CrossRef]
- Lardon, R.; Geelen, D. Natural variation in plant pluripotency and regeneration. Plants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.W.; Ye, J.L.; Zhu, K.J.; Zhang, Y.; Zhang, M.; Xu, Q.; Deng, X. A fruit ripening-associated transcription factor CsMADS5 positively regulates carotenoid biosynthesis in citrus. J. Exp. Bot. 2021, 72, 3028–3043. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Ji, H.Y.; Huang, W.K.; Zhang, Z.H.; Zhu, K.J.; Zhu, S.P.; Chai, L.J.; Ye, J.L.; Deng, X.X. Transcription factor CrWRKY42 coregulates chlorophyll degradation and carotenoid biosynthesis in citrus. Plant Physiol. 2024, 195, 728–744. [Google Scholar] [CrossRef]
- He, J.X.; Xu, Y.T.; Huang, D.; Fu, J.L.; Liu, Z.; Wang, L.; Zhang, Y.; Xu, R.W.; Li, L.; Deng, X.X.; et al. TRIPTYCHON-LIKE regulates aspects of both fruit flavor and color in citrus. J. Exp. Bot. 2022, 73, 3610–3624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, L.B.; Tao, H.H.; An, Y.Y.; Wang, L.J. Transcriptomic Profiling of Apple Calli With a Focus on the Key Genes for ALA-Induced Anthocyanin Accumulation. Front. Plant Sci. 2021, 12, 640606. [Google Scholar] [CrossRef]
- Dang, Q.Y.; Sha, H.Y.; Nie, J.Y.; Wang, Y.Z.; Yuan, Y.B.; Jia, D.J. An apple (Malus domestica) AP2/ERF transcription factor modulates carotenoid accumulation. Hortic. Res. 2021, 8, 223. [Google Scholar] [CrossRef]
- Wang, P.F.; Li, R.; Liu, X.Y.; Zhao, X.L.; Hyden, B.; Han, Y.; Zhang, X.Y.; Wang, J.H.; Chen, H.J.; Cao, H.B. Establishment of genetic transformation system of peach callus. Sci. Hortic. 2024, 323, 112501. [Google Scholar] [CrossRef]
- Shen, X.X.; Ping, Y.K.; Bao, C.N.; Liu, C.; Tahir, M.M.; Li, X.W.; Song, Y.; Xu, W.R.; Ma, F.W.; Guan, Q.M. Mdm-miR160-MdARF17-MdWRKY33 module mediates freezing tolerance in apple. Plant J. 2023, 114, 262–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.Z.; Sun, Q.; Lu, S.W.; Chai, L.J.; Ye, J.L.; Deng, X.X. Citrus transcription factor CsHB5 regulates abscisic acid biosynthetic genes and promotes senescence. Plant J. 2021, 108, 151–168. [Google Scholar] [CrossRef]
- Hurst, C.C. Notes on the origin and evolution of our garden roses. J. R. Hort. Soc. 1941, 66, 73–82. [Google Scholar]
- Martínez, M.C.; Santiago, J.L.; Boso, S.; Gago, P.; Álvarez-Acero, I.; De Vega, M.-E.; Martínez-Bartolomé, M.; Álvarez-Nogal, R.; Molíst, P.; Caser, M.; et al. Narcea-an unknown, ancient cultivated rose variety from northern Spain. Hortic. Res. 2020, 7, 44. [Google Scholar] [CrossRef]
- Feng, L.-G.; Chen, C.; Sheng, L.-X.; Liu, P.; Tao, J.; Su, J.-L.; Zhao, L.-Y. Comparative analysis of headspace volatiles of Chinese Rosa rugosa. Molecules 2010, 15, 8390–8399. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; He, J.S.; Niu, S.M.; Pi, Z.F.; Shao, W.; Liu, F.; Zhao, L. A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J. Pharm. Pharmacol. 2004, 56, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, S.C.; Choi, M.R.; Song, S.H.; Yoo, E.J.; Kim, S.H.; Miyashiro, H.; Hattori, M. Anti-HIV protease activity from rosa family plant extracts and rosamultin from Rosa rugosa. J. Med. Food. 2005, 8, 107–109. [Google Scholar] [CrossRef]
- Hill, G.P. Morphogenesis of shoot primordia in cultured stem tissue of a garden rose. Nature 1967, 216, 596. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, G.F.; Shi, X.P.; Xing, W.; Ning, G.G.; Liu, J.; Bao, M.Z. Primary and repetitive secondary somatic embryogenesis in Rosa hybrida ‘Samantha’. Plant Cell Tissue Org. 2012, 109, 411–418. [Google Scholar] [CrossRef]
- Dohm, A.; Ludwig, C.; Schilling, D.; Debener, T. Transformation of roses with genes for antifungal proteins. Acta Hortic. 2001, 547, 27–34. [Google Scholar] [CrossRef]
- Kim, S.W.; Oh, M.J.; Liu, J.R. Plant regeneration from the root derived embryonic tissues of Rosa hybrida L. cv. Charming via acombined pathway of somatic embryogenesis and organogenesis. Plant Biotechnol. Rep. 2009, 3, 341–345. [Google Scholar] [CrossRef]
- Murali, S.; Sreedhar, D.; Lokeswari, T.S. Regeneration through somatic embryogenesis from petal-derived calli of Rosa hybrida L. cv Arizona (hybrid tea). Euphytica 1996, 91, 271–275. [Google Scholar] [CrossRef]
- Li, X.Q.; Krasnyanski, S.F.; Korban, S.S. Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. Plant Physiol. 2002, 159, 313–319. [Google Scholar] [CrossRef]
- Xing, W.; Bao, Y.; Luo, P.; Bao, M.Z.; Ning, G.G. An efficient system to produce transgenic plants via cyclic leave-originated secondary somatic embryogenesis in Rosa rugosa. Acta Physiol. Plant. 2014, 36, 2013–2023. [Google Scholar] [CrossRef]
- Wei, G.; Chen, Y.D.; Wang, M.M.; Xi, Y.; Xu, Y.; Hussain, H.; Zhu, K.K.; Xu, Y.; Bai, M.J.; Wang, J.W.; et al. Integrative application of metabolomics and transcriptomics provides new insights into carotenoid biosynthesis during Rosa rugosa hips ripening. Food Biosci. 2024, 60, 104422. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I.; Hucl, P.; Fregeau-Reid, J. Identification and quantification of seed carotenoids in selected wheat species. J. Agric. Food Chem. 2007, 55, 787–794. [Google Scholar] [CrossRef]
- Shen, R.; Yang, S.P.; Zhao, G.H.; Shen, Q.; Diao, X.M. Identification of carotenoids in foxtail millet (Setaria italica) and the effects of cooking methods on carotenoid content. J. Cereal Sci. 2015, 61, 86–93. [Google Scholar] [CrossRef]
- Xu, Q.; Wen, X.P.; Deng, X.X. A simple protocol for isolating genomic DNA from chestnut rose (Rosa roxburghii tratt) for RFLP and PCR analyses. Plant Mol. Biol. Rep. 2004, 22, 301–302. [Google Scholar] [CrossRef]
- Cao, H.B.; Zhang, J.C.; Xu, J.D.; Ye, J.L.; Yun, Z.; Xu, Q.; Xu, J.; Deng, X.X. Comprehending crystalline β-carotene accumulation by comparing engineered cell models and the natural carotenoid-rich system of citrus. J. Exp. Bot. 2012, 63, 4403–4417. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.Z.; Feng, D.D.; Liu, H.; Niu, L.T.; Li, R.H.; Li, Y.J.; Chen, M.X.; Li, A.; Liu, Z.H.; He, Y.H.; et al. Evolution of the biosynthetic pathways of terpene scent compounds in roses. Curr. Biol. 2024, 34, 3550–3563. [Google Scholar] [CrossRef]
- Chen, F.; Su, L.Y.; Hu, S.Y.; Xue, J.-Y.; Liu, H.; Liu, G.H.; Jiang, Y.F.; Du, J.K.; Qiao, Y.S.; Fan, Y.N.; et al. A chromosome-level genome assembly of rugged rose (Rosa rugosa) provides insights into its evolution, ecology, and floral characteristics. Hortic. Res. 2021, 8, 141. [Google Scholar] [CrossRef]
- Hibrand Saint-Oyant, L.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef]
- Vergne, P.; Maene, M.; Gabant, G.; Chauvet, A.; Debener, T.; Bendahmane, M. Somatic embryogenesis and transformation of the diploid Rosa chinensis cv old blush. Plant Cell Tissue Org. 2010, 100, 73–81. [Google Scholar] [CrossRef]
- Akasaka, Y.; Mii, M.; Daimon, H. Morphological alterations and root nodule formation in Agrobacterium rhizogenes-mediated transgenic hairy roots of peanut (Arachis hypogaea L). Ann. Bot. 1998, 81, 355–362. [Google Scholar] [CrossRef][Green Version]
- Yan, H.J.; Shi, S.C.; Ma, N.; Cao, X.Q.; Zhang, H.; Qiu, X.Q.; Wang, Q.G.; Jian, H.Y.; Zhou, N.N.; Zhang, Z.; et al. Graft-accelerated virus-induced gene silencing facilitates functional genomics in rose flowers. J. Integr. Plant Biol. 2018, 60, 34–44. [Google Scholar] [CrossRef]
- Lu, J.; Sun, J.J.; Jiang, A.Q.; Bai, M.J.; Fan, C.G.; Liu, J.Y.; Ning, G.G.; Wang, C.Q. Alternate expression of CONSTANS-LIKE 4 in short days and CONSTANS in long days facilitates day-neutral response in Rosa chinensis. J. Exp. Bot. 2020, 71, 4057–4068. [Google Scholar] [CrossRef]
- Wang, X.N.; Yang, F.; Zhang, J.C.; Ren, Y.-R.; An, J.-P.; Chang, D.-Y.; Wang, X.-F.; You, C.-X. Ectopic expression of MmCYP1A1, a mouse cytochrome P450 gene, positively regulates stress tolerance in apple calli and Arabidopsis. Plant Physiol. Bioch. 2023, 42, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yang, X.Y.; Guo, K.; Deng, J.W.; Xu, J.; Gao, W.H.; Lindsey, K.; Zhang, X.L. ROS Homeostasis Regulates Somatic Embryogenesis via the Regulation of Auxin Signaling in Cotton. Mol. Cell Proteom. 2016, 15, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xie, L.J.; Lin, G.M.; Jiang, S.; Chen, H.B.; Li, H.P.; Takáč, T.; Šamaj, J.; Xu, C.X. Histological changes and differences in activities of some antioxidant enzymes and hydrogen peroxide content during somatic embryogenesis of Musa AAA cv. Yueyoukang 1. Sci. Hortic. 2012, 144, 87–92. [Google Scholar] [CrossRef]
- Jiang, L.W.; Liu, K.; Zhang, T.; Chen, J.; Zhao, S.Q.; Cui, Y.S.; Zhou, W.T.; Yu, Y.; Chen, S.Y.; Wang, C.Y.; et al. The RhWRKY33a-RhPLATZ9 regulatory module delays petal senescence by suppressing rapid reactive oxygen species accumulation in rose flowers. Plant J. 2023, 114, 1425–1442. [Google Scholar] [CrossRef] [PubMed]


| Medium Type | Composition |
|---|---|
| proliferation medium | MS + 1 mg/L 2, 4-D + 0.01 mg/L 6-BA + 3 g/L gel + 40 g/L sucrose |
| differentiation medium | 1/2 MS + 1 mg/L 6-BA + 3 g/L gel + 30 g/L sucrose |
| Agrobacterium infestation | 1/2 MS + 30 g/L Glucose + 100 mg/L AS |
| co-culture medium | MS + 40 g/L Glucose + 1 mg/L 2, 4-D + 0.01 mg/L 6-BA + 3 g/L gel + 200 μmol AS |
| screening medium | MS + 45 g/L Glucose + 1 mg/L 2, 4-D + 0.01 mg/L 6-BA + 3 g/L gel + 300 mg/L cef + 35 mg/L Kana |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhu, X.; Yang, Y.; Wei, G.; Feng, L.; Bai, M. Establishment of Genetic Transformation System of Non-Embryogenic Callus in Rosa rugosa. Curr. Issues Mol. Biol. 2025, 47, 894. https://doi.org/10.3390/cimb47110894
Liu X, Zhu X, Yang Y, Wei G, Feng L, Bai M. Establishment of Genetic Transformation System of Non-Embryogenic Callus in Rosa rugosa. Current Issues in Molecular Biology. 2025; 47(11):894. https://doi.org/10.3390/cimb47110894
Chicago/Turabian StyleLiu, Xinyun, Xiyang Zhu, Yating Yang, Guo Wei, Liguo Feng, and Mengjuan Bai. 2025. "Establishment of Genetic Transformation System of Non-Embryogenic Callus in Rosa rugosa" Current Issues in Molecular Biology 47, no. 11: 894. https://doi.org/10.3390/cimb47110894
APA StyleLiu, X., Zhu, X., Yang, Y., Wei, G., Feng, L., & Bai, M. (2025). Establishment of Genetic Transformation System of Non-Embryogenic Callus in Rosa rugosa. Current Issues in Molecular Biology, 47(11), 894. https://doi.org/10.3390/cimb47110894







