Targeting Ferroptosis in Sensorineural Hearing Loss: A Mechanistic Review of Therapeutic Opportunities
Abstract
1. Introduction
2. Ferroptosis and Sensorineural Hearing Loss
2.1. The Role of Ferroptosis in Age-Related Hearing Loss
2.2. The Role of Ferroptosis in Noise-Induced Hearing Loss
2.3. The Role of Ferroptosis in Ototoxic Drug-Induced Hearing Loss
2.4. The Role of Ferroptosis in Sudden Sensorineural Hearing Loss
3. Ferroptosis and Metabolic Pathways
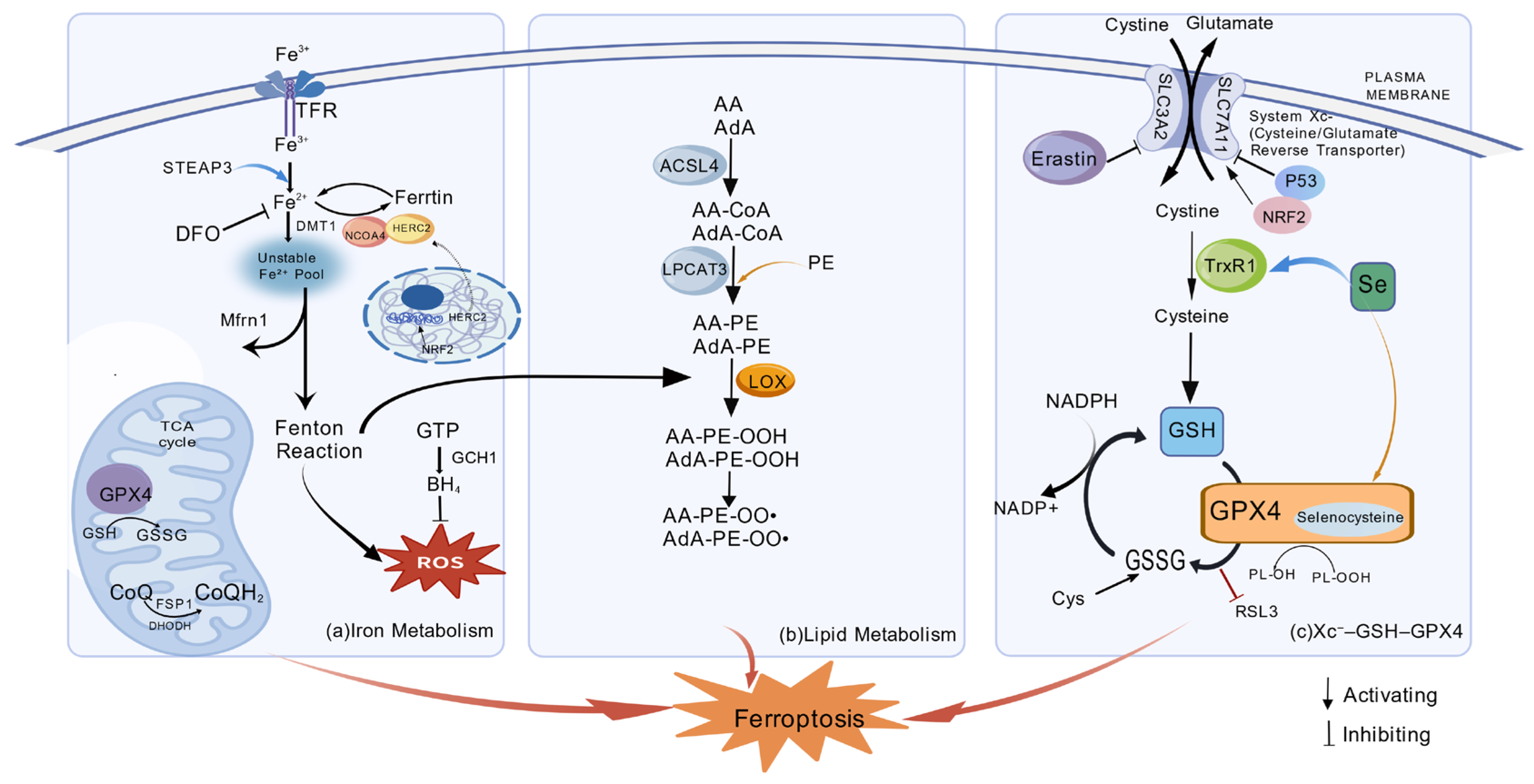
3.1. Iron Metabolism Regulates Ferroptosis
3.2. Lipid Metabolism Involved in Ferroptosis
3.3. The Xc−–GSH–GPX4 Antioxidant Axis
3.4. Pharmacological Modulation of Ferroptosis
3.4.1. Inducers of Ferroptosis as Research Probes
3.4.2. Inhibitors of Ferroptosis as Therapeutic Agents
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statements
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| ARHL | Age-related hearing loss |
| ATP | Adenosine triphosphate |
| DMT1 | Divalent metal transporter 1 |
| Fe2+ | Ferrous ion |
| Fe3+ | Ferric ion |
| Fer-1 | Ferrostatin-1 |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| LOX | Lipoxygenase |
| NIHL | Noise-induced hearing loss |
| ODIHL | Ototoxic drug-induced hearing loss |
| OHCs | Outer hair cells |
| PUFAs | Polyunsaturated fatty acids |
| RCD | Regulated cell death |
| ROS | Reactive oxygen species |
| RSL3 | RAS-selective lethal 3 |
| SLC7A11 | Solute carrier family 7 member 11 |
| SNHL | Sensorineural hearing loss |
| STEAP3 | Six-transmembrane epithelial antigen of the prostate 3 |
| TFR | Transferrin receptor |
| MUFA | Monounsaturated fatty acids |
| HMG | β-Hydroxy-β-methylglutaric acid (HMG) |
| NCOA4 | Nuclear Coactivator 4 |
| TrxR1 | Thioredoxin Reductase 1 |
| MFRN1 | Mitoferrin-1 |
| DHODH | Dihydroorotate dehydrogenase |
References
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 2021, 99, 242. [Google Scholar] [CrossRef]
- Kim, E.; Han, S.Y.; Hwang, K.; Kim, D.; Kim, E.M.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Antioxidant and cytoprotective effects of (-)-epigallocatechin-3-(3″-O-methyl) gallate. Int. J. Mol. Sci. 2019, 20, 3993. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Fetoni, A.R.; De Bartolo, P.; Eramo, S.L.; Rolesi, R.; Paciello, F.; Bergamini, C.; Fato, R.; Paludetti, G.; Petrosini, L.; Troiani, D. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: Cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci. 2013, 33, 4011–4023. [Google Scholar] [CrossRef]
- Shi, T.F.; Zhou, Z.; Jiang, W.J.; Huang, T.L.; Si, J.Q.; Li, L. Hyperglycemia-induced oxidative stress exacerbates mitochondrial apoptosis damage to cochlear stria vascularis pericytes via the ROS-mediated Bcl-2/CytC/AIF pathway. Redox Rep. 2024, 29, 2382943. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Q.; Liu, H.; Liu, J.; Yang, S.; Luo, X.; Liu, W.; Zheng, H.; Liu, Q.; Cui, Y.; et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 2022, 8, 40. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.; Liu, X.; Wang, X.; Xue, C.; Wu, X.; Zhang, X.; Xu, X.; Liu, Z.; Yao, L.; et al. LRRK2 regulates ferroptosis through the system Xc-GSH-GPX4 pathway in the neuroinflammatory mechanism of Parkinson’s disease. J. Cell. Physiol. 2024, 239, e31250. [Google Scholar] [CrossRef]
- Gong, C.; Fu, X.; Ma, Q.; He, M.; Zhu, X.; Liu, L.; Zhou, D.; Yan, S. Gastrodin: Modulating the xCT/GPX4 and ACSL4/LPCAT3 pathways to inhibit ferroptosis after ischemic stroke. Phytomedicine 2025, 136, 156331. [Google Scholar] [CrossRef]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 2020, 6, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wu, W.L.; Wan, X.R.; Wang, J.; Huang, J.N.; Jiang, Y.Y.; Sheng, Y.C.; Wu, J.C.; Liang, Z.Q.; Qin, Z.H.; et al. Regulation of FSP1 myristoylation by NADPH: A novel mechanism for ferroptosis inhibition. Redox Biol. 2024, 73, 103176. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hipp, C.; Santos Dias Mourão, A.; Borggräfe, J.; Aldrovandi, M.; Henkelmann, B.; Wanninger, J.; Mishima, E.; Lytton, E.; Emler, D.; et al. Phase separation of FSP1 promotes ferroptosis. Nature 2023, 619, 371–377. [Google Scholar] [CrossRef]
- Bi, G.; Liang, J.; Bian, Y.; Shan, G.; Huang, Y.; Lu, T.; Zhang, H.; Jin, X.; Chen, Z.; Zhao, M.; et al. Polyamine-mediated ferroptosis amplification acts as a targetable vulnerability in cancer. Nat. Commun. 2024, 15, 2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Yuan, L.; Li, W.; Li, J.Y. Ferroptosis in Parkinson’s disease: Glia-neuron crosstalk. Trends Mol. Med. 2022, 28, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Jinson, S.; Zhang, Z.; Lancaster, G.I.; Murphy, A.J.; Morgan, P.K. Iron, lipid peroxidation, and ferroptosis play pathogenic roles in atherosclerosis. Cardiovasc. Res. 2025, 121, 44–61. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Ren, Q.; Yan, B.; Zhao, Y.; Yu, P.; Gao, G.; Shi, H.; Chang, S.; Chang, Y.Z. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shen, W.; He, D.Z.; Long, K.B.; Madison, L.D.; Dallos, P. Prestin is the motor protein of cochlear outer hair cells. Nature 2000, 405, 149–155. [Google Scholar] [CrossRef]
- Fettiplace, R.; Hackney, C.M. The sensory and motor roles of auditory hair cells. Nat. Rev. Neurosci. 2006, 7, 19–29. [Google Scholar] [CrossRef]
- Liberman, M.C.; Gao, J.; He, D.Z.; Wu, X.; Jia, S.; Zuo, J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature 2002, 419, 300–304. [Google Scholar] [CrossRef]
- Marcotti, W. Functional assembly of mammalian cochlear hair cells. Exp. Physiol. 2012, 97, 438–451. [Google Scholar] [CrossRef]
- Keithley, E.M. Inner ear immunity. Hear. Res. 2022, 419, 108518. [Google Scholar] [CrossRef]
- Chen, F.Q.; Zheng, H.W.; Hill, K.; Sha, S.H. Traumatic noise activates Rho-family GTPases through transient cellular energy depletion. J. Neurosci. 2012, 32, 12421–12430. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.; Zhang, H.; Chen, C.; Zeng, M.; Yunis, J.; Wei, Y.; Wan, Y.; Wang, N.; Zhou, M.; et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021, 22, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Rance, G.; Chisari, D. Auditory neuropathy in a patient with hemochromatosis. J. Otol. 2016, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Warnke, C.; Andersen, K.; Hartung, H.P.; Hefter, H. Superficial siderosis of the central nervous system in a patient with hemochromatosis and Wilson’s disease. Dtsch. Med. Wochenschr. 2011, 136, 721–724. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, P.; Sun, Y.; Zhang, P.; Zhou, C.; Xiong, Z.; Zhang, H.; Liang, J.; Lai, B. Reducing agents enhanced Fenton-like oxidation (Fe(III)/Peroxydisulfate): Substrate specific reactivity of reactive oxygen species. Water Res. 2022, 218, 118412. [Google Scholar] [CrossRef]
- Lyu, A.R.; Kim, T.H.; Park, S.J.; Shin, S.A.; Jeong, S.H.; Yu, Y.; Huh, Y.H.; Je, A.R.; Park, M.J.; Park, Y.H. Mitochondrial damage and necroptosis in aging cochlea. Int. J. Mol. Sci. 2020, 21, 2505. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.P.; Hu, B.H.; Yang, S.; Henderson, D. Involvement of p53 and Bcl-2 in sensory cell degeneration in aging rat cochleae. Acta Otolaryngol. 2017, 137, 572–580. [Google Scholar] [CrossRef]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J. Neurosci. 2020, 40, 6357–6366. [Google Scholar] [CrossRef]
- Gupta, S.; Eavey, R.D.; Wang, M.; Curhan, S.G.; Curhan, G.C. Type 2 diabetes and the risk of incident hearing loss. Diabetologia 2019, 62, 281–285. [Google Scholar] [CrossRef]
- Someya, S.; Xu, J.; Kondo, K.; Ding, D.; Salvi, R.J.; Yamasoba, T.; Rabinovitch, P.S.; Weindruch, R.; Leeuwenburgh, C.; Tanokura, M.; et al. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc. Natl. Acad. Sci. USA 2009, 106, 19432–19437. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, Y.; Chen, X.; Zhao, J.; Han, J.; Dong, H.; Zheng, Q.; Nie, G. Ferrostatin-1 protects auditory hair cells from cisplatin-induced ototoxicity in vitro and in vivo. Biochem. Biophys. Res. Commun. 2020, 533, 1442–1448. [Google Scholar] [CrossRef]
- Pham, T.B.; Boussaty, E.C.; Currais, A.; Maher, P.; Schubert, D.R.; Manor, U.; Friedman, R.A. Attenuation of age-related hearing impairment in Senescence-Accelerated Mouse Prone 8 (SAMP8) mice treated with Fatty Acid Synthase Inhibitor CMS121. J. Mol. Neurosci. 2023, 73, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.C.; Mondorf, A.; Beifuß, J.; Jung, M.; Brüne, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Keithley, E.M.; Housley, G.D.; Ryan, A.F.; Wong, A.C. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017, 349, 129–137. [Google Scholar] [CrossRef]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Primary neural degeneration in noise-exposed human cochleas: Correlations with outer hair cell loss and word-discrimination scores. J. Neurosci. 2021, 41, 4439–4447. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Ma, P.W.; Wang, W.L.; Chen, J.W.; Yuan, H.; Lu, P.H.; Gao, W.; Ding, X.R.; Lun, Y.Q.; Liang, R.; He, Z.H.; et al. Treatment with the ferroptosis inhibitor ferrostatin-1 attenuates noise-induced hearing loss by suppressing ferroptosis and apoptosis. Oxid. Med. Cell. Longev. 2022, 2022, 3373828. [Google Scholar] [CrossRef]
- Nicotera, T.M.; Hu, B.H.; Henderson, D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 466–477. [Google Scholar] [CrossRef]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, lipid peroxidation, and cell death: Discoveries, rediscoveries, and open issues. Antioxid. Redox Signal. 2018, 29, 61–74. [Google Scholar] [CrossRef]
- Leis, J.A.; Rutka, J.A.; Gold, W.L. Aminoglycoside-induced ototoxicity. CMAJ 2015, 187, E52. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, T.; Chen, C.; Lin, T.; Wu, X.; Chen, Y.; Lin, Y.; Luo, X.; Zeng, C.; Lin, C. Sarsasapogenin protects hair cells from cisplatin-induced ototoxicity by attenuating apoptosis and ferroptosis via alleviating oxidative stress. Front. Pharmacol. 2025, 16, 1641174. [Google Scholar] [CrossRef] [PubMed]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J.; et al. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997, 18, 559–571. [Google Scholar] [PubMed]
- Ramkumar, V.; Mukherjea, D.; Dhukhwa, A.; Rybak, L.P. Oxidative stress and inflammation caused by cisplatin ototoxicity. Antioxidants 2021, 10, 1919. [Google Scholar] [CrossRef]
- Das, A.; Ash, D.; Fouda, A.Y.; Sudhahar, V.; Kim, Y.M.; Hou, Y.; Hudson, F.Z.; Stansfield, B.K.; Caldwell, R.B.; McMenamin, M.; et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. Cell Biol. 2022, 24, 35–50. [Google Scholar] [CrossRef]
- Maier, J.; Niello, M.; Rudin, D.; Daws, L.C.; Sitte, H.H. The interaction of organic cation transporters 1-3 and PMAT with psychoactive substances. Handb. Exp. Pharmacol. 2021, 266, 199–214. [Google Scholar] [CrossRef]
- Masuda, Y.; Futamura, M.; Kamino, H.; Nakamura, Y.; Kitamura, N.; Ohnishi, S.; Miyamoto, Y.; Ichikawa, H.; Ohta, T.; Ohki, M.; et al. The potential role of DFNA5, a hearing impairment gene, in p53-mediated cellular response to DNA damage. J. Hum. Genet. 2006, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Mao, H.; Zhao, L.; Li, X.; Liao, Y.; Li, W.; Li, H.; Chen, Y. Nuciferine protects cochlear hair cells from ferroptosis through inhibiting NCOA4-mediated ferritinophagy. Antioxidants 2024, 13, 714. [Google Scholar] [CrossRef]
- Hazlitt, R.A.; Min, J.; Zuo, J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J. Med. Chem. 2018, 61, 5512–5524. [Google Scholar] [CrossRef]
- Takahashi, K.; Kamiya, K.; Urase, K.; Suga, M.; Takizawa, T.; Mori, H.; Yoshikawa, Y.; Ichimura, K.; Kuida, K.; Momoi, T. Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain Res. 2001, 894, 359–367. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Wang, D.; Wang, Y.; Zhou, Z.; Ma, X.; Liu, X.; Dong, Y. Cisplatin-induced ototoxicity: From signaling network to therapeutic targets. Biomed. Pharmacother. 2023, 157, 114045. [Google Scholar] [CrossRef]
- Hu, K.; Li, K.; Lv, J.; Feng, J.; Chen, J.; Wu, H.; Cheng, F.; Jiang, W.; Wang, J.; Pei, H.; et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Investig. 2020, 130, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Hong, S.J.; Kang, S.H.; Park, Y.; Kim, S.K. Alpha-lipoic acid attenuates apoptosis and ferroptosis in cisplatin-induced ototoxicity via the reduction of intracellular lipid droplets. Int. J. Mol. Sci. 2022, 23, 10981. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, B.E.; Agrup, C.; Haskard, D.O.; Luxon, L.M. Sudden sensorineural hearing loss. Lancet 2010, 375, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Newsted, D.; Bajin, M.D.; You, P. Sudden sensorineural hearing loss. CMAJ 2025, 197, E68. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Goebel, B.; Boll, M.; Adhikari, A.; Maurer, V.; Steinhilber, D.; Culmsee, C. Mitochondrial reactive oxygen species formation determines ACSL4/LPCAT2-mediated ferroptosis. Antioxidants 2023, 12, 1590. [Google Scholar] [CrossRef]
- Bai, X.; Chen, S.; Xu, K.; Jin, Y.; Niu, X.; Xie, L.; Qiu, Y.; Liu, X.Z.; Sun, Y. N-acetylcysteine combined with dexamethasone treatment improves sudden sensorineural hearing loss and attenuates hair cell death caused by ROS stress. Front. Cell Dev. Biol. 2021, 9, 659486. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Böttcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Park, E.; Chung, S.W. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019, 10, 822. [Google Scholar] [CrossRef]
- Krainz, T.; Gaschler, M.M.; Lim, C.; Sacher, J.R.; Stockwell, B.R.; Wipf, P. A mitochondrial-targeted nitroxide is a potent inhibitor of ferroptosis. ACS Cent. Sci. 2016, 2, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pang, J.; Xu, L.; Niu, W.; Zhang, Y.; Li, S.; Li, X. Hedyotis diffusa injection induces ferroptosis via the Bax/Bcl2/VDAC2/3 axis in lung adenocarcinoma. Phytomedicine 2022, 104, 154319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, M.; Zhang, K.; Zhang, J.; Gao, T.; Connell, D.O.; Yao, F.; Mu, C.; Cai, B.; Shang, Y.; et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat. Commun. 2020, 11, 433. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed cell-death by ferroptosis: Antioxidants as mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef]
- Amaral, E.P.; Foreman, T.W.; Namasivayam, S.; Hilligan, K.L.; Kauffman, K.D.; Barbosa Bomfim, C.C.; Costa, D.L.; Barreto-Duarte, B.; Gurgel-Rocha, C.; Santana, M.F.; et al. GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection. J. Exp. Med. 2022, 219, e20220504. [Google Scholar] [CrossRef]
- Touret, N.; Martin-Orozco, N.; Paroutis, P.; Furuya, W.; Lam-Yuk-Tseung, S.; Forbes, J.; Gros, P.; Grinstein, S. Molecular and cellular mechanisms underlying iron transport deficiency in microcytic anemia. Blood 2004, 104, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Schorpp, K.; Jin, J.; Yozwiak, C.E.; Hoffstrom, B.G.; Decker, A.M.; Rajbhandari, P.; Stokes, M.E.; Bender, H.G.; Csuka, J.M.; et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020, 30, 3411–3423.e7. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorg. Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef]
- Pantopoulos, K. Iron metabolism and the IRE/IRP regulatory system: An update. Ann. N. Y. Acad. Sci. 2004, 1012, 1–13. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Gao, M.; Dai, M.; Zheng, Y.; Qu, L.; Zhang, J.; Gong, G. A comparative study of the efficiency of mitochondria-targeted antioxidants MitoTEMPO and SKQ1 under oxidative stress. Free Radic. Biol. Med. 2024, 224, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Gurrin, L.C.; Constantine, C.C.; Osborne, N.J.; Delatycki, M.B.; Nicoll, A.J.; McLaren, C.E.; Bahlo, M.; Nisselle, A.E.; Vulpe, C.D.; et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N. Engl. J. Med. 2008, 358, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Retamal, C.; Schupper, D.; Vergara-Hernández, D.; Saha, S.; Profumo, E.; Buttari, B.; Saso, L. Antioxidant cardioprotection against reperfusion injury: Potential therapeutic roles of resveratrol and quercetin. Molecules 2022, 27, 2564. [Google Scholar] [CrossRef]
- Dixon, S.J.; Winter, G.E.; Musavi, L.S.; Lee, E.D.; Snijder, B.; Rebsamen, M.; Superti-Furga, G.; Stockwell, B.R. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 2015, 10, 1604–1609. [Google Scholar] [CrossRef]
- Soupene, E.; Fyrst, H.; Kuypers, F.A. Mammalian acyl-CoA: Lysophosphatidylcholine acyltransferase enzymes. Proc. Natl. Acad. Sci. USA 2008, 105, 88–93. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Das, N.K.; Solanki, S.; Jain, C.; El-Derany, M.O.; Koo, I.; Bell, H.N.; Aabed, N.; Singhal, R.; et al. Fibroblast lipid metabolism through ACSL4 regulates epithelial sensitivity to ferroptosis in IBD. Nat. Metab. 2025, 7, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Sato, H.; Tamba, M.; Ishii, T.; Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999, 274, 11455–11458. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Seiler, A.; Perisic, T.; Kölle, P.; Banjac Canak, A.; Förster, H.; Weiss, N.; Kremmer, E.; Lieberman, M.W.; Bannai, S.; et al. System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J. Biol. Chem. 2010, 285, 22244–22253. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Shi, J.; Li, W.; Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017, 292, 14240–14249. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, Y.; Ren, K.; Qiao, Y.; Sun, Z.; Pan, S.; Liu, F.; Liu, Y.; Huo, J.; Liu, D.; et al. Broadening horizons: The multifaceted functions of ferroptosis in kidney diseases. Int. J. Biol. Sci. 2023, 19, 3726–3743. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Zhou, X.; Zhao, Z.; Zhao, R.; Xu, X.; Kong, X.; Ren, J.; Yao, X.; Wen, Q.; et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J. Neuroinflammation 2021, 18, 249. [Google Scholar] [CrossRef]
- Sun, Y.; Berleth, N.; Wu, W.; Schlütermann, D.; Deitersen, J.; Stuhldreier, F.; Berning, L.; Friedrich, A.; Akgün, S.; Mendiburo, M.J.; et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell Death Dis. 2021, 12, 1028. [Google Scholar] [CrossRef]
- Lu, P.H.; Ma, P.W.; Wang, W.L.; Gao, W.; Chen, J.W.; Yuan, H.; Ding, X.R.; Lun, Y.Q.; Liang, R.; Li, S.Y.; et al. Deferoxamine protects cochlear hair cells and hair cell-like HEI-OC1 cells against tert-butyl hydroperoxide-induced ototoxicity. Biochim. Biophys. Acta, Mol. Basis Dis. 2024, 1870, 167024. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Hong, G.; Bi, X.; Hu, J.; Zhang, T.; An, Y.; Guo, N.; Dong, F.; Xiao, Y.; et al. Inhibition of Gpx4-mediated ferroptosis alleviates cisplatin-induced hearing loss in C57BL/6 mice. Mol. Ther. 2024, 32, 1387–1406. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Skibska, B.; Kochan, E.; Stanczak, A.; Lipert, A.; Skibska, A. Antioxidant and anti-inflammatory effects of α-lipoic acid on lipopolysaccharide-induced oxidative stress in rat kidney. Arch. Immunol. Ther. Exp. 2023, 71, 16. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Li, G.; Xiao, H.; Zuo, C.; Xie, H.; Wang, X.; Wang, J.; Liu, Y.; Hou, Q.; Sun, G.; Tian, Y. N-butylphthalide (NBP) and ligustrazine (TMP) triazole hybrids target the KEAP1-NRF2 pathway to inhibit ferroptosis and exert brain neuroprotectivity. Redox Biol. 2025, 86, 103835. [Google Scholar] [CrossRef]
- Wang, T.T.; Yu, L.L.; Zheng, J.M.; Han, X.Y.; Jin, B.Y.; Hua, C.J.; Chen, Y.S.; Shang, S.S.; Liang, Y.Z.; Wang, J.R. Berberine inhibits ferroptosis and stabilizes atherosclerotic plaque through NRF2/SLC7A11/GPX4 pathway. Chin. J. Integr. Med. 2024, 30, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pi, D.; Zhou, S.; Yi, Z.; Dong, Y.; Wang, W.; Ye, H.; Chen, Y.; Zuo, Q.; Ouyang, M. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim. Biophys. Sin. 2023, 55, 587–600. [Google Scholar] [CrossRef]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chu, X.; Liao, M.; Wang, J.; Zhang, H.; Han, L. Targeting Ferroptosis in Sensorineural Hearing Loss: A Mechanistic Review of Therapeutic Opportunities. Curr. Issues Mol. Biol. 2025, 47, 876. https://doi.org/10.3390/cimb47110876
Liu H, Chu X, Liao M, Wang J, Zhang H, Han L. Targeting Ferroptosis in Sensorineural Hearing Loss: A Mechanistic Review of Therapeutic Opportunities. Current Issues in Molecular Biology. 2025; 47(11):876. https://doi.org/10.3390/cimb47110876
Chicago/Turabian StyleLiu, Han, Xinlei Chu, Meiqi Liao, Jie Wang, Hongbing Zhang, and Lei Han. 2025. "Targeting Ferroptosis in Sensorineural Hearing Loss: A Mechanistic Review of Therapeutic Opportunities" Current Issues in Molecular Biology 47, no. 11: 876. https://doi.org/10.3390/cimb47110876
APA StyleLiu, H., Chu, X., Liao, M., Wang, J., Zhang, H., & Han, L. (2025). Targeting Ferroptosis in Sensorineural Hearing Loss: A Mechanistic Review of Therapeutic Opportunities. Current Issues in Molecular Biology, 47(11), 876. https://doi.org/10.3390/cimb47110876




