The Tumor-Suppressive Role of SAT2 in Pancreatic Cancer: Involvement in PI3K/Akt-MAPK Pathways and Immune Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. PC Sample Source
2.2. Pathway and Gene Enrichment Analyses
2.3. Analysis of Infiltration of Immune Cells
2.4. Cell Culture and Transfection
2.5. qRT-PCR Assay
2.6. CCK-8 Assay
2.7. EdU (5-Ethynyl-2′-Deoxyuridine) Assay
2.8. Wound Healing Assay
2.9. Transwell Assay
2.10. Western Blotting
2.11. Co-Culture System
2.12. Cytotoxicity Detection
2.13. Enzyme-Linked Immunosorbnent Assay (ELISA)
2.14. Xenograft Tumor Model
2.15. Hematoxylin and Eosin (HE) Staining
2.16. Immunohistochemistry (IHC)
2.17. TUNEL Assay
2.18. Statistical Analysis
3. Results
3.1. SAT2 Expression Is Low and Related to PC Tumor Size
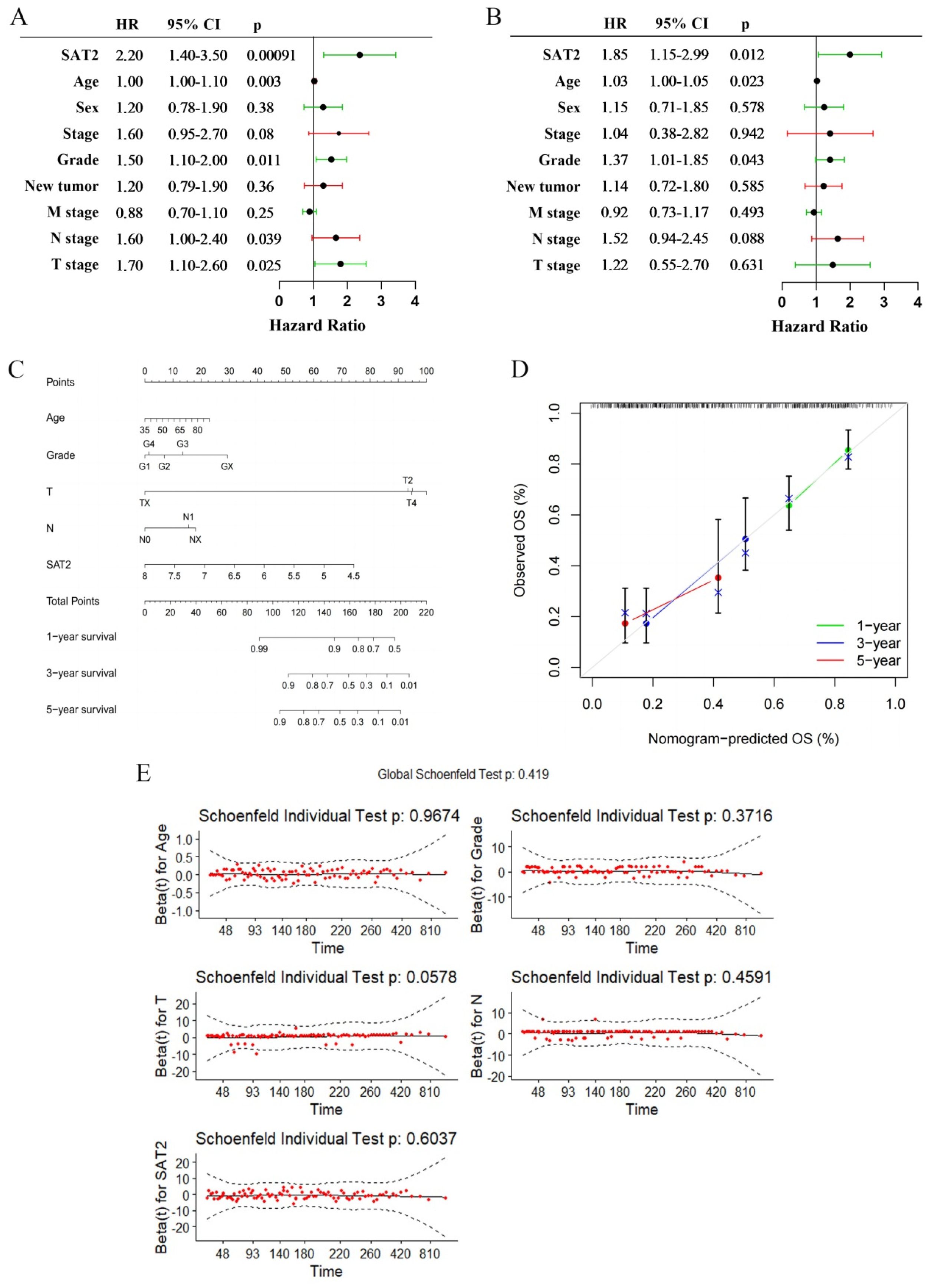
3.2. Low Expression of SAT2 Predicts Poor PC Prognostic Outcome
3.3. SAT2 Expression Has Independent Prognostic Value in PC
3.4. SAT2 Is Related to PI3K/Akt and MAPK Pathways Through GSEA Analysis
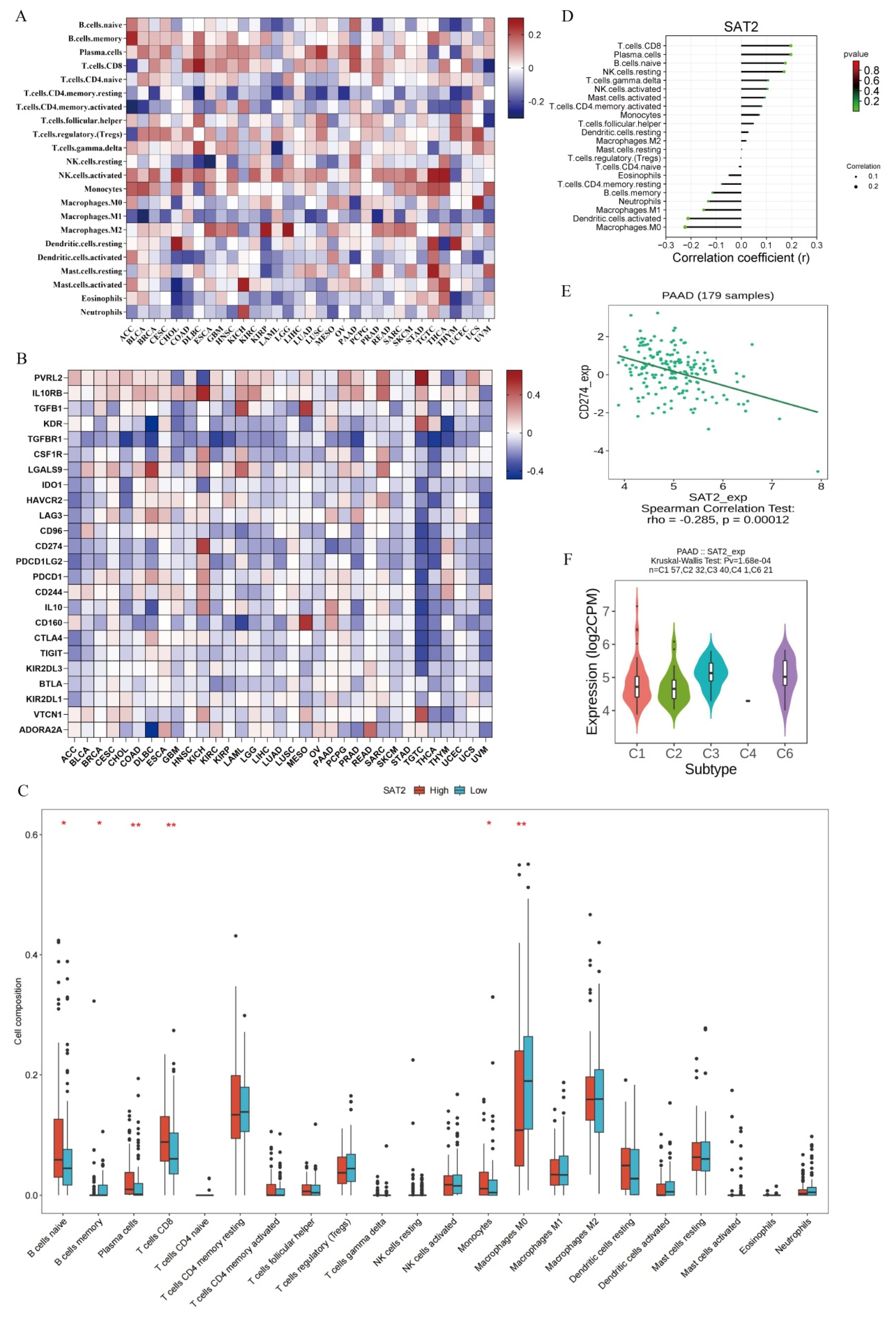
3.5. SAT2 Level Is Related to Immune Cell Infiltration Within PC Samples
3.6. Overexpression of SAT2 Inhibits PC Cell Growth, Invasion, and Migration
3.7. Overexpression of SAT2 Inhibits MAPK and PI3K/AKT Pathway Activation and PD-L1 Expression in PC Cells
3.8. Overexpression of SAT2 Inhibits the Growth of PC Xenografts in Nude Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Vujcic, S.; Liang, P.; Diegelman, P.; Kramer, D.L.; Porter, C.W. Genomic identification and biochemical characterization of a second spermidine/spermine N1-acetyltransferase. Biochem. J. 2003, 373, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kramer, D.L.; Jell, J.; Vujcic, S.; Porter, C.W. Small interfering RNA suppression of polyamine analog-induced spermidine/spermine n1-acetyltransferase. Mol. Pharmacol. 2003, 64, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.S.; Stanley, B.A.; Jones, A.D.; Pegg, A.E. Spermidine/spermine-N1-acetyltransferase-2 (SSAT2) acetylates thialysine and is not involved in polyamine metabolism. Biochem. J. 2004, 384, 139–148. [Google Scholar] [CrossRef]
- Pegg, A.E. Spermidine/spermine-N(1)-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Baek, J.H.; Liu, Y.V.; McDonald, K.R.; Wesley, J.B.; Zhang, H.; Semenza, G.L. Spermidine/spermine N(1)-acetyltransferase-1 binds to hypoxia-inducible factor-1alpha (HIF-1alpha) and RACK1 and promotes ubiquitination and degradation of HIF-1alpha. J. Biol. Chem. 2007, 282, 33358–33366. [Google Scholar] [CrossRef]
- Baek, J.H.; Liu, Y.V.; McDonald, K.R.; Wesley, J.B.; Hubbi, M.E.; Byun, H.; Semenza, G.L. Spermidine/spermine-N1-acetyltransferase 2 is an essential component of the ubiquitin ligase complex that regulates hypoxia-inducible factor 1alpha. J. Biol. Chem. 2007, 282, 23572–23580. [Google Scholar] [CrossRef]
- Gornati, R.; Chini, V.; Rimoldi, S.; Meregalli, M.; Schiaffino, E.; Bernardini, G. Evaluation of SAT-1, SAT-2 and GalNAcT-1 mRNA in colon cancer by real-time PCR. Mol. Cell. Biochem. 2007, 298, 59–68. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Edwards, N.J.; Oberti, M.; Thangudu, R.R.; Cai, S.; McGarvey, P.B.; Jacob, S.; Madhavan, S.; Ketchum, K.A. The CPTAC Data Portal: A Resource for Cancer Proteomics Research. J. Proteome Res. 2015, 14, 2707–2713. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Zhang, L.; Cao, Y.; Gao, W.; Zhao, C.; Fang, Y.; Zahedi, K.; Soleimani, M.; Lu, X.; Fang, Z.; et al. Spermidine/spermine N1-acetyltransferase-mediated polyamine catabolism regulates beige adipocyte biogenesis. Metabolism 2018, 85, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Chang, D.K.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Jones, M.D.; Colvin, E.K.; Nagrial, A.; Chin, V.T.; Chantrill, L.A.; et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann. Oncol. 2012, 23, 1713–1722. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Mortazavi, M.; Moosavi, F.; Martini, M.; Giovannetti, E.; Firuzi, O. Prospects of targeting PI3K/AKT/mTOR pathway in pancreatic cancer. Crit. Rev. Oncol. Hematol. 2022, 176, 103749. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, M.; Sil, S.; Malagon-Lopez, J.; Nieman, L.T.; Misale, S.; Di Pilato, M.; Ebright, R.Y.; Karabacak, M.N.; Kulkarni, A.S.; Liu, A.; et al. Stromal Microenvironment Shapes the Intratumoral Architecture of Pancreatic Cancer. Cell 2019, 178, 160–175.e127. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh Valilou, S.; Keshavarz-Fathi, M.; Silvestris, N.; Argentiero, A.; Rezaei, N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018, 39, 46–61. [Google Scholar] [CrossRef]
- Morrison, A.H.; Byrne, K.T.; Vonderheide, R.H. Immunotherapy and Prevention of Pancreatic Cancer. Trends Cancer 2018, 4, 418–428. [Google Scholar] [CrossRef]
- Zhang, A.; Qian, Y.; Ye, Z.; Chen, H.; Xie, H.; Zhou, L.; Shen, Y.; Zheng, S. Cancer-associated fibroblasts promote M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Cancer Med. 2017, 6, 463–470. [Google Scholar] [CrossRef]
- Fukunaga, A.; Miyamoto, M.; Cho, Y.; Murakami, S.; Kawarada, Y.; Oshikiri, T.; Kato, K.; Kurokawa, T.; Suzuoki, M.; Nakakubo, Y.; et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004, 28, e26–e31. [Google Scholar] [CrossRef]
- Wölfle, S.J.; Strebovsky, J.; Bartz, H.; Sähr, A.; Arnold, C.; Kaiser, C.; Dalpke, A.H.; Heeg, K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur. J. Immunol. 2011, 41, 413–424. [Google Scholar] [CrossRef]
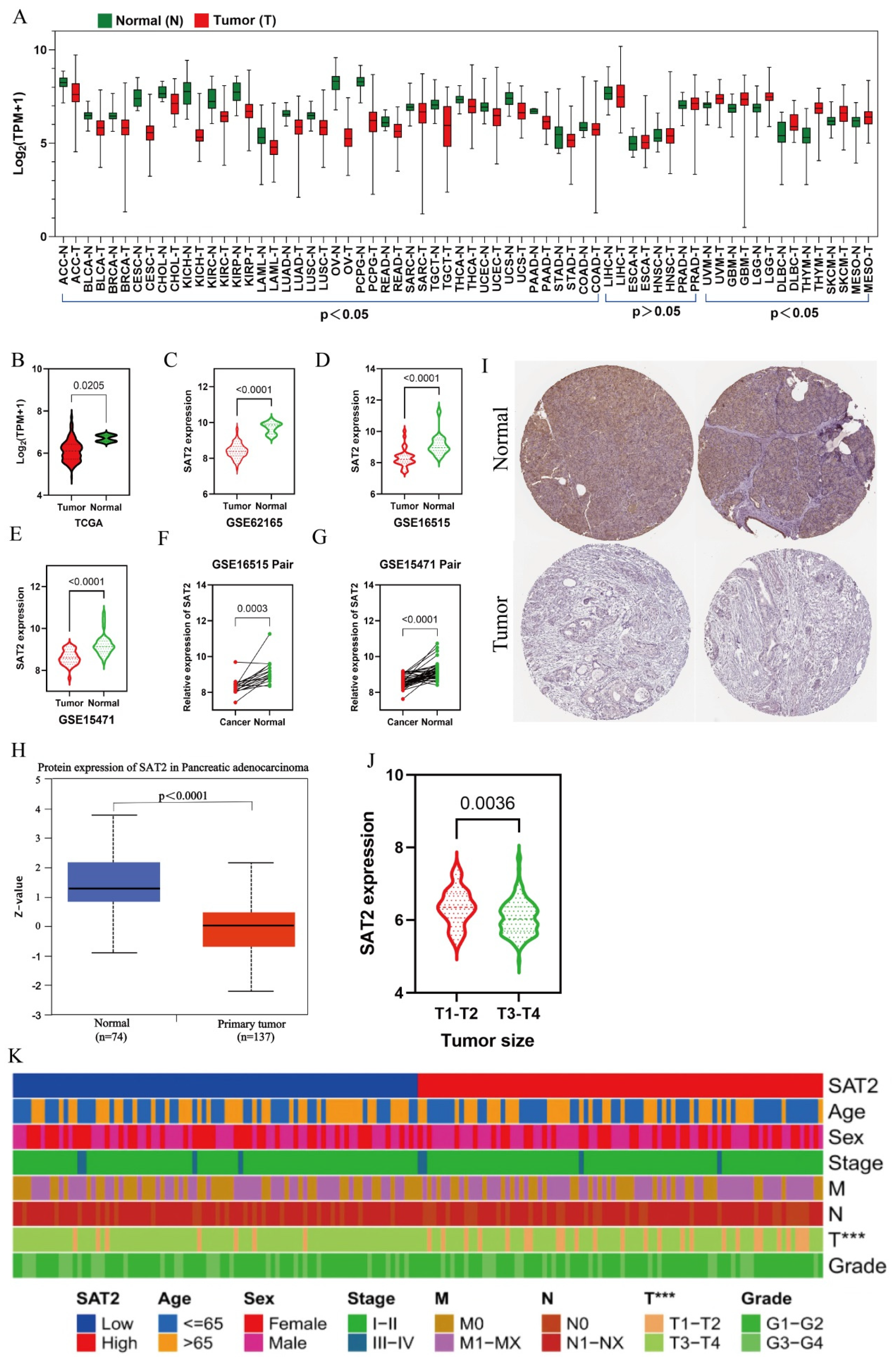






| Name | Detail | Tumor (TCGA) | Normal (Source) |
|---|---|---|---|
| ACC | Adrenocortical carcinoma | 77 | 77 (Adrenal gland , GTEx) |
| BLCA | Bladder urothelial carcinoma | 406 | 19 (TCGA) |
| BRCA | Breast invasive carcinoma | 1085 | 99 (TCGA) |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma | 306 | 13 (Cervix uteri, GTEx) |
| CHOL | Cholangio carcinoma | 36 | 9 (TCGA) |
| COAD | Colon adenocarcinoma | 448 | 41 (TCGA) |
| DLBC | Lymphoid neoplasm diffuse large B-cell lymphoma | 47 | 47 (Whole blood, GTEx) |
| ESCA | Esophageal carcinoma | 182 | 13 (TCGA) |
| GBM | Glioblastoma multiforme | 167 | 163 (Brain cortex, GTEx) |
| HNSC | Head and neck squamous cell carcinoma | 519 | 44 (TCGA) |
| KICH | Kidney chromophobe | 66 | 25 (TCGA) |
| KIRC | Kidney renal clear cell carcinoma | 531 | 25 (TCGA) |
| KIRP | Kidney renal papillary cell carcinoma | 286 | 32 (TCGA) |
| LAML | Acute myeloid Leukemia | 173 | 173 (Whole blood, GTEx) |
| LGG | Brain lower grade glioma | 524 | 255 (Brain cortex, GTEx) |
| LIHC | Liver hepatocellular carcinoma | 369 | 50 (TCGA) |
| LUAD | Lung adenocarcinoma | 513 | 59 (TCGA) |
| LUSC | Lung squamous cell carcinoma | 486 | 50 (TCGA) |
| MESO | Mesothelioma | 87 | 87 (Heart atrial appendage, GTEx) |
| OV | Ovarian serous cystadenocarcinoma | 426 | 180 (Ovary, GTEx) |
| PAAD | Pancreatic adenocarcinoma | 178 | 4 (TCGA) |
| PCPG | Pheochromocytoma and paraganglioma | 183 | 182 (Adrenal gland, GTEx) |
| PRAD | Prostate adenocarcinoma | 499 | 52 (TCGA) |
| READ | Rectum adenocarcinoma | 158 | 10 (TCGA) |
| SARC | Sarcoma | 262 | 262 (Adipose subcutaneous, GTEx) |
| SKCM | Skin cutaneous melanoma | 461 | 461 (Skin sun exposed lower, GTEx) |
| STAD | Stomach adenocarcinoma | 408 | 36 (TCGA) |
| TGCT | Testicular germ cell tumors | 139 | 137 (Testis, GTEx) |
| THCA | Thyroid carcinoma | 512 | 59 (TCGA) |
| THYM | Thymoma | 118 | 118 (Whole blood, GTEx) |
| UCEC | Uterine corpus endometrial carcinoma | 544 | 35 (TCGA) |
| UCS | Uterine carcinosarcoma | 57 | 57 (Uterus, GTEx) |
| UVM | Uveal melanoma | 80 | 79 (EyeGEx retina, GTEx) |
| GSE15471 | 69 (GEO) | 61 (GEO) | |
| GSE16515 | 36 (GEO) | 16 (GEO) | |
| GSE62165 | 118 (GEO) | 13 (GEO) | |
| PAAD | ||||
|---|---|---|---|---|
| Variable | Cases | High | Low | p Value |
| Age (years) | 0.2334 | |||
| <60 | 55 (31.07%) | 15 (21.43%) | 40 (72.73%) | |
| ≥60 | 122 (68.93%) | 30 (24.59%) | 92 (75.41%) | |
| Gender | 0.8908 | |||
| Female | 80 (45.20%) | 22 (27.50%) | 58 (72.50%) | |
| Male | 97 (54.80%) | 23 (23.71%) | 74 (76.29%) | |
| TNM stage | 0.4029 | |||
| I–II | 167 (94.35%) | 42 (25.15%) | 125 (74.85%) | |
| III–IV | 8 (5.65%) | 2 (25.00%) | 6 (75.00%) | |
| T stage | 0.0036 | |||
| T1–T2 | 31 (17.61%) | 13 (41.94%) | 18 (58.06%) | |
| T3–T4 | 145 (82.39%) | 31 (21.38%) | 114 (78.62%) | |
| N stage | 0.3670 | |||
| N0 | 49 (65.80%) | 12 (62.61%) | 37 (37.39%) | |
| N1 | 123 (34.20%) | 32 (60.82%) | 91 (39.18%) | |
| M stage | 0.2503 | |||
| M0 | 79 (45.66%) | 15 (18.99%) | 64 (81.01%) | |
| MX | 94 (54.34%) | 29 (16.76%) | 65 (83.24%) | |
| Grade | 0.386 | |||
| G1–G2 | 126 (71.19%) | 36 (28.57%) | 90 (71.43%) | |
| G3–G4 | 51 (28.81%) | 8 (15.69%) | 43 (84.31%) | |
| New tumor | 0.0674 | |||
| No | 86 (55.13%) | 25 (26.07%) | 61 (73.93%) | |
| Yes | 70 (44.87%) | 13 (18.57%) | 47 (81.43%) | |
| Smoking history | 0.6803 | |||
| ≤15 years | 17 (29.82%) | 3 (17.65%) | 14 (82.35%) | |
| >15 years | 40 (70.18%) | 9 (22.50%) | 31 (77.50%) | |
| Alcohol history | 0.5174 | |||
| Yes | 102 (61.45%) | 27 (26.47%) | 75 (73.53%) | |
| No | 64 (38.55%) | 16 (25.00%) | 48 (75.00%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Wang, L.; Fang, R.; Luo, X.; Zhang, L. The Tumor-Suppressive Role of SAT2 in Pancreatic Cancer: Involvement in PI3K/Akt-MAPK Pathways and Immune Modulation. Curr. Issues Mol. Biol. 2025, 47, 872. https://doi.org/10.3390/cimb47100872
Zhao B, Wang L, Fang R, Luo X, Zhang L. The Tumor-Suppressive Role of SAT2 in Pancreatic Cancer: Involvement in PI3K/Akt-MAPK Pathways and Immune Modulation. Current Issues in Molecular Biology. 2025; 47(10):872. https://doi.org/10.3390/cimb47100872
Chicago/Turabian StyleZhao, Ben, Lu Wang, Rui Fang, Xiaoxiao Luo, and Lu Zhang. 2025. "The Tumor-Suppressive Role of SAT2 in Pancreatic Cancer: Involvement in PI3K/Akt-MAPK Pathways and Immune Modulation" Current Issues in Molecular Biology 47, no. 10: 872. https://doi.org/10.3390/cimb47100872
APA StyleZhao, B., Wang, L., Fang, R., Luo, X., & Zhang, L. (2025). The Tumor-Suppressive Role of SAT2 in Pancreatic Cancer: Involvement in PI3K/Akt-MAPK Pathways and Immune Modulation. Current Issues in Molecular Biology, 47(10), 872. https://doi.org/10.3390/cimb47100872





