The Linkage Between Inflammation and the Progression of Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biochemical Parameters and Cytokines
2.3. MicroRNA RT qPCR Assays
2.4. Statistical Analysis
2.5. Ethical Declaration
3. Results
3.1. Inflammatory Cytokines and Adipokines
3.2. MicroRNA Expression Profiles
3.3. Diagnostic Performance of Biomarkers
3.4. Biomarkers Associated with Diabetic Kidney Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF DIABETES ATLAS [Internet], 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar] [PubMed]
- O’Hearn, M.; Lara-Castor, L.; Cudhea, F.; Miller, V.; Reedy, J.; Shi, P.; Zhang, J.; Wong, J.B.; Economos, C.D.; Micha, R.; et al. Global Dietary Database. Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat. Med. 2023, 29, 982–995. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadísticas y Censos (INEC). Boletín Técnico Registro Estadístico de Defunciones Generales. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Poblacion_y_Demografia/Defunciones_Generales/2023/Boletin_EDG_2023.pdf (accessed on 1 September 2025).
- World Health Organization (WHO). Definition, Diagnosis, and Classification of Diabetes Mellitus and Its Complications; World Health Organization (WHO), Department of Noncommunicable Disease Surveillance: Geneva, Switzerland, 1999; pp. 1–66. [Google Scholar]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pevida, B.; Llavero, M.; Gargallo, J.; Escalada, J. Complicaciones microvasculares de la diabetes. Med—Programa Form. Médica Contin. Acreditado 2016, 12, 958–970. [Google Scholar] [CrossRef]
- Ritz, E.; Rychlík, I.; Locatelli, F.; Halimi, S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1999, 34, 795–808. [Google Scholar] [CrossRef]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. Lond. Engl. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Ferri, C.M.; Sánchez-Quintana, F.; Pérez-Castro, A.; González-Luis, A.; Martín-Núñez, E.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory Cytokines in Diabetic Kidney Disease: Pathophysiologic and Therapeutic Implications. Front. Med. 2020, 7, 628289. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, Y.; Liu, F. Transforming Growth Factor-Beta1 in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 187. [Google Scholar] [CrossRef]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, J.; Li, Y.F. Clinical significance of serum interleukin-8 and soluble tumor necrosis factor-like weak inducer of apoptosis levels in patients with diabetic nephropathy. J. Diabetes Investig. 2018, 9, 1182–1188. [Google Scholar] [CrossRef]
- Hung, P.H.; Hsu, Y.C.; Chen, T.H.; Lin, C.L. Recent Advances in Diabetic Kidney Diseases: From Kidney Injury to Kidney Fibrosis. Int. J. Mol. Sci. 2021, 22, 11857. [Google Scholar] [CrossRef]
- Boldin, M.P.; Taganov, K.D.; Rao, D.S.; Yang, L.; Zhao, J.L.; Kalwani, M.; Garcia-Flores, Y.; Luong, M.; Devrekanli, A.; Xu, J.; et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 2011, 208, 1189. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, B.; Thomas, A.A.; Chakrabarti, S. miR-146a regulates glucose induced upregulation of inflammatory cytokines extracellular matrix proteins in the retina and kidney in diabetes. PLoS ONE 2017, 12, e0173918. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Kahn, D.; Gibson, W.S.J.; Round, J.L.; Scholz, R.L.; Chaudhuri, A.A.; Kahn, M.E.; Rao, D.S.; Baltimore, D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 2010, 33, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Kavanagh, M.; Jimenez-Castilla, L.; Pardines, M.; Lazaro, I.; Herrero del Real, I.; Flores-Muñoz, M.; Egido, J.; Lopez-Franco, O.; Gomez-Guerrero, C. A mutual regulatory loop between miR-155 and SOCS1 influences renal inflammation and diabetic kidney disease. Mol. Ther. Nucleic Acids 2023, 34, 102041. [Google Scholar] [CrossRef]
- D’Marco, L.; Puchades, M.J.; Gorriz, J.L.; Romero-Parra, M.; Lima-Martínez, M.; Soto, C.; Bermudez, V.; Raggi, P. Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 978. [Google Scholar] [CrossRef]
- Korczynska, J.; Czumaj, A.; Chmielewski, M.; Swierczynski, J.; Sledzinski, T. The Causes and Potential Injurious Effects of Elevated Serum Leptin Levels in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2021, 22, 4685. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2011, 34, S62. [Google Scholar] [CrossRef]
- Knijff, E.M.; Breunis, M.N.; van Geest, M.C.; Kupka, R.W.; Ruwhof, C.; de Wit, H.J.; Nolen, W.; Drexhage, H.A. A relative resistance of T cells to dexamethasone in bipolar disorder. Bipolar Disord. 2006, 8, 740–750. [Google Scholar] [CrossRef]
- Eswar, S.; Rajagopalan, B.; Ete, K.; Nageswara Rao Gattem, S. Serum Tumor Necrosis Factor Alpha (TNF-α) Levels in Obese and Overweight Adults: Correlations With Metabolic Syndrome and Inflammatory Markers. Cureus 2024, 16, e64619. [Google Scholar] [CrossRef]
- Pérez-Galarza, J.; Baldeón, L.; Franco, O.H.; Muka, T.; Drexhage, H.A.; Voortman, T.; Freire, W.B. Prevalence of overweight and metabolic syndrome, and associated sociodemographic factors among adult Ecuadorian populations: The ENSANUT-ECU study. J. Endocrinol. Investig. 2021, 44, 63–74. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.D.; Anghel, A.E.; Neagu, M.; Nicolae, I. Interleukin 8 and diabetic nephropathy. HVM Bioflux 2015, 7, 370–374. [Google Scholar]
- Loretelli, C.; Rocchio, F.; D’Addio, F.; Ben Nasr, M.; Castillo-Leon, E.; Dellepiane, S.; Vergani, A.; Abdelsalam, A.; Assi, E.; Maestroni, A.; et al. The IL-8-CXCR1/2 axis contributes to diabetic kidney disease. Metabolism 2021, 121, 154804. [Google Scholar] [CrossRef]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Feigerlová, E.; Battaglia-Hsu, S.F. IL-6 signaling in diabetic nephropathy: From pathophysiology to therapeutic perspectives. Cytokine Growth Factor. Rev. 2017, 37, 57–65. [Google Scholar] [CrossRef]
- Jo, H.A.; Kim, J.Y.; Yang, S.H.; Han, S.S.; Joo, K.W.; Kim, Y.S.; Kim, D.K. The role of local IL6/JAK2/STAT3 signaling in high glucose-induced podocyte hypertrophy. Kidney Res. Clin. Pract. 2016, 35, 212–218. [Google Scholar] [CrossRef]
- Kim, D.I.; Park, S.H. Sequential signaling cascade of IL-6 and PGC-1α is involved in high glucose-induced podocyte loss and growth arrest. Biochem. Biophys. Res. Commun. 2013, 435, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Hanai, K.; Babazono, T.; Mugishima, M.; Yoshida, N.; Nyumura, I.; Toya, K.; Bouchi, R.; Tanaka, N.; Uchigata, Y. Association of serum leptin levels with progression of diabetic kidney disease in patients with type 2 diabetes. Diabetes Care 2011, 34, 2557–2559. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Nelson, R.; Nicolson, M.; Pratley, R. Plasma leptin concentrations: No difference between diabetic Pima Indians with and without nephropathy. Diabetologia 1998, 41, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yang, J.W.; Han, B.G.; Choi, S.O.; Kim, J.S. Adiponectin for the treatment of diabetic nephropathy. Korean J. Intern. Med. 2019, 34, 480–491. [Google Scholar] [CrossRef]
- Jia, T.; Carrero, J.J.; Lindholm, B.; Stenvinkel, P. The complex role of adiponectin in chronic kidney disease. Biochimie 2012, 94, 2150–2156. [Google Scholar] [CrossRef]
- Szostak, J.; Gorący, A.; Durys, D.; Dec, P.; Modrzejewski, A.; Pawlik, A. The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 6214. [Google Scholar] [CrossRef]
- Baldeón-Rojas, L.; Weigelt, K.; de Wit, H.; Ozcan, B.; van Oudenaren, A.; Sempértegui, F.; Sijbrands, E.; Grosse, L.; Freire, W.; Drexhage, H.A.; et al. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PLoS ONE 2014, 9, e115209. [Google Scholar] [CrossRef]
- Xie, Y.; Chu, A.; Feng, Y.; Chen, L.; Shao, Y.; Luo, Q.; Deng, X.; Wu, M.; Shi, X.; Chen, Y. MicroRNA-146a: A Comprehensive Indicator of Inflammation and Oxidative Stress Status Induced in the Brain of Chronic T2DM Rats. Front. Pharmacol. 2018, 9, 478. [Google Scholar] [CrossRef]
- Ghaffari, M.; Razi, S.; Zalpoor, H.; Nabi-Afjadi, M.; Mohebichamkhorami, F.; Zali, H. Association of MicroRNA-146a with Type 1 and 2 Diabetes and their Related Complications. J. Diabetes Res. 2023, 2023, 2587104. [Google Scholar] [CrossRef]
- Bhatt, K.; Lanting, L.L.; Jia, Y.; Yadav, S.; Reddy, M.A.; Magilnick, N.; Boldin, M.; Natarajan, R. Anti-Inflammatory Role of MicroRNA-146a in the Pathogenesis of Diabetic Nephropathy. J. Am. Soc. Nephrol. JASN 2016, 27, 2277–2288. [Google Scholar] [CrossRef]
- Gilyazova, I.; Asadullina, D.; Kagirova, E.; Sikka, R.; Mustafin, A.; Ivanova, E.; Bakhtiyarova, K.; Gilyazova, G.; Gupta, S.; Khusnutdinova, E.; et al. MiRNA-146a—A Key Player in Immunity and Diseases. Int. J. Mol. Sci. 2023, 24, 12767. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, V.; Näär, A.M. MicroRNAs in Metabolism and Metabolic Disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef]
- Wu, D.; Xi, Q.Y.; Cheng, X.; Dong, T.; Zhu, X.T.; Shu, G.; Wan, L.N.; Jiang, Q.Y.; Zhang, Y.L. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J. Lipid Res. 2016, 57, 1360–1372. [Google Scholar] [CrossRef]
- Baldeón-Rojas, L.; Weigelt, K.; de Wit, H.; Ozcan, B.; van Oudenaren, A.; Sempértegui, F.; Sijbrands, E.; Grosse, L.; van Zonneveld, A.J.; Drexhage, H.A.; et al. Study on inflammation-related genes and microRNAs, with special emphasis on the vascular repair factor HGF and miR-574–3p, in monocytes and serum of patients with T2D. Diabetol. Metab. Syndr. 2016, 8, 6. [Google Scholar] [CrossRef]
- Maratni, N.P.T.; Saraswati, M.R.; Ayu Dewi, N.N.; Suastika, K. MIRNA146a And Diabetes-Related Complications: A Review. Curr. Diabetes Rev. 2023, 19, e141022209958. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
| NDC (n = 49) | C-T2D (n = 50) | NC-T2D (n = 50) | DKD (n = 49) | p-Value | ||
|---|---|---|---|---|---|---|
| Female (%) | 39(79.52) | 40 (80.0) | 45 (90.0) | 45(91.84) | 0.175 | |
| Male (%) | 10(20.41) | 10(20.0) | 5(10.0) | 4(8.16) | ||
| Age (years) | 59 ± 8 | 60 ± 8 | 59 ± 10 | 72 ± 11 | 0.000 NDC vs. DKD p = 0.000 C-T2D vs. DKD p = 0.000 NC-T2D vs. DKD p = 0.000 | |
| Exercise | Yes | 6 (12.24) | 41 (83.67) | 38 (79.17) | 39 (79.59) | 0.649 |
| Not | 43 (87.76) | 8 (16.33) | 10 (20.83) | 10 (20.41) | ||
| Smoker | Yes | 7 (14.29) | 4 (8.16) | 6 (12.24) | 5 (10.20) | 0.795 |
| Not | 42 (85.71) | 45 (91.84) | 43 (87.76) | 44 (89.80) | ||
| Length of illness (years) | 8 ± 5 | 9 ± 7 | 16 ± 11 | 0.000 C-T2D vs. NC-T2D p = 0.042 C-T2D vs. DKD p = 0.000 NC-T2D vs. DKD p = 0.000 | ||
| Age at diagnosis (years) | 53 ± 10 | 54 ± 12 | 52 ± 14 | 0.005 NC-T2D vs. C-T2D p = 0.018 NC-T2D vs. DKD p = 0.012 C-T2D vs. DKD p = 1.00 | ||
| Medication | Metformin | 39 (79.59) | 17 (36.17) | 3 (6.12) | 0.000 C-T2D vs. Metf p = 0.000 DKD vs. Metf p = 0.000 | |
| Metformin/Gilbenclamide | 3 (6.12) | 12 (25.53) | 15 (30.61) | |||
| Insulin | 0 (0) | 1 (2.13) | 6 (12.24) | |||
| Insulin/Metformine | 7 (14.29) | 16 (34.04) | 23 (46.95) | |||
| Gilbenclamide/Insulin/Metformine | 0 (0) | 1 (2.13) | 2 (4.08) | |||
| Family history | Yes | 39 (79.59) | 32 (65.31) | 29 (58) | 26 (54.17) | 0.006 |
| Not | 10 (20.41) | 17 (34.69) | 21 (42) | 22 (45.83) | ||
| BMI (kg/m2) | 31.1 ± 4.2 | 30.8 ± 5.6 | 30.4 ± 4.4 | 27.2 ± 4.2 | 0.001 NDC vs. DKD p = 0.007 C-T2D vs. DKD p = 0.002 NC-T2D vs. DKD p = 0.029 | |
| SBP (mmHg) | 121 ± 12 | 121 ± 10 | 121 ± 12 | 124 ± 16 | 0.427 | |
| DBP (mmHg) | 74 ± 6 | 74 ± 7 | 73 ± 74.74 | 70 ± 8 | 0.03 NDC vs. DKD p = 0.036 | |
| Glucose (mg/dL) | 82 ± 9 | 100 ± 15 | 141 ± 84.84 | 161 ± 46 | 0.000 NDC vs. DKD p = 0.000 C-T2D vs. DKD p = 0.000 NC-T2D vs. DKD p = 0.000 | |
| HbA1C (%) | 5.6 ± 0.5 | 5.8 ± 0.4 | 7.5 ± 0.3 | 8.8 ± 0.9 | 0.000 NDC vs. DKD p = 0.000 C-T2D vs. DKD p = 0.000 NC-T2D vs. DKD p = 0.000 | |
| Cholesterol (mg/dL) | 193 ± 37 | 185 ± 38 | 174 ± 37.37 | 200 ± 37 | 0.136 | |
| Triglycerides (mg/dL) | 174 ± 68 | 161 ± 85 | 168 ± 68.68 | 171 ± 76 | 0.801 | |
| HDL (mg/dL) | 47 ± 13 | 48 ± 10 | 45 ± 8 | 48 ± 13 | 0.653 | |
| LDL (mg/dL) | 106 ± 33 | 104 ± 35 | 92 ± 33 | 105 ± 31 | 0.369 | |
| SGOT (U/L) | 21.90 ± 12.96 | 22.35 ± 13.93 | 23.25 ± 10.23 | 21.00 ± 24.08 | 0.583 | |
| SGPT (U/L) | 12.85 ± 10.73 | 13.40 ± 7.61 | 15.20 ± 6.24 | 11.00 ± 7.21 | 0.657 | |
| Urea (mg/dL) | 33.00 ± 8.90 | 32.00 ± 10.16 | 27.50 ± 9.27 | 40.00 ± 45.01 | 0.000 NDC vs. NC-T2D p= 0.020 NC-T2D vs. DKD p = 0.000 C-T2D vs. DKD p = 0.002 | |
| Creatine (mg/dL) | 0.90 ± 0.15 | 0.90 ± 0.15 | 0.85 ± 0.0 | 1.10 ± 1.02 | 0.000 NDC vs. DKD p = 0.000 NC-T2D vs. DKD p = 0.000 C-T2D vs. DKD p = 0.002 | |
| MDRD (mL/min) | 70.10 ± 11.04 | 73.35 ± 11.48 | 76.45 ± 10.75 | 52.80 ± 17.56 | 0.000 NDC vs. DKD p = 0.000 C-T2D vs. DKD p = 0.000 NC-T2D vs. DKD p = 0.000 | |
| Variable | NDC (n = 20) | C-T2D (n = 50) | NC-T2D (n = 50) | DKD (n = 49) | p-Value |
|---|---|---|---|---|---|
| IL-8 (pg/mL) | 14.91 ± 12.05 | 30.66 ± 27.45 | 30.38 ± 27.94 | 30.66 ± 24.78 | 0.000 NDC vs. C-T2D p = 0.000 NDC vs. NC-T2D p = 0.000 NDC vs. DKD p = 0.000 |
| IL-6 (pg/mL) | 3.09 ± 10.63 | 11.62 ± 9.10 | 10.48± 14.13 | 12.16 ± 13.29 | 0.000 NDC vs. C-T2D p = 0.001 NDC vs. NC-T2D p = 0.001 NDC vs. DKD p = 0.000 |
| TNF-α (pg/mL) | 9.28 ± 3.17 | 6.73 ± 3.43 | 6.90 ± 2.70 | 5.58 ± 3.94 | 0.001 NDC vs. C-T2D p = 0.011 NDC vs. NC-T2D p = 0.011 NDC vs. DKD p = 0.002 |
| Leptin (ng/mL) | 1.32 ± 4.46 | 11.14 ± 6.96 | 9.84 ± 7.85 | 8.65 ± 8.12 | 0.000 NDC vs. C-T2D p = 0.000 NDC vs. NC-T2D p = 0.000 NDC vs. DKD p = 0.000 |
| Adiponectin (ug/mL) | 6.17 ± 5.54 | 9.30 ± 5.43 | 6.65 ± 4.12 | 11.30 ± 6.55 | 0.000 NDC vs. C-T2D p = 0.006 NDC vs. NC-T2D p = 0.000 NDC vs. DKD p = 0.001 |
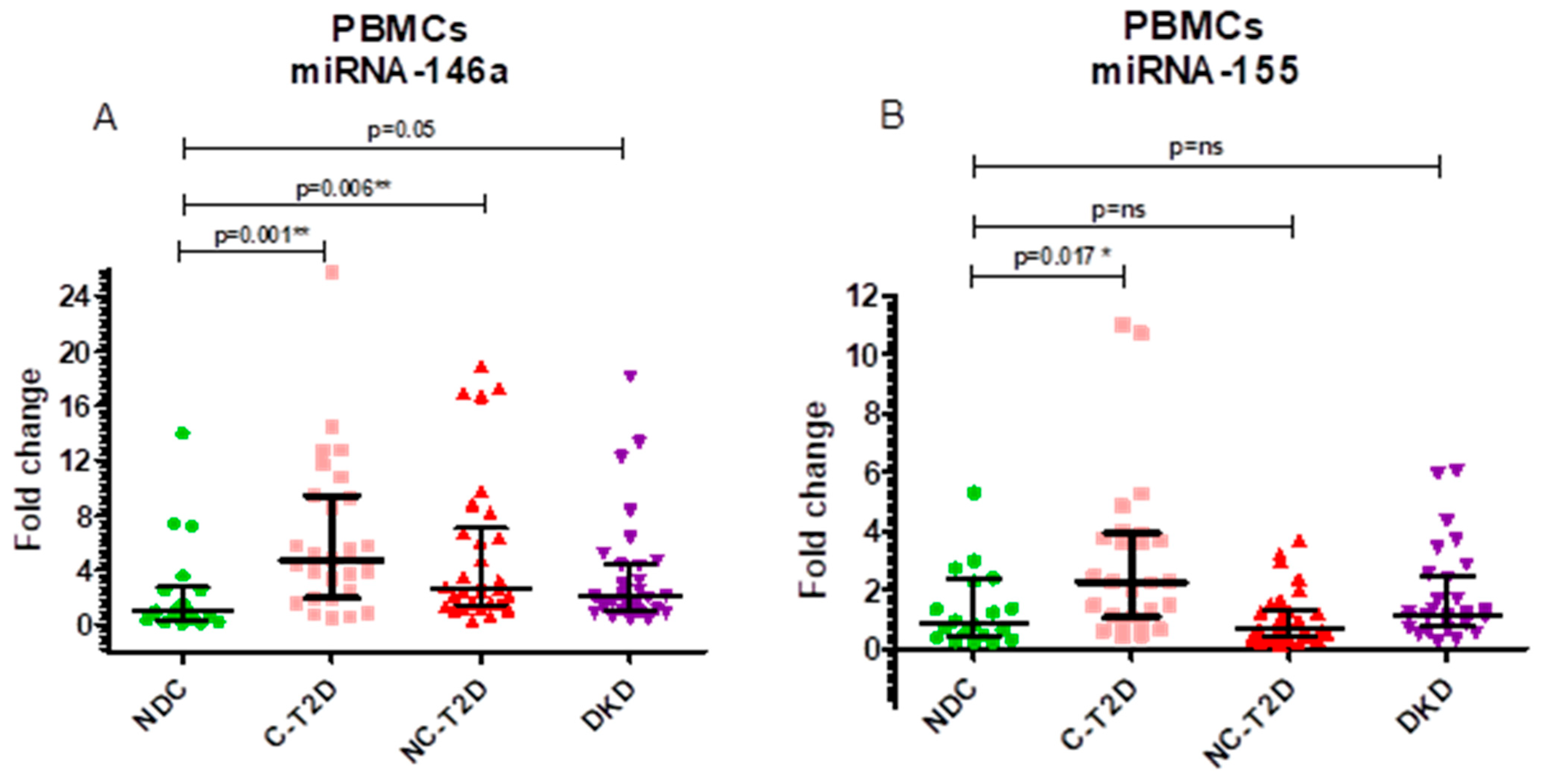
| miR-146a (fold change) | 1.04 ± 2.45 | 4.75 ± 7.51 | 2.68 ± 5.65 | 2.07 ± 3.43 | 0.003 NDC vs. C-T2D p = 0.001 NDC vs. NC-T2D p = 0.006 NDC vs. DKD p = 0.050 |
| miR-155 (fold change) | 0.89 ± 1.99 | 2.28 ± 2.87 | 0.65 ± 0.94 | 1.15 ± 1.66 | 0.000 NDC vs. C-T2D p = 0.017 NDC vs. NC-T2D p = 0.422 NDC vs. DKD p = 0.263 |
| Variable | p-Value | B | OR | OR 95% CI | |
|---|---|---|---|---|---|
| LI | HI | ||||
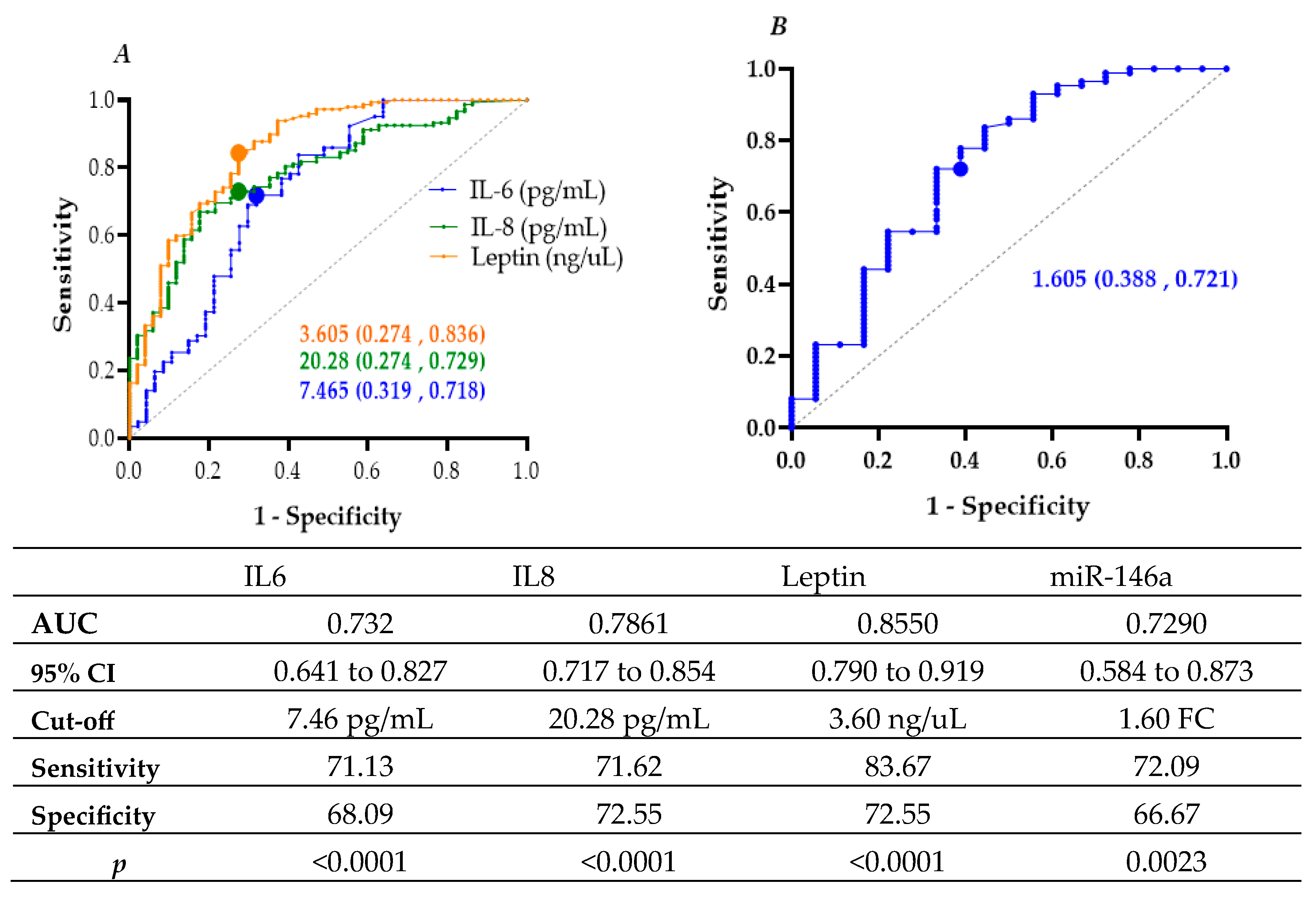
| IL-8 ≥ 20.28 (pg/mL) | 0.000 | 1.907 | 6.733 | 3.307 | 13.709 |
| IL-6 ≥ 7.465 (pg/mL) | 0.001 | 1.490 | 4.436 | 2.266 | 8.987 |
| Leptin ≥ 3.605 (ng/mL) | 0.001 | 2.622 | 13.765 | 6.474 | 29.267 |
| miR-146a ≥ 1.605 (fold change) | 0.003 | 1.580 | 4.857 | 1.714 | 13.767 |
| Variable | p-Value | B | OR | OR 95% CI | |
|---|---|---|---|---|---|
| LI | HI | ||||
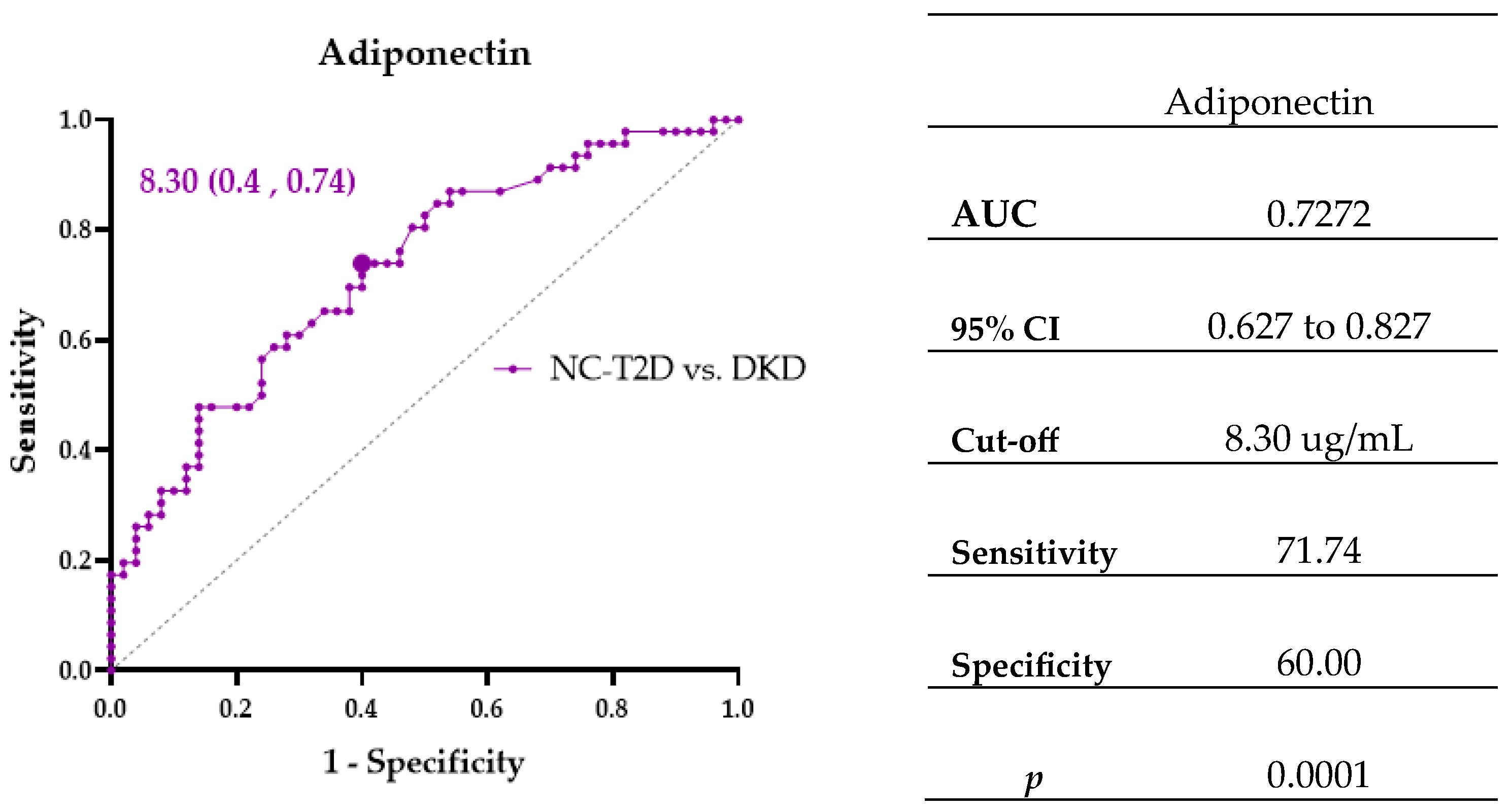
| Adiponectin ≥ 8.30 (ug/mL) NC-T2D vs. DKD | 0.001 | 1.424 | 4.154 | 1.776 | 9.718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldeón-Rojas, L.; Alulema, V.; Barrera-Guarderas, F.; Aguirre-Villacís, D.; Cañadas-Herrera, C.; Bedón-Galarza, R.; Pérez-Tasigchana, F.; Pérez-Galarza, J. The Linkage Between Inflammation and the Progression of Type 2 Diabetes Mellitus. Curr. Issues Mol. Biol. 2025, 47, 859. https://doi.org/10.3390/cimb47100859
Baldeón-Rojas L, Alulema V, Barrera-Guarderas F, Aguirre-Villacís D, Cañadas-Herrera C, Bedón-Galarza R, Pérez-Tasigchana F, Pérez-Galarza J. The Linkage Between Inflammation and the Progression of Type 2 Diabetes Mellitus. Current Issues in Molecular Biology. 2025; 47(10):859. https://doi.org/10.3390/cimb47100859
Chicago/Turabian StyleBaldeón-Rojas, Lucy, Valeria Alulema, Francisco Barrera-Guarderas, Diana Aguirre-Villacís, Cristina Cañadas-Herrera, Ricardo Bedón-Galarza, Francisco Pérez-Tasigchana, and Jorge Pérez-Galarza. 2025. "The Linkage Between Inflammation and the Progression of Type 2 Diabetes Mellitus" Current Issues in Molecular Biology 47, no. 10: 859. https://doi.org/10.3390/cimb47100859
APA StyleBaldeón-Rojas, L., Alulema, V., Barrera-Guarderas, F., Aguirre-Villacís, D., Cañadas-Herrera, C., Bedón-Galarza, R., Pérez-Tasigchana, F., & Pérez-Galarza, J. (2025). The Linkage Between Inflammation and the Progression of Type 2 Diabetes Mellitus. Current Issues in Molecular Biology, 47(10), 859. https://doi.org/10.3390/cimb47100859







