Equal Prevalence of Genotypes ON1 and BA of Human Orthopneumovirus in Riyadh, Saudi Arabia, in 2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Collection
2.3. RNA Extraction, cDNA Synthesis, and Orthopneumovirus Detection
2.4. Amplification and Sequencing of G Gene
2.5. Phylogenetic Analysis
2.6. Amino Acid Sequence and Selection Pressure Analysis
2.7. Entropy Analysis
2.8. N- and O-Linked Glycosylation Sites
2.9. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Orthopneumovirus Prevalence, DNA Sequencing, and GenBank Accession Numbers
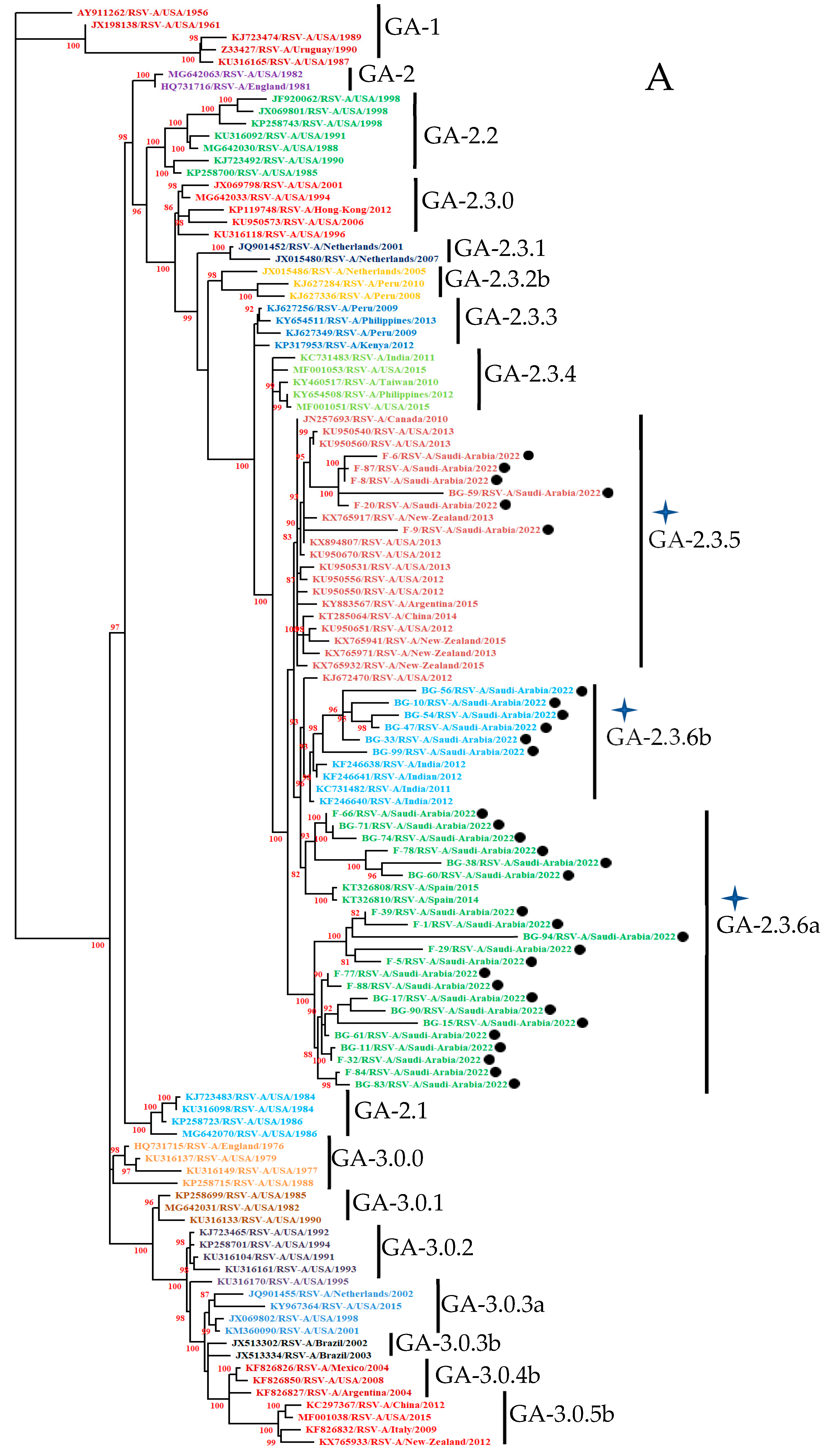
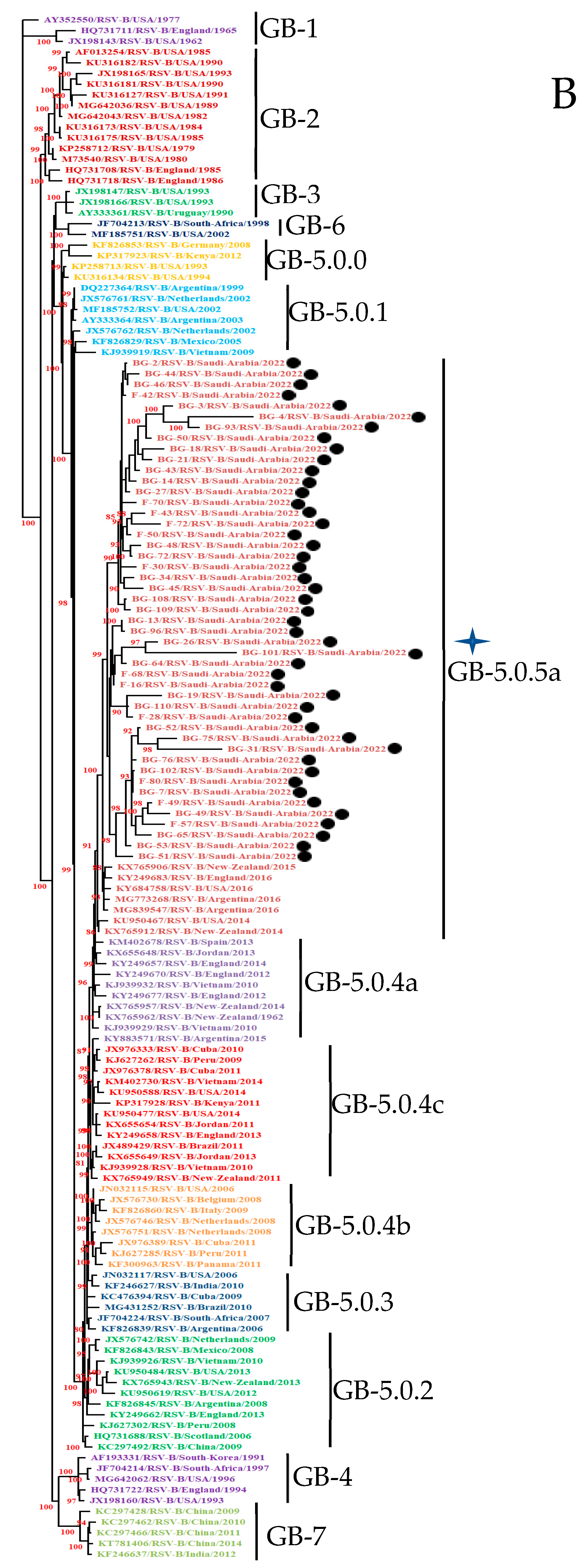
3.3. Phylogenetic Analysis
3.3.1. HOPV-A Strains
3.3.2. HOPV-B Strains
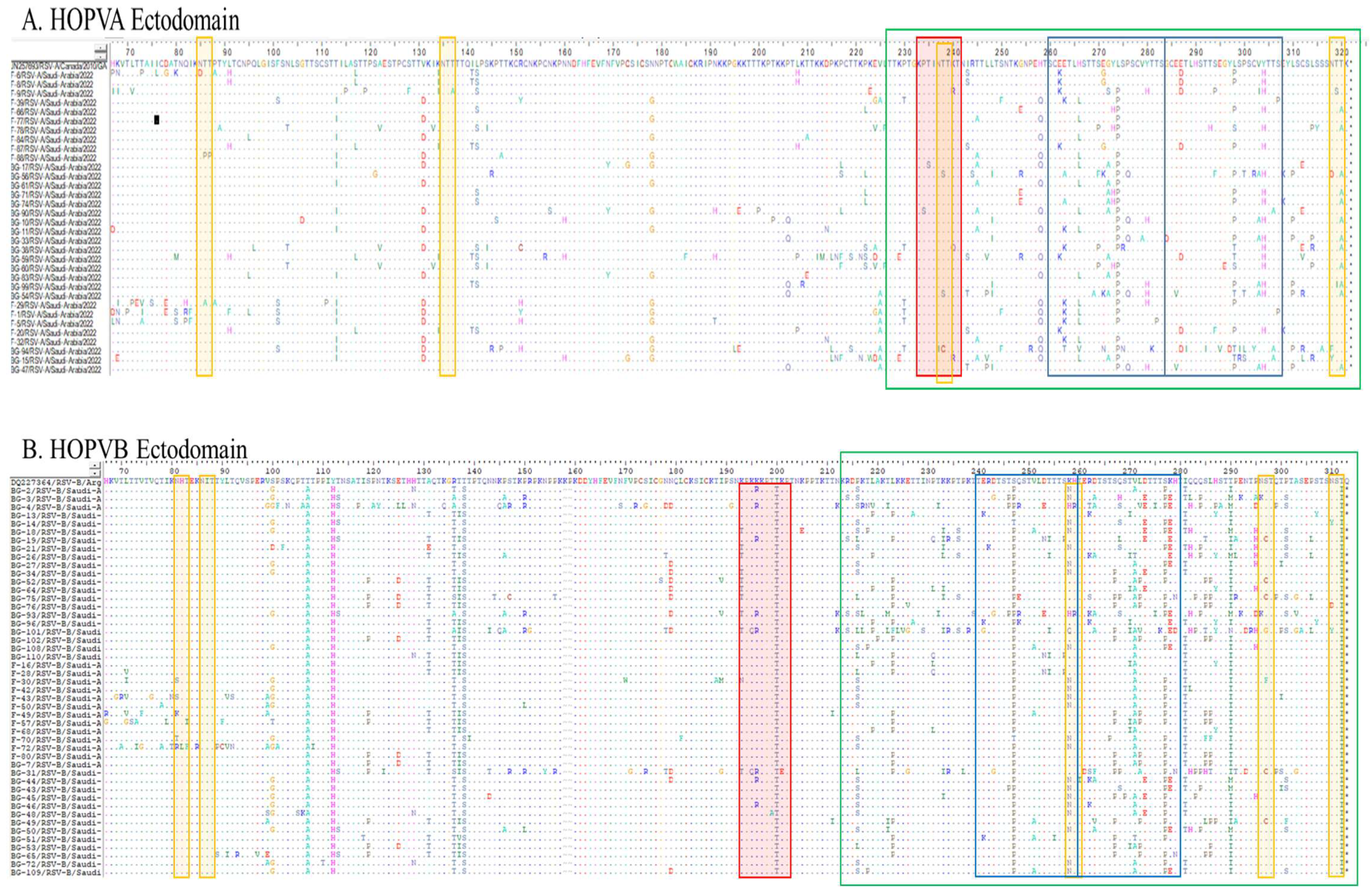
3.4. Mutational Analysis
3.4.1. Deduced Amino Acid Sequences and Mutational Analysis of HOPVA-ON1 Genotypes
3.4.2. Deduced Amino Acid Sequences and Mutational Analysis of HOPVB-BA Genotypes
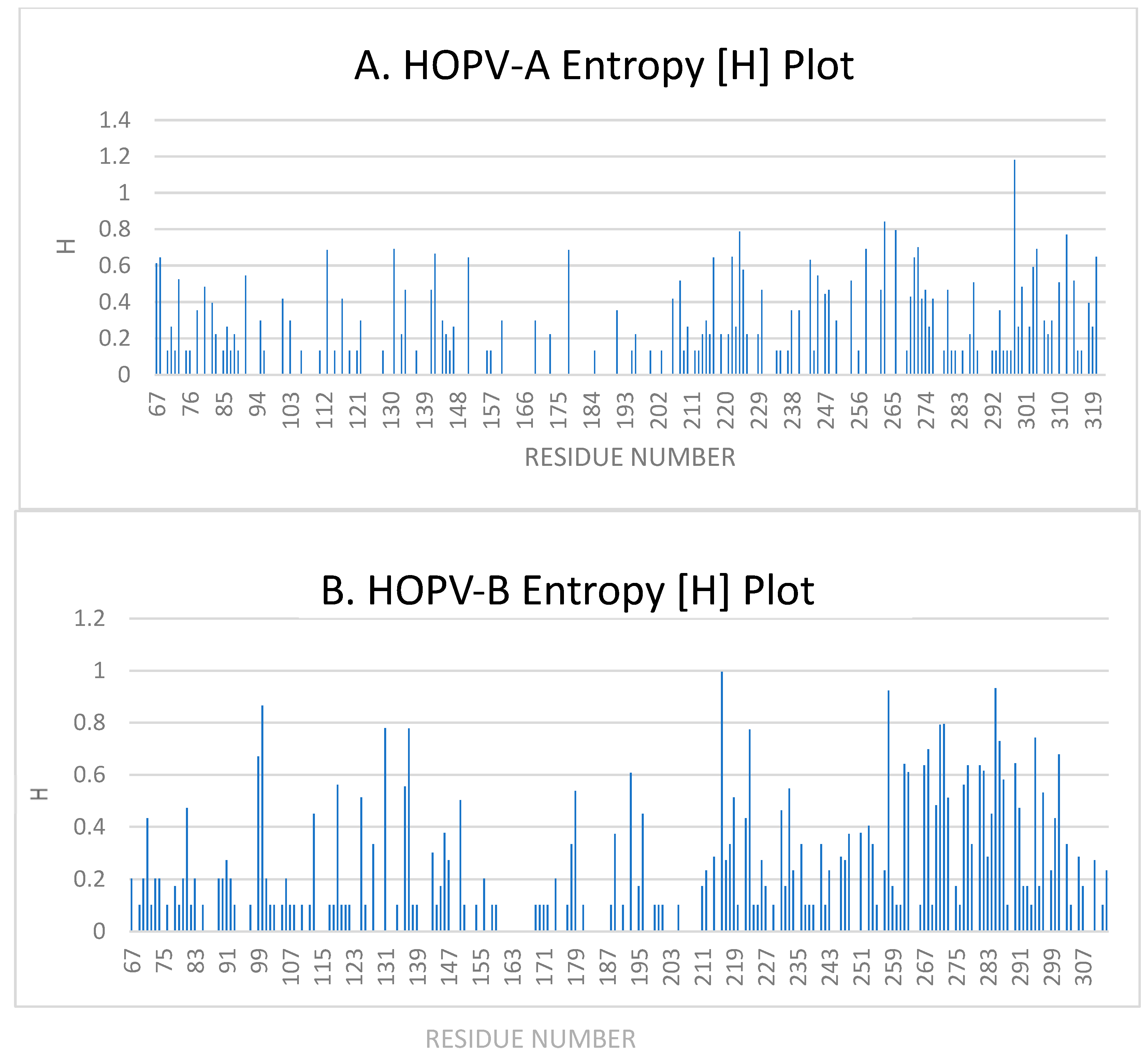
3.5. Entropy Analysis
3.5.1. Entropy Analysis of HOPVA-ON1 Genotypes
3.5.2. Entropy Analysis of HOPVB-BA Genotypes
3.6. O- and N-Linked Glycosylation Sites in HOPV-A and HOPV-B Genotypes
3.6.1. O- and N-Linked Glycosylation Sites in HOPVA-ON1 Genotypes
3.6.2. O- and N-Linked Glycosylation Sites in HOPV-BA Genotypes
3.7. Selection Pressure Analysis
3.7.1. ON1 Genotype
3.7.2. BA Genotype
3.8. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; A Madhi, S.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger Than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38. [Google Scholar] [CrossRef]
- Tan, L.; Coenjaerts, F.E.J.; Houspie, L.; Viveen, M.C.; van Bleek, G.M.; Wiertz, E.J.H.J.; Martin, D.P.; Lemey, P. The comparative genomics of human respiratory syncytial virus subgroups A and B: Genetic variability and molecular evolutionary dynamics. J. Virol. 2013, 87, 8213–8226. [Google Scholar] [CrossRef]
- Sullender, W.M. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef]
- Anderson, L.J.; Bingham, P.; Hierholzer, J.C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 1988, 62, 4232–4238. Available online: http://www.ncbi.nlm.nih.gov/pubmed/2459412 (accessed on 19 August 2025).
- Ahmed, A.; Haider, S.H.; Parveen, S.; Arshad, M.; Alsenaidy, H.A.; Baaboud AOSullender, W. Co-Circulation of 72bp Duplication Group A and 60bp Duplication Group B Respiratory Syncytial Virus (RSV) Strains in Riyadh, Saudi Arabia during 2014. PLoS ONE 2016, 11, e0166145. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Alhetheel, A.; Parveen, S.; Almajhdi, F.N.; Alkubaisi, N.A.; Al-Saadi, M.M. Human RSVA-ON1, The Only Genotype Present During 2019–2020 Winter Season in Riyadh, Saudi Arabia: A Retrospective Study. Future Virol. 2023, 18, 1043–1055. [Google Scholar] [CrossRef]
- Al-Hassinah, S.; Parveen, S.; Somily, A.M.; AlSaadi, M.M.; Alamery, S.F.; Haq, S.H.; Alsenaidy, H.A.; Ahmed, A. Evolutionary analysis of the ON1 genotype of subtype a respiratory syncytial virus in Riyadh during 2008–2016. Infect. Genet. Evol. 2020, 79, 104153. [Google Scholar] [CrossRef] [PubMed]
- Trento, A.; Ábrego, L.; Rodriguez-Fernandez, R.; González-Sánchez, M.I.; González-Martínez, F.; Delfraro, A.; Pascale, J.M.; Arbiza, J.; Melero, J.A. Conservation of G-Protein Epitopes in Respiratory Syncytial Virus (Group A) Despite Broad Genetic Diversity: Is Antibody Selection Involved in Virus Evolution? J. Virol. 2015, 89, 7776–7785. [Google Scholar] [CrossRef]
- Agoti, C.N.; Mayieka, L.M.; Otieno, J.R.; A Ahmed, J.; Fields, B.S.; Waiboci, L.W.; Nyoka, R.; Eidex, R.B.; Marano, N.; Burton, W.; et al. Examining strain diversity and phylogeography in relation to an unusual epidemic pattern of respiratory syncytial virus (RSV) in a long-term refugee camp in Kenya. BMC Infect. Dis. 2014, 14, 178. [Google Scholar] [CrossRef]
- Goya, S.; Galiano, M.; Nauwelaers, I.; Trento, A.; Openshaw, P.J.; Mistchenko, A.S.; Zambon, M.; Viegas, M. Toward unified molecular surveillance of RSV: A proposal for genotype definition. Influenza Other Respir. Viruses 2020, 14, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Trento, A.; Galiano, M.; Videla, C.; Carballal, G.; Garcia-Barreno, B.; Melero, J.A.; Palomo, C. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 2003, 84, 3115–3120. [Google Scholar] [CrossRef]
- Eshaghi, A.; Duvvuri, V.R.; Lai, R.; Nadarajah, J.T.; Li, A.; Patel, S.N.; E Low, D.; Gubbay, J.B. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: A novel genotype with a 72 nucleotide G gene duplication. PLoS ONE 2012, 7, e32807. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.H.; Khan, W.H.; Deeba, F.; Ali, S.; Ahmed, A.; Naqvi, I.H.; Dohare, R.; A Alsenaidy, H.; Alsenaidy, A.M.; Broor, S.; et al. BA9 lineage of respiratory syncytial virus from across the globe and its evolutionary dynamics. PLoS ONE 2018, 13, e0193525. [Google Scholar] [CrossRef]
- Liang, X.; Liu, D.H.; Chen, D.; Guo, L.; Yang, H.; Shi, Y.S.; Wang, Y.J.; Wang, W.K.; Xie, Z.P.; Gao, H.C.; et al. Gradual replacement of all previously circulating respiratory syncytial virus A strain with the novel ON1 genotype in Lanzhou from 2010 to 2017. Medicine 2019, 98, e15542. [Google Scholar] [CrossRef]
- Schobel, S.A.; Stucker, K.M.; Moore, M.L.; Anderson, L.J.; Larkin, E.K.; Shankar, J.; Bera, J.; Puri, V.; Shilts, M.H.; Rosas-Salazar, C.; et al. Respiratory Syncytial Virus whole-genome sequencing identifies convergent evolution of sequence duplication in the C-terminus of the G gene. Sci. Rep. 2016, 6, 26311. [Google Scholar] [CrossRef]
- Malasao, R.; Okamoto, M.; Chaimongkol, N.; Imamura, T.; Tohma, K.; Dapat, I.; Dapat, C.; Suzuki, A.; Saito, M.; Saito, M.; et al. Molecular Characterization of Human Respiratory Syncytial Virus in the Philippines, 2012–2013. PLoS ONE 2015, 10, e0142192. [Google Scholar] [CrossRef]
- Cane, P.A. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 2001, 11, 103–116. [Google Scholar] [CrossRef]
- Pangesti, K.N.A.; Abd El Ghany, M.; Walsh, M.G.; Kesson, A.M.; Hill-Cawthorne, G.A. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 2018, 28, e1968. [Google Scholar] [CrossRef]
- Choi, E.H.; Lee, H.J. Genetic diversity and molecular epidemiology of the G protein of subgroups A and B of respiratory syncytial viruses isolated over 9 consecutive epidemics in Korea. J. Infect. Dis. 2000, 181, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Peret, T.C.T.; Hall, C.B.; Hammond, G.W.; Piedra, P.A.; Storch, G.A.; Sullender, W.M.; Tsou, C.; Anderson, L.J. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J. Infect. Dis. 2000, 181, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Papillard-Marechal, S.; Enouf, V.; Schnuriger, A.; Vabret, A.; Macheras, E.; Rameix-Welti, M.-A.; Page, B.; Freymuth, F.; van der Werf, S.; Garbarg-Chenon, A.; et al. Monitoring epidemic viral respiratory infections using one-step real-time triplex RT-PCR targeting influenza A and B viruses and respiratory syncytial virus. J. Med. Virol. 2011, 83, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Agoti, C.N.; Mwihuri, A.G.; Sande, C.J.; Onyango, C.O.; Medley, G.F.; Cane, P.A.; Nokes, D.J. Genetic relatedness of infecting and reinfecting respiratory syncytial virus strains identified in a birth cohort from rural Kenya. J. Infect. Dis. 2012, 206, 1532–1541. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Pond, S.L.; Frost, S.D. Datamonkey: Rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 2005, 21, 2531–2533. [Google Scholar] [CrossRef]
- Malasao, R.; Chaiut, W.; Tantipetcharawan, W.; Tongphung, R.; Charoensri, N.; Takarn, P.; Sudjaritruk, T.; Maneekarn, N. Predominance of ON1 and BA9 genotypes of human respiratory syncytial virus in children with acute respiratory infection in Chiang Mai, Thailand, 2020–2021. J. Infect. Public Health 2023, 16, 1418–1426. [Google Scholar] [CrossRef]
- Julenius, K.; Molgaard, A.; Gupta, R.; Brunak, S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 2005, 15, 153–164. [Google Scholar] [CrossRef]
- Zomer-Kooijker, K.; Uiterwaal, C.S.; van der Gugten, A.C.; Wilbrink, B.; Bont, L.J.; van der Ent, C.K. Decreased lung function precedes severe respiratory syncytial virus infection and post-respiratory syncytial virus wheeze in term infants. Eur. Respir. J. 2014, 44, 666–674. [Google Scholar] [CrossRef]
- Salman, A.; Muslamani, A.; Farid, E. Clinical Profiles of Infants Hospitalized with Acute Respiratory Syncytial Virus Bronchiolitis IN Bahrain. J. Bahrain Med. Soc. 2006, 18, 169–173. [Google Scholar]
- Schweitzer, J.W.; Justice, N.A. Respiratory syncytial virus infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459215/ (accessed on 19 August 2025).
- Yassine, H.M.; Sohail, M.U.; Younes, N.; Nasrallah, G.K. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms 2020, 8, 713. [Google Scholar] [CrossRef]
- Van de Steen, O.; Miri, F.; Gunjaca, M.; Klepac, V.; Gross, B.; Notario, G.; Wegzyn, C.M. The Burden of Severe Respiratory Syncytial Virus Disease Among Children Younger than 1 Year in Central and Eastern Europe. Infect. Dis. Ther. 2016, 5, 125–137. [Google Scholar] [CrossRef]
- Bashir, U.; Alam, M.M.; Sadia, H.; Zaidi, S.S.; Kazi, B.M. Correction: Molecular Characterization of Circulating Respiratory Syncytial Virus (RSV) Genotypes in Gilgit Baltistan Province of Pakistan during 2011–2012 Winter Season. PLoS ONE 2015, 10, e0145599. [Google Scholar] [CrossRef]
- Janahi, I.; Abdulkayoum, A.; Almeshwesh, F.; Alkuwari, M.; Al Hammadi, A.; Alameri, M. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Infect. Dis. 2017, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Eifan, S.A.; Hanif, A.; AlJohani, S.M.; Atif, M. Respiratory Tract Viral Infections and Coinfections Identified by Anyplex II RV16 Detection Kit in Pediatric Patients at a Riyadh Tertiary Care Hospital. Biomed. Res. Int. 2017, 2017, 1928795. [Google Scholar] [CrossRef]
- Alkharsah, K.R. The Scope of Respiratory Syncytial Virus Infection in a Tertiary Hospital in the Eastern Province of Saudi Arabia and the Change in Seasonal Pattern during and after the COVID-19 Pandemic. Medicina 2022, 58, 1623. [Google Scholar] [CrossRef]
- Asseri, A.A.; Al-Qahtani, S.M.; A Alzaydani, I.; Al-Jarie, A.; Alyazidi, N.S.; A Alrmelawi, A.; Alqahtani, A.M.; Alsulayyim, R.S.; Alzailaie, A.K.; Abdullah, D.M.; et al. Clinical and epidemiological characteristics of respiratory syncytial virus, SARS-CoV-2 and influenza paediatric viral respiratory infections in southwest Saudi Arabia. Ann. Med. 2025, 57, 2445791. [Google Scholar] [CrossRef]
- Farrag, M.A.; Amer, H.M.; Aziz, I.M.; Alsaleh, A.N.; Almajhdi, F.N. The emergence of subgenotype ON-1 of Human orthopneumovirus type A in Riyadh, Saudi Arabia: A new episode of the virus epidemiological dynamic. J. Med. Virol. 2020, 92, 1133–1140. [Google Scholar] [CrossRef]
- Ahmed, A.; Parveen, S.; Al-Hassinah, S.M.; Al-Amery, S.F. An overview of respiratory syncytial virus infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 929–936. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Farrag, M.A.; Amer, H.M. Genetic diversity in the G protein gene of group A human respiratory syncytial viruses circulating in Riyadh, Saudi Arabia. Arch. Virol. 2014, 159, 73–81. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Farrag, M.A.; Amer, H.M. Group B strains of human respiratory syncytial virus in Saudi Arabia: Molecular and phylogenetic analysis. Virus Genes 2014, 48, 252–259. [Google Scholar] [CrossRef]
- Al-Sharif, H.A.; El-Kafrawy, S.A.; Yousef, J.M.; Kumosani, T.A.; Kamal, M.A.; Khathlan, N.A.; Kaki, R.M.; Alnajjar, A.A.; Azhar, E.I. Dominance of the ON1 Genotype of RSV-A and BA9 Genotype of RSV-B in Respiratory Cases from Jeddah, Saudi Arabia. Genes 2020, 11, 1323. [Google Scholar] [CrossRef]
- Abdulhaq, A.A.; Basode, V.K.; Hashem, A.M.; Alshrari, A.S.; Badroon, N.A.; Hassan, A.M.; Alsubhi, T.L.; Solan, Y.; Ejeeli, S.; Azhar, E.I. Patterns of Human Respiratory Viruses and Lack of MERS-Coronavirus in Patients with Acute Upper Respiratory Tract Infections in Southwestern Province of Saudi Arabia. Adv. Virol. 2017, 2017, 4247853. [Google Scholar] [CrossRef]
- Korsun, N.; Trifonova, I.; Madzharova, I.; Alexiev, I.; Uzunova, I.; Ivanov, I.; Velikov, P.; Tcherveniakova, T.; Christova, I. Resurgence of respiratory syncytial virus with dominance of RSV-B during the 2022–2023 season. Front. Microbiol. 2024, 15, 1376389. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Sjaarda, C.P.; Rand, B.; Roberts, D.; Tozer, K.; Fattouh, R.; Kozak, R.; Sheth, P.M. The molecular epidemiology of respiratory syncytial virus in Ontario, Canada from 2022–2024 using a custom whole genome sequencing assay and analytics package. J. Clin. Virol. 2025, 176, 105759. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Y.; Chmaisse, A.; Boutros, C.; Chamseddine, S.; Fayad, D.; Zaraket, H.; Dbaibo, G. The burden of Respiratory Syncytial Virus (RSV) infection in the Middle East and North Africa (MENA) region across age groups: A systematic review. Vaccine 2021, 39, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Madi, N.; Sadeq, M.; Safar, H.A.; Al-Adwani, A.; Al-Turab, M. Circulation of new lineages of RSV-A and RSV-B in Kuwait shows high diversity in the N- and O-linked glycosylation sites in the G protein between 2020 and 2022. Front. Cell. Infect. Microbiol. 2024, 16, 1445115. [Google Scholar] [CrossRef]
- Essa, S.; Owayed, A.; Altawalah, H.; Khadadah, M.; Behbehani, N.; Al-Nakib, W. Mixed viral infections circulating in hospitalized patients with respiratory tract infections in kuwait. Adv. Virol. 2015, 2015, 714062. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Alhammadi, A.H.; Chandra, P.; Muneer, E.; Khalifa, M.S. Identifying agents triggering bronchiolitis in the State of Qatar. Int. J. Gen. Med. 2018, 10, 143–149. [Google Scholar] [CrossRef]
- Al-Romaihi, H.E.; Smatti, M.K.; Al-Khatib, H.A.; Coyle, P.V.; Ganesan, N.; Nadeem, S.; Farag, E.A.; Al Thani, A.A.; Al Khal, A.; Al Ansari, K.M.; et al. Molecular epidemiology of influenza, RSV, and other respiratory infections among children in Qatar: A six years report (2012–2017). Int. J. Infect. Dis. 2020, 95, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hibino, A.; Saito, R.; Taniguchi, K.; Zaraket, H.; Shobugawa, Y.; Matsui, T.; Suzuki, H.; Japanese HRSV Collaborative Study Group. Molecular epidemiology of human respiratory syncytial virus among children in Japan during three seasons and hospitalization risk of genotype ON1. PLoS ONE 2018, 13, e0192085. [Google Scholar] [CrossRef] [PubMed]
- Otieno, J.R.; Kamau, E.M.; Agoti, C.N.; Lewa, C.; Otieno, G.; Bett, A.; Ngama, M.; Cane, P.A.; Nokes, D.J. Spread and Evolution of Respiratory Syncytial Virus A Genotype ON1, Coastal Kenya, 2010–2015. Emerg. Infect. Dis. 2017, 23, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, A.I.; Himsawi, N.; Sammour, A.; Abu Safieh, H.; Burayzat, S.; Al-Momani, H.; Alotaibi, M.R.; Al Shboul, S.; Saleh, T. Molecular characterization of human respiratory syncytial virus strains circulating among hospitalized children in Jordan. BMC Infect. Dis. 2024, 24, 1347. [Google Scholar] [CrossRef]
- Na, B.; Park, Y.J.; Seo, J.; Park, M.; Baek, J.Y.; Lee, J.Y.; Kim, M.; Ahn, J.G.; Lee, S.T.; Kang, J.M. Genotype Analysis of Respiratory Syncytial Virus Before and After the COVID-19 Pandemic Using Whole-Genome Sequencing: A Prospective, Single-Center Study in Korea From 2019 to 2022. J. Korean Med. Sci. 2024, 39, e206. [Google Scholar] [CrossRef]
- Nuttens, C.; Moyersoen, J.; Curcio, D.; Aponte-Torres, Z.; Baay, M.; Vroling, H.; Gessner, B.D.; Begier, E. Differences Between RSV A and RSV B Subgroups and Implications for Pharmaceutical Preventive Measures. Infect. Dis. Ther. 2024, 13, 1725–1742. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Y.; Wang, H.; Shi, J.; Sun, L.; Zhang, X.; Yang, Z.; Guan, W.; Zhang, H.; Yu, P.; et al. Emergence of ON1 genotype of human respiratory syncytial virus subgroup A in China between 2011 and 2015. Sci. Rep. 2017, 14, 5501. [Google Scholar] [CrossRef]
- Hirano, E.; Kobayashi, M.; Tsukagoshi, H.; Yoshida, L.M.; Kuroda, M.; Noda, M.; Ishioka, T.; Kozawa, K.; Ishii, H.; Yoshida, A.; et al. Molecular evolution of human respiratory syncytial virus attachment glycoprotein (G) gene of new genotype ON1 and ancestor NA1. Infect. Genet. Evol. 2014, 28, 183–191. [Google Scholar] [CrossRef]
- Ogunsemowo, O.; Olaleye, D.O.; Odaibo, G.N. Genetic diversity of human respiratory syncytial virus circulating among children in Ibadan, Nigeria. PLoS ONE 2018, 13, e0191494. [Google Scholar] [CrossRef]
- Botosso, V.F.; Zanotto, P.M.d.A.; Ueda, M.; Arruda, E.; Gilio, A.E.; Vieira, S.E.; Stewien, K.E.; Peret, T.C.T.; Jamal, L.F.; Pardini, M.I.d.M.C.; et al. Positive selection results in frequent reversible amino acid replacements in the G protein gene of human respiratory syncytial virus. PLoS Pathog. 2009, 5, e1000254. [Google Scholar] [CrossRef]
- Martínez, I.; Dopazo, J.; Melero, J.A. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J. Gen. Virol. 1997, 78, 2419–2429. [Google Scholar] [CrossRef]
- Rueda, P.; Delgado, T.; Portela, A.; Melero, J.A.; Garcia-barreno, B. Premature Stop Codons in the G Glycoprotein of Human Respiratory Syncytial Viruses Resistant to Neutralization by Monoclonal Antibodies. J. Virol. 1991, 65, 3374–3378. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Piralla, A.; Zampiero, A.; Bianchini, S.; Di Pietro, G.; Scala, A.; Pinzani, R.; Fossali, E.; Baldanti, F.; Principi, N. Characteristics and Their Clinical Relevance of Respiratory Syncytial Virus Types and Genotypes Circulating in Northern Italy in Five Consecutive Winter Seasons. PLoS ONE 2015, 5, e0129369. [Google Scholar] [CrossRef]
- Zlateva, K.T.; Lemey, P.; Vandamme, A.M.; Van Ranst, M. Molecular evolution and circulation patterns of human respiratory syncytial virus subgroup a: Positively selected sites in the attachment g glycoprotein. J. Virol. 2004, 78, 4675–4683. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wu, T.H.; Fang, Y.P.; Chang, J.C.; Wang, H.C.; Lin, S.J.; Mai, C.H.; Chang, Y.C.; Chou, T.Y. Delayed respiratory syncytial virus outbreak in 2020 in Taiwan was correlated with two novel RSV-A genotype ON1 variants. Influenza Other Respir. Viruses 2022, 16, 511–520. [Google Scholar] [CrossRef]
| Age Group (Months) | No. of Clinical Cases | Gender | Symptoms (%) | RSV | |||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Fever | Cough | Nasal Discharge | Sore Throat | +Ve | −Ve | ||
| 1–6 | 290 | 167 | 123 | 283 | 281 | 284 | 273 | 45 | 245 |
| 7–12 | 205 | 123 | 82 | 204 | 200 | 189 | 192 | 31 | 174 |
| 13–24 | 145 | 93 | 52 | 133 | 131 | 132 | 136 | 22 | 123 |
| Total | 640 | 383 | 257 | 620 | 612 | 605 | 601 | 98 | 542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Alhetheel, A.; Almajhdi, F.N.; Parveen, S.; AlSaadi, M.M.; Al-Mobaireek, K.F. Equal Prevalence of Genotypes ON1 and BA of Human Orthopneumovirus in Riyadh, Saudi Arabia, in 2022. Curr. Issues Mol. Biol. 2025, 47, 826. https://doi.org/10.3390/cimb47100826
Ahmed A, Alhetheel A, Almajhdi FN, Parveen S, AlSaadi MM, Al-Mobaireek KF. Equal Prevalence of Genotypes ON1 and BA of Human Orthopneumovirus in Riyadh, Saudi Arabia, in 2022. Current Issues in Molecular Biology. 2025; 47(10):826. https://doi.org/10.3390/cimb47100826
Chicago/Turabian StyleAhmed, Anwar, Abdulkarim Alhetheel, Fahad N. Almajhdi, Shama Parveen, Muslim M. AlSaadi, and Khalid F. Al-Mobaireek. 2025. "Equal Prevalence of Genotypes ON1 and BA of Human Orthopneumovirus in Riyadh, Saudi Arabia, in 2022" Current Issues in Molecular Biology 47, no. 10: 826. https://doi.org/10.3390/cimb47100826
APA StyleAhmed, A., Alhetheel, A., Almajhdi, F. N., Parveen, S., AlSaadi, M. M., & Al-Mobaireek, K. F. (2025). Equal Prevalence of Genotypes ON1 and BA of Human Orthopneumovirus in Riyadh, Saudi Arabia, in 2022. Current Issues in Molecular Biology, 47(10), 826. https://doi.org/10.3390/cimb47100826







