The Interventional Effects and Mechanisms of Lonidamine in Combination with Apigenin on Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture
2.2. Antibodies and Reagents
2.3. Cell Viability and Proliferation Assay
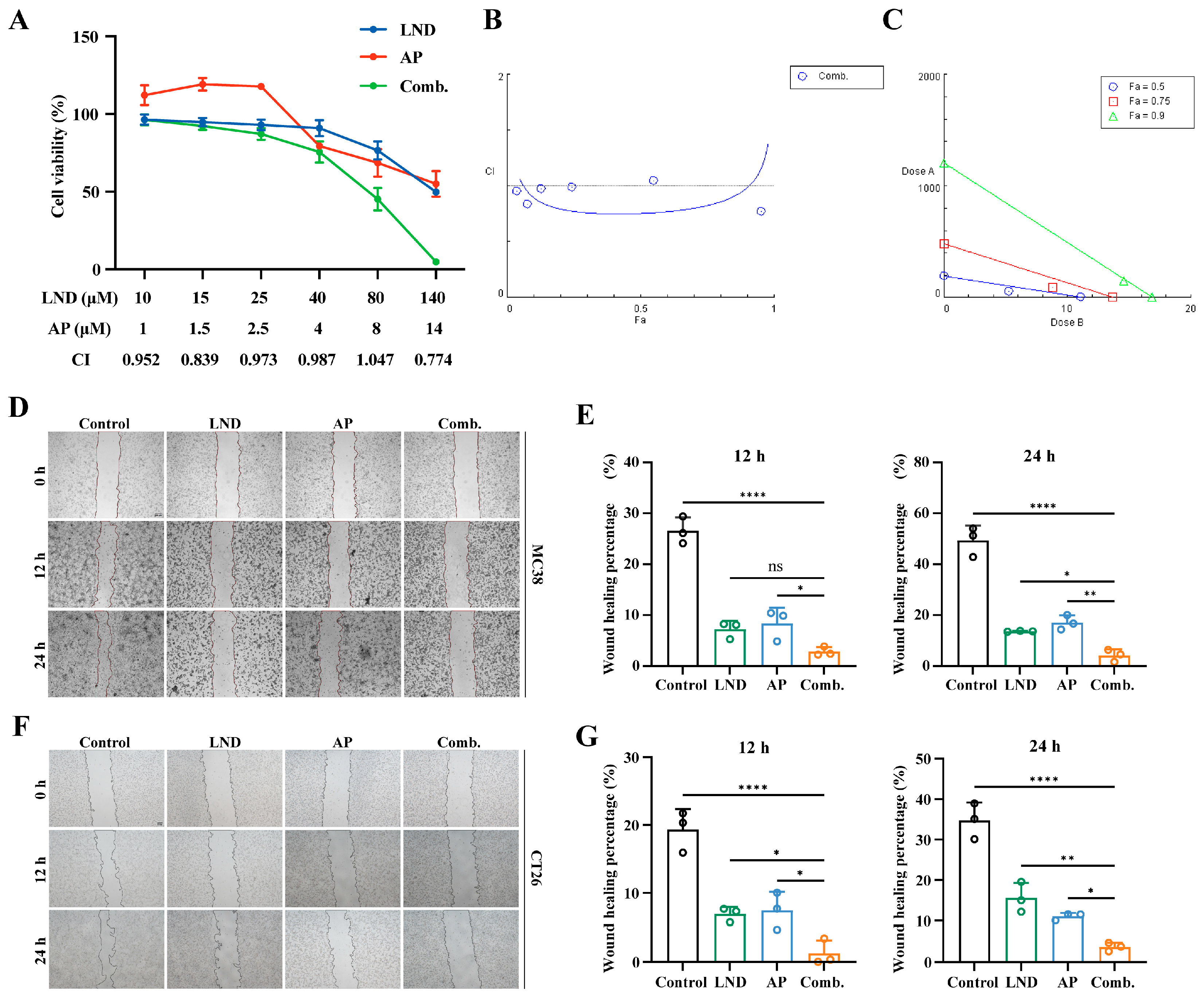
2.4. Combination Index (CI) Analysis
2.5. Cell Migration Assay
2.6. Cell Cycle Analysis
2.7. Annexin V/PI Staining
2.8. Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
2.9. Western Blot Analysis
2.10. Metabolite Measurement Using Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry (UPLC-MS/MS)
2.11. In Vivo Experiment
2.12. Immunohistochemical (IHC) Staining
2.13. Statistical Analysis
3. Results
3.1. Lonidamine and Apigenin Co-Treatment Synergistically Inhibits Proliferation and Migration of Colon Cancer Cells
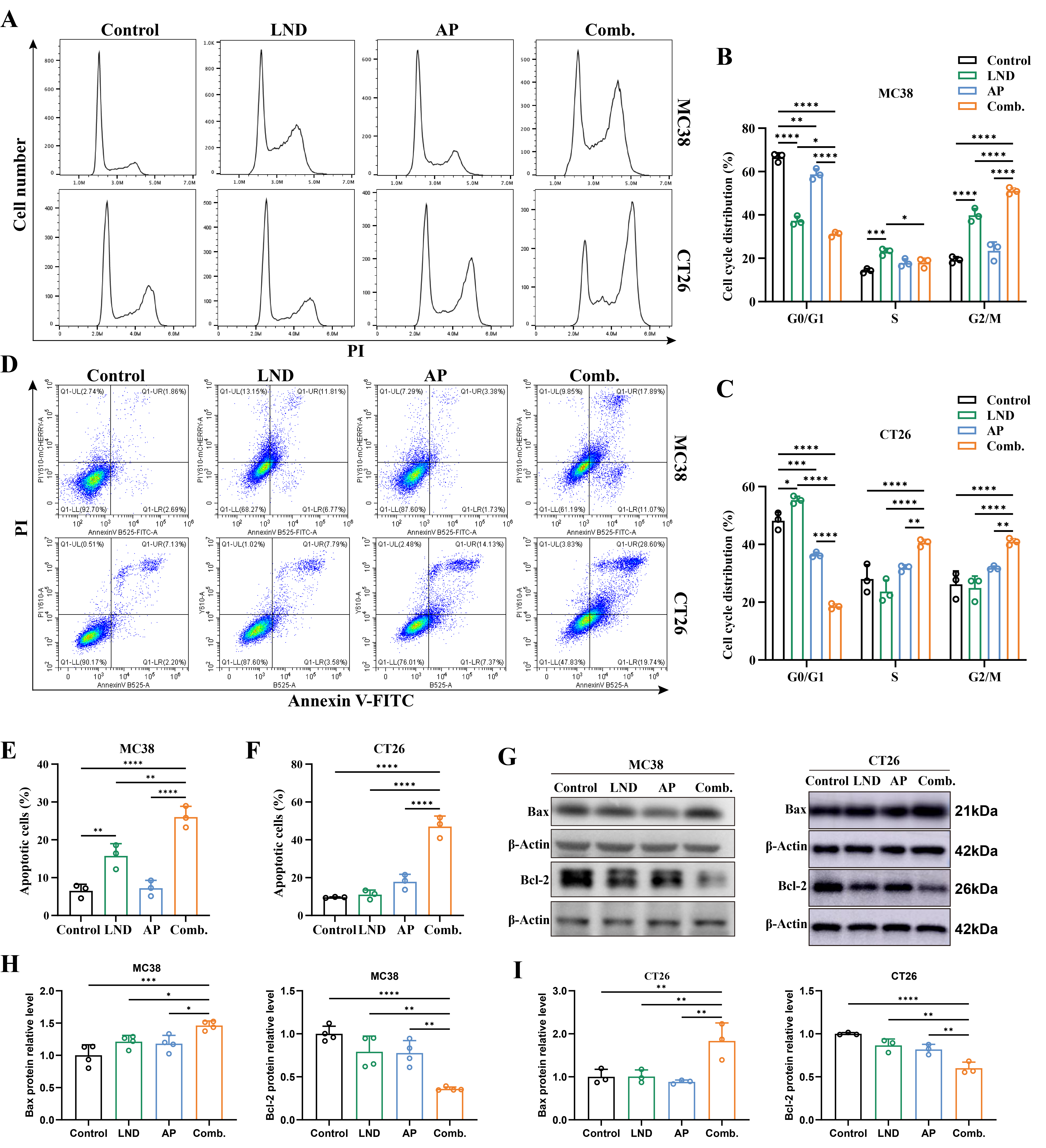
3.2. Combination Treatment of Lonidamine and Apigenin Induced Apoptosis and Cell Cycle Arrest
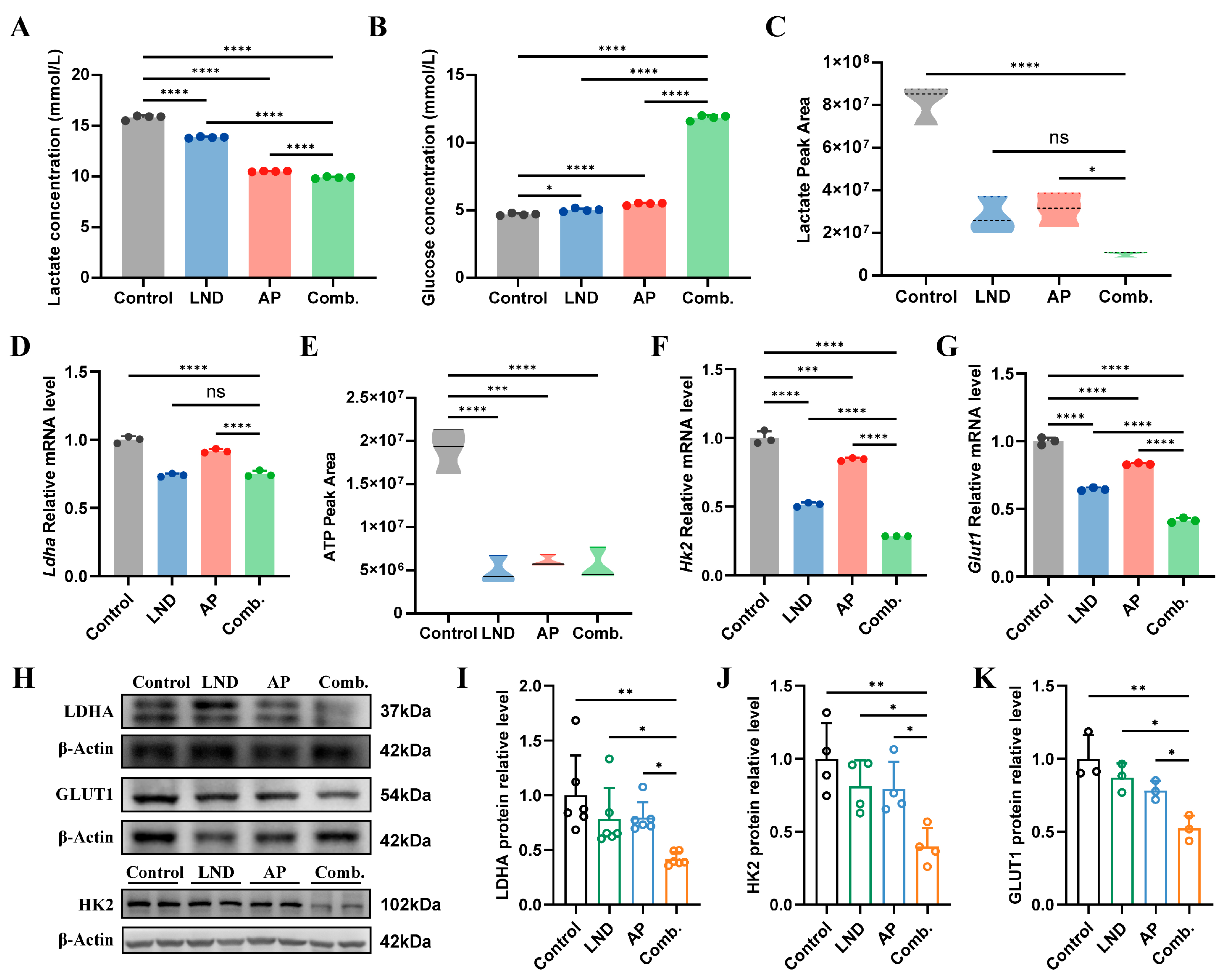
3.3. Inhibition of Glycolytic Pathways and Energy Production
3.4. Impact of Lonidamine and Apigenin on Nucleotide Depletion and NAD Disruption
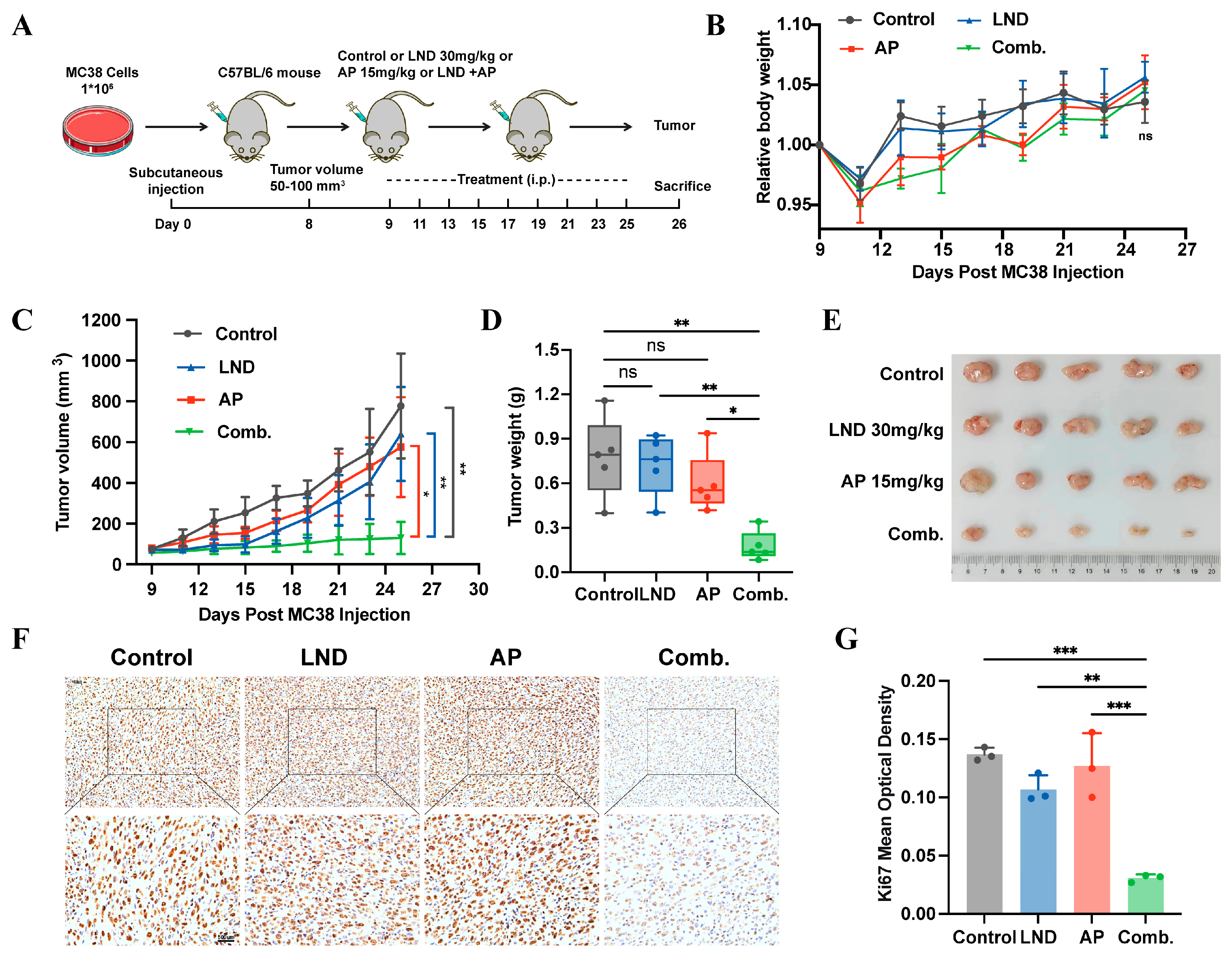
3.5. Lonidamine Combined with Apigenin Suppressed Tumor Growth In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Apigenin |
| Comb. | Combination |
| CRC | Colorectal cancer |
| GLUT1 | Glucose transporter 1 |
| HK2 | Hexokinase 2 |
| LDHA | Lactate dehydrogenase A |
| LND | Lonidamine |
| PDX | Patient-derived xenografts |
| PRPP | Phosphoribosyl pyrophosphate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef]
- Al Bitar, S.; El-Sabban, M.; Doughan, S.; Abou-Kheir, W. Molecular mechanisms targeting drug-resistance and metastasis in colorectal cancer: Updates and beyond. World J. Gastroenterol. 2023, 29, 1395–1426. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Icard, P.; Shulman, S.; Farhat, D.; Steyaert, J.M.; Alifano, M.; Lincet, H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist. Updates 2018, 38, 1–11. [Google Scholar]
- Cioli, V.; Bellocci, B.; Putzolu, S.; Malorni, W.; Demartino, C. Anti-Spermogenic Activity of Lonidamine (AF-1890) in Rabbit. Ultramicroscopy 1980, 5, 418. [Google Scholar]
- Nath, K.; Nelson, D.S.; Heitjan, D.F.; Leeper, D.B.; Zhou, R.; Glickson, J.D. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015, 28, 281–290. [Google Scholar]
- Nath, K.; Roman, J.; Nelson, D.S.; Guo, L.; Lee, S.C.; Orlovskiy, S.; Muriuki, K.; Heitjan, D.F.; Pickup, S.; Leeper, D.B.; et al. Effect of Differences in Metabolic Activity of Melanoma Models on Response to Lonidamine plus Doxorubicin. Sci. Rep. 2018, 8, 14654. [Google Scholar] [CrossRef]
- Lu, H.; Tong, W.; Jiang, M.; Liu, H.; Meng, C.; Wang, K.; Mu, X. Mitochondria-Targeted Multifunctional Nanoprodrugs by Inhibiting Metabolic Reprogramming for Combating Cisplatin-Resistant Lung Cancer. ACS Nano 2024, 18, 21156–21170. [Google Scholar]
- Huang, Y.; Sun, G.; Sun, X.; Li, F.; Zhao, L.; Zhong, R.; Peng, Y. The Potential of Lonidamine in Combination with Chemotherapy and Physical Therapy in Cancer Treatment. Cancers 2020, 12, 3332. [Google Scholar] [CrossRef]
- Meijer, T.W.H.; Peeters, W.J.M.; Dubois, L.J.; van Gisbergen, M.W.; Biemans, R.; Venhuizen, J.H.; Span, P.N.; Bussink, J. Targeting glucose and glutamine metabolism combined with radiation therapy in non-small cell lung cancer. Lung Cancer 2018, 126, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Li, F.; Zeng, R.; Zhang, X.; Wang, X.; Zhao, S.; Weng, J.; Li, Z.; Sun, L. Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles. Drug Deliv. 2022, 29, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Wang, T.; Zhang, J.; Zhou, X.; Niu, Y.; Liu, F.; Zhong, Y.; Huang, D.; Chen, W. Mitochondrial Targeting and pH-Responsive Nanogels for Co-Delivery of Lonidamine and Paclitaxel to Conquer Drug Resistance. Front. Bioeng. Biotechnol. 2021, 9, 787320. [Google Scholar]
- Sánchez, Y.; Simón, G.P.; Calviño, E.; de Blas, E.; Aller, P. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell lines. J. Pharmacol. Exp. Ther. 2010, 335, 114–123. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, J.; Deng, X.; Shen, L.; Wu, S.; Fan, H.; Liu, L. Curcumin and nanodelivery systems: New directions for targeted therapy and diagnosis of breast cancer. Biomed. Pharmacother. 2024, 180, 117404. [Google Scholar] [CrossRef]
- Lin, G.; Wu, Y.; Cai, F.; Li, Z.; Su, S.; Wang, J.; Cao, J.; Ma, L. Matrine Promotes Human Myeloid Leukemia Cells Apoptosis Through Warburg Effect Mediated by Hexokinase 2. Front. Pharmacol. 2019, 10, 1069. [Google Scholar] [CrossRef]
- You, L.; Yang, C.; Du, Y.; Wang, W.; Sun, M.; Liu, J.; Ma, B.; Pang, L.; Zeng, Y.; Zhang, Z.; et al. A Systematic Review of the Pharmacology, Toxicology and Pharmacokinetics of Matrine. Front. Pharmacol. 2020, 11, 01067. [Google Scholar] [CrossRef]
- De Cesare, M.; Pratesi, G.; Giusti, A.; Polizzi, D.; Zunino, F. Stimulation of the apoptotic response as a basis for the therapeutic synergism of lonidamine and cisplatin in combination in human tumour xenografts. Br. J. Cancer 1998, 77, 434–439. [Google Scholar] [CrossRef]
- Ozkan, E.; Bakar-Ates, F. Potentiation of the Effect of Lonidamine by Quercetin in MCF-7 human breast cancer cells through downregulation of MMP-2/9 mRNA Expression. An. Acad. Bras. Cienc. 2020, 92, e20200548. [Google Scholar] [CrossRef]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef]
- Ali, F.; Rahul, R.; Naz, F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.R.; Lee, D.G. Apigenin promotes antibacterial activity via regulation of nitric oxide and superoxide anion production. J. Basic Microbiol. 2020, 60, 862–872. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, J.X.; Yang, S.F.; Yang, C.K.; Chen, T.H.; Hsiao, Y.H. Anticancer Effects and Molecular Mechanisms of Apigenin in Cervical Cancer Cells. Cancers 2022, 14, 1824. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Song, Y.U.; Yao, J.; Huang, K.; Zhu, X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef]
- Yang, C.; Song, J.; Hwang, S.; Choi, J.; Song, G.; Lim, W. Apigenin enhances apoptosis induction by 5-fluorouracil through regulation of thymidylate synthase in colorectal cancer cells. Redox Biol. 2021, 47, 102144. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, W.; Chen, M.; Huang, L.; Li, S.; Li, Z.; Pan, Q.; Lv, S.; Yang, X.; Wang, Y.; et al. Apigenin suppresses tumor angiogenesis and growth via inhibiting HIF-1α expression in non-small cell lung carcinoma. Chem. Biol. Interact. 2022, 361, 109966. [Google Scholar] [CrossRef]
- Shukla, S.; Bhaskaran, N.; Babcook, M.A.; Fu, P.; Maclennan, G.T.; Gupta, S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis 2014, 35, 452–460. [Google Scholar] [CrossRef]
- Ujiki, M.B.; Ding, X.Z.; Salabat, M.R.; Bentrem, D.J.; Golkar, L.; Milam, B.; Talamonti, M.S.; Bell, R.H., Jr.; Iwamura, T.; Adrian, T.E. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol. Cancer 2006, 5, 76. [Google Scholar] [CrossRef]
- Huang, C.; Wei, Y.X.; Shen, M.C.; Tu, Y.H.; Wang, C.C.; Huang, H.C. Chrysin, Abundant in Morinda citrifolia Fruit Water-EtOAc Extracts, Combined with Apigenin Synergistically Induced Apoptosis and Inhibited Migration in Human Breast and Liver Cancer Cells. J. Agric. Food Chem. 2016, 64, 4235–4245. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Shafiee, S.; Burcher, J.T.; Lagoa, R.; Farzaei, M.H.; Bishayee, A. Anticancer Potential of Apigenin and Isovitexin with Focus on Oncogenic Metabolism in Cancer Stem Cells. Metabolites 2023, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, G.; Oh, T.I.; Kim, B.M.; Shim, D.W.; Lee, K.H.; Kim, Y.J.; Lim, B.O.; Lim, J.H. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 2016, 48, 399–408. [Google Scholar] [CrossRef]
- Yu, M.; Xue, J.; Liu, N.; Ren, X.; Tan, L.; Fu, C.; Wu, Q.; Niu, M.; Zou, J.; Meng, X. Apigenin and doxorubicin loaded zeolitic imidazole frameworks for triple-combination therapy of breast cancer. J. Colloid Interface Sci. 2025, 697, 137979. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Y.; Wang, L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J. Biol. Chem. 2009, 284, 31921–31927. [Google Scholar] [CrossRef]
- Golding, J.P.; Wardhaugh, T.; Patrick, L.; Turner, M.; Phillips, J.B.; Bruce, J.I.; Kimani, S.G. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br. J. Cancer 2013, 109, 976–982. [Google Scholar] [CrossRef]
- Villa, R.; Zaffaroni, N.; Orlandi, L.; Bearzatto, A.; Costa, A.; Silvestrini, R. In vitro effect of lonidamine on the cytotoxicity of mitomycin C and BCNU in human colon adenocarcinoma cells. Eur. J. Cancer 1994, 30, 1534–1540. [Google Scholar] [CrossRef]
- Gornati, D.; Zaffaroni, N.; Orlandi, L.; Difronzo, G.; Silvestrini, R. Combined effects of lonidamine and tamoxifen in human breast-cancer cell-lines. Int. J. Oncol. 1995, 6, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Rosbe, K.W.; Brann, T.W.; Holden, S.A.; Teicher, B.A.; Frei, E., 3rd. Effect of lonidamine on the cytotoxicity of four alkylating agents in vitro. Cancer Chemother. Pharmacol. 1989, 25, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Floridi, A.; Paggi, M.G.; D’Atri, S.; De Martino, C.; Marcante, M.L.; Silvestrini, B.; Caputo, A. Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res. 1981, 41, 4661–4666. [Google Scholar] [PubMed]
- Wang, B.; Zhang, M.; Guo, J.; Liu, Z.; Zhou, R.; Guo, F.; Li, K.; Mu, Y. The Effects of Flavonoid Apigenin on Male Reproductive Health: Inhibition of Spermatogonial Proliferation through Downregulation of Prmt7/Akt3 Pathway. Int. J. Mol. Sci. 2021, 22, 12209. [Google Scholar] [CrossRef]
- Wang, S.M.; Yang, P.W.; Feng, X.J.; Zhu, Y.W.; Qiu, F.J.; Hu, X.D.; Zhang, S.H. Apigenin Inhibits the Growth of Hepatocellular Carcinoma Cells by Affecting the Expression of microRNA Transcriptome. Front. Oncol. 2021, 11, 657665. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Chen, F.; Lu, Z. Combined effect of chrysin and apigenin on inhibiting the development and progression of colorectal cancer by suppressing the activity of P38-MAPK/AKT pathway. IUBMB Life 2021, 73, 774–783. [Google Scholar] [CrossRef]
- Hong, J.; Fristiohady, A.; Nguyen, C.H.; Milovanovic, D.; Huttary, N.; Krieger, S.; Hong, J.; Geleff, S.; Birner, P.; Jäger, W.; et al. Apigenin and Luteolin Attenuate the Breaching of MDA-MB231 Breast Cancer Spheroids Through the Lymph Endothelial Barrier in Vitro. Front. Pharmacol. 2018, 9, 220. [Google Scholar] [CrossRef]
- Lei, L.; Dai, W.; Man, J.; Hu, H.; Jin, Q.; Zhang, B.; Tang, Z. Lonidamine liposomes to enhance photodynamic and photothermal therapy of hepatocellular carcinoma by inhibiting glycolysis. J. Nanobiotechnol. 2023, 21, 482. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; He, X.; Jin, L.; Zhu, Y.; Hu, L.; Feng, M.; Zhu, J.; Wang, L.; Zheng, Y.; et al. Impaired microglial glycolysis promotes inflammatory responses after intracerebral haemorrhage via HK2-,dependent mitochondrial dysfunction. J. Adv. Res. 2024, 73, 575–591. [Google Scholar] [CrossRef]
- Milane, L.; Duan, Z.; Amiji, M. Therapeutic efficacy and safety of paclitaxel/lonidamine loaded EGFR-targeted nanoparticles for the treatment of multi-drug resistant cancer. PLoS ONE 2011, 6, e24075. [Google Scholar] [CrossRef]
- Korga, A.; Ostrowska, M.; Jozefczyk, A.; Iwan, M.; Wojcik, R.; Zgorka, G.; Herbet, M.; Vilarrubla, G.G.; Dudka, J. Apigenin and hesperidin augment the toxic effect of doxorubicin against HepG2 cells. BMC Pharmacol. Toxicol. 2019, 20, 22. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de novo synthesis increases breast cancer stemness and metastasis via cGMP-PKG-MAPK signaling pathway. PLoS Biol. 2020, 18, e3000872. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Astapova, I.; Zhang, G.; Cangelosi, A.L.; Ilkayeva, O.; Marchuk, H.; Muehlbauer, M.J.; George, T.; Brozinick, J.; Herman, M.A.; et al. Integration of metabolomic and transcriptomic analyses reveals novel regulatory functions of the ChREBP transcription factor in energy metabolism. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Huang, K.C.; Huang, T.C.; Chen, H.Y.; Ali, M.; Al-Hendy, A.; Huang, P.S.; Hsia, S.M. Regulatory roles of NAMPT and NAD(+) metabolism in uterine leiomyoma progression: Implications for ECM accumulation, stemness, and microenvironment. Redox Biol. 2024, 78, 103411. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Holden, S.A.; Ara, G.; Menon, K. Whole-body hyperthermia and lonidamine as adjuvant therapy to treatment with cisplatin with or without local radiation in mouse bearing the Lewis lung carcinoma. Int. J. Hyperth. 1995, 11, 637–645. [Google Scholar] [CrossRef]
- Medhat, A.M.; Azab, K.S.; Said, M.M.; El Fatih, N.M.; El Bakary, N.M. Antitumor and radiosensitizing synergistic effects of apigenin and cryptotanshinone against solid Ehrlich carcinoma in female mice. Tumour Biol. 2017, 39, 1010428317728480. [Google Scholar] [CrossRef]
- Mansi, J.L.; de Graeff, A.; Newell, D.R.; Glaholm, J.; Button, D.; Leach, M.O.; Payne, G.; Smith, I.E. A phase II clinical and pharmacokinetic study of Lonidamine in patients with advanced breast cancer. Br. J. Cancer 1991, 64, 593–597. [Google Scholar] [CrossRef]
- Nozhat, Z.; Heydarzadeh, S.; Memariani, Z.; Ahmadi, A. Chemoprotective and chemosensitizing effects of apigenin on cancer therapy. Cancer Cell Int. 2021, 21, 574. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, X.; Wang, F.; Tan, H.; Hu, J.; Bai, W.; Wang, X.; Wang, B.; Hu, J. Inhibitory effects of flavonoids on glucose transporter 1 (GLUT1): From library screening to biological evaluation to structure-activity relationship. Toxicology 2023, 488, 153475. [Google Scholar] [CrossRef]
- Fang, J.; Bao, Y.Y.; Zhou, S.H.; Fan, J. Apigenin inhibits the proliferation of adenoid cystic carcinoma via suppression of glucose transporter-1. Mol. Med. Rep. 2015, 12, 6461–6466. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Li, Y.; Ziwen, X.; Zhang, P.; Song, Q.; Yao, Y.; Pei, H. De novo nucleotide biosynthetic pathway and cancer. Genes Dis. 2023, 10, 2331–2338. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Z.; Wang, X.; Peng, J.; Sun, Q.; Li, J.; Wang, M.; Niu, L.; Zhang, Z.; Cai, G.; et al. Crystal and EM structures of human phosphoribosyl pyrophosphate synthase I (PRS1) provide novel insights into the disease-associated mutations. PLoS ONE 2015, 10, e0120304. [Google Scholar] [CrossRef][Green Version]
- Hove-Jensen, B.; Andersen, K.R.; Kilstrup, M.; Martinussen, J.; Switzer, R.L.; Willemoës, M. Phosphoribosyl Diphosphate (PRPP): Biosynthesis, Enzymology, Utilization, and Metabolic Significance. Microbiol. Mol. Biol. Rev. 2017, 81, e00040-16. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Shi, J.; Zhang, M.; Yang, H.; Fei, J. The Interventional Effects and Mechanisms of Lonidamine in Combination with Apigenin on Colorectal Cancer. Curr. Issues Mol. Biol. 2025, 47, 825. https://doi.org/10.3390/cimb47100825
Zhou Y, Shi J, Zhang M, Yang H, Fei J. The Interventional Effects and Mechanisms of Lonidamine in Combination with Apigenin on Colorectal Cancer. Current Issues in Molecular Biology. 2025; 47(10):825. https://doi.org/10.3390/cimb47100825
Chicago/Turabian StyleZhou, Yi, Jiahao Shi, Mengjie Zhang, Hua Yang, and Jian Fei. 2025. "The Interventional Effects and Mechanisms of Lonidamine in Combination with Apigenin on Colorectal Cancer" Current Issues in Molecular Biology 47, no. 10: 825. https://doi.org/10.3390/cimb47100825
APA StyleZhou, Y., Shi, J., Zhang, M., Yang, H., & Fei, J. (2025). The Interventional Effects and Mechanisms of Lonidamine in Combination with Apigenin on Colorectal Cancer. Current Issues in Molecular Biology, 47(10), 825. https://doi.org/10.3390/cimb47100825






