Molecular Mechanisms and Clinical Implications of Fibroblast Growth Factor Receptor 2 Signaling in Gastrointestinal Stromal Tumors
Abstract
1. Introduction
2. Methods
3. FGFR2 and the Characteristics Related to GIST
3.1. FGFR2 Structure, Isoforms, and Basic Function in GIST Context
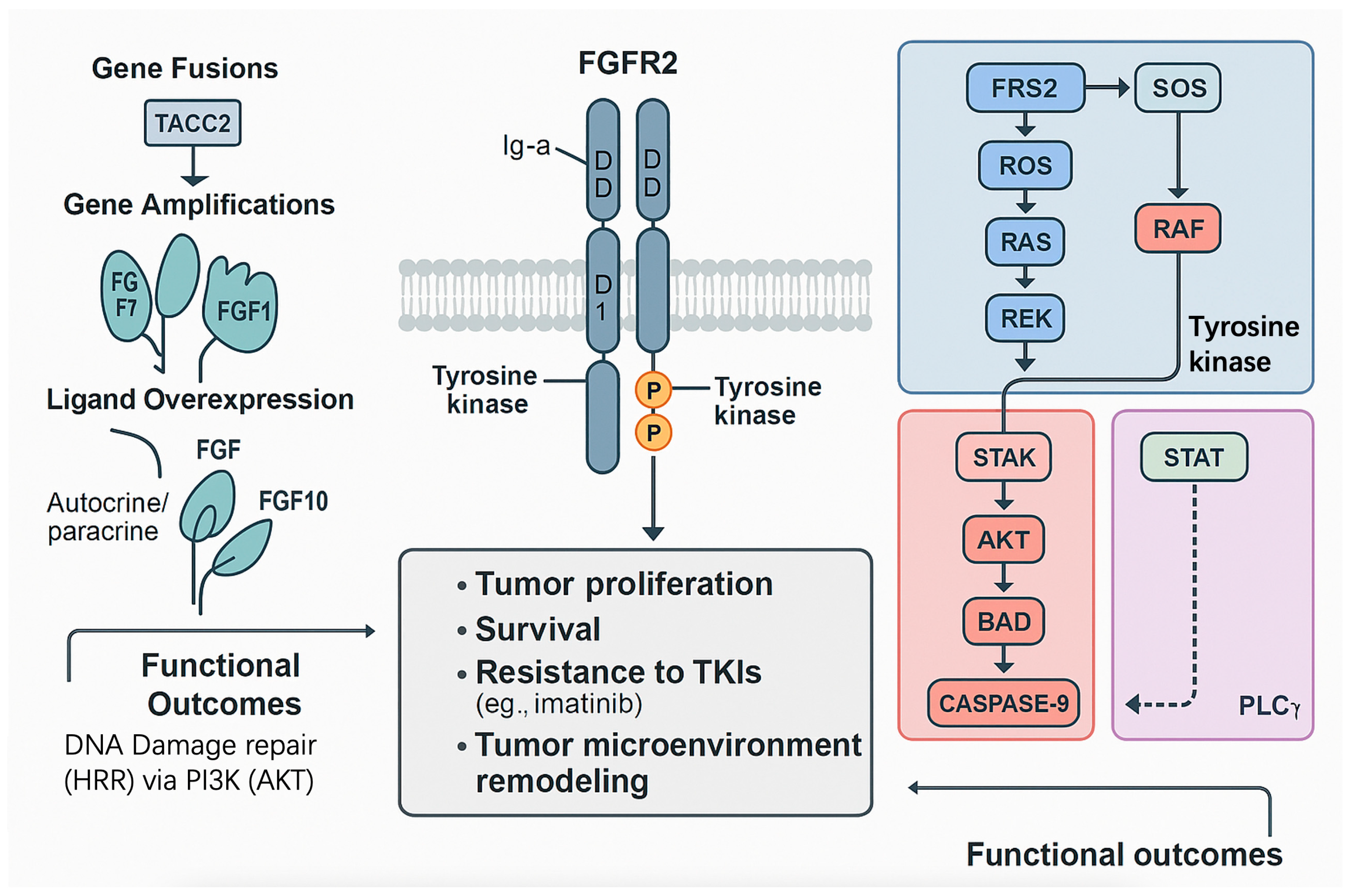
3.1.1. Molecular Structure and Isoforms
3.1.2. Physiological Roles and Signaling Pathways
3.1.3. Physiological and Pathological Relevance
3.2. Genomic Alteration Spectrum of FGFR2 in GISTs
3.2.1. Prevalence and Types of FGFR2 Alterations
3.2.2. Molecular Characteristics and Mechanisms
3.2.3. Point Mutations and Their Rarity
3.3. FGFR2-Mediated Signaling Pathway Activation in GISTs
3.3.1. Mechanisms of FGFR2 Activation
3.3.2. Key Downstream Signaling Pathways
3.3.3. Role in TKI Resistance
3.3.4. Critical Evaluation and Limitations
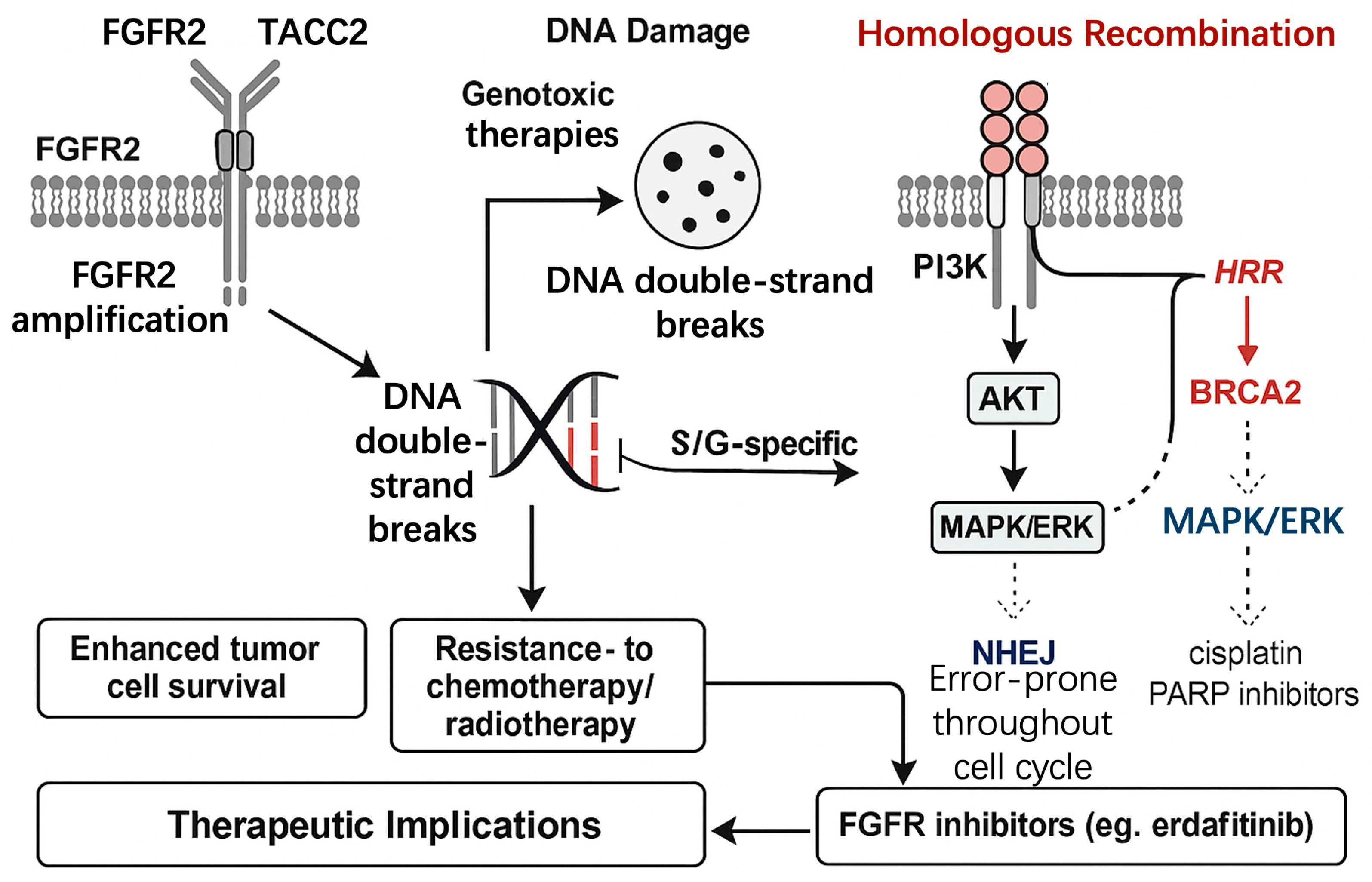
3.4. FGFR2 and DNA Damage Repair (DDR) in GISTs
3.4.1. FGFR2’s Role in Homologous Recombination Repair (HRR)
3.4.2. FGFR2 and Nonhomologous End Joining (NHEJ)
3.4.3. Implications for Therapeutic Resistance
3.4.4. Therapeutic Opportunities: Combining FGFR2 Inhibitors with DNA-Damaging Agents
3.4.5. Critical Evaluation and Research Gaps
4. Clinical Significance and Treatment Strategies of FGFR2
4.1. FGFR2 as a Key Bypass Mechanism in TKIs Resistance in GISTs
4.1.1. Molecular Mechanisms of FGFR2-Mediated TKI Resistance
4.1.2. Clinical Evidence of FGFR2-Driven Resistance
4.1.3. Therapeutic Implications
4.1.4. Critical Evaluation and Research Gaps
- Variable detection methods (DNA vs. RNA NGS, FISH sensitivity) may underestimate true prevalence [73].
- Lack of GIST-specific pre-clinical models (e.g., FGFR2-driven cell lines, patient-derived xenografts) hampers functional validation [74].
- Risk of secondary FGFR2 mutations (e.g., gatekeeper V564F) conferring resistance to erdafitinib, as observed in cholangiocarcinoma [61], may extend to GIST but remains unquantified.
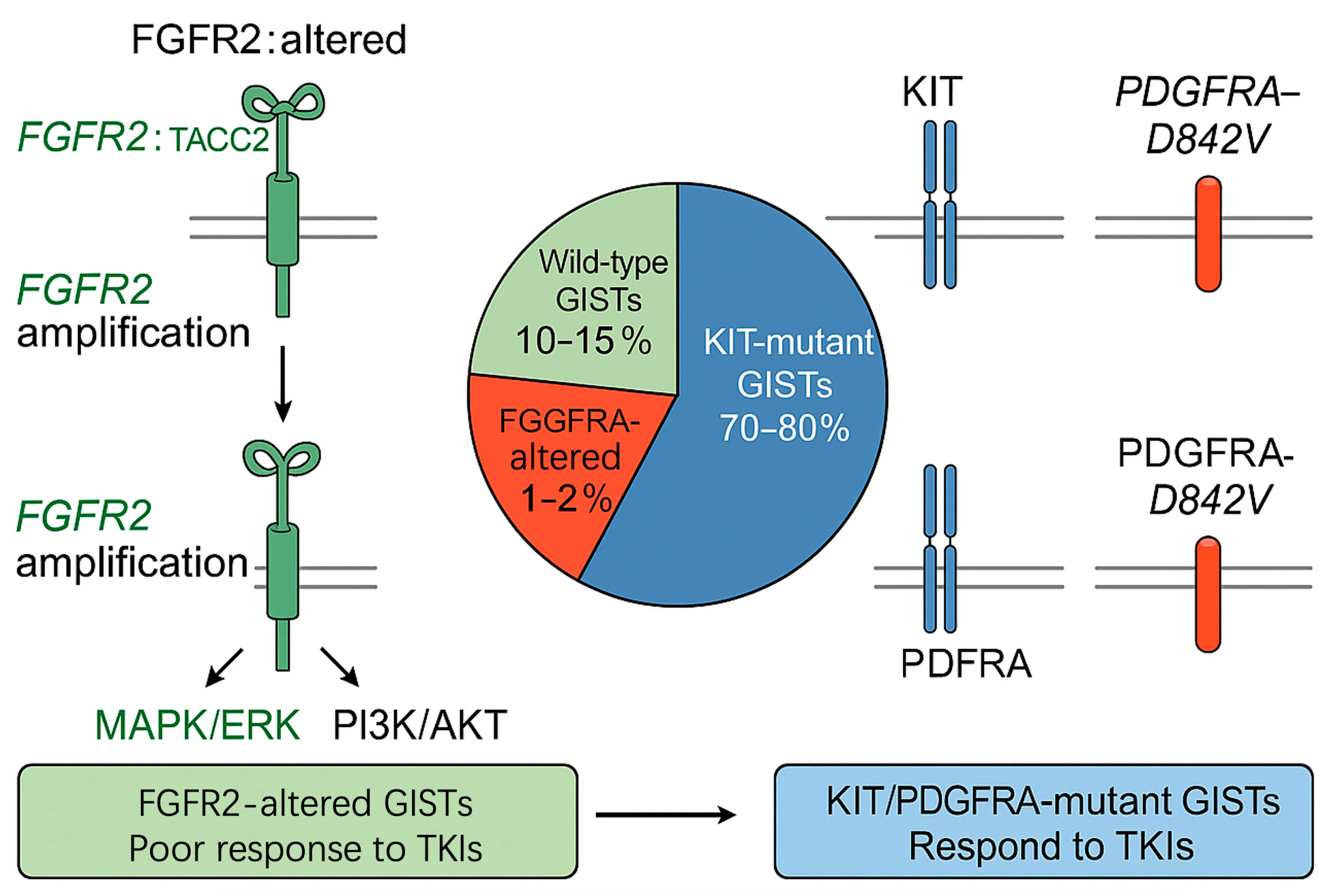
4.2. Mutual Exclusivity of FGFR2 with KIT and PDGFRA Mutations in GISTs
4.2.1. Molecular Basis of Mutual Exclusivity
4.2.2. Clinical Evidence Supporting Mutual Exclusivity
4.2.3. Implications for Pathogenesis and Treatment
| Aspect | Description | References |
|---|---|---|
| Pathogenesis | In wild-type GISTs, FGFR2 alterations likely serve as primary oncogenic drivers, activating MAPK/ERK and PI3K/AKT pathways to mimic the effects of KIT/PDGFRA mutations. This suggests the existence of distinct molecular subtypes of GISTs, with FGFR2-driven tumors representing a rare but clinically significant subgroup. | [8,76,78] |
| Treatment Response | The presence of FGFR2 alterations predicts poor response to standard TKIs such as imatinib, necessitating alternative therapies. FGFR inhibitors, such as erdafitinib and pemigatinib, approved for other FGFR-driven cancers, show preclinical promise in FGFR2-altered GISTs. | [11,12,79] |
| Combination Therapies | The mutual exclusivity suggests that dual inhibition of FGFR2 and KIT/PDGFRA pathways may be unnecessary in most cases. However, combination therapies targeting FGFR2 and downstream pathways (e.g., MAPK or PI3K inhibitors) could enhance efficacy in FGFR2-driven GISTs. | [14] |
4.2.4. Critical Evaluation and Research Gaps
4.3. Diagnostic Methods and Clinical Applications of FGFR2 in GISTs
4.3.1. Molecular Pathology Standardization
4.3.2. Tissue vs. Liquid Biopsy
4.3.3. Clinical Applications
4.3.4. Critical Evaluation
4.4. Therapeutic Potential of Targeting FGFR2 in GISTs
| Study/Trial | Year | Population Setting | Intervention (Comparator) | Primary Endpoint(s) | Key Findings Pertinent to FGFR2-Altered GIST | References |
|---|---|---|---|---|---|---|
| PEMIGIST basket cohort NCT04595747 | 2021–2024 | TKI-refractory FGFR2-fusion or amp GIST (n = 7) | Pemigatinib ± olaparib | Safety, ORR, ctDNA clearance | DCR 100% (3 PR, 4 SD); median ΔctDNA −92% at C2; V564F gatekeeper detected at PD | [88,89] |
| KIN-3248 phase I solid-tumour NCT05136028 | 2022–2024 | Mixed solid tumours incl. 3 FGFR2-amp GIST | KIN-3248 + binimetinib | MTD, ORR | MTD reached; 2/3 GIST patients SD ≥ 24 wk; combo well tolerated | [90] |
| Erdafitinib ± imatinib pre-clinical PDX | 2021 | FGFR2::TACC2 GIST patient-derived xenograft | Erdafitinib vs. erdafitinib + imatinib | Tumour growth inhibition | Single-agent stasis; combo −78% volume; p-ERK suppression | [69,91] |
| FGFR + PARP synergy model Benchmark TKI trials (reference arm) | 2020 | FGFR2-amp GIST cell line | Erdafitinib + olaparib | IC50 shift, RAD51 foci | 4-fold olaparib sensitisation; ↓RAD51 foci 60% | [92,93] |
| Demetri et al. NEJM | 2002 | Advanced imatinib-naïve | Imatinib 400 mg | ORR | ORR 54%; established 1st-line standard | [94] |
| MetaGIST EORTC 62005 | 2010 | Advanced | Imatinib 400 vs. 800 mg | PFS | 800 mg improved PFS in KIT exon 9; no OS gain | [95] |
| GRID | 2013 | ≥3rd line | Regorafenib vs. placebo | PFS | PFS 4.8 mo vs. 0.9 mo; HR 0.27 | [47] |
| INVICTUS | 2020 | ≥4th line | Ripretinib vs. placebo | PFS, OS | PFS 6.3 mo vs. 1.0 mo; OS HR 0.36 | [96] |
5. Discussion and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GIST | Gastrointestinal Stromal Tumor |
| FGFR2 | Fibroblast Growth Factor Receptor 2 |
| KIT | KIT Proto-Oncogene, Receptor Tyrosine Kinase |
| PDGFRA | Platelet-Derived Growth Factor Receptor Alpha |
| TKI | Tyrosine Kinase Inhibitor |
| AKT | Protein Kinase B (Akt) |
| ERK | Extracellular Signal-Regulated Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| PI3K | Phosphoinositide 3-Kinase |
| DNA | Deoxyribonucleic Acid |
| HRR | Homologous Recombination Repair |
| RAD51 | RAD51 Recombinase (a protein involved in HRR) |
| NGS | Next-Generation Sequencing |
| FISH | Fluorescence In Situ Hybridization |
| PFS | Progression-Free Survival |
| VEGF | Vascular Endothelial Growth Factor |
| RTK | Receptor Tyrosine Kinase |
| FGF | Fibroblast Growth Factor |
| DDR | DNA Damage Repair |
| DSB | Double-Strand Break |
| NHEJ | Nonhomologous End Joining |
| PARP | Poly (ADP-Ribose) Polymerase |
References
- Nilsson, B.; Bumming, P.; Meis-Kindblom, J.M.; Oden, A.; Dortok, A.; Gustavsson, B.; Sablinska, K.; Kindblom, L.G. Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-A population-based study in western Sweden. Cancer 2005, 103, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H. Second line therapies for the treatment of gastrointestinal stromal tumor. Curr. Opin. Oncol. 2007, 19, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Thuneberg, L.; Kluppel, M.; Malysz, J.; Mikkelsen, H.B.; Bernstein, A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373, 347–349. [Google Scholar] [CrossRef]
- Kindblom, L.G.; Remotti, H.E.; Aldenborg, F.; Meis-Kindblom, J.M. Gastrointestinal pacemaker cell tumor (GIPACT): Gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am. J. Pathol. 1998, 152, 1259–1269. [Google Scholar]
- Heinrich, M.C.; Corless, C.L.; Duensing, A.; McGreevey, L.; Chen, C.J.; Joseph, N.; Singer, S.; Griffith, D.J.; Haley, A.; Town, A.; et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003, 299, 708–710. [Google Scholar] [CrossRef]
- Hirota, S.; Isozaki, K.; Moriyama, Y.; Hashimoto, K.; Nishida, T.; Ishiguro, S.; Kawano, K.; Hanada, M.; Kurata, A.; Takeda, M.; et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998, 279, 577–580. [Google Scholar] [CrossRef]
- Dematteo, R.P.; Heinrich, M.C.; El-Rifai, W.M.; Demetri, G. Clinical management of gastrointestinal stromal tumors: Before and after STI-571. Hum. Pathol. 2002, 33, 466–477. [Google Scholar] [CrossRef]
- Dematteo, R.P.; Ballman, K.V.; Antonescu, C.R.; Maki, R.G.; Pisters, P.W.; Demetri, G.D.; Blackstein, M.E.; Blanke, C.D.; von Mehren, M.; Brennan, M.F.; et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: A randomised, double-blind, placebo-controlled trial. Lancet 2009, 373, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Kee, D.; Zalcberg, J.R. Current and emerging strategies for the management of imatinib-refractory advanced gastrointestinal stromal tumors. Ther. Adv. Med. Oncol. 2012, 4, 255–270. [Google Scholar] [CrossRef]
- Bauer, S.; Joensuu, H. Emerging Agents for the Treatment of Advanced, Imatinib-Resistant Gastrointestinal Stromal Tumors: Current Status and Future Directions. Drugs 2015, 75, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; Schroeder, A.; Griffith, D.; Town, A.; McGreevey, L.; Harrell, P.; Shiraga, S.; Bainbridge, T.; Morich, J.; Heinrich, M.C. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J. Clin. Oncol. 2005, 23, 5357–5364. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Besmer, P.; Guo, T.; Arkun, K.; Hom, G.; Koryotowski, B.; Leversha, M.A.; Jeffrey, P.D.; Desantis, D.; Singer, S.; et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin. Cancer Res. 2005, 11, 4182–4190. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L.; Blanke, C.D.; Demetri, G.D.; Joensuu, H.; Roberts, P.J.; Eisenberg, B.L.; von Mehren, M.; Fletcher, C.D.; Sandau, K.; et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J. Clin. Oncol. 2006, 24, 4764–4774. [Google Scholar] [CrossRef]
- Serrano, C.; Martin-Broto, J.; Asencio-Pascual, J.M.; Lopez-Guerrero, J.A.; Rubio-Casadevall, J.; Bague, S.; Garcia-Del-Muro, X.; Fernandez-Hernandez, J.A.; Herrero, L.; Lopez-Pousa, A.; et al. 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther. Adv. Med. Oncol. 2023, 15, 17588359231192388. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nakamura, Y.; Komatsu, Y.; Yuki, S.; Takahashi, N.; Okano, N.; Hirano, H.; Ohtsubo, K.; Ohta, T.; Oki, E.; et al. Different efficacy of tyrosine kinase inhibitors by KIT and PGFRA mutations identified in circulating tumor DNA for the treatment of refractory gastrointestinal stromal tumors. BJC Rep. 2024, 2, 54. [Google Scholar] [CrossRef]
- Yip, D.; Zalcberg, J.; Blay, J.Y.; Eriksson, M.; Espinoza, D.; Price, T.; Marreaud, S.; Italiano, A.; Steeghs, N.; Boye, K.; et al. Imatinib alternating with regorafenib compared to imatinib alone for the first-line treatment of advanced gastrointestinal stromal tumor: The AGITG ALT-GIST intergroup randomized phase II trial. Br. J. Cancer 2025, 132, 897–904. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L.; Demetri, G.D.; Blanke, C.D.; von Mehren, M.; Joensuu, H.; McGreevey, L.S.; Chen, C.J.; Van den Abbeele, A.D.; Druker, B.J.; et al. Kinase Mutations and Imatinib Response in Patients With Metastatic Gastrointestinal Stromal Tumor. J. Clin. Oncol. 2023, 41, 4829–4836. [Google Scholar] [CrossRef]
- Debiec-Rychter, M.; Dumez, H.; Judson, I.; Wasag, B.; Verweij, J.; Brown, M.; Dimitrijevic, S.; Sciot, R.; Stul, M.; Vranck, H.; et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 2004, 40, 689–695, Correction in Lancet Oncol. 2020, 21, e341. [Google Scholar] [CrossRef]
- Wardelmann, E.; Merkelbach-Bruse, S.; Pauls, K.; Thomas, N.; Schildhaus, H.U.; Heinicke, T.; Speidel, N.; Pietsch, T.; Buettner, R.; Pink, D.; et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin. Cancer Res. 2006, 12, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.; Shankar, S.; Heinrich, M.C.; Fletcher, J.A.; Fletcher, C.D.; Manola, J.; Morgan, J.A.; Corless, C.L.; George, S.; Tuncali, K.; et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin. Cancer Res. 2007, 13, 5398–5405. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Kanda, T.; Nishitani, A.; Takahashi, T.; Nakajima, K.; Ishikawa, T.; Hirota, S. Secondary mutations in the kinase domain of the KIT gene are predominant in imatinib-resistant gastrointestinal stromal tumor. Cancer Sci. 2008, 99, 799–804. [Google Scholar] [CrossRef]
- Corless, C.L.; Barnett, C.M.; Heinrich, M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer 2011, 11, 865–878. [Google Scholar] [CrossRef]
- Ohshima, K.; Nagashima, T.; Fujiya, K.; Hatakeyama, K.; Watanabe, Y.; Morimoto, K.; Kamada, F.; Shimoda, Y.; Ohnami, S.; Naruoka, A.; et al. Whole-genome and Epigenomic Landscapes of Malignant Gastrointestinal Stromal Tumors Harboring KIT Exon 11 557-558 Deletion Mutations. Cancer Res. Commun. 2023, 3, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Galembikova, A.; Dunaev, P.; Valeeva, E.; Shagimardanova, E.; Gusev, O.; Khaiboullina, S. A Novel Receptor Tyrosine Kinase Switch Promotes Gastrointestinal Stromal Tumor Drug Resistance. Molecules 2017, 22, 2152. [Google Scholar] [CrossRef] [PubMed]
- Li, D.G.; Jiang, J.P.; Chen, F.Y.; Wu, W.; Fu, J.; Wang, G.H.; Li, Y.B. Insulin-like growth factor 2 targets IGF1R signaling transduction to facilitate metastasis and imatinib resistance in gastrointestinal stromal tumors. World J. Gastrointest. Oncol. 2024, 16, 3585–3599. [Google Scholar] [CrossRef]
- Kelly, C.M.; Shoushtari, A.N.; Qin, L.X.; D’Angelo, S.P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; McFadyen, C.; Sjoberg, A.; Singer, S.; et al. A phase Ib study of BGJ398, a pan-FGFR kinase inhibitor in combination with imatinib in patients with advanced gastrointestinal stromal tumor. Invest. New Drugs 2019, 37, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, N.Y.; Guan, R.Y.; Zhu, Y.K.; Jiang, F.Q.; Piao, D. Identification of critical microRNAs in gastrointestinal stromal tumor patients treated with Imatinib. Neoplasma 2018, 65, 683–692. [Google Scholar] [CrossRef]
- Cui, Z.; Sun, H.; Gao, Z.; Li, C.; Xiao, T.; Bian, Y.; Liu, Z.; Gu, T.; Zhang, J.; Li, T.; et al. TRIM21/USP15 balances ACSL4 stability and the imatinib resistance of gastrointestinal stromal tumors. Br. J. Cancer 2024, 130, 526–541. [Google Scholar] [CrossRef]
- Shima, T.; Taniguchi, K.; Tokumaru, Y.; Inomata, Y.; Arima, J.; Lee, S.W.; Takabe, K.; Yoshida, K.; Uchiyama, K. Glucose transporter-1 inhibition overcomes imatinib resistance in gastrointestinal stromal tumor cells. Oncol. Rep. 2022, 47, 7. [Google Scholar] [CrossRef]
- Rausch, J.L.; Ali, A.A.; Lee, D.M.; Gebreyohannes, Y.K.; Mehalek, K.R.; Agha, A.; Patil, S.S.; Tolstov, Y.; Wellens, J.; Dhillon, H.S.; et al. Differential antitumor activity of compounds targeting the ubiquitin-proteasome machinery in gastrointestinal stromal tumor (GIST) cells. Sci. Rep. 2020, 10, 5178. [Google Scholar] [CrossRef]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Abrams, T.J.; Lee, L.B.; Murray, L.J.; Pryer, N.K.; Cherrington, J.M. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol. Cancer Ther. 2003, 2, 471–478. [Google Scholar] [PubMed]
- Mendel, D.B.; Laird, A.D.; Xin, X.; Louie, S.G.; Christensen, J.G.; Li, G.; Schreck, R.E.; Abrams, T.J.; Ngai, T.J.; Lee, L.B.; et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003, 9, 327–337. [Google Scholar] [PubMed]
- Mulet-Margalef, N.; Garcia-Del-Muro, X. Sunitinib in the treatment of gastrointestinal stromal tumor: Patient selection and perspectives. Onco Targets Ther. 2016, 9, 7573–7582. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Maki, R.G.; Corless, C.L.; Antonescu, C.R.; Harlow, A.; Griffith, D.; Town, A.; McKinley, A.; Ou, W.B.; Fletcher, J.A.; et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J. Clin. Oncol. 2008, 26, 5352–5359. [Google Scholar] [CrossRef]
- Judson, I.R. Prognosis, imatinib dose, and benefit of sunitinib in GIST: Knowing the genotype. J. Clin. Oncol. 2008, 26, 5322–5325. [Google Scholar] [CrossRef]
- Shirao, K.; Nishida, T.; Doi, T.; Komatsu, Y.; Muro, K.; Li, Y.; Ueda, E.; Ohtsu, A. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest. New Drugs 2010, 28, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.; Zhang, T.; Liu, H.; Zhong, J.; Wang, Z.; Wang, L.; Hong, L. Second-line sunitinib for Chinese patients with advanced gastrointestinal stromal tumor: 37.5 mg schedule outperformed 50 mg schedule in adherence and prognosis. Transl. Cancer Res. 2021, 10, 3206–3217. [Google Scholar] [CrossRef]
- Sasaki, K.; Kanda, T.; Matsumoto, Y.; Ishikawa, T.; Hirota, S.; Saijo, Y. Sunitinib therapy for imatinib-resistant and/or intolerant gastrointestinal stromal tumors: Comparison of safety and efficacy between standard and reduced dosage regimens. Jpn. J. Clin. Oncol. 2023, 53, 297–303. [Google Scholar] [CrossRef]
- Rutkowski, P.; Bylina, E.; Klimczak, A.; Switaj, T.; Falkowski, S.; Kroc, J.; Lugowska, I.; Brzeskwiniewicz, M.; Melerowicz, W.; Osuch, C.; et al. The outcome and predictive factors of sunitinib therapy in advanced gastrointestinal stromal tumors (GIST) after imatinib failure—One institution study. BMC Cancer 2012, 12, 107. [Google Scholar] [CrossRef]
- Guo, T.; Hajdu, M.; Agaram, N.P.; Shinoda, H.; Veach, D.; Clarkson, B.D.; Maki, R.G.; Singer, S.; Dematteo, R.P.; Besmer, P.; et al. Mechanisms of sunitinib resistance in gastrointestinal stromal tumors harboring KITAY502-3ins mutation: An in vitro mutagenesis screen for drug resistance. Clin. Cancer Res. 2009, 15, 6862–6870. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; George, S.; Gelderblom, H.; Schoffski, P.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.K.; Razak, A.A.; Trent, J.; et al. Ripretinib versus sunitinib in gastrointestinal stromal tumor: ctDNA biomarker analysis of the phase 3 INTRIGUE trial. Nat. Med. 2024, 30, 498–506. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Blay, J.Y.; Chi, P.; Jones, R.L.; Serrano, C.; Somaiah, N.; Gelderblom, H.; Zalcberg, J.R.; Reichmann, W.; Sprott, K.; et al. The INSIGHT study: A randomized, Phase III study of ripretinib versus sunitinib for advanced gastrointestinal stromal tumor with KIT exon 11 + 17/18 mutations. Future Oncol. 2024, 20, 1973–1982. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schutz, G.; Thierauch, K.H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef]
- Mross, K.; Frost, A.; Steinbild, S.; Hedbom, S.; Buchert, M.; Fasol, U.; Unger, C.; Kratzschmar, J.; Heinig, R.; Boix, O.; et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin. Cancer Res. 2012, 18, 2658–2667. [Google Scholar] [CrossRef]
- Ferraro, D.; Zalcberg, J. Regorafenib in gastrointestinal stromal tumors: Clinical evidence and place in therapy. Ther. Adv. Med. Oncol. 2014, 6, 222–228. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.K.; Blay, J.Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef]
- Bauer, S.; George, S.; von Mehren, M.; Heinrich, M.C. Early and Next-Generation KIT/PDGFRA Kinase Inhibitors and the Future of Treatment for Advanced Gastrointestinal Stromal Tumor. Front. Oncol. 2021, 11, 672500. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nishida, T.; Kudo, T.; Boku, N.; Honma, Y.; Komatsu, Y.; Nakatsumi, H.; Matsumoto, K.; Onoe, T.; Oki, E.; et al. Regorafenib as second-line therapy for imatinib-resistant gastrointestinal stromal tumor (GIST). J. Clin. Oncol. 2020, 38, 823. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Valverde, C.; Hindi, N.; Vincenzi, B.; Martinez-Trufero, J.; Grignani, G.; Italiano, A.; Lavernia, J.; Vallejo, A.; Tos, P.D.; et al. REGISTRI: Regorafenib in first-line of KIT/PDGFRA wild type metastatic GIST: A collaborative Spanish (GEIS), Italian (ISG) and French Sarcoma Group (FSG) phase II trial. Mol. Cancer 2023, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schoffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 923–934, Correction in Lancet Oncol. 2020, 21, e341. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Kaufman, M.D.; Lu, W.P.; Gupta, A.; Leary, C.B.; Wise, S.C.; Rutkoski, T.J.; Ahn, Y.M.; Al-Ani, G.; Bulfer, S.L.; et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019, 35, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Jones, R.L.; Blay, J.Y.; Gelderblom, H.; George, S.; Schoffski, P.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.K.; Razak, A.A.; et al. Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, 40, 3918–3928. [Google Scholar] [CrossRef]
- Gelderblom, H.; Jones, R.L.; Blay, J.Y.; George, S.; von Mehren, M.; Zalcberg, J.R.; Kang, Y.K.; Razak, A.A.; Trent, J.; Attia, S.; et al. Patient-reported outcomes and tolerability in patients receiving ripretinib versus sunitinib after treatment with imatinib in INTRIGUE, a phase 3, open-label study. Eur. J. Cancer 2023, 192, 113245. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schoffski, P.; Serrano, C.; Kang, Y.K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef]
- Henriques-Abreu, M.; Serrano, C. Avapritinib in unresectable or metastatic gastrointestinal stromal tumor with PDGFRA exon 18 mutation: Safety and efficacy. Expert. Rev. Anticancer. Ther. 2021, 21, 1081–1088. [Google Scholar] [CrossRef]
- Kang, Y.K.; George, S.; Jones, R.L.; Rutkowski, P.; Shen, L.; Mir, O.; Patel, S.; Zhou, Y.; von Mehren, M.; Hohenberger, P.; et al. Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study. J. Clin. Oncol. 2021, 39, 3128–3139. [Google Scholar] [CrossRef]
- Adenis, A.; Blay, J.Y.; Bui-Nguyen, B.; Bouche, O.; Bertucci, F.; Isambert, N.; Bompas, E.; Chaigneau, L.; Domont, J.; Ray-Coquard, I.; et al. Masitinib in advanced gastrointestinal stromal tumor (GIST) after failure of imatinib: A randomized controlled open-label trial. Ann. Oncol. 2014, 25, 1762–1769. [Google Scholar] [CrossRef]
- Abbaspour Babaei, M.; Kamalidehghan, B.; Saleem, M.; Huri, H.Z.; Ahmadipour, F. Receptor tyrosine kinase (c-Kit) inhibitors: A potential therapeutic target in cancer cells. Drug Des. Devel Ther. 2016, 10, 2443–2459. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Griffith, D.; McKinley, A.; Patterson, J.; Presnell, A.; Ramachandran, A.; Debiec-Rychter, M. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin. Cancer Res. 2012, 18, 4375–4384. [Google Scholar] [CrossRef]
- Joensuu, H.; De Braud, F.; Grignagni, G.; De Pas, T.; Spitalieri, G.; Coco, P.; Spreafico, C.; Boselli, S.; Toffalorio, F.; Bono, P.; et al. Vatalanib for metastatic gastrointestinal stromal tumour (GIST) resistant to imatinib: Final results of a phase II study. Br. J. Cancer 2011, 104, 1686–1690. [Google Scholar] [CrossRef]
- Blay, J.Y.; Shen, L.; Kang, Y.K.; Rutkowski, P.; Qin, S.; Nosov, D.; Wan, D.; Trent, J.; Srimuninnimit, V.; Papai, Z.; et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): A randomised phase 3 trial. Lancet Oncol. 2015, 16, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Jones, R.L.; Blay, J.-Y.; George, S.; Schöffski, P.; Mehren, M.v.; Zalcberg, J.R.; Kang, Y.-K.; Razak, A.R.A.; Trent, J.C.; et al. Patient reported outcomes and tolerability in patients receiving ripretinib versus sunitinib after imatinib treatment in INTRIGUE: A phase 3 open-label study. J. Clin. Oncol. 2022, 40, 11541. [Google Scholar] [CrossRef]
- Cauchi, C.; Somaiah, N.; Engstrom, P.F.; Litwin, S.; Lopez, M.; Lee, J.; Davey, M.; Bove, B.; von Mehren, M. Evaluation of nilotinib in advanced GIST previously treated with imatinib and sunitinib. Cancer Chemother. Pharmacol. 2012, 69, 977–982. [Google Scholar] [CrossRef]
- Komatsu, Y.; Doi, T.; Sawaki, A.; Kanda, T.; Yamada, Y.; Kuss, I.; Demetri, G.D.; Nishida, T. Regorafenib for advanced gastrointestinal stromal tumors following imatinib and sunitinib treatment: A subgroup analysis evaluating Japanese patients in the phase III GRID trial. Int. J. Clin. Oncol. 2015, 20, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Westerdijk, K.; Desar, I.M.E.; Steeghs, N.; van der Graaf, W.T.A.; van Erp, N.P.; Dutch, P.; Oncology, G. Imatinib, sunitinib and pazopanib: From flat-fixed dosing towards a pharmacokinetically guided personalized dose. Br. J. Clin. Pharmacol. 2020, 86, 258–273. [Google Scholar] [CrossRef]
- Zheng, S.; Shu, Y.; Lu, Y.; Sun, Y. Chloroquine Combined with Imatinib Overcomes Imatinib Resistance in Gastrointestinal Stromal Tumors by Inhibiting Autophagy via the MAPK/ERK Pathway. Onco Targets Ther. 2020, 13, 6433–6441. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.; Kang, S.Y.; Ahn, S.; Kim, K.M. Peritoneal Seeding Is More Common in Gastric Cancer Patients with FGFR2 Amplification or High Tumor Mutation Burden. Diagnostics 2022, 12, 2355. [Google Scholar] [CrossRef]
- Dermawan, J.K.; Vanderbilt, C.M.; Chang, J.C.; Untch, B.R.; Singer, S.; Chi, P.; Tap, W.D.; Antonescu, C.R. FGFR2::TACC2 fusion as a novel KIT-independent mechanism of targeted therapy failure in a multidrug-resistant gastrointestinal stromal tumor. Genes. Chromosomes Cancer 2022, 61, 412–419. [Google Scholar] [CrossRef]
- Anastassiadis, T.; Deacon, S.W.; Devarajan, K.; Ma, H.; Peterson, J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1039–1045. [Google Scholar] [CrossRef]
- Le Cesne, A.; Blay, J.Y.; Bui, B.N.; Bouche, O.; Adenis, A.; Domont, J.; Cioffi, A.; Ray-Coquard, I.; Lassau, N.; Bonvalot, S.; et al. Phase II study of oral masitinib mesilate in imatinib-naive patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST). Eur. J. Cancer 2010, 46, 1344–1351. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Kefeli, U.; Benekli, M.; Sevinc, A.; Yildiz, R.; Kaplan, M.A.; Ciltas, A.; Balakan, O.; Isikdogan, A.; Coskun, U.; Dane, F.; et al. Efficacy of sorafenib in patients with gastrointestinal stromal tumors in the third- or fourth-line treatment: A retrospective multicenter experience. Oncol. Lett. 2013, 6, 605–611. [Google Scholar] [CrossRef]
- Park, S.H.; Ryu, M.H.; Ryoo, B.Y.; Im, S.A.; Kwon, H.C.; Lee, S.S.; Park, S.R.; Kang, B.Y.; Kang, Y.K. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: A phase II study of Korean gastrointestinal stromal tumors study group. Invest. New Drugs 2012, 30, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Blay, J.Y.; Comandone, A.; Martin-Broto, J.; Fumagalli, E.; Grignani, G.; Del Muro, X.G.; Adenis, A.; Valverde, C.; Pousa, A.L.; et al. Dovitinib in patients with gastrointestinal stromal tumour refractory and/or intolerant to imatinib. Br. J. Cancer 2017, 117, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Ganjoo, K.N.; Villalobos, V.M.; Kamaya, A.; Fisher, G.A.; Butrynski, J.E.; Morgan, J.A.; Wagner, A.J.; D’Adamo, D.; McMillan, A.; Demetri, G.D.; et al. A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann. Oncol. 2014, 25, 236–240. [Google Scholar] [CrossRef]
- Nascimento, M.; Moura, S.; Parra, L.; Vasconcellos, V.; Costa, G.; Leite, D.; Dias, M.; Fernandes, T.V.A.; Hoelz, L.; Pimentel, L.; et al. Ponatinib: A Review of the History of Medicinal Chemistry behind Its Development. Pharmaceuticals 2024, 17, 1361. [Google Scholar] [CrossRef]
- Schoffski, P.; Mir, O.; Kasper, B.; Papai, Z.; Blay, J.Y.; Italiano, A.; Benson, C.; Kopeckova, K.; Ali, N.; Dileo, P.; et al. Activity and safety of the multi-target tyrosine kinase inhibitor cabozantinib in patients with metastatic gastrointestinal stromal tumour after treatment with imatinib and sunitinib: European Organisation for Research and Treatment of Cancer phase II trial 1317 ’CaboGIST’. Eur. J. Cancer 2020, 134, 62–74. [Google Scholar] [CrossRef]
- Markowitz, J.N.; Fancher, K.M. Cabozantinib: A Multitargeted Oral Tyrosine Kinase Inhibitor. Pharmacotherapy 2018, 38, 357–369. [Google Scholar] [CrossRef] [PubMed]
- George, S.; von Mehren, M.; Fletcher, J.A.; Sun, J.; Zhang, S.; Pritchard, J.R.; Hodgson, J.G.; Kerstein, D.; Rivera, V.M.; Haluska, F.G.; et al. Phase II Study of Ponatinib in Advanced Gastrointestinal Stromal Tumors: Efficacy, Safety, and Impact of Liquid Biopsy and Other Biomarkers. Clin. Cancer Res. 2022, 28, 1268–1276. [Google Scholar] [CrossRef]
- Glod, J.; Arnaldez, F.I.; Wiener, L.; Spencer, M.; Killian, J.K.; Meltzer, P.; Dombi, E.; Derse-Anthony, C.; Derdak, J.; Srinivasan, R.; et al. A Phase II Trial of Vandetanib in Children and Adults with Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumor. Clin. Cancer Res. 2019, 25, 6302–6308. [Google Scholar] [CrossRef]
- Lindauer, M.; Hochhaus, A. Dasatinib. Recent. Results Cancer Res. 2010, 184, 83–102. [Google Scholar] [CrossRef]
- Zeng, C.; Zhu, L.; Jia, X.; Pang, Y.; Li, Z.; Lu, X.; Xie, F.; Duan, L.; Wang, Y. Spectrum of activity of dasatinib against mutant KIT kinases associated with drug-sensitive and drug-resistant gastrointestinal stromal tumors. Gastric Cancer 2020, 23, 837–847. [Google Scholar] [CrossRef]
- Montemurro, M.; Cioffi, A.; Domont, J.; Rutkowski, P.; Roth, A.D.; von Moos, R.; Inauen, R.; Toulmonde, M.; Burkhard, R.O.; Knuesli, C.; et al. Long-term outcome of dasatinib first-line treatment in gastrointestinal stromal tumor: A multicenter, 2-stage phase 2 trial (Swiss Group for Clinical Cancer Research 56/07). Cancer 2018, 124, 1449–1454. [Google Scholar] [CrossRef]
- Wood, J.M.; Bold, G.; Buchdunger, E.; Cozens, R.; Ferrari, S.; Frei, J.; Hofmann, F.; Mestan, J.; Mett, H.; O’Reilly, T.; et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res 2000, 60, 2178–2189. [Google Scholar] [PubMed]
- Zhou, Y.; Zhang, X.; Wu, X.; Zhou, Y.; Zhang, B.; Liu, X.; Wu, X.; Li, Y.; Shen, L.; Li, J. A prospective multicenter phase II study on the efficacy and safety of dasatinib in the treatment of metastatic gastrointestinal stromal tumors failed by imatinib and sunitinib and analysis of NGS in peripheral blood. Cancer Med. 2020, 9, 6225–6233. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Mukohara, T.; Kuwata, T.; Sunami, K.; Naito, Y. Efficacy of Trametinib in Neurofibromatosis Type 1-Associated Gastrointestinal Stromal Tumors: A Case Report. JCO Precis. Oncol. 2024, 8, e2300649. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of Rogaratinib in Patients with Sarcomas with FGFR Alterations Including SDH-Deficient GIST. ClinicalTrials.gov Identifier: NCT04595747. Available online: https://clinicaltrials.gov/ct2/show/NCT04595747 (accessed on 27 August 2025).
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Study of KIN-3248 in Patients with Advanced Solid Tumors Harboring FGFR Alterations. ClinicalTrials.gov Identifier: NCT05136028. Available online: https://clinicaltrials.gov/ct2/show/NCT05136028 (accessed on 27 August 2025).
- ClinicalTrials.gov. A Study of Erdafitinib in Participants with Advanced Solid Tumors and FGFR Gene Alterations. ClinicalTrials.gov Identifier: NCT04083976.. Available online: https://clinicaltrials.gov/ct2/show/NCT04083976 (accessed on 27 August 2025).
- Lai, S.W.; Bamodu, O.A.; Chen, J.H.; Wu, A.T.; Lee, W.H.; Chao, T.Y.; Yeh, C.T. Targeted PARP inhibition combined with FGFR1 blockade is synthetically lethal to malignant cells. Mol. Cancer Ther. 2020, 19, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Castroviejo-Bermejo, M.; Gutiérrez-Enríquez, S.; Llop-Guevara, A.; Ibrahim, Y.H.; Gris-Oliver, A.; Bonache, S.; Morancho, B.; Bruna, A.; Rueda, O.M.; et al. RAD51 foci as a biomarker of PARP inhibitor response and synthetic lethality with FGFR inhibition. Cancers 2020, 12, 2119. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; von Mehren, M.; Blanke, C.D.; Van den Abbeele, A.D.; Eisenberg, B.; Roberts, P.J.; Heinrich, M.C.; Tuveson, D.A.; Singer, S.; Janicek, M.; et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002, 347, 472–480. [Google Scholar] [CrossRef] [PubMed]
- MetaGIST Trial Investigators. Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1640 patients. J. Clin. Oncol. 2010, 28, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Serrano, C.; Heinrich, M.C.; Zalcberg, J.; Bauer, S.; Gelderblom, H.; Schöffski, P.; Jones, R.L.; Attia, S.; D’Amato, G.; et al. Ripretinib in patients with advanced gastrointestinal stromal tumors (INVICTUS): A phase III, randomised, placebo-controlled trial. Lancet Oncol. 2020, 21, 923–934. [Google Scholar] [CrossRef] [PubMed]
| Pathway | Function | Reference |
|---|---|---|
| MAPK/ERK | Promotes cell proliferation and differentiation by activating transcription factors such as c-Jun and c-Fos | [20] |
| PI3K/AKT | Enhances cell survival and inhibits apoptosis through regulation of BCL-2 family proteins | [21] |
| STAT | Modulates gene expression related to cell growth and immune responses | [22] |
| PLCγ | Regulates calcium signaling and cytoskeletal dynamics, contributing to cell migration | [23] |
| Type of Alteration | Approximate Frequency in GISTs | Molecular Mechanism | Clinical Significance |
|---|---|---|---|
| FGFR2 fusions (e.g., FGFR2::TACC2, FGFR2::BICC1) | <1% [9,31,33] | Retain FGFR2 kinase domain → constitutive dimerization and activation of MAPK/ERK and PI3K/AKT pathways | Drive oncogenesis and resistance to TKIs (e.g., imatinib); generally mutually exclusive with KIT/PDGFRA mutations |
| FGFR2 amplifications | 1–2% [2,35,36] | Increased gene copy number → receptor overexpression and enhanced downstream signaling | Associated with higher tumor grade, aggressive behavior, and TKI resistance; potential biomarker for FGFR inhibitor sensitivity |
| FGFR2 point mutations | <0.1% [37,38] | Rare missense mutations (reported in other cancers, not recurrent in GISTs) | No confirmed clinical significance in GIST; uncertain therapeutic relevance |
| Polymorphisms (e.g., SNPs such as rs2981582) | Not established in GIST; reported in breast and gastric cancer [37] | Germline variants linked to cancer susceptibility in other malignancies | No proven association with GIST incidence or outcome; requires further investigation |
| Mechanism | Description | References |
|---|---|---|
| Gene Fusions | FGFR2::TACC2 fusions, which retain the FGFR2 kinase domain, result in constitutive dimerization and autophosphorylation, independent of fibroblast growth factor (FGF) ligands. This leads to the sustained activation of downstream pathways, thereby enhancing tumor growth and tyrosine kinase inhibitor resistance. | [9,31,40] |
| Gene Amplifications | FGFR2 amplifications increase receptor density on the cell membrane, amplifying signaling even with low ligand levels. Overexpression of FGF ligands (e.g., FGF7, FGF10) further enhances FGFR2 activation in amplified cases. | [36,41] |
| Ligand Overexpression | In some GISTs, the autocrine or paracrine overexpression of FGF7 and FGF10, which is specific to the FGFR2b isoform, drives pathway activation, particularly in mesenchymal-derived tumors. | [42] |
| Pathway | Key Effectors | Functional Outcome | Evidence in GIST | Key Refs. |
|---|---|---|---|---|
| MAPK/ERK | RAS → RAF → MEK → ERK | Proliferation, transcriptional reprogramming | Phospho ERK high in FGFR2 fusion tumours; bypasses imatinib blockade | [20,40,43] |
| PI3K/AKT | PI3K → PDK1 → AKT → mTOR | Survival, protein synthesis, chemo resistance | AKT phosphorylation variable; synergistic lethality with MEK inhibitors | [3,21,44] |
| PLCγ | PLCγ → IP3/DAG → Ca2+/PKC | Cytoskeletal remodelling, migration | Detected in cell lines only; not validated in patient tissue | [22] |
| JAK/STAT | JAK → STAT1/3 | Immune evasion, cytokine feed forward | Inferred from transcriptomic signatures; no phospho data available | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Wang, X.; Shou, C.; Liu, X. Molecular Mechanisms and Clinical Implications of Fibroblast Growth Factor Receptor 2 Signaling in Gastrointestinal Stromal Tumors. Curr. Issues Mol. Biol. 2025, 47, 822. https://doi.org/10.3390/cimb47100822
Hong Y, Wang X, Shou C, Liu X. Molecular Mechanisms and Clinical Implications of Fibroblast Growth Factor Receptor 2 Signaling in Gastrointestinal Stromal Tumors. Current Issues in Molecular Biology. 2025; 47(10):822. https://doi.org/10.3390/cimb47100822
Chicago/Turabian StyleHong, Yanyun, Xiaodong Wang, Chunhui Shou, and Xiaosun Liu. 2025. "Molecular Mechanisms and Clinical Implications of Fibroblast Growth Factor Receptor 2 Signaling in Gastrointestinal Stromal Tumors" Current Issues in Molecular Biology 47, no. 10: 822. https://doi.org/10.3390/cimb47100822
APA StyleHong, Y., Wang, X., Shou, C., & Liu, X. (2025). Molecular Mechanisms and Clinical Implications of Fibroblast Growth Factor Receptor 2 Signaling in Gastrointestinal Stromal Tumors. Current Issues in Molecular Biology, 47(10), 822. https://doi.org/10.3390/cimb47100822






