Effects of a Natural Polyherbal Extract on Alleviating Scopolamine-Induced Memory Deficits in C57BL/6 Mice via Enhancing Cholinergic Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Treatment Schedule in C57BL/6 Mice
2.2. Components of NPX
2.3. Animals and Treatments
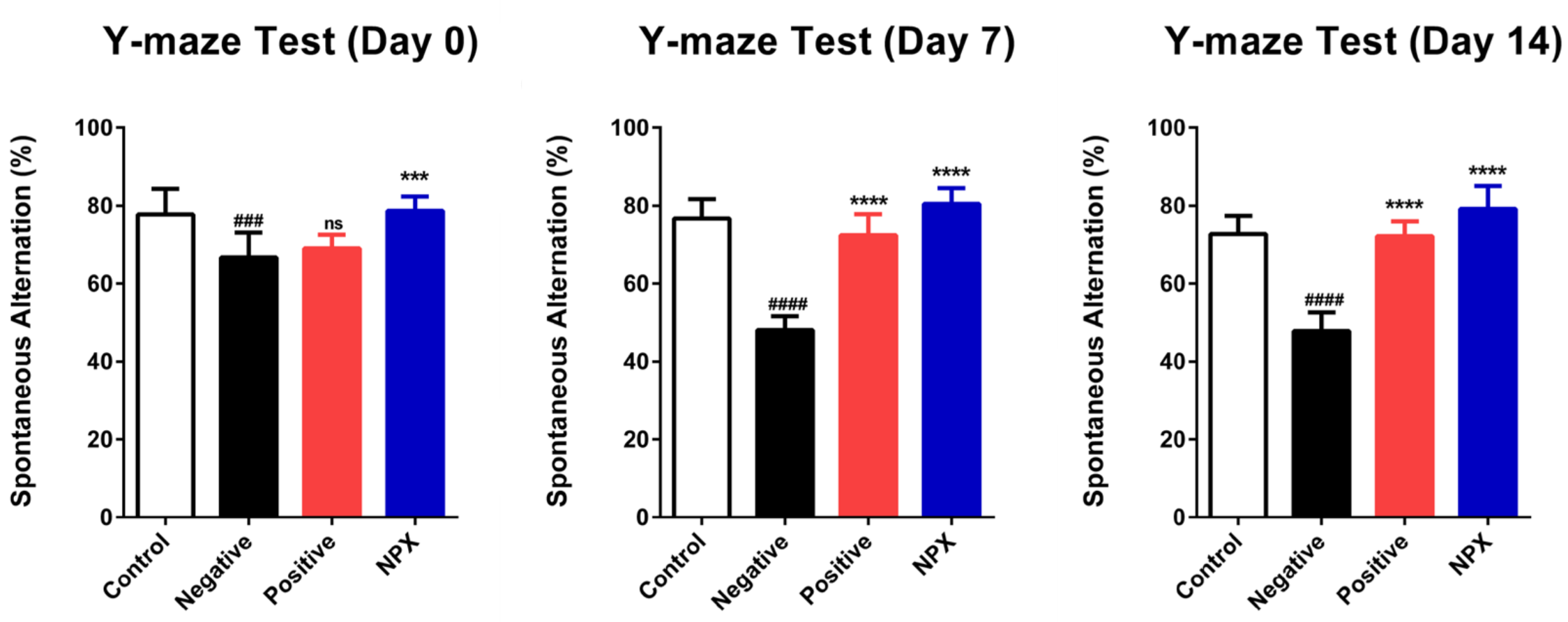
2.4. Y-Maze Test
2.5. Serum Measurement Methods
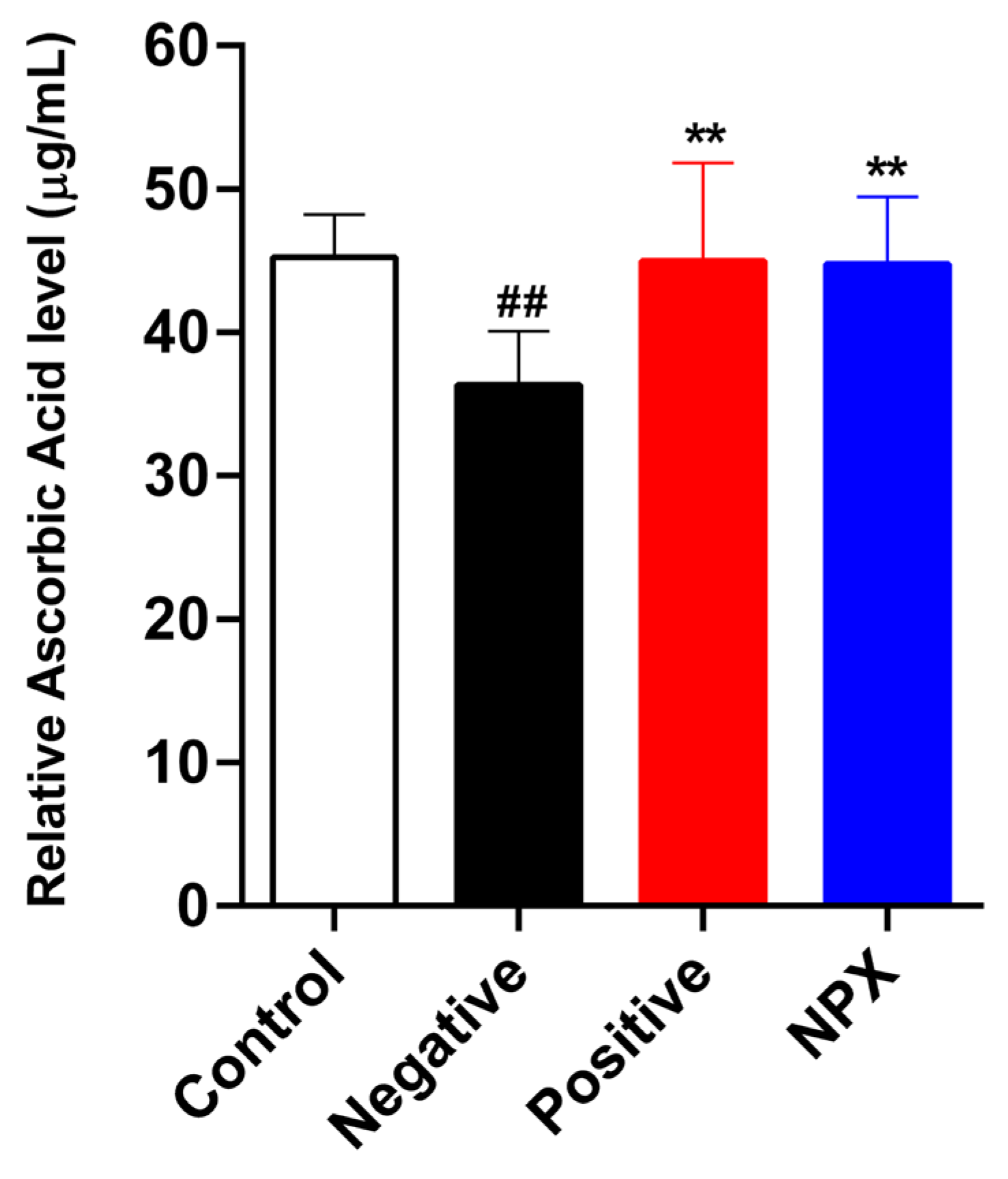
2.5.1. Serum Ascorbic Acid Concentration Analysis
2.5.2. Serum AChE Activity Analysis
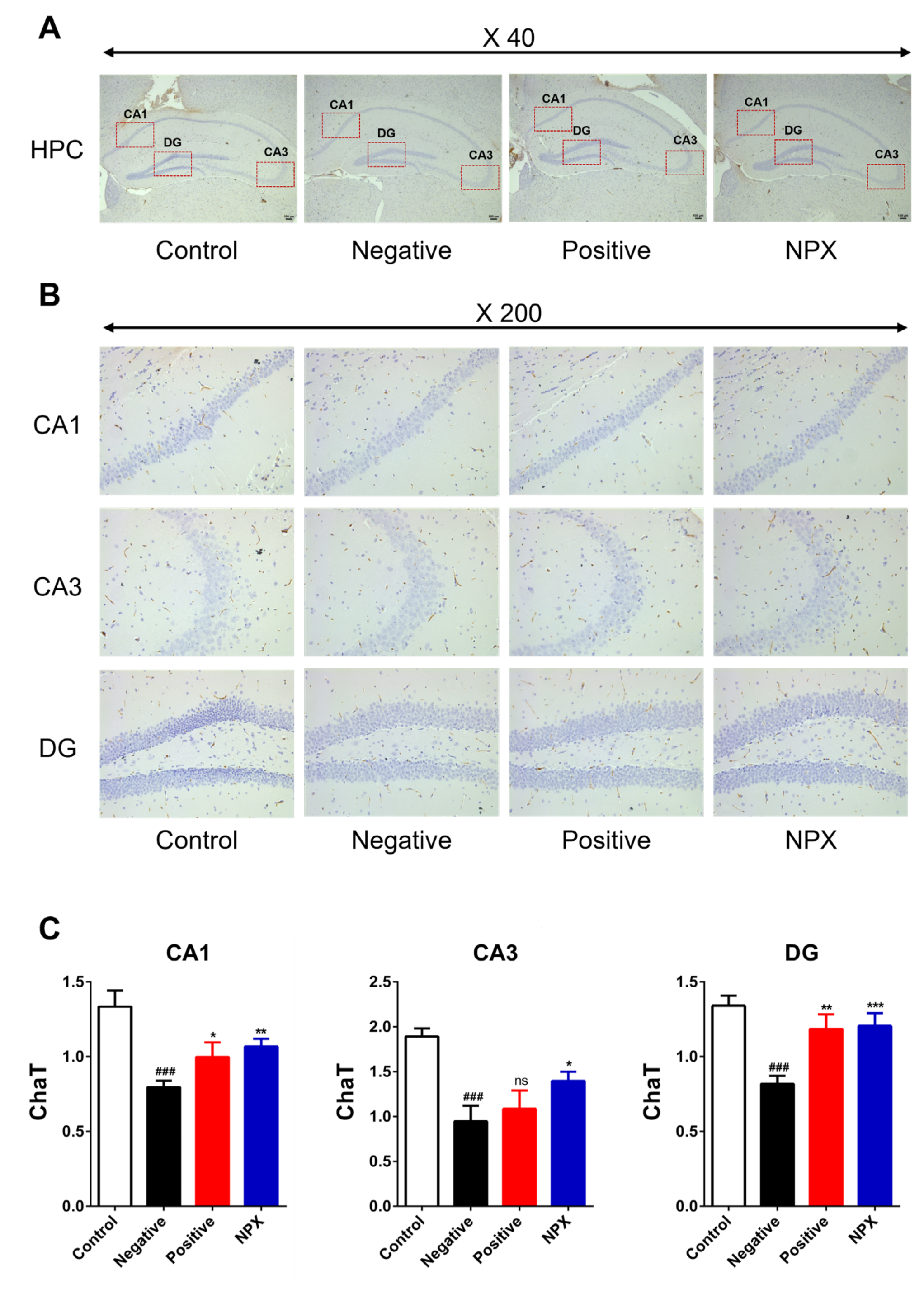
2.6. Immunohistochemistry Staining Method
2.7. Statistical Analysis
3. Results
3.1. Improving Cognitive Function by Increasing Y-Maze Scores
3.2. Measuring Serum
3.2.1. Ascorbic Acid Concentration
3.2.2. Acetylcholinesterase Activity
3.3. Immunohistochemistry Staining
3.3.1. Choline Acetyltransferase (ChaT)
3.3.2. Amyloid β-Peptide (Aβ)
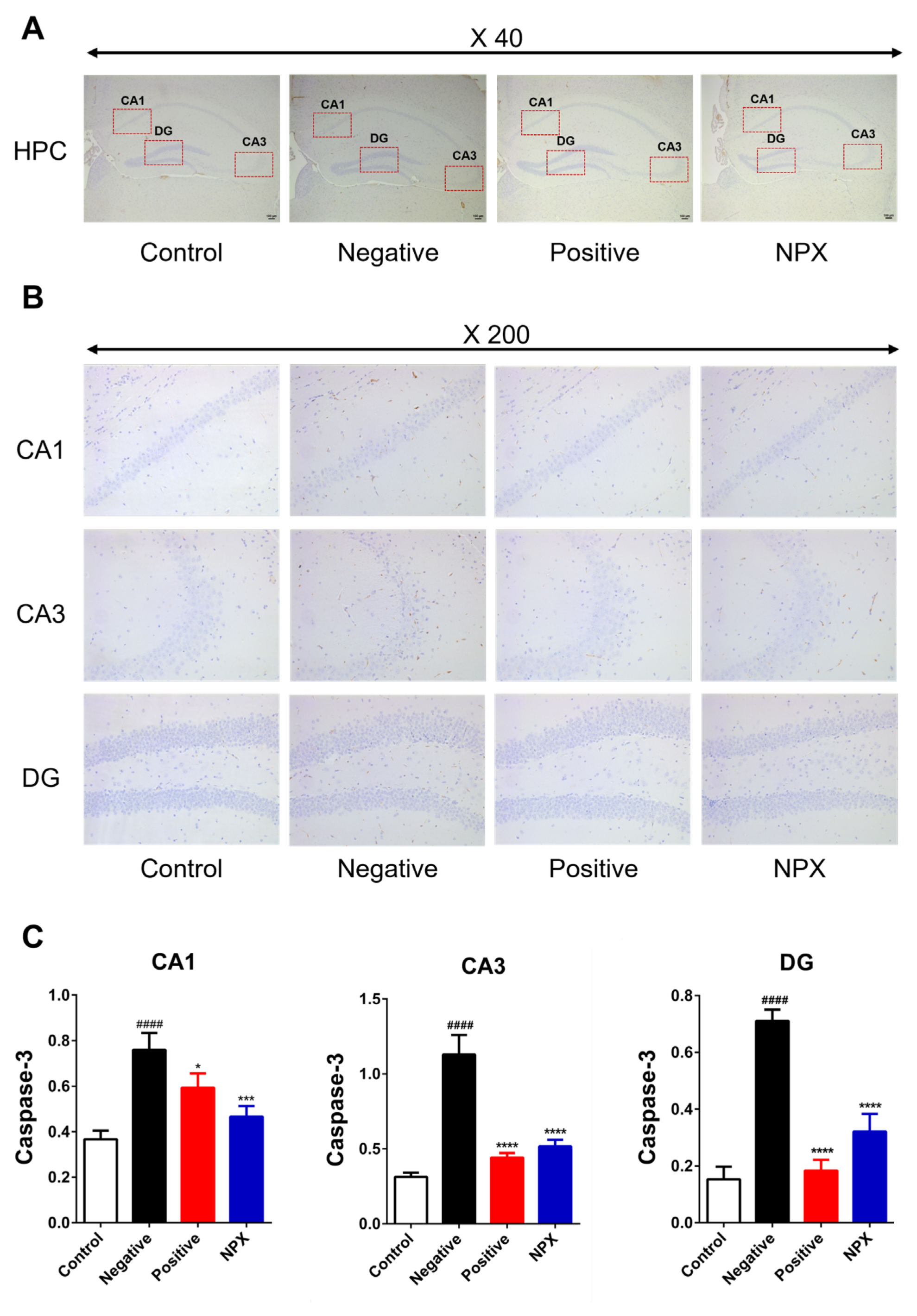
3.3.3. Caspase-3 (Cas-3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Cas-3 | Caspase-3 |
| ChaT | Choline acetyltransferase |
| AChE | Acetylcholinesterase |
| ACh | Acetylcholine |
| HPC | Hippocampus |
| Acetyl-CoA | Acetyl-coenzyme A |
| Aβ | Beta-amyloid |
| NPX | Natural polyherbal extract |
| NC | Negative control |
| PC | Positive control |
| RT | Room temperature |
References
- 2025 Alzheimer’s disease facts and figures. Alzheimers Dement 2025, 21, e70235. [CrossRef]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease; Professional Edition E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2014. [Google Scholar]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. The Cholinergic System, the Adrenergic System and the Neuropathology of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1273. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- E Hasselmo, M.; Sarter, M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology 2010, 36, 52–73. [Google Scholar] [CrossRef]
- Falsafi, S.K.; Deli, A.; Höger, H.; Pollak, A.; Lubec, G. Scopolamine Administration Modulates Muscarinic, Nicotinic and NMDA Receptor Systems. PLoS ONE 2012, 7, e32082. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Mewaldt, S.P. Effects of diazepam and scopolamine on storage, retrieval and organizational processes in memory. Psychopharmacology 1975, 44, 257–262. [Google Scholar] [CrossRef]
- Kloog, Y.; Sokolovsky, M. Studies on muscarinic acetylcholine receptors from mouse brain: Characterization of the interaction with antagonists. Brain Res. 1978, 144, 31–48. [Google Scholar] [CrossRef]
- Safar, M.M.; Arab, H.H.; Rizk, S.M.; El-Maraghy, S.A. Bone Marrow-Derived Endothelial Progenitor Cells Protect Against Scopolamine-Induced Alzheimer-Like Pathological Aberrations. Mol. Neurobiol. 2014, 53, 1403–1418. [Google Scholar] [CrossRef]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a Toxin-Induced Experimental Model, Used for Research in Alzheimer’s Disease. CNS Neurol. Disord. - Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Lalonde, R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef]
- Cleal, M.; Fontana, B.D.; Ranson, D.C.; McBride, S.D.; Swinny, J.D.; Redhead, E.S.; Parker, M.O. The Free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behav. Res. Methods 2020, 53, 536–557. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. In Pre-Clinical Models: Techniques and Protocols, Methods in Molecular BIOLOGY; Guest, P.C., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 105–111. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-Q.; Wu, D.-W.; Zhang, C.-X.; Yan, R.; Yang, C.; Rong, C.-P.; Zhang, L.; Chang, X.; Su, R.-Y.; Zhang, S.-J.; et al. Bushen-Yizhi formula ameliorates cognition deficits and attenuates oxidative stress-related neuronal apoptosis in scopolamine-induced senescence in mice. Int. J. Mol. Med. 2014, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yang, Y.; Cui, Z.; Zheng, L.; Zeng, Z.; Zhang, H. Novel peptide VIP-TAT with higher affinity for PAC1 inhibited scopolamine induced amnesia. Peptides 2014, 60, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Li, X.; Zheng, K.; Chen, H.; Li, W. Ginsenoside Re Regulates Oxidative Stress through the PI3K/Akt/Nrf2 Signaling Pathway in Mice with Scopolamine-Induced Memory Impairments. Curr. Issues Mol. Biol. 2024, 46, 11359–11374. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 2–7. [Google Scholar] [CrossRef]
- Monacelli, F.; Acquarone, F.M.E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer’s Disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef]
- Crismon, M.L. Tacrine: First Drug Approved for Alzheimer’s Disease. Ann. Pharmacother. 1994, 28, 744–751. [Google Scholar] [CrossRef]
- Mitra, S.; Muni, M.; Shawon, N.J.; Das, R.; Bin Emran, T.; Sharma, R.; Chandran, D.; Islam, F.; Hossain, J.; Safi, S.Z.; et al. Tacrine Derivatives in Neurological Disorders: Focus on Molecular Mechanisms and Neurotherapeutic Potential. Oxidative Med. Cell. Longev. 2022, 2022, 7252882. [Google Scholar] [CrossRef]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD005593. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Holubova, K.; Nepovimova, E.; Krusek, J.; Kaniakova, M.; Korabecny, J.; Vyklicky, L.; Kuca, K.; Stuchlik, A.; Ricny, J.; et al. The pharmacology of tacrine at N -methyl- d -aspartate receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ríos, C.d.L.; Marco-Contelles, J. Tacrines for Alzheimer’s disease therapy. III. The PyridoTacrines. Eur. J. Med. Chem. 2019, 166, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kara, S.; Polat, S.; Akillioglu, K.; Saker, D.; Evlice, A.T.; Sencar, L.; Aydın, U.F.; Polat, S. Effects of TGF-β1 on Aβ-40 and α-β-γ secretase expression in hippocampus and prefrontal cortex in experimental Alzheimer’s disease. Behav. Brain Res. 2025, 482, 115432. [Google Scholar] [CrossRef]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimer’s Dis. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, E.-B.; Jang, H.-H.; Cha, Y.-S.; Park, Y.-S.; Lee, S.-H. Allium hookeri Extracts Improve Scopolamine-Induced Cognitive Impairment via Activation of the Cholinergic System and Anti-Neuroinflammation in Mice. Nutrients 2021, 13, 2890. [Google Scholar] [CrossRef]
- Aydin, E.; Hritcu, L.; Dogan, G.; Hayta, S.; Bagci, E. The Effects of Inhaled Pimpinella peregrina Essential Oil on Scopolamine-Induced Memory Impairment, Anxiety, and Depression in Laboratory Rats. Mol. Neurobiol. 2016, 53, 6557–6567. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, Y.; Gao, C.; Li, J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017, 490, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, W.; Niu, L.; Luo, X.; Li, J.; Zhang, Y.; Liu, H.; He, J.; Wan, W. Amelioration of scopolamine-induced learning and memory impairment by the TRPV4 inhibitor HC067047 in ICR mice. Neurosci. Lett. 2022, 767, 136209. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef]
- Harrison, F.; Hosseini, A.; Dawes, S.; Weaver, S.; May, J. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav. Brain Res. 2009, 205, 550–558. [Google Scholar] [CrossRef]
- Joseph, E.; Villalobos-Acosta, D.M.Á.; Torres-Ramos, M.A.; Farfán-García, E.D.; Gómez-López, M.; Miliar-García, Á.; Fragoso-Vázquez, M.J.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Neuroprotective Effects of Apocynin and Galantamine During the Chronic Administration of Scopolamine in an Alzheimer’s Disease Model. J. Mol. Neurosci. 2019, 70, 180–193. [Google Scholar] [CrossRef]
- Pezze, M.-A.; Marshall, H.J.; Cassaday, H.J. Scopolamine Impairs Appetitive But Not Aversive Trace Conditioning: Role of the Medial Prefrontal Cortex. J. Neurosci. 2017, 37, 6289–6298. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Parrish, W.R.; Rosas-Ballina, M.; Ochani, M.; Puerta, M.; Ochani, K.; Chavan, S.; Al-Abed, Y.; Tracey, K.J. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain, Behav. Immun. 2009, 23, 41–45. [Google Scholar] [CrossRef]
- Ishola, I.O.; Tota, S.; Adeyemi, O.O.; Agbaje, E.O.; Narender, T.; Shukla, R. Protective effect of Cnestis ferruginea and its active constituent on scopolamine-induced memory impairment in mice: A behavioral and biochemical study. Pharm. Biol. 2013, 51, 825–835. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.; Armstrong, E.; Moses, A.; Fahlman, R.; Koosha, H.; Yager, J.Y. Broccoli, Kale, and Radish Sprouts: Key Phytochemical Constituents and DPPH Free Radical Scavenging Activity. Molecules 2023, 28, 4266. [Google Scholar] [CrossRef]
- Moreno, D.; Carvajal, M.; López-Berenguer, C.; García-Viguera, C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J. Pharm. Biomed. Anal. 2006, 41, 1508–1522. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, J.-H.; Oh, J.-W.; Shin, G.-H.; Lee, J.S.; Cho, J.-H.; Park, J.-J.; Lim, J.-H.; Lee, O.-H. Antioxidant and Anti-adipogenic Effects of Kohlrabi and Radish Sprout Extracts. Korean J. Food Sci. Technol. 2014, 46, 531–537. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, J.; Zhang, H.; Liu, Y.; Kan, M.; Xiu, Z.; Chen, X.; Lan, X.; Li, X.; Shi, X.; et al. Ginsenoside Compound K Regulates Amyloid β via the Nrf2/Keap1 Signaling Pathway in Mice with Scopolamine Hydrobromide-Induced Memory Impairments. J. Mol. Neurosci. 2018, 67, 62–71. [Google Scholar] [CrossRef]
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng berry aqueous extract prevents scopolamine-induced memory impairment in mice. Exp. Ther. Med. 2019, 18, 4388–4396. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A Potential Neuroprotective Agent. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Yin, J.; Zhang, Y.; Huang, R.; Lou, Y.; Jiang, H.; Sun, L.; Jia, J.; Zeng, X. Neuroprotective Mechanisms of Ginsenoside Rb1 in Central Nervous System Diseases. Front. Pharmacol. 2022, 13, 914352. [Google Scholar] [CrossRef]
- Zhang, M.; Niu, H.; Li, Q.; Jiao, L.; Li, H.; Wu, W. Active Compounds of Panax ginseng in the Improvement of Alzheimer’s Disease and Application of Spatial Metabolomics. Pharmaceuticals 2023, 17, 38. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Park, S.-B.; Yu, D.-Y.; Cho, J.-Y.; Lee, W.-W.; Park, M.-R.; Lee, J.-W.; Jeon, Y.-D. Neuroprotective Effect of the Mixture of Gastrodiae elata and Glycyrrhizae uralensis In Vitro. Appl. Sci. 2022, 13, 190. [Google Scholar] [CrossRef]
- Tan, W.; Qi, L.; Hu, X.; Tan, Z. Research progress in traditional Chinese medicine in the treatment of Alzheimer’s disease and related dementias. Front. Pharmacol. 2022, 13, 921794. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, H.; Zhou, X.; Yang, W.; Lin, Y. Pharmacological effects of natural medicine ginsenosides against Alzheimer’s disease. Front. Pharmacol. 2022, 13, 952332. [Google Scholar] [CrossRef]
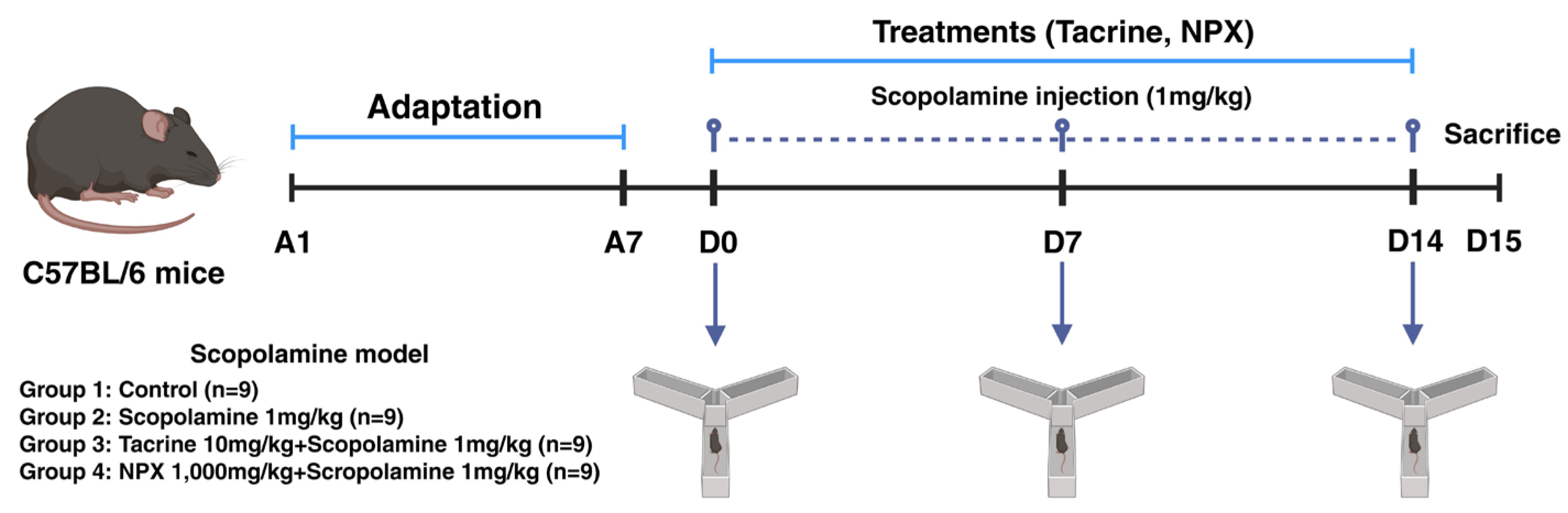


| Group No. | Designation | Treatment | n |
|---|---|---|---|
| Group 1 | Con | Saline | 9 |
| Group 2 | NC | SCP 1 mg/kg | 9 |
| Group 3 | PC | SCP 1 mg/kg + Tacrine 10 mg/kg | 9 |
| Group 4 | NPX | SCP 1 mg/kg + NPX 1000 mg/kg | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Kwon, M.H.; Jeong, M.; Kim, Y.; Yoon, H.-G.; Ju, Y.; Hyun, K.-Y.; Choi, G.-E. Effects of a Natural Polyherbal Extract on Alleviating Scopolamine-Induced Memory Deficits in C57BL/6 Mice via Enhancing Cholinergic Function. Curr. Issues Mol. Biol. 2025, 47, 817. https://doi.org/10.3390/cimb47100817
Kwon H, Kwon MH, Jeong M, Kim Y, Yoon H-G, Ju Y, Hyun K-Y, Choi G-E. Effects of a Natural Polyherbal Extract on Alleviating Scopolamine-Induced Memory Deficits in C57BL/6 Mice via Enhancing Cholinergic Function. Current Issues in Molecular Biology. 2025; 47(10):817. https://doi.org/10.3390/cimb47100817
Chicago/Turabian StyleKwon, Hyeokjin, Min Ho Kwon, Myeongguk Jeong, Yeeun Kim, Hae-Gyung Yoon, Yeongdon Ju, Kyung-Yae Hyun, and Go-Eun Choi. 2025. "Effects of a Natural Polyherbal Extract on Alleviating Scopolamine-Induced Memory Deficits in C57BL/6 Mice via Enhancing Cholinergic Function" Current Issues in Molecular Biology 47, no. 10: 817. https://doi.org/10.3390/cimb47100817
APA StyleKwon, H., Kwon, M. H., Jeong, M., Kim, Y., Yoon, H.-G., Ju, Y., Hyun, K.-Y., & Choi, G.-E. (2025). Effects of a Natural Polyherbal Extract on Alleviating Scopolamine-Induced Memory Deficits in C57BL/6 Mice via Enhancing Cholinergic Function. Current Issues in Molecular Biology, 47(10), 817. https://doi.org/10.3390/cimb47100817







