Reticuline and Coclaurine Exhibit Vitamin D Receptor-Dependent Anticancer and Pro-Apoptotic Activities in the Colorectal Cancer Cell Line HCT116
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Establishment of the CRC-VDR/Knockout Cell Line (HCT116-VDR/KO)
2.3. Cell Culture and Treatment
2.4. Immunofluorescence Staining
2.5. Cell Growth Rate
2.6. Scratch-Wound-Healing Assay
2.7. MTT Assay
2.8. Flow Cytometry
2.9. Preparation of Cell Lysates and Western Blot Analysis
2.10. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.11. Molecular Docking
2.12. Statistical Analysis
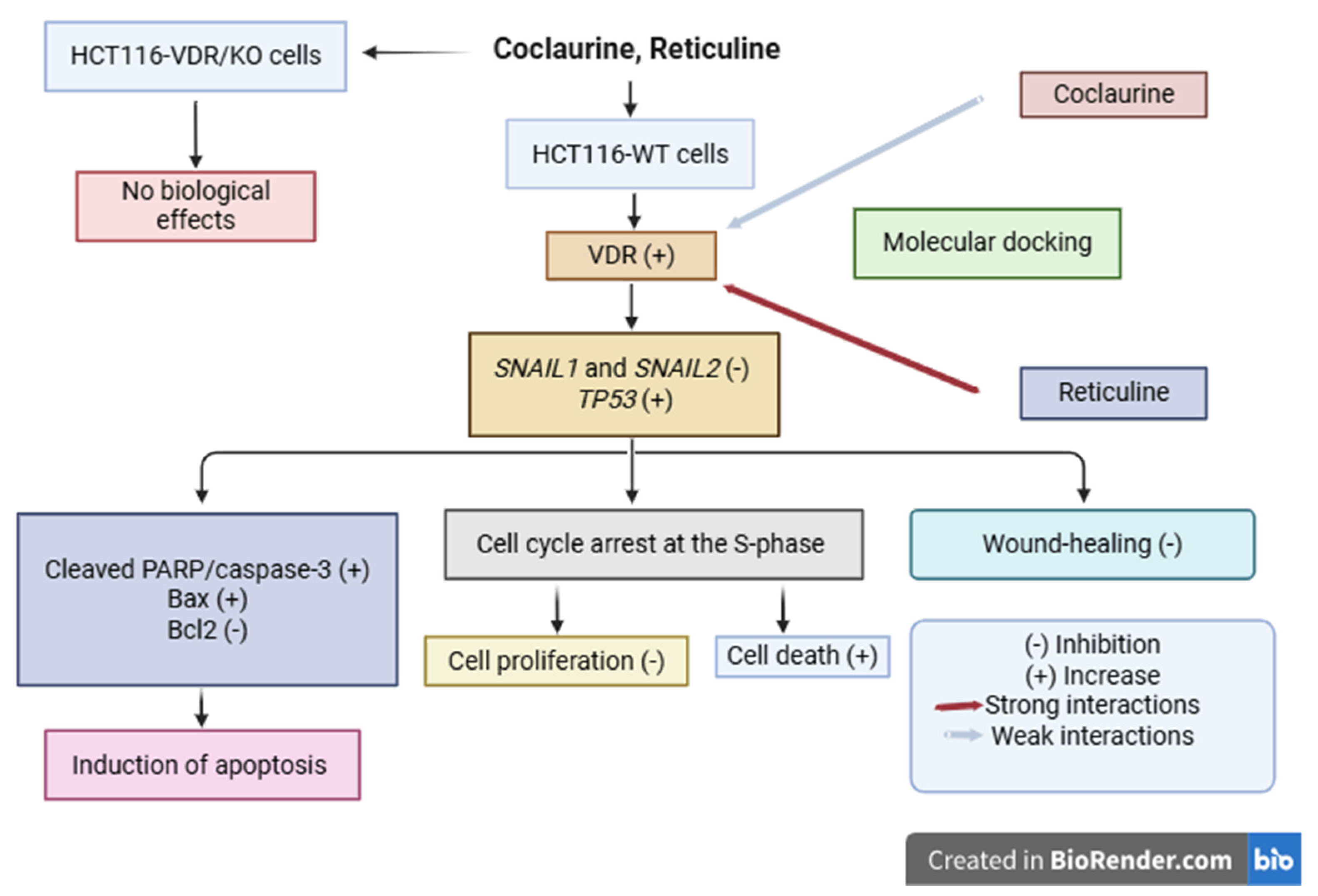
3. Results
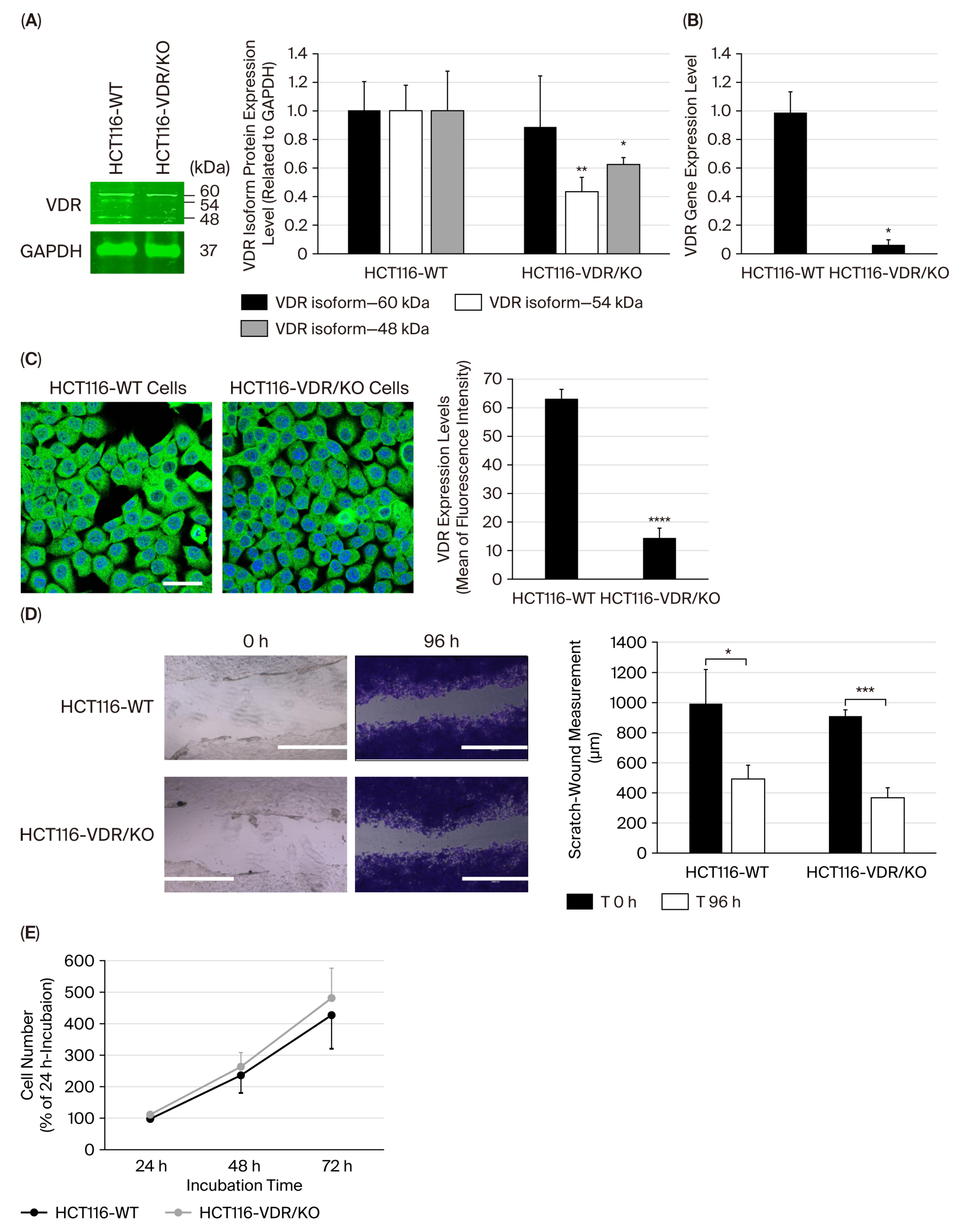
3.1. Loss of VDR Expression Level in CRC HCT116-VDR/KO Cells and Its Functional Impact
3.2. Coclaurine and Reticuline Decrease the CRC Cell Viability Through VDR
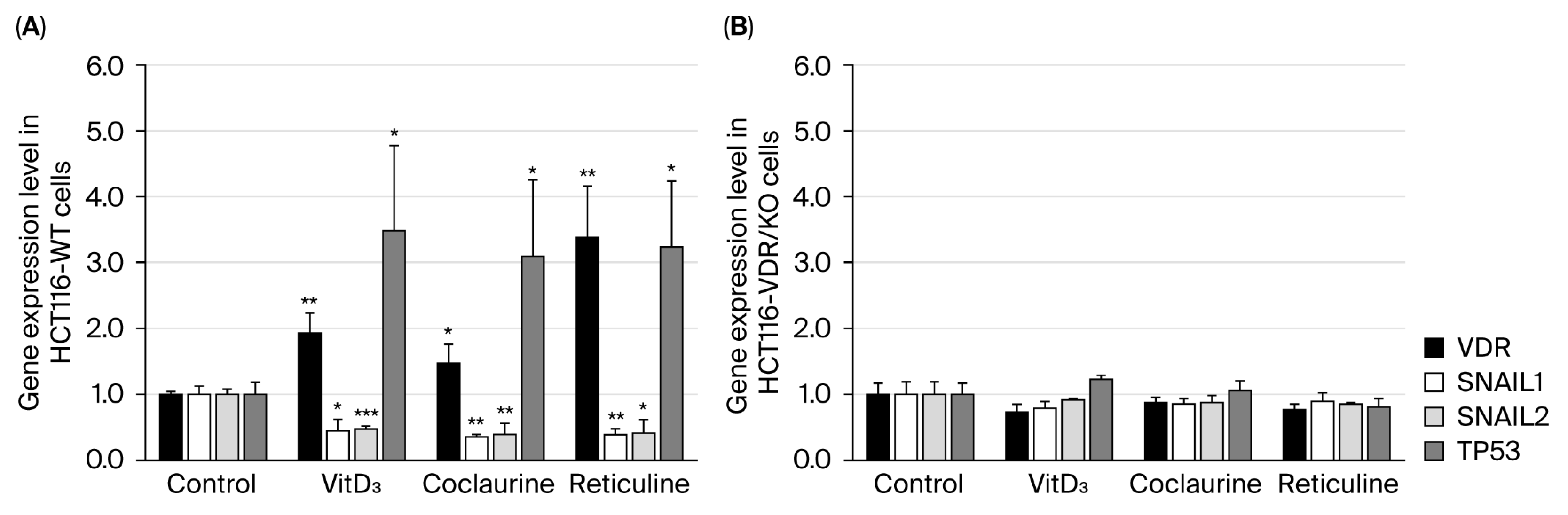
3.3. Coclaurine and Reticuline Increase Nuclear VDR Expression, Upregulate VDR and TP53, and Downregulate SNAIL in HCT116-WT Cells but Not in HCT116-VDR/KO Cells
3.4. Coclaurine and Reticuline Reduce the CRC Wound-Healing Process Through VDR
3.5. Coclaurine and Reticuline Induce Late Apoptosis and Modulate Apoptosis-Related Proteins in CRC Cells Through VDR
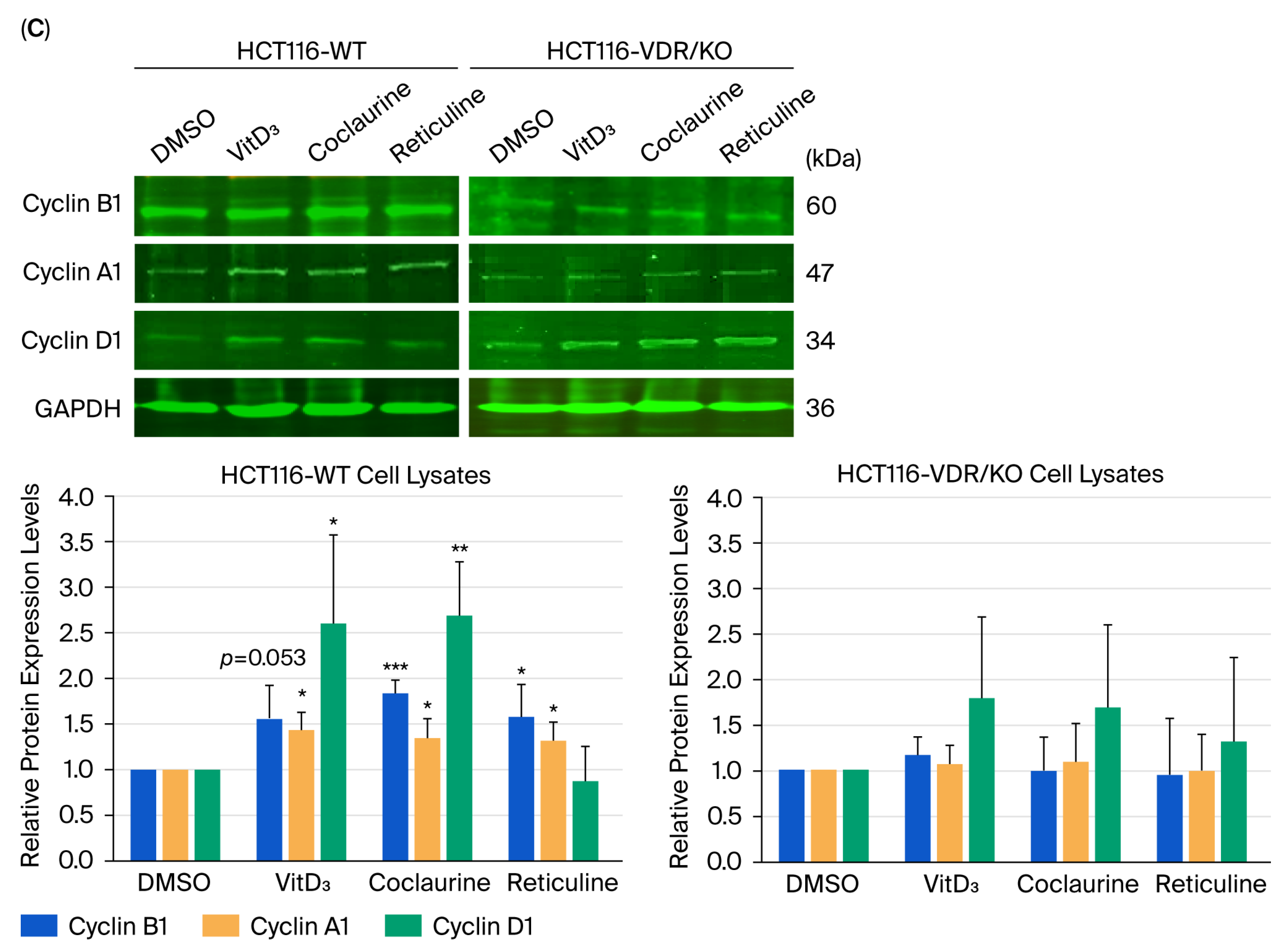
3.6. Coclaurine and Reticuline Cause CRC Cell Growth Arrest in the S-Phase Through VDR
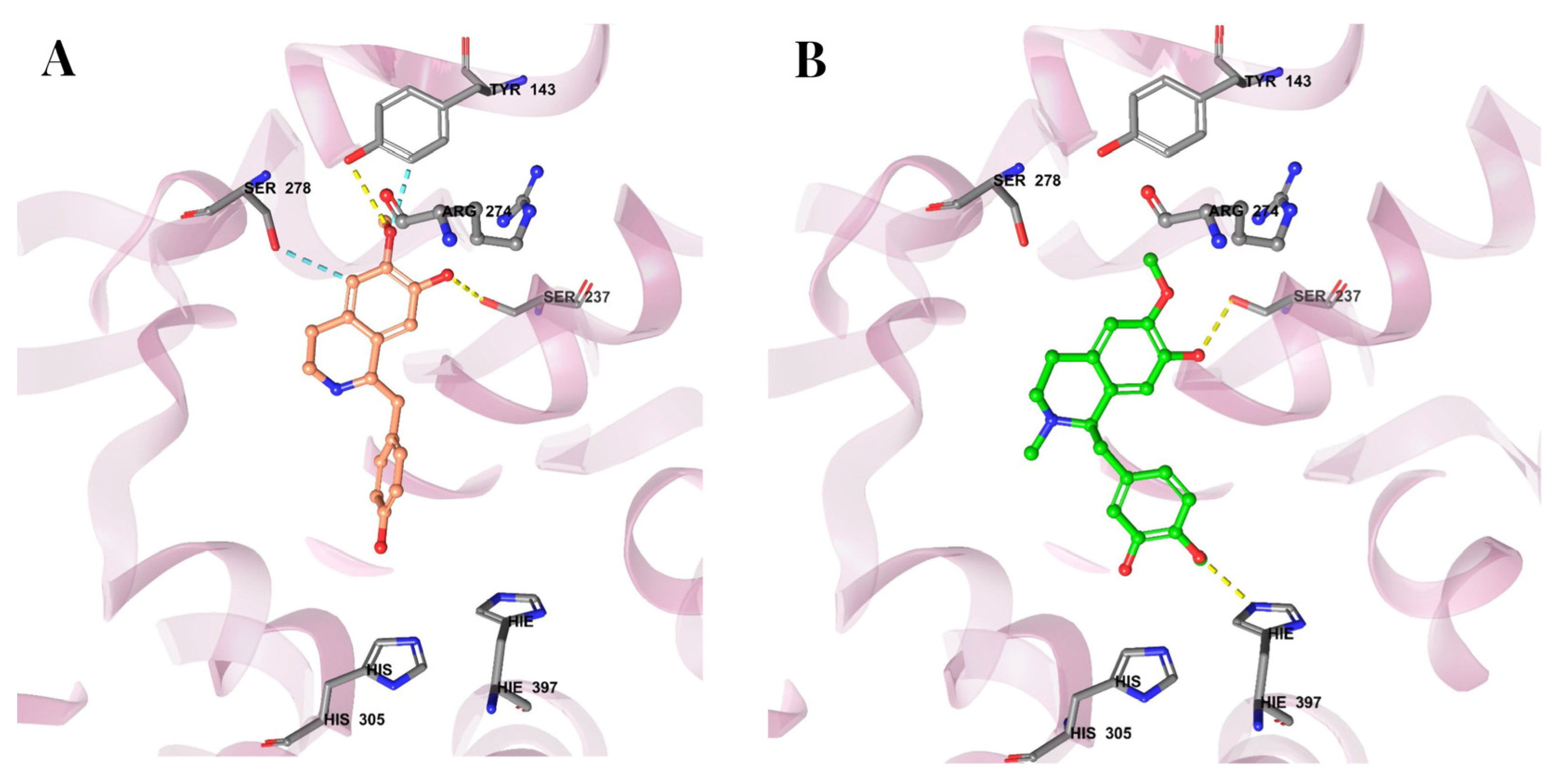
3.7. Molecular Docking Reveals Binding Interactions Between Coclaurine, Reticuline, and VDR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Latacz, M.; Rozmus, D.; Fiedorowicz, E.; Snarska, J.; Jarmołowska, B.; Kordulewska, N.; Savelkoul, H.; Cieślińska, A. Vitamin D receptor (VDR) gene polymorphism in patients diagnosed with colorectal cancer. Nutrients 2021, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Z.; Lengyel, C.G. Challenges in the management of colorectal cancer in low- and middle-income countries. Cancer Treat. Res. Commun. 2024, 35, 100705. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.P.; Ruan, W.J.; Wang, W. Colorectal cancer screening: The value of early detection and modern challenges. World J. Clin. Oncol. 2024, 30, 2726–2730. [Google Scholar] [CrossRef]
- Shi, Q.; Han, X.P.; Yu, J.; Peng, H.; Chen, Y.Z.; Li, F.; Cui, X.B. Decreased vitamin D receptor protein expression is associated with progression and poor prognosis of colorectal cancer patients. Int. J. Clin. Exp. Pathol. 2020, 13, 746–755. [Google Scholar] [PubMed]
- Yang, M.; Ji, W.; Xu, N.; Zong, C.; Gu, J.; Guo, X.; Zhang, L. Association of vitamin D receptor polymorphisms with colorectal cancer susceptibility: A systematic meta-analysis. Medicine 2023, 102, e32575. [Google Scholar] [CrossRef]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An overview of gene regulation, ranging from metabolism to genomics effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef]
- García-Martínez, J.M.; Chocarro-Calvo, A.; Martínez-Useros, J.; Fernández-Aceñero, M.J.; Fiuza, M.C.; Cáceres-Rentero, J.; De la Vieja, A.; Barbachano, A.; Munoz, A.; Larriba, M.J.; et al. Vitamin D induces SIRT1 activation through K610 deacetylation in colon cancer. eLife 2023, 12, RP86913. [Google Scholar] [CrossRef]
- Rong, K.; He, Q.; Chen, S.; Yu, Y.; Mei, L.; Mi, Y.; Mu, L.; Zhu, M.; Nan, M.; Zhang, X.; et al. The mechanism of vitamin D3 in preventing colorectal cancer through network pharmacology. Front. Pharmacol. 2023, 14, 1192210. [Google Scholar] [CrossRef]
- McCullough, M.L.; Zoltick, E.S.; Weinstein, S.J.; Fedirko, V.; Wang, M.; Cook, N.R.; Eliassen, A.H.; Zeleniuch-Jacquotte, A.; Agnoli, C.; Albanes, D.; et al. Circulating vitamin D and colorectal cancer risk: An international pooling project of 17 cohorts. J. Natl. Cancer Inst. 2019, 111, 158–169. [Google Scholar] [CrossRef]
- Na, S.Y.; Kim, K.B.; Lim, Y.J.; Song, H.J. Vitamin D and colorectal cancer: Current perspectives and future directions. J. Cancer Prev. 2022, 27, 147–156. [Google Scholar] [CrossRef]
- Yao, M.; Oduro, P.K.; Akintibu, A.M.; Yan, H. Modulation of the vitamin D receptor by traditional Chinese medicines and bioactive compounds: Potential therapeutic applications in VDR-dependent diseases. Front. Pharmacol. 2024, 15, 1298181. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, H.; Li, A.M.; Liu, Y.T.; Liu, Y.; Zhang, W.; Yang, C.; Song, N.; Zhan, M.; Yang, S. VDR regulates mitochondrial function as a protective mechanism against renal tubular cell injury in diabetic rats. Redox Biol. 2024, 70, 103062. [Google Scholar] [CrossRef] [PubMed]
- Kottila, R.; Hena, J.V. Phytochemical properties and therapeutic applications of Annona muricata: A comprehensive review. J. Young Pharm. 2024, 16, 642–652. [Google Scholar] [CrossRef]
- Chan, W.J.J.; Beale, P.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The safety and tolerability of Annona muricata leaf product in people living with cancer: Study protocol. Adv. Integr. Med. 2024, 11, 143–148. [Google Scholar] [CrossRef]
- Samaratunga, S.; Katuwavila, N.P. Evaluation of the anticancer properties of the phytochemicals present in Annona muricata. Ceylon J. Sci. 2024, 53, 585–597. [Google Scholar] [CrossRef]
- Ganapathy, D. Health benefits of Annona muricata—A review. Int. J. Dent. Oral Sci. 2021, 8, 2965–2967. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline alkaloid metabolism: A century of discovery and a brave new world. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef]
- Al-ghazzawi, A.M. Anti-cancer activity of new benzyl isoquinoline alkaloid from Saudi plant Annona squamosa. BMC Chem. 2019, 13, 13. [Google Scholar] [CrossRef]
- Al-Zahrani, M.H.; Alghamdi, R.A. In silico molecular docking analysis of the potential role of reticuline and coclaurine as anti-colorectal cancer alkaloids. J. Pharm. Res. Int. 2022, 34, 33–42. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Liu, J.L.; Chen, G.X.; Shen, D.T.; Zhu, W.; Chen, X.L.; Liu, F.B.; Hou, Q.K. Berberine enhances intestinal mucosal barrier function by promoting vitamin D receptor activity. Chin. J. Integr. Med. 2024, 30, 143–151. [Google Scholar] [CrossRef]
- Mubeen, M.; Ali, H.; Zehra, S.S.; Khan, A. Harnessing the power of vitexin as a vitamin D receptor agonist in colorectal cancer: A new frontier. Balkan Med. J. 2025, 42, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Teske, K.; Feleke, B.; Yuan, N.Y.; Guthrie, M.L.; Fernstrum, G.B.; Vyas, N.D.; Han, L.; Preston, J.; Bogart, J.W.; et al. Anticancer activity of VDR-coregulator inhibitor PS121912. Cancer Chemother. Pharmacol. 2014, 74, 787–788. [Google Scholar] [CrossRef]
- El-Obeid, A.; Maashi, Y.; AlRoshody, R.; Alatar, G.; Aljudayi, M.; Al-Eidi, H.; AlGaith, N.; Khan, A.H.; Hassib, A.; Matou-Nasri, S. Herbal melanin modulated PGE2 and IL-6 gastroprotective markers through COX-2 and TLR4 signaling in the gastric cancer cell lines AGS. BMC Complement. Med. Ther. 2023, 23, 305. [Google Scholar] [CrossRef]
- Al-Nasser, S.; Abdulla, M.H.; Alhassan, N.; Vaali-Mohammed, M.A.; Al-Omar, S.; Hamdi, N.; Elnakady, Y.; Matou-Nasri, S.; Mansour, L. A benzimidazole-based N-heterocyclic carbene derivative exhibits potent antiproliferative and apoptotic effects against colorectal cancer. Medicina 2024, 60, 1379. [Google Scholar] [CrossRef] [PubMed]
- Maashi, Y.; Almutairi, S.; Aldawood, M.; Al-Eidi, H.; AlRoshody, R.; Alghamdi, H.A.; Bahattab, S.; Alsaleh, A.A.; Alkadi, H.; Alghamdi, S.; et al. In vitro oncogenic effects of glycated albumin in human colorectal cancer cell lines HT29 and SW620 revealing EpCAM and Galectin-3 upregulation in Type 2 diabetic colorectal cancer tissues as potential biomarkers. J. Oncol. Res. Ther. 2024, 9, 10227. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shaw, D.E.; Shelley, M.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision Glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Kumaran, T. A review of recent studies on the phytochemical and pharmacological activity of Annona muricata. For. Agric. Rev. 2021, 2, 1–5. [Google Scholar] [CrossRef]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids used as medicines: Structural phytochemistry meets biodiversity—An update and forward look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Tabakam, T.G.; Makhafola, T.J. Plant-derived alkaloids as a potential source of treatment for colorectal cancer over the past five years: A comprehensive review. Plants 2024, 13, 2723. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, Q.; Hui, Y.; Xu, J.; Shi, P.; Chen, Y.; Chen, Y. Vitamin D receptor prevents tumour development by regulating the Wnt/β-catenin signalling pathway in human colorectal cancer. BMC Cancer 2023, 23, 336. [Google Scholar] [CrossRef] [PubMed]
- Merchan, B.B.; Morcillo, S.; Martin-Nunez, G.; Tinahones, F.J.; Macias-Gonzalez, M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J. Steroid Biochem. Mol. Biol. 2017, 167, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Salman, P.; Oliveira-Cruz, L.; Soza-Ried, C. Unraveling the complex link between vitamin D levels and cancer: A crucial understanding for designing future supplementation approaches. Braz. J. Pharm. Sci. 2023, 59, e23319. [Google Scholar] [CrossRef]
- Al-Ghafari, A.B.; Balamash, K.S.; Al Doghaither, H.A. Serum vitamin D receptor (VDR) levels as a potential diagnostic marker for colorectal cancer. Saudi J. Biol. Sci. 2020, 27, 827–832. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Wang, W.; Jin, J.; Zhou, Z.; Wang, Y.; Min, K.; Zuo, X.; Jiang, J.; Zhou, Y.; Shi, J. Snail inhibits metastasis via upregulation of E-cadherin and is associated with prognosis in colorectal cancer. Oncol. Lett. 2023, 25, 271. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Kornmann, M.; Traub, B. Role of epithelial to mesenchymal transition in colorectal cancer. Int. J. Mol. Sci. 2023, 24, 14815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ng, A.S.; Cai, S.; Li, Q.; Yang, L.; Kerr, D. Novel therapeutic strategies: Targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 2021, 22, e358–e368. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Xu, Y.; Hu, X.; Liu, B.; Yu, J. Vitamin D receptor knockdown attenuates the antiproliferative, pro-apoptotic and anti-invasive effect of vitamin D by activating the Wnt/β-catenin signaling pathway in papillary thyroid cancer. Mol. Med. Rep. 2020, 22, 4135–4142. [Google Scholar] [CrossRef]
- Tsukahara, T.; Haniu, H.; Matsuda, Y. Cyclic phosphatidic acid induces G0/G1 arrest, inhibits AKT phosphorylation, and downregulates cyclin D1 expression in colorectal cancer cells. Cell Mol. Biol. Lett. 2015, 20, 38–47. [Google Scholar] [CrossRef]
- Sun, L.; Xing, J.; Zhou, X.; Sang, X.; Gao, S. Wnt/β-catenin signaling, epithelial-mesenchymal transition in colorectal cancer cells. Biomed. Pharmacother. 2024, 175, 116685. [Google Scholar] [CrossRef]
- Salimizadeh, Z.; Tahmasebi Enferadi, S.; Majidizadeh, T.; Mahjoubi, F. Cytotoxicity of alkaloids isolated from Peganum harmala seeds on HCT116 human colon cancer cells. Mol. Biol. Rep. 2024, 51, 732. [Google Scholar] [CrossRef]
- Kim, G.D. Harmine hydrochloride triggers G2/M cell cycle arrest and apoptosis in HCT116 cells through ERK and PI3K/AKT/mTOR signaling pathways. Prev. Nutr. Food Sci. 2021, 26, 445–452. [Google Scholar] [CrossRef]
- Liu, J.; Sun, S.; Zhou, C.; Sun, Z.; Wang, Q.; Sun, C. In vitro and in vivo anticancer activity of lycorine in prostate cancer by inhibiting NF-κB signaling pathway. J. Cancer 2022, 13, 3151–3159. [Google Scholar] [CrossRef]
- Al-Obeed, O.; Vaali-Mohammed, M.A.; Eldehna, W.M.; Al-Khayal, K.; Mahmood, A.; Abdel-Aziz, H.A.; Zubaidi, A.; Alafeefy, A.; Abdulla, M.; Ahmad, R. Novel quinazoline-based sulfonamide derivative (3D) induces apoptosis in colorectal cancer by inhibiting JAK2–STAT3 pathway. Onco Targets Ther. 2018, 11, 3313–3322. [Google Scholar] [CrossRef]
- Zhu, S.; Li, T.; Tan, J.; Yan, X.; Zhang, D.; Zheng, C.; Chen, Y.; Xiang, Z.; Cui, H. Bax is essential for death receptor-mediated apoptosis in human colon cancer cells. Cancer Biother. Radiopharm. 2012, 27, 577–581. [Google Scholar] [CrossRef]
- Cherbonnel-Lasserre, C.; Dosanjh, M.K. Suppression of apoptosis by overexpression of Bcl-2 or Bcl-xL promotes survival and mutagenesis after oxidative damage. Biochimie 1997, 79, 613–617. [Google Scholar] [CrossRef]
- Xing, X.; Huang, A.; Xing, Y.; Lan, L.; Yi, Z.; He, P. Lycorine inhibits breast cancer growth and metastasis via inducing apoptosis and blocking Src/FAK-involved pathway. Sci. China Life Sci. 2017, 60, 417–428. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Xing, G. Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway. Acta Biochim. Biophys. Sin. 2017, 49, 771–779. [Google Scholar] [CrossRef]
- Li, C.; Deng, C.; Pan, G.; Wang, X.; Zhang, K.; Dong, Z.; Zhao, G.; Tan, M.; Hu, X.; Shi, S.; et al. Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J. Exp. Clin. Cancer Res. 2020, 39, 230. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qiu, Y.; Pang, X.; Li, J.; Wu, S.; Yin, S.; Han, L.; Zhang, Y.; Jin, C.; Gao, X.; et al. Lycorine promotes autophagy and apoptosis via TCRP1/Akt/mTOR axis inactivation in human hepatocellular carcinoma. Mol. Cancer Ther. 2017, 16, 2711–2723. [Google Scholar] [CrossRef]
- El-Far, M.; El-Newary, S.; Sayed, R.H. Autophagy, apoptosis, vitamin D, and vitamin D receptor in hepatocellular carcinoma associated with hepatitis C virus. Medicine 2018, 97, e0172. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Bartek, J. Cell cycle-targeted cancer therapies. Annu. Rev. Cancer Biol. 2017, 1, 41–57. [Google Scholar] [CrossRef]
- Pfeffer, C.; Singh, A. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, Y.R.; Lee, S.Y.; Jin, Y.; Han, D.C.; Kwon, B.M. Ginkgetin induces G2-phase arrest in HCT116 colon cancer cells through the modulation of b-Myb and miRNA34a expression. Int. J. Oncol. 2017, 51, 1331–1342. [Google Scholar] [CrossRef]
- Ji, P.; Agrawal, S.; Diederichs, S.; Bäumer, N.; Becker, A.; Cauvet, T.; Kowski, S.; Beger, C.; Welte, K.; Berdel, W.E.; et al. Cyclin A1, the alternative A-type cyclin, contributes to G1/S cell cycle progression in somatic cells. Oncogene 2005, 24, 2739–2744. [Google Scholar] [CrossRef]
- Thoma, O.M.; Neurath, M.F.; Waldner, M.J. Cyclin-dependent kinase inhibitors and their therapeutic potential in colorectal cancer treatment. Front. Pharmacol. 2021, 12, 757120. [Google Scholar] [CrossRef]
- Bhoora, S.; Punchoo, R. Policing cancer: Vitamin D arrests the cell cycle. Int. J. Mol. Sci. 2020, 21, 9296. [Google Scholar] [CrossRef]





| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) | PCR Product Size (bp) |
|---|---|---|---|
| VDR | CCAGTTCGTGTGAATGATGG | GTCGTCCATGGTGAAGGA | 384 |
| SNAIL1 | GCTCCACAAGCACCAAGAGT | ATTCCATGGCAGTGAGAAGG | 145 |
| SNAIL2 | GAGCATTTGCAGACAGGTCA | GCTTCGGAGTGAAGAAATGC | 200 |
| TP53 | GAGATGTTCCGAGAGCTGAATGAGGC | TCTTGAACATGAGTTTTTTATGGCGGGAGG | 1063 |
| ACTB | GCTCGTCGTCGACAACGGCTC | CAAACATGATCTGGGTCATCTTCT | 352 |
| Compounds | IC50 Values (μM) |
|---|---|
| VitD3 | 15.7 |
| Coclaurine | 26.2 |
| Reticuline | 17.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, H.A.; Alghamdi, S.S.; Al-Zahrani, M.H.; Trivilegio, T.; Bahattab, S.; AlRoshody, R.; Alhaidan, Y.; Alghamdi, R.A.; Matou-Nasri, S. Reticuline and Coclaurine Exhibit Vitamin D Receptor-Dependent Anticancer and Pro-Apoptotic Activities in the Colorectal Cancer Cell Line HCT116. Curr. Issues Mol. Biol. 2025, 47, 810. https://doi.org/10.3390/cimb47100810
Alghamdi HA, Alghamdi SS, Al-Zahrani MH, Trivilegio T, Bahattab S, AlRoshody R, Alhaidan Y, Alghamdi RA, Matou-Nasri S. Reticuline and Coclaurine Exhibit Vitamin D Receptor-Dependent Anticancer and Pro-Apoptotic Activities in the Colorectal Cancer Cell Line HCT116. Current Issues in Molecular Biology. 2025; 47(10):810. https://doi.org/10.3390/cimb47100810
Chicago/Turabian StyleAlghamdi, Hind A., Sahar S. Alghamdi, Maryam Hassan Al-Zahrani, Thadeo Trivilegio, Sara Bahattab, Rehab AlRoshody, Yazeid Alhaidan, Rana A. Alghamdi, and Sabine Matou-Nasri. 2025. "Reticuline and Coclaurine Exhibit Vitamin D Receptor-Dependent Anticancer and Pro-Apoptotic Activities in the Colorectal Cancer Cell Line HCT116" Current Issues in Molecular Biology 47, no. 10: 810. https://doi.org/10.3390/cimb47100810
APA StyleAlghamdi, H. A., Alghamdi, S. S., Al-Zahrani, M. H., Trivilegio, T., Bahattab, S., AlRoshody, R., Alhaidan, Y., Alghamdi, R. A., & Matou-Nasri, S. (2025). Reticuline and Coclaurine Exhibit Vitamin D Receptor-Dependent Anticancer and Pro-Apoptotic Activities in the Colorectal Cancer Cell Line HCT116. Current Issues in Molecular Biology, 47(10), 810. https://doi.org/10.3390/cimb47100810






