Cinnamomum migao H.W. Li Ethanol-Water Extract Suppresses IL-6 Production in Cardiac Fibroblasts: Mechanisms Elucidated via UPLC-Q-TOF-MS, Network Pharmacology, and Experimental Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Ethanol-Water Extract of MG
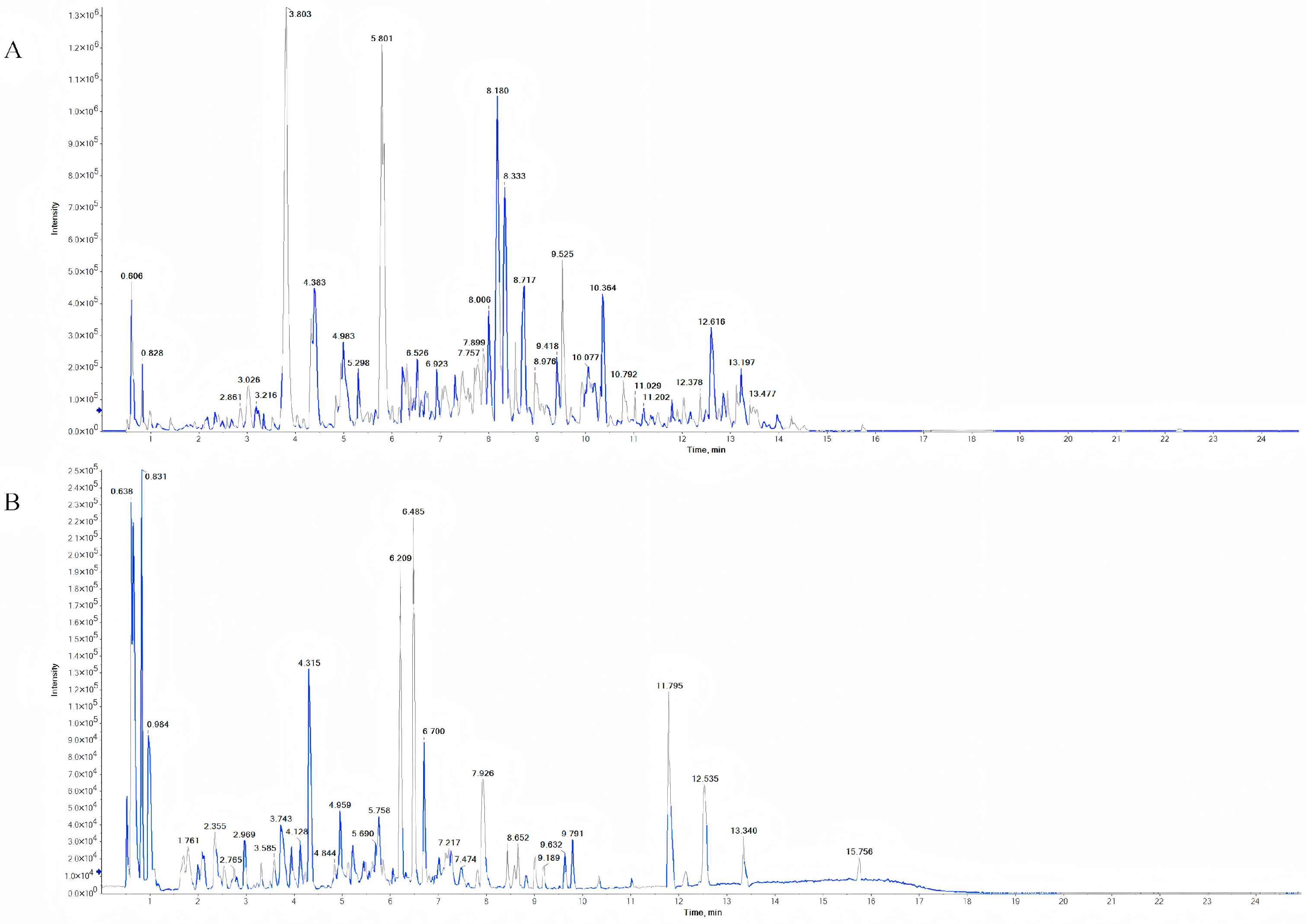
2.3. UPLC-Q-TOF-MS/MS Analysis
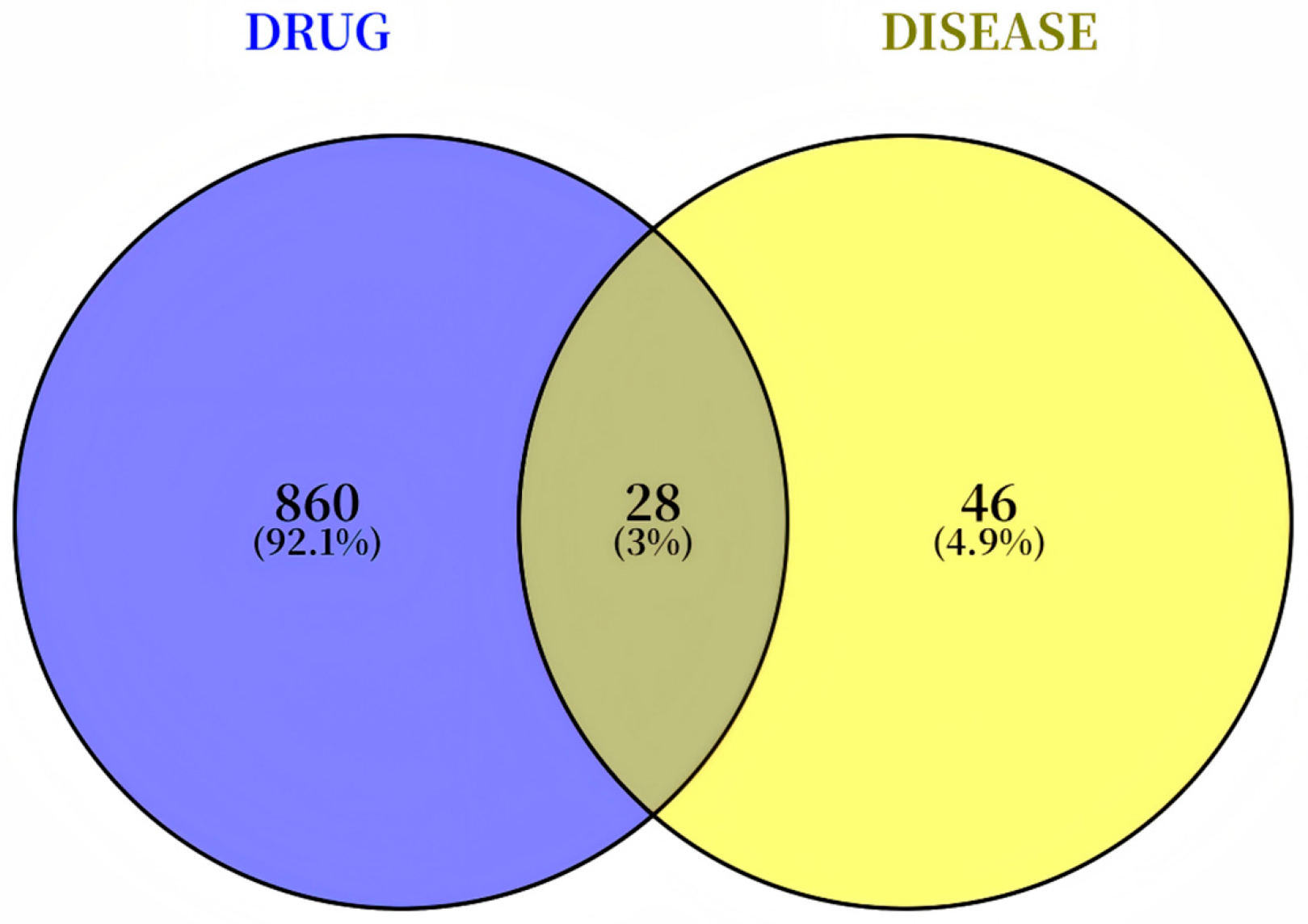
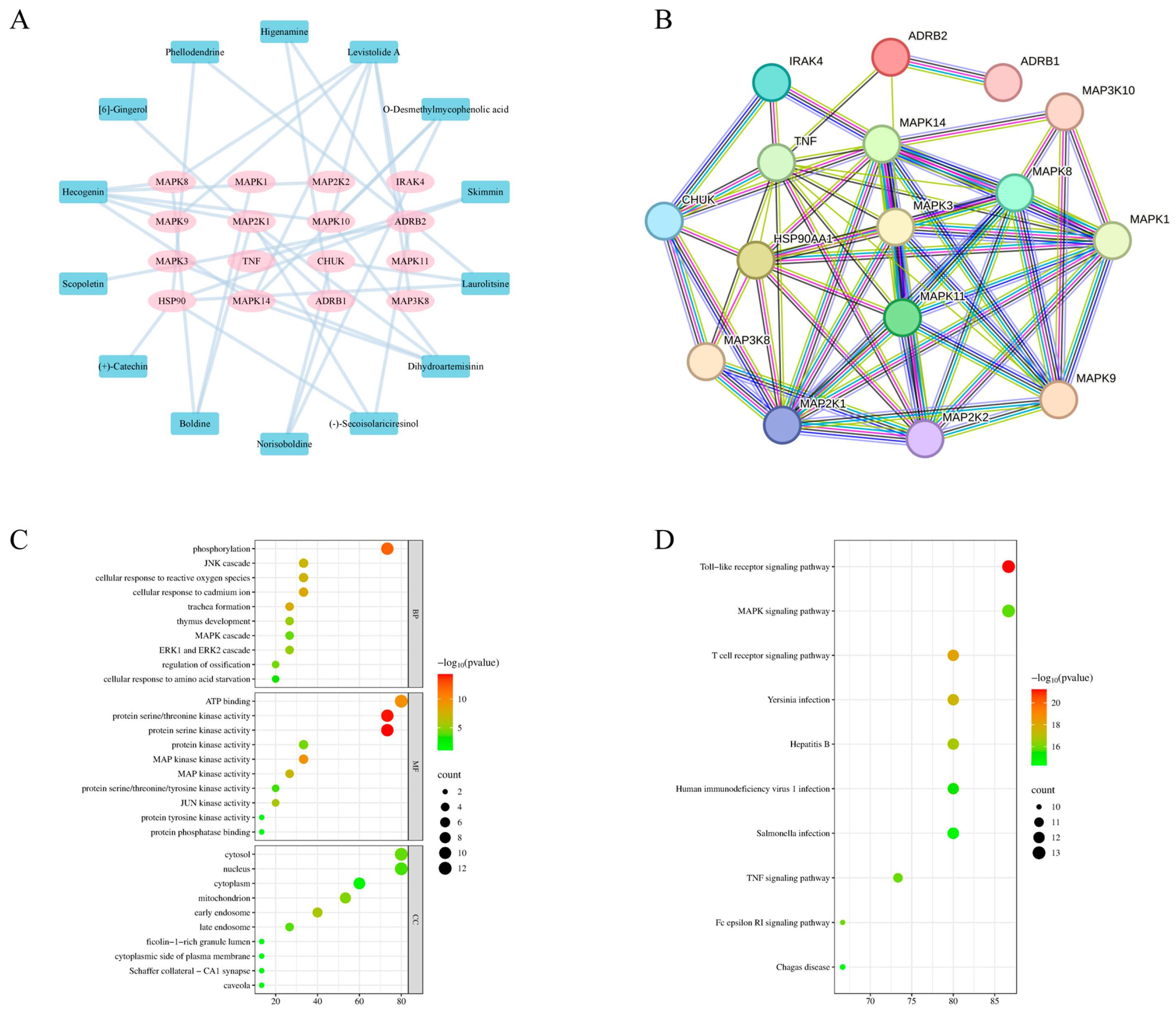
2.4. Network Pharmacology
2.5. Molecular Docking
2.6. Cell Culture and Model Establishment
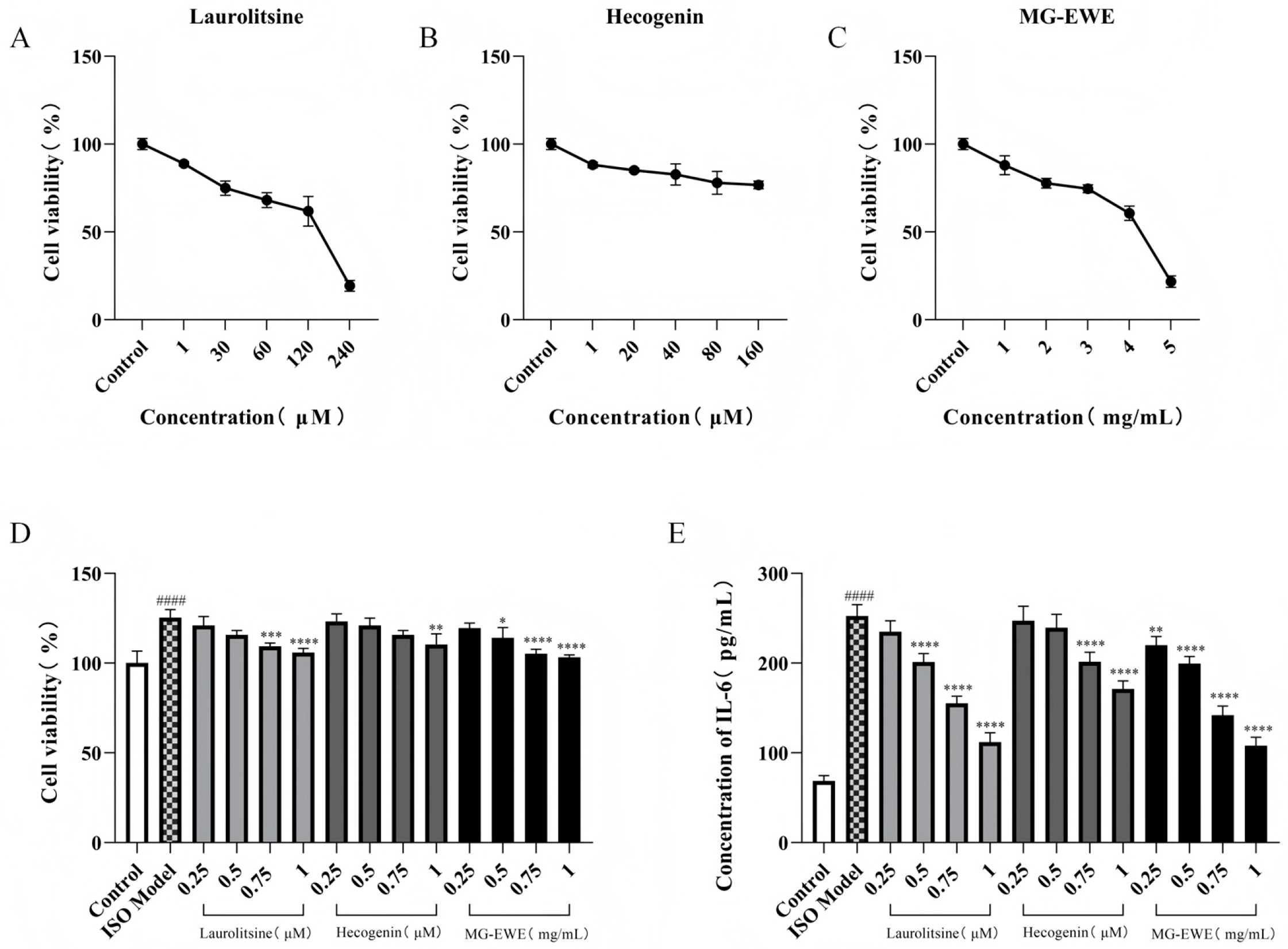
2.7. Drug Concentration Screening
2.7.1. CCK8 Assay
2.7.2. ELISA Analysis
2.8. Experimental Grouping and Intervention
2.9. Detection of Hydroxyproline (Hyp) Content
2.10. Transwell Cell Migration Assay
2.11. Real-Time Fluorescence Quantitative PCR
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. Identification of Chemical Components in MG-EWE
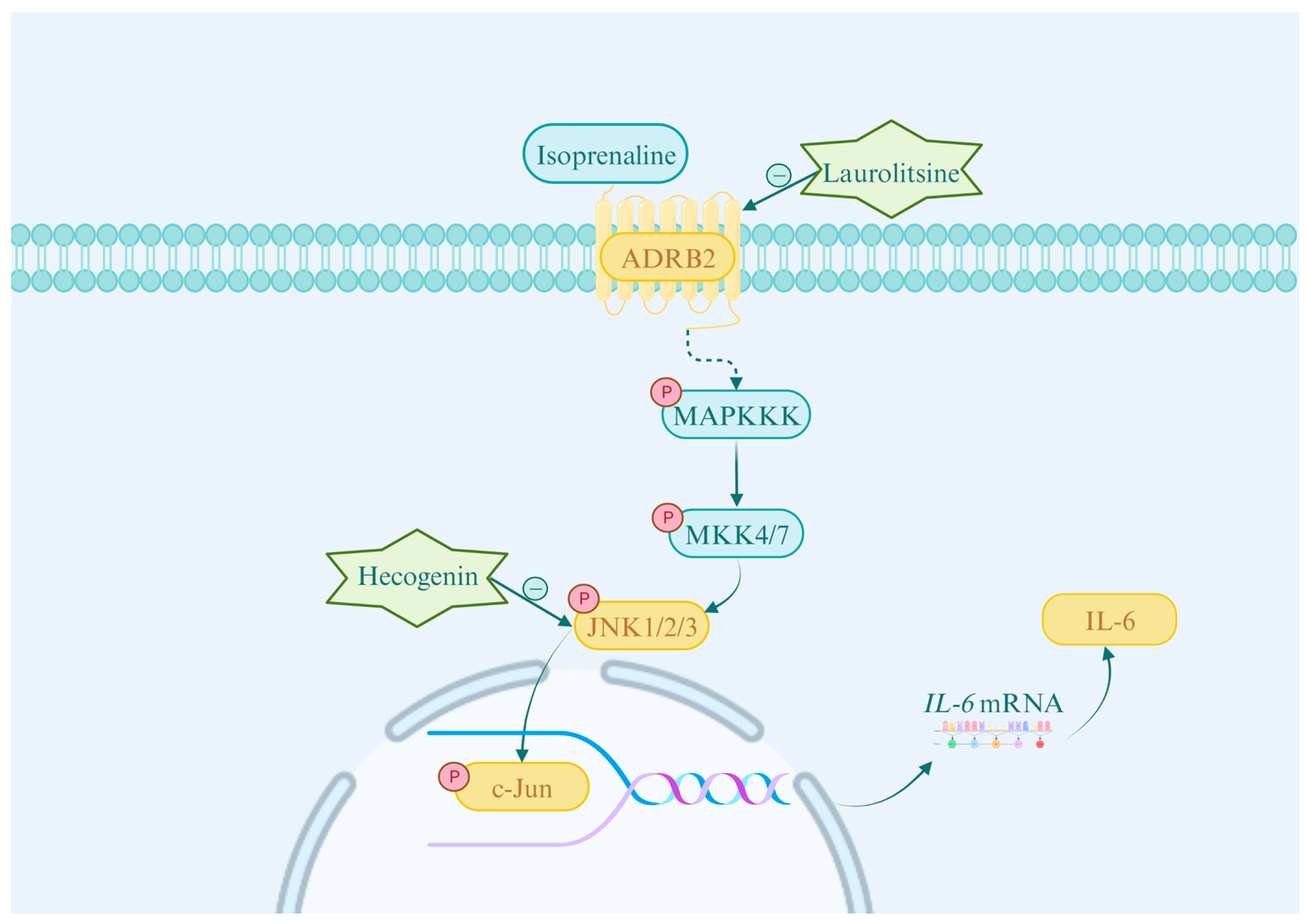
3.2. Network Pharmacology Reveals That MG-EWE Inhibits IL-6 Production in Cardiac Fibroblasts via the ADRB2/JNK Pathway
3.3. Screening of Optimal Intervention Concentration for MG-EWE and Its Active Components
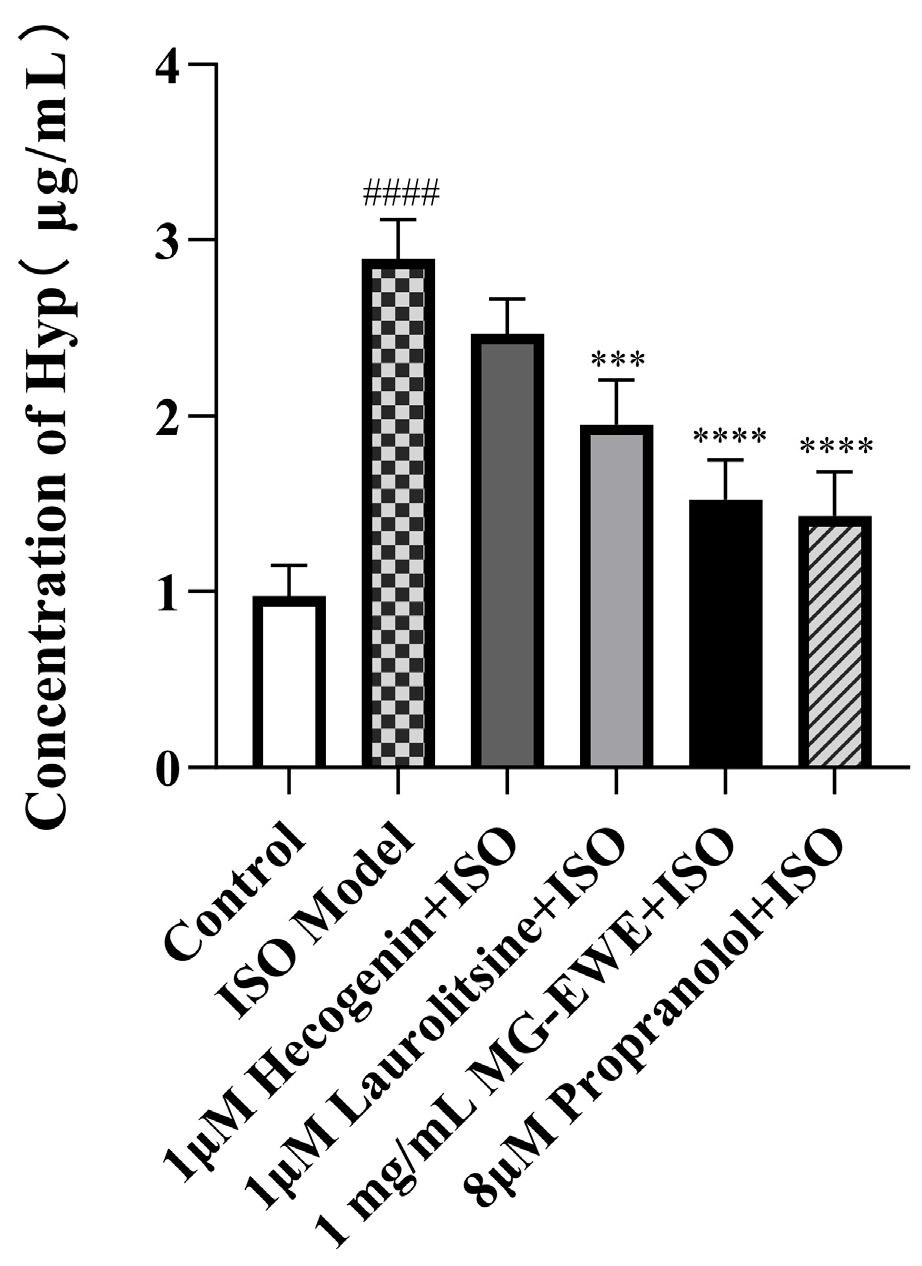
3.4. MG-EWE, Laurolitsine, and Hecogenin Reduce the Ability of ISO-Induced CFs to Secrete Hyp
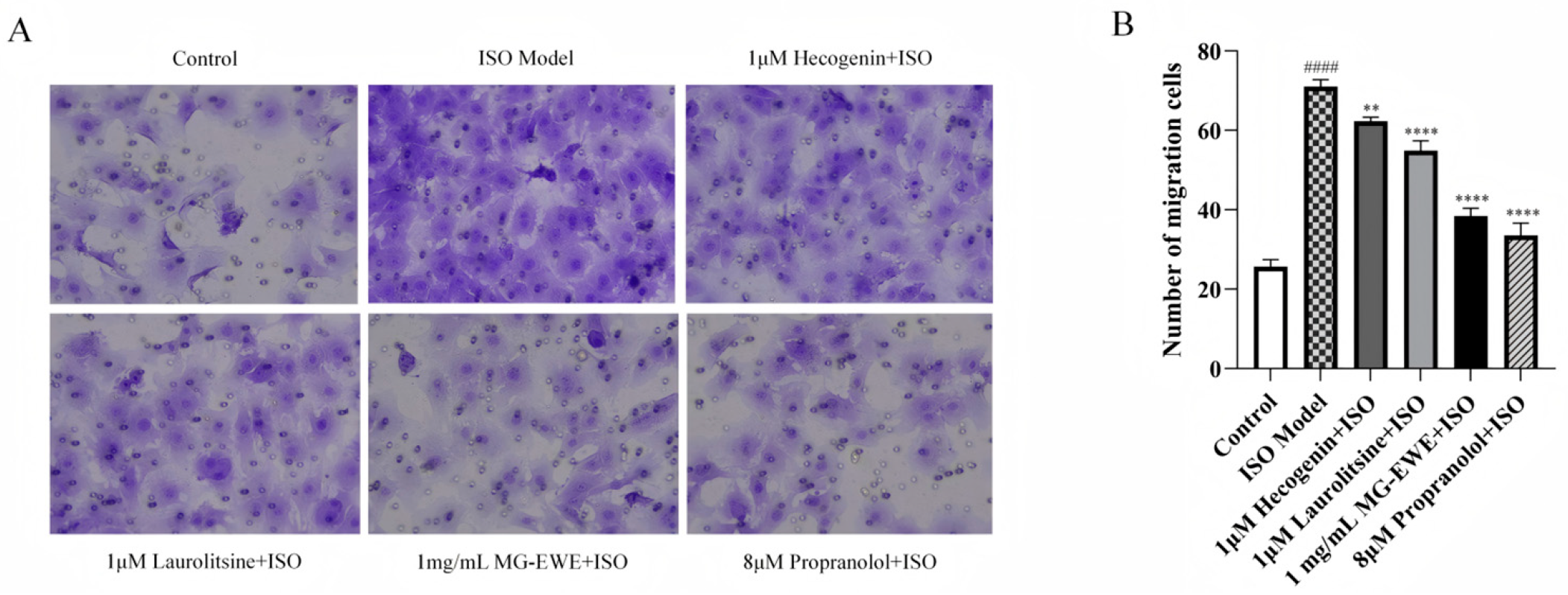
3.5. Inhibitory Effects of MG-EWE, Laurolitsine, and Hecogenin on ISO-Induced Migration of Rat CFs
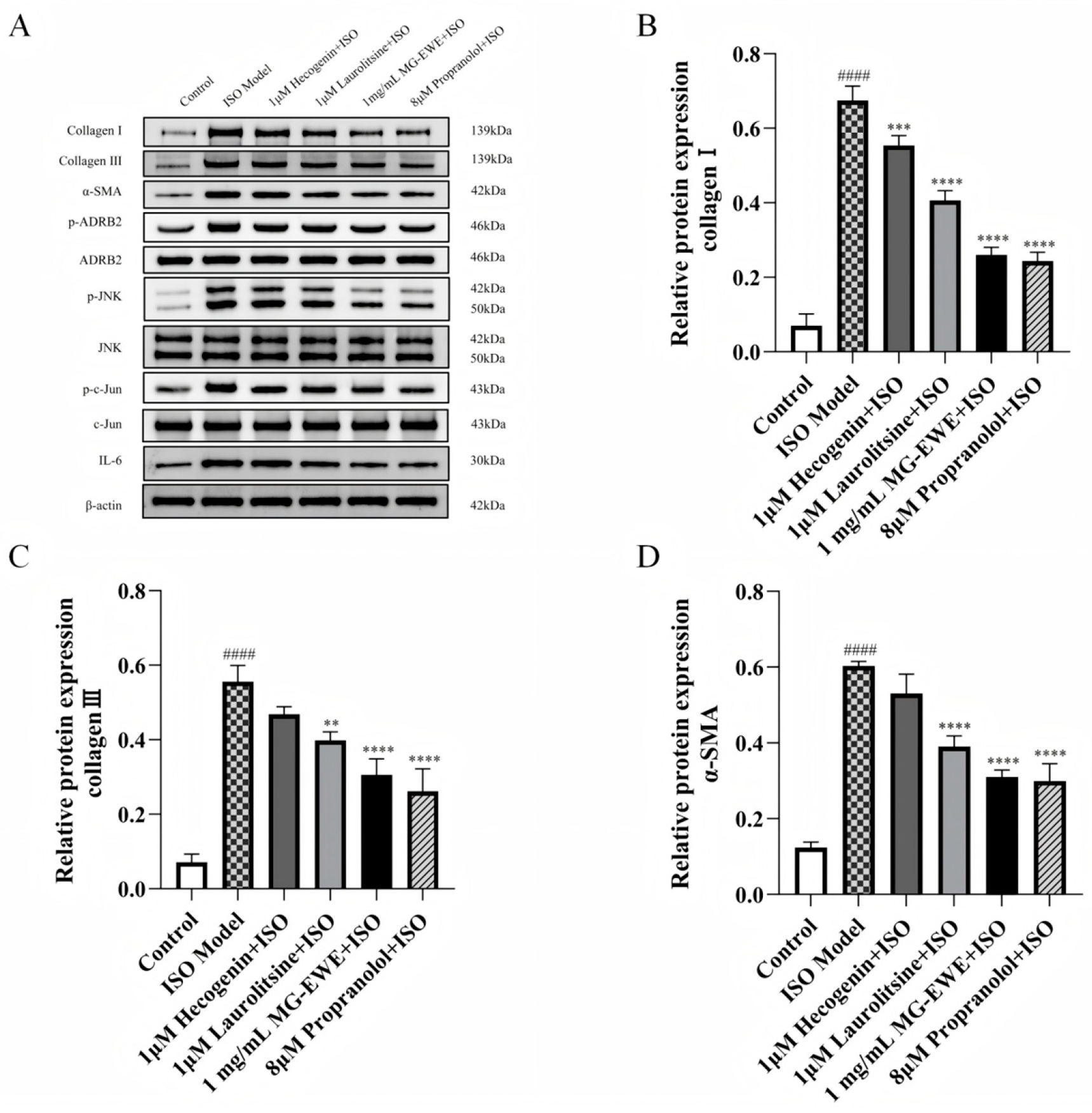
3.6. MG-EWE, Laurolitsine, and Hecogenin Reduce the Expression of Collagen I, Collagen III, and α-SMA Proteins in ISO-Induced Rat CFs
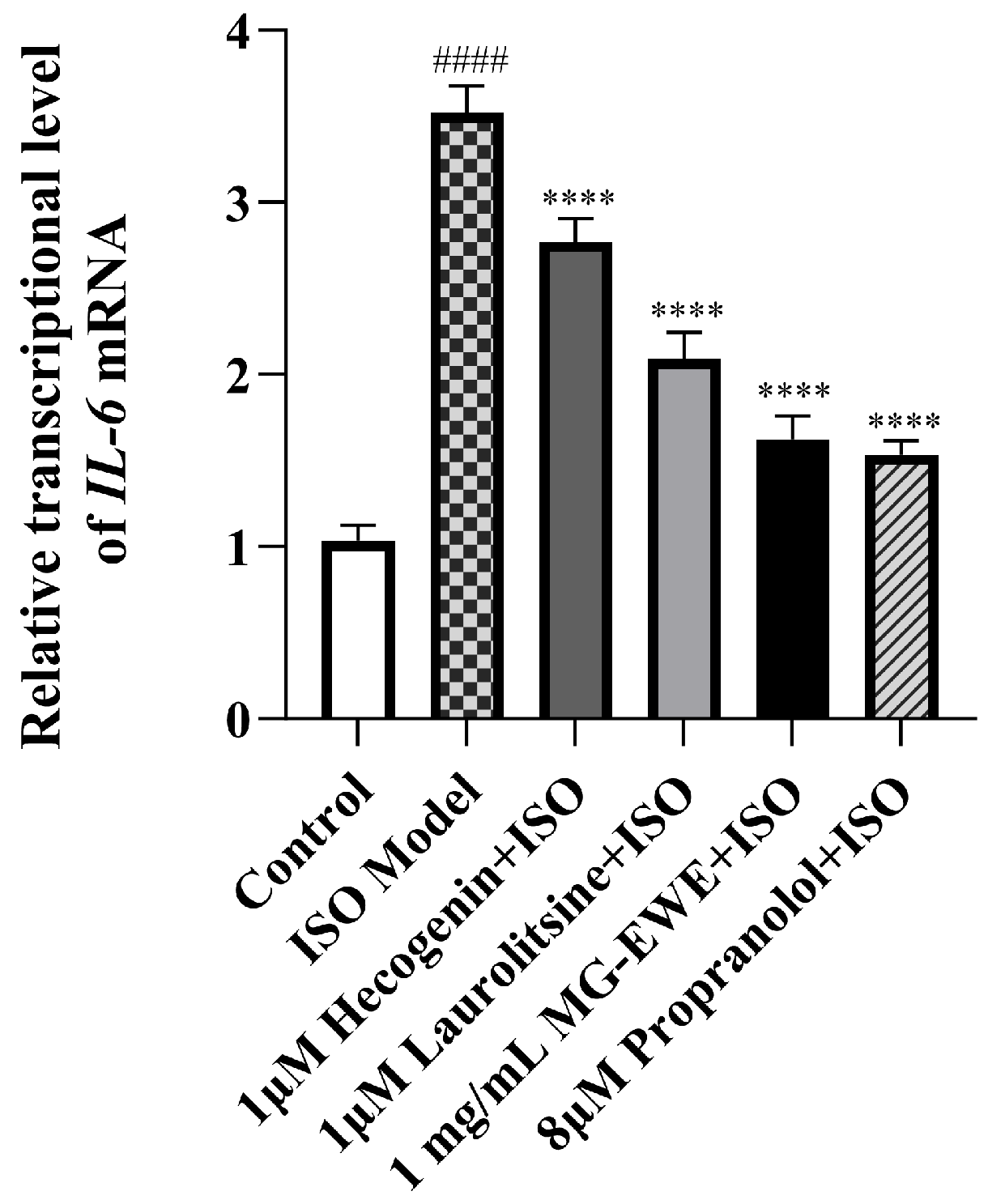
3.7. MG-EWE, Laurolitsine, and Hecogenin Reduce the Expression Level of IL-6 mRNA in ISO-Induced Rat CFs
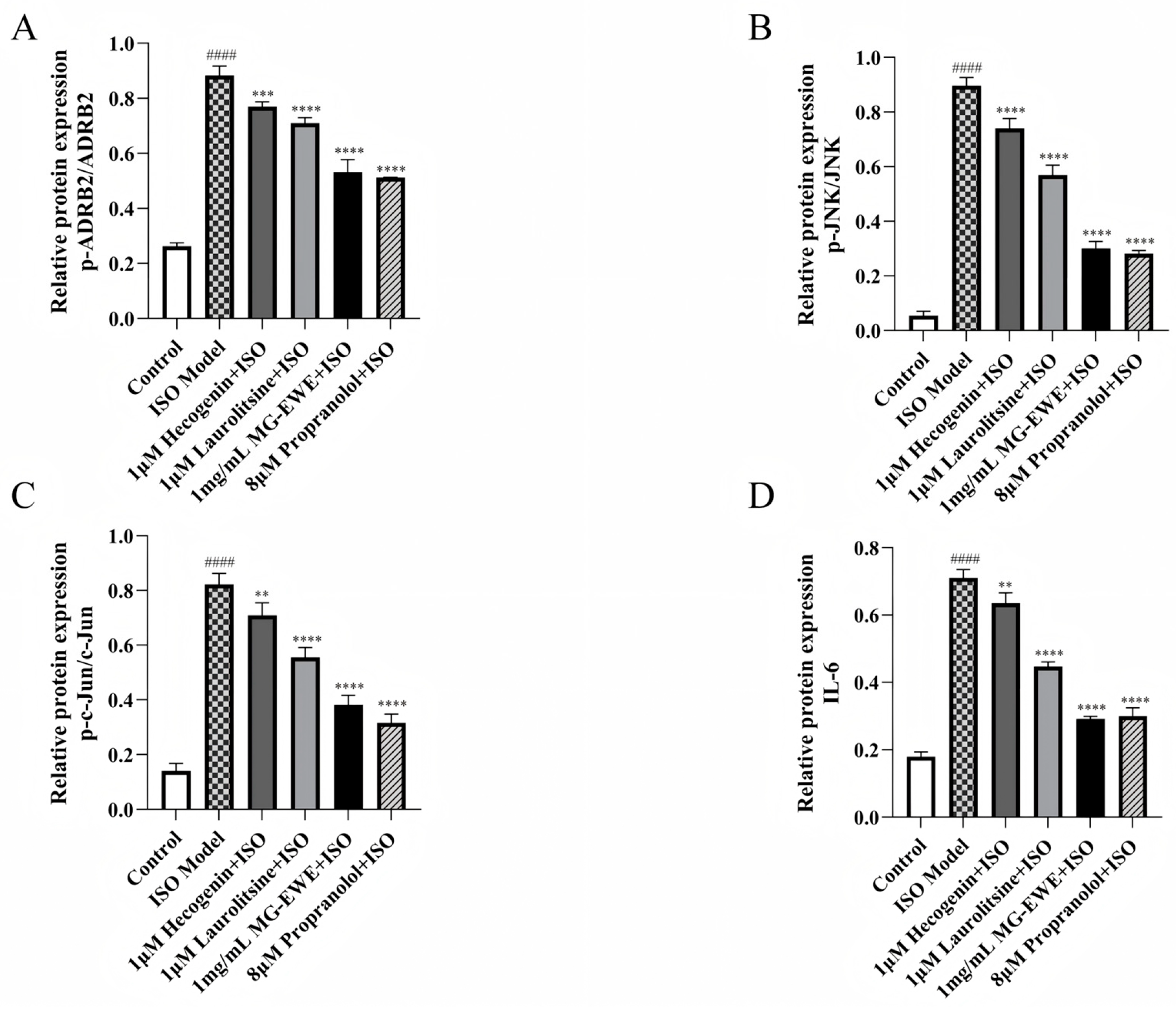
3.8. MG-EWE, Laurolitsine, and Hecogenin Significantly Attenuate the Phosphorylation of ADRB2, JNK and c-Jun Proteins, as Well as the Expression of IL-6 Protein in ISO-Induced Rat CFs
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFs | Cardiac Fibroblasts |
| IL-6 | Interleukin-6 |
| MF | Myocardial Fibrosis |
| MG | Cinnamomum migao H.W. Li |
| MG-EWE | Ethanol-water Extract of Cinnamomum migao H.W. Li |
| CCK8 | Cell Counting Kit-8 |
| ELISA | Enzyme-linked Immunosorbent Assay |
| TCMSP | Traditional Chinese Medicine System Pharmacology Database and Analysis Platform |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PDB | Protein Data Bank |
| ECM | Extracellular Matrix |
| MyoFbs | Myofibroblasts |
| α-SMA | α-Smooth Muscle Actin |
| CVF | Collagen Volume Fraction |
| ISO | Isoproterenol |
| PPI | Protein–protein Interaction |
| Hyp | Hydroxyproline |
| PBS | Phosphate-buffered Saline |
| qPCR | Real-Time Fluorescence Quantitative PCR |
| SDS-PAGE | Sodium Dodecyl Sulfate-polyacrylamide Gel |
| PVDF | Polyvinylidene Difluoride |
| BPs | Biological Processes |
| MFs | Molecular Functions |
| CCs | Cellular Components |
| TLR | Toll-like Receptor |
| CK-MB | Creatine Kinase-MB |
| cTnT | Cardiac Troponin T |
| cTnI | Cardiac Troponin I |
| HW/BW | Heart Weight/Body Weight Ratio |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| MAPKKK | MAP kinase kinase kinase |
| MAPKK | MAP kinase kinase |
| UPLC-Q-TOF-MS | Ultra-performance Liquid Chromatography-quadrupole Time-of-flight Mass Spectrometry |
References
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2020, 13, 1221–1234. [Google Scholar] [CrossRef]
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac Fibrosis in Myocardial Infarction-from Repair and Remodeling to Regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Liu, M.; López de Juan Abad, B.; Cheng, K. Cardiac Fibrosis: Myofibroblast-Mediated Pathological Regulation and Drug Delivery Strategies. Adv. Drug Deliv. Rev. 2021, 173, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac Fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, G.; Zheng, N.; Cheng, W.; Ouyang, K.; Lin, H.; Liao, Y.; Liu, J. HIMF (Hypoxia-Induced Mitogenic Factor)-IL (Interleukin)-6 Signaling Mediates Cardiomyocyte-Fibroblast Crosstalk to Promote Cardiac Hypertrophy and Fibrosis. Hypertension 2019, 73, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, G.C.; McLarty, J.L.; Levick, S.P.; Du, Y.; Janicki, J.S.; Brower, G.L. Interleukin 6 Mediates Myocardial Fibrosis, Concentric Hypertrophy, and Diastolic Dysfunction in Rats. Hypertension 2010, 56, 225–231. [Google Scholar] [CrossRef]
- Han, J.; Hou, J.; Liu, Y.; Liu, P.; Zhao, T.; Wang, X. Using Network Pharmacology to Explore the Mechanism of Panax Notoginseng in the Treatment of Myocardial Fibrosis. J. Diabetes Res. 2022, 2022, 8895950. [Google Scholar] [CrossRef] [PubMed]
- Scridon, A.; Balan, A.I. Targeting Myocardial Fibrosis-A Magic Pill in Cardiovascular Medicine? Pharmaceutics 2022, 14, 1599. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally Occurring Anti-Cancer Compounds: Shining from Chinese Herbal Medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Tong, B.; Wang, D.; Liu, J.; Liao, X.; Sun, Q. Effects of Rhizosphere Fungi on the Chemical Composition of Fruits of the Medicinal Plant Cinnamomum migao Endemic to Southwestern China. BMC Microbiol. 2021, 21, 206. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Tong, B.; Wang, D.; Tian, X.; Liu, J.; Chen, J.; Xiao, X.; Wang, S. Rhizobacterial Compositions and Their Relationships with Soil Properties and Medicinal Bioactive Ingredients in Cinnamomum migao. Front. Microbiol. 2023, 14, 1078886. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xu, L.; Mu, D.; Wang, D.; Tan, S.; Liu, L.; Li, Y.; Chai, H.; Hou, Y. Ethanol Extracts of Cinnamomum migao H.W. Li Attenuates Neuroinflammation in Cerebral Ischemia-Reperfusion Injury via Regulating TLR4-PI3K-Akt-NF-κB Pathways. J. Ethnopharmacol. 2025, 339, 119150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, F.; Yang, L.; Peng, M.; Pan, X.; Lou, H.; Yang, J.; Yang, X.; Li, Q. Vicinal Diol Sesquiterpenes from Cinnamomum migao with Neuroprotective Effects in PC12 Cells. Int. J. Mol. Sci. 2024, 25, 12693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Pan, X.; Chen, F.-J.; Peng, M.; Wang, Y.; Gao, M.; Yang, L.-S.; Li, L.-Q.; Yang, J.; Yang, X.-S.; et al. Targeted Discovery of Oxygen-Bridged Sesquiterpenes from Cinnamomum migao by MS/MS-Based Molecular Networking and Their Neuroprotective Activities. Phytochemistry 2025, 236, 114519. [Google Scholar] [CrossRef]
- Muhammad, I.; Luo, W.; Shoaib, R.M.; Li, G.-L.; Shams Ul Hassan, S.; Yang, Z.-H.; Xiao, X.; Tu, G.-L.; Yan, S.-K.; Ma, X.-P.; et al. Guaiane-Type Sesquiterpenoids from Cinnamomum migao H. W. Li: And Their Anti-Inflammatory Activities. Phytochemistry 2021, 190, 112850. [Google Scholar] [CrossRef]
- Muhammad, I.; Hassan, S.S.U.; Xu, W.-J.; Tu, G.-L.; Yu, H.-J.; Xiao, X.; Yan, S.-K.; Jin, H.-Z.; Bungau, S. An Extensive Pharmacological Evaluation of Novel Anti-Nociceptive and IL-6 Targeted Anti-Inflammatory Guaiane-Type Sesquiterpenoids from Cinnamomum migao H. W. Li through in-Depth in-Vitro, ADMET, and Molecular Docking Studies. Biomed. Pharmacother. 2023, 164, 114946. [Google Scholar] [CrossRef]
- Sun, M.; Yu, H.; Zhang, Y.; Li, Z.; Gao, W. MicroRNA-214 Mediates Isoproterenol-Induced Proliferation and Collagen Synthesis in Cardiac Fibroblasts. Sci. Rep. 2015, 5, 18351. [Google Scholar] [CrossRef]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef] [PubMed]
- Humeres, C.; Frangogiannis, N.G. Fibroblasts in the Infarcted, Remodeling, and Failing Heart. JACC Basic. Transl. Sci. 2019, 4, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, Y.; Zhang, Y.; Zhang, C.; Chen, N.; Zhang, X. Total Synthesis of the Racemate of Laurolitsine. Molecules 2024, 29, 745. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Ruiqi, W.; Yanan, Y.; Ning, M.; Zhi, Z.; Yinfeng, T.; Lin, D.; Yiying, L.; Weiying, L.; Chongming, W.; et al. Laurolitsine Ameliorates Type 2 Diabetes by Regulating the Hepatic LKB1-AMPK Pathway and Gut Microbiota. Phytomedicine 2022, 106, 154423. [Google Scholar] [CrossRef]
- Borges, M.A.d.H.; Passos, F.R.S.; Quintans, J.d.S.S.; Azeredo, F.J. Hecogenin and Its Derivates: A Pharmacology Review. Biomed. Pharmacother. 2023, 159, 114251. [Google Scholar] [CrossRef]
- Pei, R.; Duan, C.; Vijayalakshmi, A.; Vijayalakshmi, A. Hecogenin Attenuates Isoproterenol-Induced Myocardial Infarction through Nuclear Factor-Kappa B-Mediated Signaling Pathway in Rats. Pharmacogn. Mag. 2022, 18, 463–469. [Google Scholar]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast: One Function, Multiple Origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The Role of α-Smooth Muscle Actin in Fibroblast-Mediated Matrix Contraction and Remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac Fibroblasts, Fibrosis and Extracellular Matrix Remodeling in Heart Disease. Fibrogenesis Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Xie, Y.; Zhang, Y.; Dong, F.; Wu, K.; Yang, L.; Lv, H. Exploring the Anti-oxidative Mechanisms of Rhodiola Rosea in Ameliorating Myocardial Fibrosis through Network Pharmacology and in Vitro Experiments. Mol. Med. Rep. 2024, 30, 214. [Google Scholar] [CrossRef]
- Li, F.; Li, S.-S.; Chen, H.; Zhao, J.-Z.; Hao, J.; Liu, J.-M.; Zu, X.-G.; Cui, W. miR-320 Accelerates Chronic Heart Failure with Cardiac Fibrosis through Activation of the IL6/STAT3 Axis. Aging 2021, 13, 22516–22527. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Bansal, T.; Rana, S.; Datta, K.; Datta Chaudhuri, R.; Chawla-Sarkar, M.; Sarkar, S. Myocyte-Derived Hsp90 Modulates Collagen Upregulation via Biphasic Activation of STAT-3 in Fibroblasts during Cardiac Hypertrophy. Mol. Cell Biol. 2017, 37, e00611-16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cheng, G.; Jin, R.; Afzal, M.R.; Samanta, A.; Xuan, Y.-T.; Girgis, M.; Elias, H.K.; Zhu, Y.; Davani, A.; et al. Deletion of Interleukin-6 Attenuates Pressure Overload-Induced Left Ventricular Hypertrophy and Dysfunction. Circ. Res. 2016, 118, 1918–1929, Erratum in Circ. Res. 2020, 126, e35. [Google Scholar] [CrossRef]
- González, G.E.; Rhaleb, N.-E.; D’Ambrosio, M.A.; Nakagawa, P.; Liu, Y.; Leung, P.; Dai, X.; Yang, X.-P.; Peterson, E.L.; Carretero, O.A. Deletion of Interleukin-6 Prevents Cardiac Inflammation, Fibrosis and Dysfunction without Affecting Blood Pressure in Angiotensin II-High Salt-Induced Hypertension. J. Hypertens. 2015, 33, 144–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-H.; Zhang, Y.-Y.; Wang, Y.-Z.; Wang, J.; Zhao, Y.; Jin, X.-X.; Xue, G.-L.; Li, P.-H.; Sun, Y.-L.; et al. Deletion of Interleukin-6 Alleviated Interstitial Fibrosis in Streptozotocin-Induced Diabetic Cardiomyopathy of Mice through Affecting TGFβ1 and miR-29 Pathways. Sci. Rep. 2016, 6, 23010. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling Pathway of MAPK/ERK in Cell Proliferation, Differentiation, Migration, Senescence and Apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic Stress Promotes Gastric Cancer Progression and Metastasis: An Essential Role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef]
- Hao, H.; Yuan, T.; Li, Z.; Zhang, C.; Liu, J.; Liang, G.; Feng, L.; Pan, Y. Curcumin Analogue C66 Ameliorates Mouse Cardiac Dysfunction and Structural Disorders after Acute Myocardial Infarction via Suppressing JNK Activation. Eur. J. Pharmacol. 2023, 946, 175629. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Sun, Y.; Zhang, S.; Pan, R.; Xie, Y.; Chen, J.; Shi, L.; Chen, Y.; Sun, Z.; et al. Insulin-Like Growth Factor 1 Receptor Deficiency Alleviates Angiotensin II-Induced Cardiac Fibrosis Through the Protein Kinase B/Extracellular Signal-Regulated Kinase/Nuclear Factor-κB Pathway. J. Am. Heart Assoc. 2023, 12, e029631. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Jouenne, F.; Tazi, A. The MAPK Pathway in Pulmonary Langerhans Cell Histiocytosis. Arch. Bronconeumol. 2023, 59, 347–349. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.G. Role of JNK Signaling in Oral Cancer: A Mini Review. Tumour Biol. 2017, 39, 1010428317711659. [Google Scholar] [CrossRef] [PubMed]
- Bubici, C.; Papa, S. JNK Signalling in Cancer: In Need of New, Smarter Therapeutic Targets. Br. J. Pharmacol. 2014, 171, 24–37. [Google Scholar] [CrossRef]
- Whitham, M.; Chan, M.H.S.; Pal, M.; Matthews, V.B.; Prelovsek, O.; Lunke, S.; El-Osta, A.; Broenneke, H.; Alber, J.; Brüning, J.C.; et al. Contraction-Induced Interleukin-6 Gene Transcription in Skeletal Muscle Is Regulated by c-Jun Terminal Kinase/Activator Protein-1. J. Biol. Chem. 2012, 287, 10771–10779. [Google Scholar] [CrossRef]
- Rohrbach, S.; Engelhardt, S.; Lohse, M.J.; Werdan, K.; Holtz, J.; Muller-Werdan, U. Activation of AP-1 Contributes to the Beta-Adrenoceptor-Mediated Myocardial Induction of Interleukin-6. Mol. Med. 2007, 13, 605–614. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, X.-J.; Zhang, W.-P.; Fang, T.-T.; Hu, J.; Ma, S.-F.; Gao, Q. The Protective Effect of Low-Dose Ethanol on Myocardial Fibrosis through Downregulating the JNK Signaling Pathway in Diabetic Rats. J. Diabetes Res. 2016, 2016, 3834283. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-C.; Chen, C.-L.; Zhang, Y.; Mellor, R.L.; Kanter, E.M.; Fang, Y.; Wang, H.-C.; Hung, C.-T.; Nong, J.-Y.; Chen, H.-J.; et al. Endoplasmic Reticulum Protein TXNDC5 Augments Myocardial Fibrosis by Facilitating Extracellular Matrix Protein Folding and Redox-Sensitive Cardiac Fibroblast Activation. Circ. Res. 2018, 122, 1052–1068. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Jin, W.; Liu, W.; Li, Z.; Fan, Z.; Zou, L.; Chen, S.; Zhu, L.; Zhao, Q.; et al. 2-APQC, a Small-Molecule Activator of Sirtuin-3 (SIRT3), Alleviates Myocardial Hypertrophy and Fibrosis by Regulating Mitochondrial Homeostasis. Signal Transduct. Target. Ther. 2024, 9, 133. [Google Scholar] [CrossRef]

| Gene | Forward | Reverse |
|---|---|---|
| IL-6 | CGATGATGCACTGTCAGAAAAC | ACTCCAGGTAGAAACGGAACTC |
| β-actin | TGACGTTGACATCCGTAAAGACC | GTGCTAGGAGCCAGGGCAGTAA |
| Ingredients | CAS Number | Targets | Score (kcal/mol) |
|---|---|---|---|
| Laurolitsine | 5890-18-6 | ADRB2 | −10.2 |
| Hecogenin | 467-55-0 | MAPK9 | −10.1 |
| Skimmin | 93-39-0 | HSP90 | −9.9 |
| Hecogenin | 467-55-0 | MAPK14 | −9.5 |
| Levistolide A | 88182-33-6 | MAPK11 | −9.5 |
| Higenamine | 5843-65-2 | ADRB2 | −9.4 |
| Hecogenin | 467-55-0 | MAPK8 | −9.4 |
| (+)-Catechin | 154-23-4 | HSP90 | −9.4 |
| Norisoboldine | 23599-69-1 | IRAK4 | −9.3 |
| Dihydroartemisinin | 71939-50-9 | MAPK14 | −9.1 |
| Phellodendrine | 104112-82-5 | HSP90 | −9.1 |
| Norisoboldine | 23599-69-1 | CHUK | −9.0 |
| Boldine | 476-70-0 | MAP2K1 | −9.0 |
| Levistolide A | 88182-33-6 | MAPK14 | −8.9 |
| Hecogenin | 467-55-0 | MAP2K2 | −8.9 |
| (−)-Secoisolariciresinol | 29388-59-8 | HSP90 | −8.8 |
| Higenamine | 5843-65-2 | ADRB1 | −8.7 |
| Hecogenin | 467-55-0 | MAP2K1 | −8.7 |
| Hecogenin | 467-55-0 | MAPK10 | −8.7 |
| O-Desmethylmycophenolic acid | 31858-65-8 | MAPK10 | −8.7 |
| Levistolide A | 88182-33-6 | MAP3K8 | −8.7 |
| Dihydroartemisinin | 71939-50-9 | MAPK3 | −8.7 |
| Dihydroartemisinin | 71939-50-9 | MAPK10 | −8.6 |
| Levistolide A | 88182-33-6 | MAPK8 | −8.5 |
| [6]-Gingerol | 23513-14-6 | ADRB1 | −8.4 |
| Laurolitsine | 5890-18-6 | CHUK | −8.4 |
| (−)-Secoisolariciresinol | 29388-59-8 | MAP2K1 | −8.4 |
| Dihydroartemisinin | 71939-50-9 | MAP2K1 | −8.4 |
| Levistolide A | 88182-33-6 | MAPK9 | −8.4 |
| Boldine | 476-70-0 | MAPK1 | −8.3 |
| Boldine | 476-70-0 | MAPK8 | −8.3 |
| O-Desmethylmycophenolic acid | 31858-65-8 | IRAK4 | −8.3 |
| Laurolitsine | 5890-18-6 | HSP90 | −8.3 |
| (−)-Secoisolariciresinol | 29388-59-8 | ADRB2 | −8.2 |
| Phellodendrine | 104112-82-5 | ADRB2 | −8.2 |
| Scopoletin | 92-61-5 | ADRB2 | −8.2 |
| Skimmin | 93-39-0 | TNF | −8.2 |
| O-Desmethylmycophenolic acid | 31858-65-8 | MAPK14 | −8.2 |
| Levistolide A | 88182-33-6 | MAPK10 | −8.2 |
| Norisoboldine | 23599-69-1 | ADRB1 | −8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Li, Y.; Zhu, C.; Guo, M.; Liu, X.; Chen, X. Cinnamomum migao H.W. Li Ethanol-Water Extract Suppresses IL-6 Production in Cardiac Fibroblasts: Mechanisms Elucidated via UPLC-Q-TOF-MS, Network Pharmacology, and Experimental Assays. Curr. Issues Mol. Biol. 2025, 47, 798. https://doi.org/10.3390/cimb47100798
Lu Y, Li Y, Zhu C, Guo M, Liu X, Chen X. Cinnamomum migao H.W. Li Ethanol-Water Extract Suppresses IL-6 Production in Cardiac Fibroblasts: Mechanisms Elucidated via UPLC-Q-TOF-MS, Network Pharmacology, and Experimental Assays. Current Issues in Molecular Biology. 2025; 47(10):798. https://doi.org/10.3390/cimb47100798
Chicago/Turabian StyleLu, Yuxin, Yaofeng Li, Can Zhu, Mengyue Guo, Xia Liu, and Xiangyun Chen. 2025. "Cinnamomum migao H.W. Li Ethanol-Water Extract Suppresses IL-6 Production in Cardiac Fibroblasts: Mechanisms Elucidated via UPLC-Q-TOF-MS, Network Pharmacology, and Experimental Assays" Current Issues in Molecular Biology 47, no. 10: 798. https://doi.org/10.3390/cimb47100798
APA StyleLu, Y., Li, Y., Zhu, C., Guo, M., Liu, X., & Chen, X. (2025). Cinnamomum migao H.W. Li Ethanol-Water Extract Suppresses IL-6 Production in Cardiac Fibroblasts: Mechanisms Elucidated via UPLC-Q-TOF-MS, Network Pharmacology, and Experimental Assays. Current Issues in Molecular Biology, 47(10), 798. https://doi.org/10.3390/cimb47100798





