Antipsychotic Chlorpromazine Suppresses STAT5 Signaling, Overcomes Resistance Mediated by the Gatekeeper Mutation FLT3-ITD/F691L, and Synergizes with Quizartinib in FLT3-ITD-Positive Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies (Abs)
2.2. Cell Lines and Cell Cultures
- Forward: 5′-cat ctc gag cac cat gga tgc ggg cgt tgg-3′
- Forward (for replacement): 5′-tca cca tag caa caa tat tcc aaa atc aag-3′
- Reverse (for replacement): 5′-ggc cag tgt act tga ttt tgg aat att gtt-3′
- Reverse: 5′-cat gtt aac cta act tct ttc tcc gtg aat ctt-3′
2.3. Cell Viability Assays
2.4. Interactive Analysis and Consensus Interpretation of Multi-Drug Synergies
2.5. Immunoblotting
2.6. Statistical Analyses
3. Results
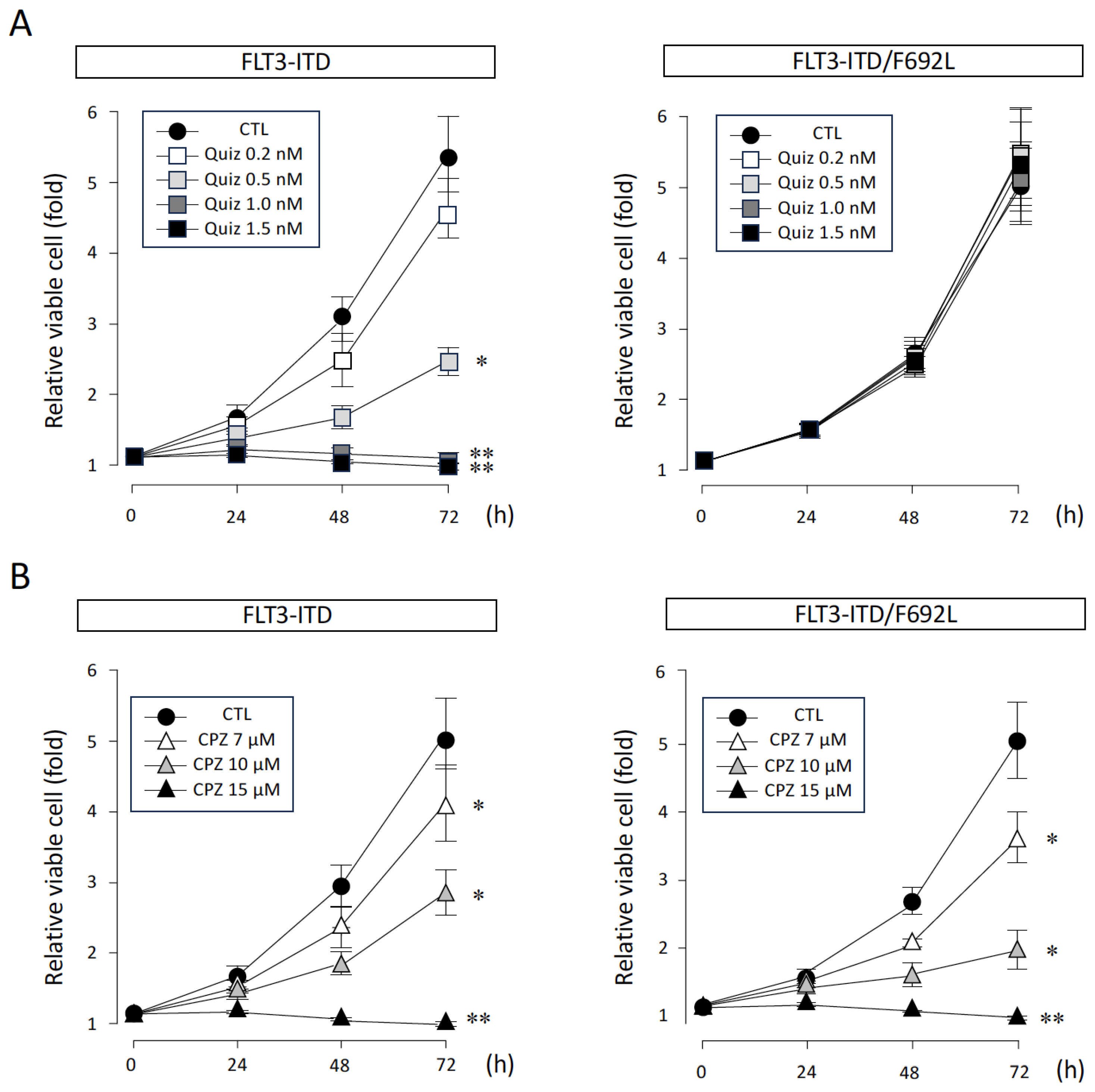
3.1. Effects of CPZ and Quiz on FLT3-ITD-Dependent Proliferation of Ba/F3 Cells
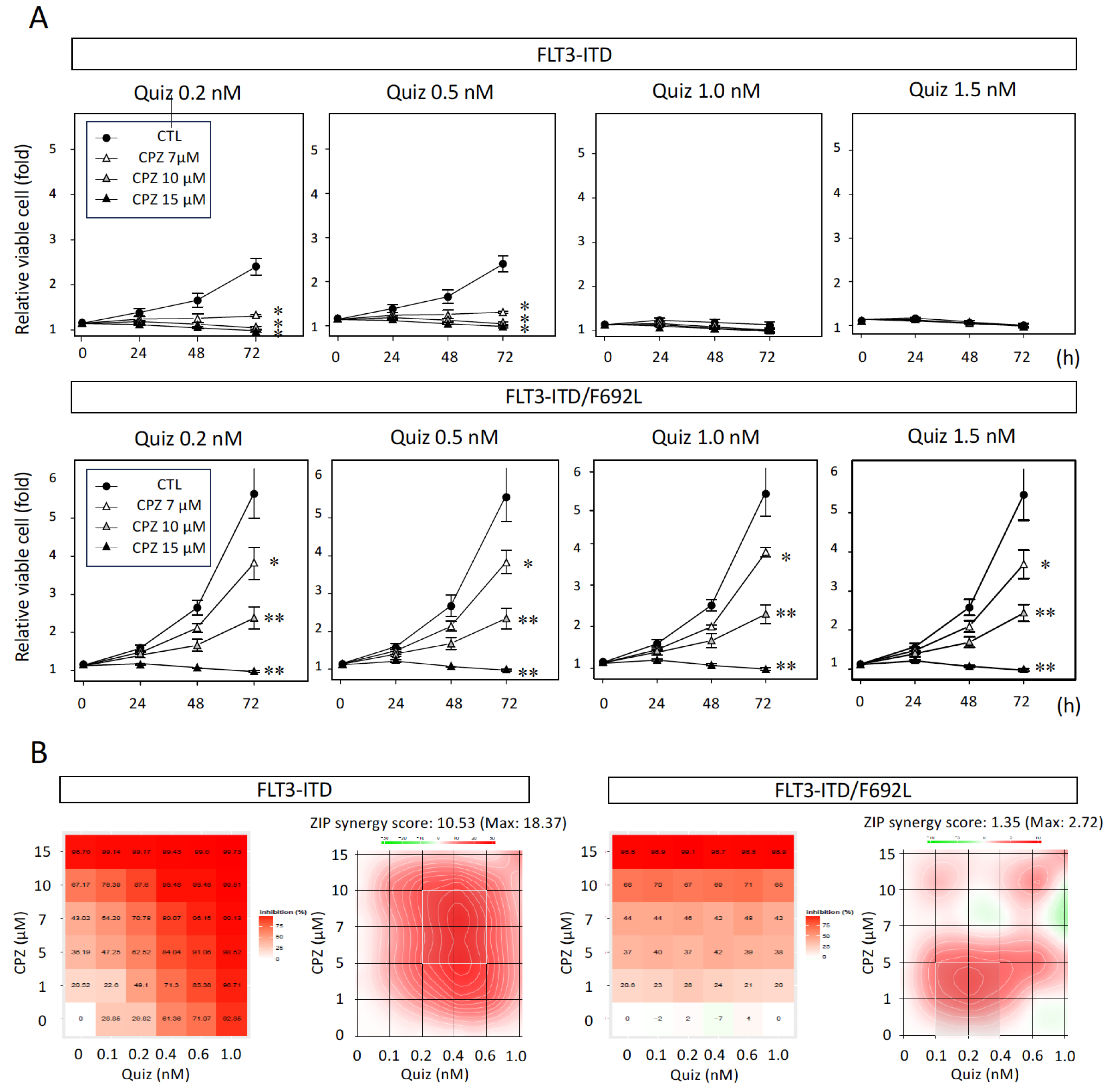
3.2. Synergistic Inhibitory Effect of CPZ with Quiz in FLT3-ITD-Expressing Cells
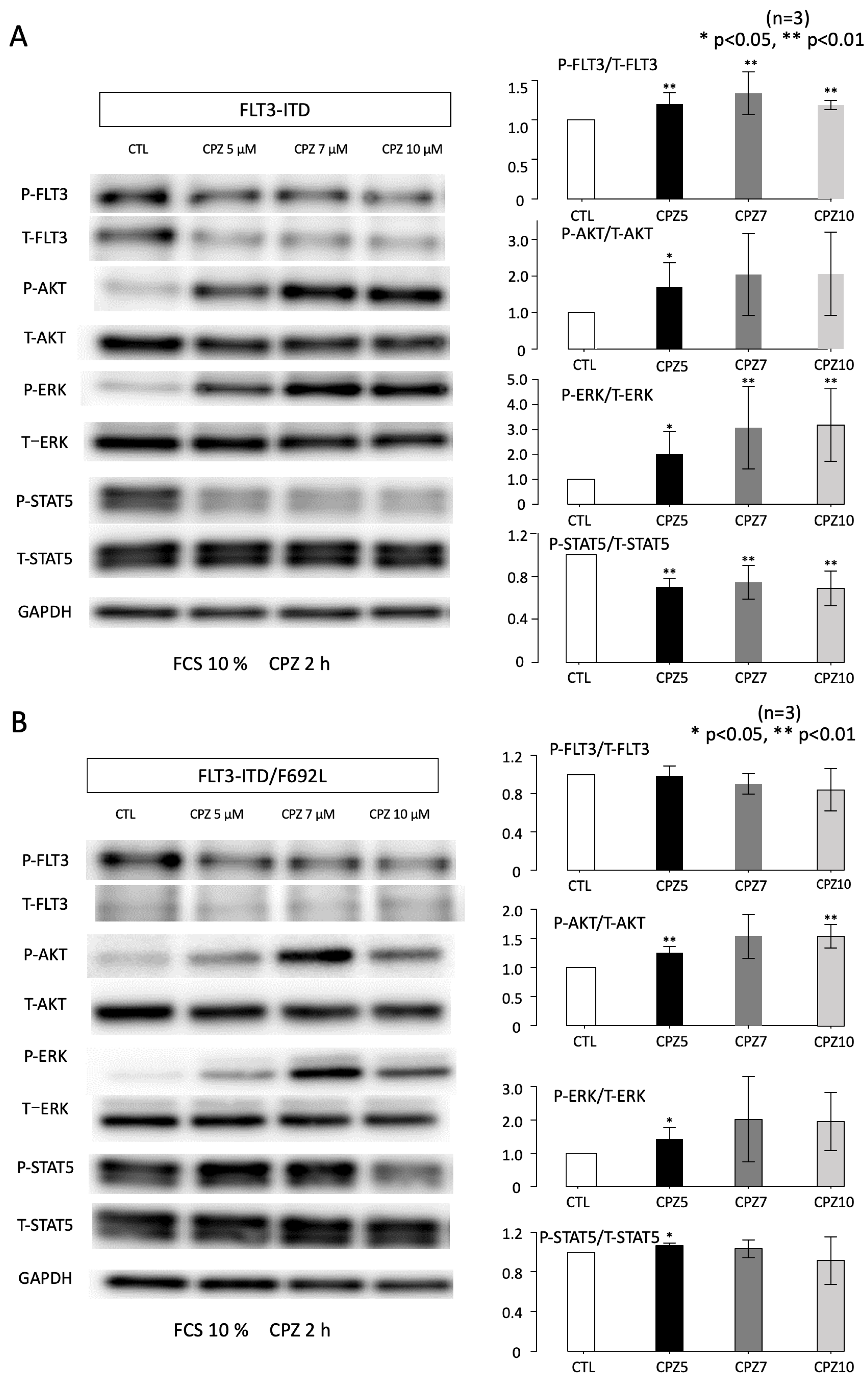
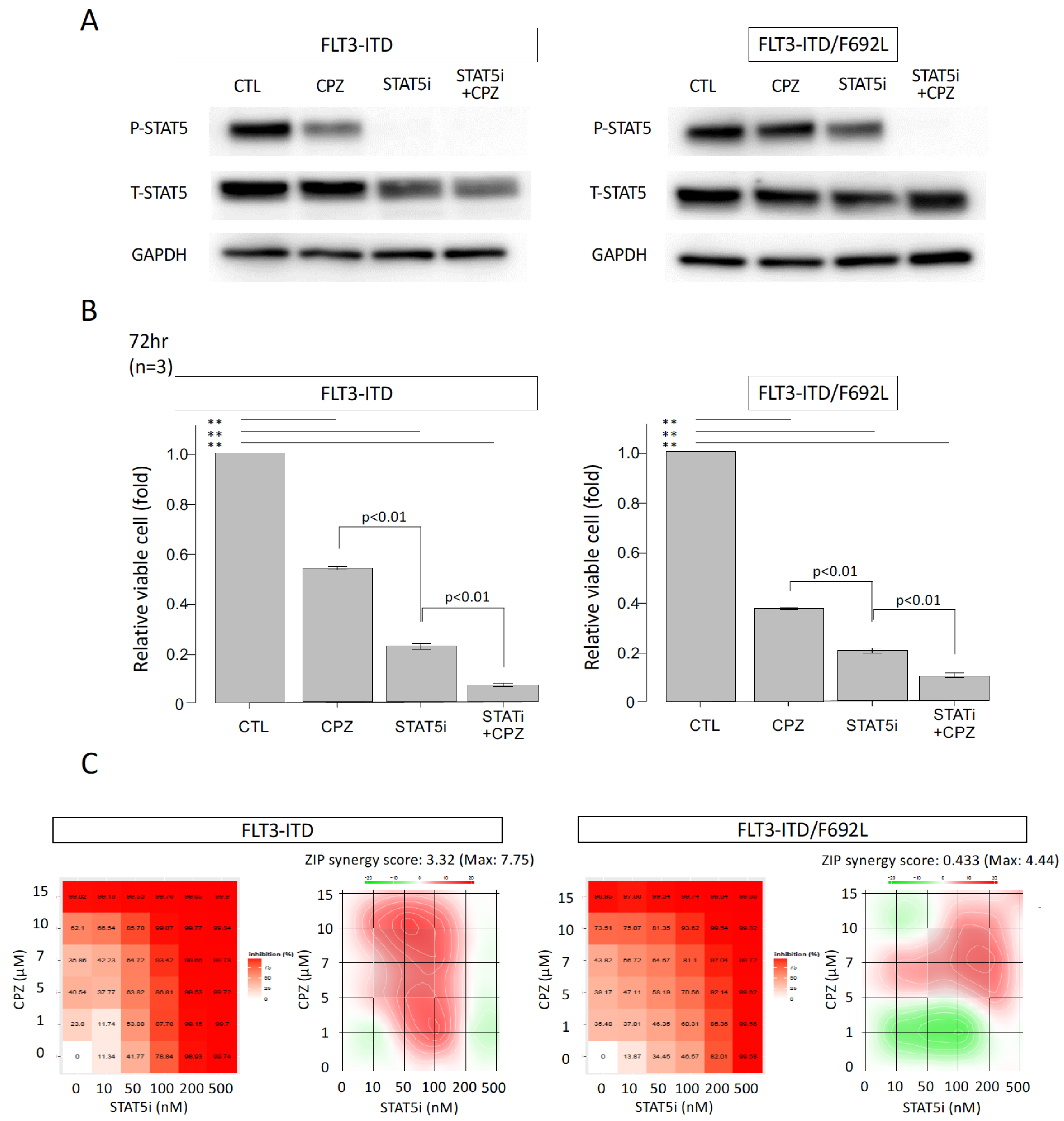
3.3. Effects of CPZ on AKT and STAT5 Activities
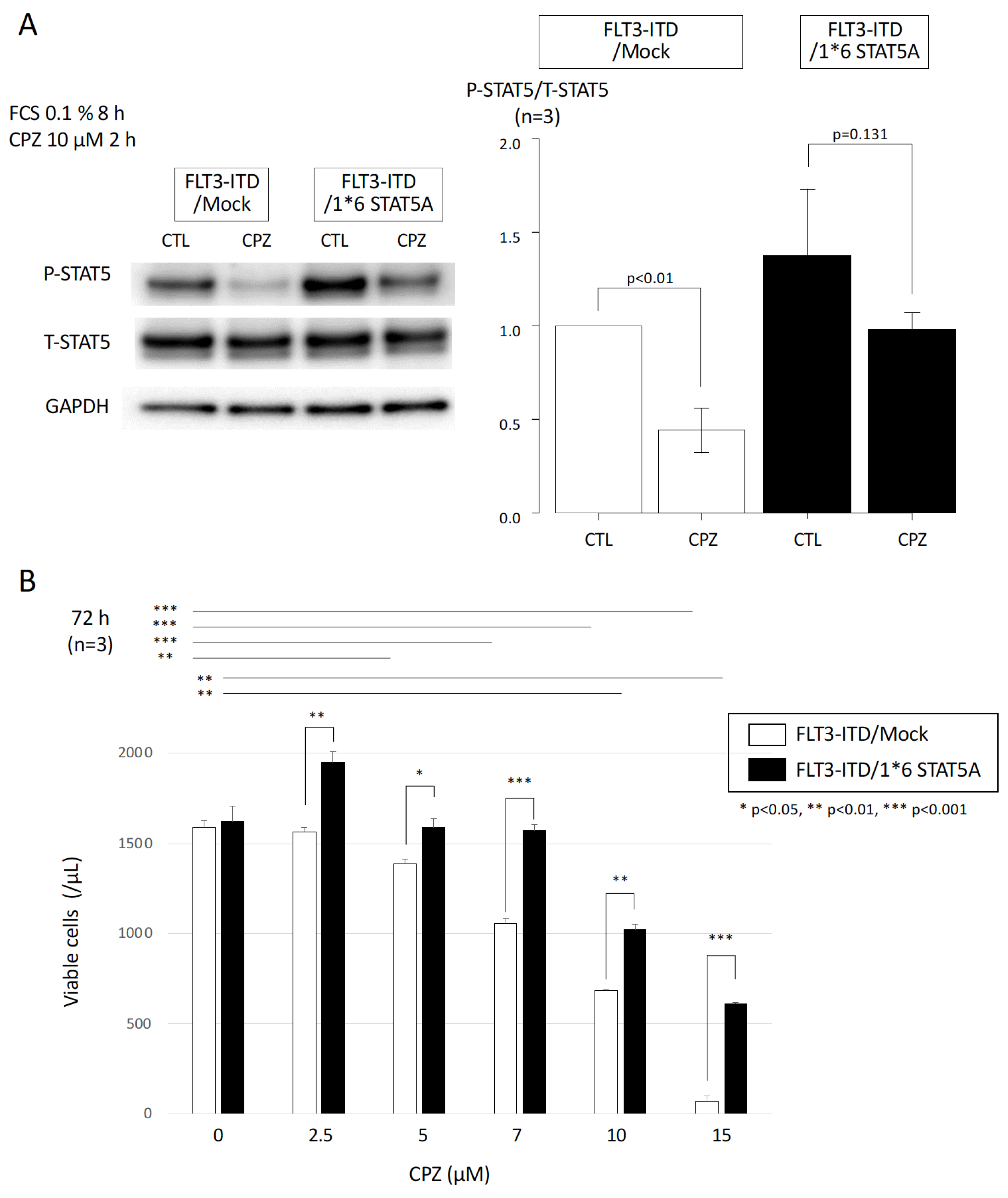
3.4. Effect of Constitutively Active STAT5 on CPZ-Mediated Growth Inhibition in FLT3-ITD-Expressing Cells
3.5. Additive Growth Inhibitory Effect of CPZ and a STAT5 Inhibitor in FLT3-ITD-Expressing and FLT3-ITD/F692L-Expressing Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| FLT3 | FMS-like tyrosine kinase 3 |
| ITD | internal tandem duplication |
| TKD | tyrosine kinase domain |
| TKIs | tyrosine kinase inhibitors |
| Gil | gilteritinib |
| Quiz | quizartinib |
| GEF | gefitinib |
| CPZ | Chlorpromazine |
| STAT5 | signal transducer and activator of transcription 5 |
| RTKs | receptor tyrosine kinases |
References
- Kottaridis, P.D.; Gale, R.E.; Frew, M.E.; Harrison, G.; Langabeer, S.E.; Belton, A.A.; Walker, H.; Wheatley, K.; Bowe, D.T.; Burnett, A.K.; et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001, 98, 1752–1759. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Smith, C.C. Flt3mutations in acute myeloid leukemia: Key concepts and emerging controversies. Front. Oncol. 2020, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.J.; Linch, D.C.; Medical Research Council Adult Leukaemia Working Party. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.; Montesinos, P.; Thomas, X.; Griskevicius, L.; Cluzeau, T.; Caillot, D.; Legrand, O.; Minotti, C.; Luppi, M.; Farkas, F.; et al. Midostaurin plus daunorubicin or idarubicin for young and older adults with FLT3-mutated AML: A phase 3b trial. Blood Adv. 2023, 7, 6441–6450. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolin, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2022, 381, 1728–1740, Erratum in N. Engl. J. Med. 2022, 386, 1868. [Google Scholar] [CrossRef] [PubMed]
- Erba, H.P.; Montesinos, P.; Kim, H.J.; Patkowska, E.; Vrhovac, R.; Žák, P.; Wang, P.N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1571–1583. [Google Scholar] [CrossRef]
- Mohamed Jiffry, M.Z.; Kloss, R.; Ahmed-Khan, M.; Carmona-Pires, F.; Okam, N.; Weeraddana, P.; Dharmaratna, D.; Dandwani, M.; Moin, K. A review of treatment options employed in relapsed/refractory AML. Hematology 2023, 28, 2196482. [Google Scholar] [CrossRef]
- Thol, F.; Döhner, H.; Ganser, A. How I treat refractory and relapsed acute myeloid leukemia. Blood 2024, 143, 11–20. [Google Scholar] [CrossRef]
- Erba, H.P. A study to learn how well quizartinib with chemotherapy works and how safe it is in people with acute myeloid leukemia that is FLT3-ITD-positive: A plain language summary of the QuANTUM-First study. Future Oncol. 2025, 21, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Pollye, D.A.; Altma, J.K.; Assi, R.; Bixby, D.; Fathi, A.T.; Foran, J.M.; Gojo, I.; Hall, A.C.; Jonas, B.N.A.; Kishtagari, A.; et al. Acute Myeloid Leukemia, Version 3.2023, NCCN Clinical Practice Guidelinesin Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Levis, M. FLT3 mutations in acute myeloid leukemia: What is the best approach in 2013? Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 220–226. [Google Scholar] [CrossRef]
- Smith, C.C.; Wang, Q.; Chin, C.S.; Salerno, S.; Damon, L.E.; Levis, M.J.; Perl, A.E.; Travers, K.J.; Wang, S.; Hunt, J.P.; et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acutemyeloid leukaemia. Nature 2012, 485, 260–263. [Google Scholar] [CrossRef]
- Smith, C.C.; Lin, K.; Stecula, A.; Sali, A.; Shah, N.P. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia 2015, 29, 2390–2392. [Google Scholar] [CrossRef]
- Capelli, D.; Menotti, D.; Fiorentini, A.; Saraceni, F.; Olivieri, A. Overcoming Resistance: FLT3 Inhibitors Past, Present, Future and the Challenge of Cure. Cancers 2022, 14, 4315. [Google Scholar] [CrossRef]
- Kiyoi, H.; Kawashima, N.; Ishikawa, Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020, 111, 312–322. [Google Scholar] [CrossRef]
- Desikan, S.P.; Daver, N.; DiNardo, C.; Kadia, T.; Konopleva, M.; Ravandi, F. Resistance to targeted therapies: Delving into FLT3 and IDH. Blood Cancer J. 2022, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Garciaz, S.; Hospital, M.A. FMS-Like Tyrosine Kinase 3 Inhibitors in the Treatment of Acute Myeloid Leukemia: An Update on the Emerging Evidence and Safety Profile. Onco Targets Ther. 2023, 16, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Scholl, S.; Fleischmann, M.; Schnetzke, U.; Heidel, F.H. Molecular Mechanisms of Resistance to FLT3 Inhibitors in Acute MyeloidLeukemia: Ongoing Challenges and Future Treatments. Cells 2020, 9, 2493. [Google Scholar] [CrossRef] [PubMed]
- Traer, E.; Martinez, J.; Javidi-Sharifi, N.; Agarwal, A.; Dunlap, J.; English, I.; Kovacsovics, T.; Tyner, J.W.; Wong, M.; Druker, B.J. FGF2 from Marrow Microenvironment Promotes Resistance to FLT3 Inhibitors in Acute Myeloid Leukemia. Cancer Res. 2016, 76, 6471–6482. [Google Scholar] [CrossRef]
- Chang, Y.T.; Hernandez, D.; Alonso, S.; Gao, M.; Su, M.; Ghiaur, G.; Levis, M.J.; Jones, R.J. Role of CYP3A4 in bone marrow microenvironment-mediated protection of FLT3/ITD AML from tyrosine kinase inhibitors. Blood Adv. 2019, 3, 908–916. [Google Scholar] [CrossRef]
- Zhou, J.; Chng, W.J. Resistance to FLT3 inhibitors in acute myeloid leukemia: Molecularmechanisms and resensitizing strategies. World J. Clin. Oncol. 2018, 9, 90–97. [Google Scholar] [CrossRef]
- Dumas, P.Y.; Naudin, C.; Martin-Lannerée, S.; Izac, B.; Casetti, L.; Mansier, O.; Rousseau, B.; Artus, A.; Dufossée, M.; Giese, A.; et al. Hematopoietic niche drives FLT3-ITD acute myeloid leukemia resistance to quizartinib via STAT5-and hypoxia-dependent upregulation of AXL. Haematologica 2019, 104, 2017–2027. [Google Scholar] [CrossRef]
- Miaczynska, M. Effects of membrane trafficking on signaling by receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a009035. [Google Scholar] [CrossRef]
- Goh, L.K.; Sorkin, A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a017459. [Google Scholar] [CrossRef]
- Critchley, W.R.; Pellet-Many, C.; Ringham-Terry, B.; Harrison, M.A.; Zachary, I.C.; Ponnambalam, S. Receptor Tyrosine Kinase Ubiquitination and De-Ubiquitination in Signal Transduction and Receptor Trafficking. Cells 2018, 7, 22. [Google Scholar] [CrossRef]
- Xiang, Z.; Kreisel, F.; Cain, J.; Colson, A.; Tomasson, M.H. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signaling. Mol. Cell Biol. 2007, 27, 267–282. [Google Scholar] [CrossRef]
- Obata, Y.; Toyoshima, S.; Wakamatsu, E.; Suzuki, S.; Ogawa, S.; Esumi, H.; Abe, R. Oncogenic Kit signals on endolysosomes and endoplasmic reticulum are essential for neoplastic mast cell proliferation. Nat. Commun. 2014, 5, 5715. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Olsen, J.V.; Brandts, C.; Cox, J.; Reddy, P.N.; Böhmer, F.D.; Gerke, V.; Schmidt-Arras, D.E.; Berdel, W.E.; Müller-Tidow, C.; et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell 2009, 36, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, K.; Shiina, I.; Murata, T.; Tateyama, S.; Maekawa, Y.; Niwa, M.; Shimonaka, M.; Okamoto, K.; Suzuki, T.; Nishida, T.; et al. FLT3-ITD transduces autonomous growth signals during its biosynthetic trafficking in acute myelogenous leukemia cells. Sci. Rep. 2021, 11, 22678. [Google Scholar] [CrossRef]
- Joffre, C.; Barrow, R.; Ménard, L.; Calleja, V.; Hart, I.R.; Kermorgant, S. A direct role for Met endocytosis in tumorigenesis. Nat. Cell Biol. 2011, 13, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Tanaka, H.; Suzuki, M.; Ogoh, H.; Taniguchi, Y.; Morita, Y.; Shimada, T.; Tanimura, A.; Matsui, K.; Yokota, T.; et al. Clathrin assembly protein CALM plays a critical role in KIT signaling by regulating its cellular transport from early to late endosomes in hematopoietic cells. PLoS ONE 2014, 9, e109441. [Google Scholar] [CrossRef]
- Rai, S.; Tanaka, H.; Suzuki, M.; Espinoza, J.L.; Kumode, T.; Tanimura, A.; Yokota, T.; Oritani, K.; Watanabe, T.; Kanakura, Y.; et al. Chlorpromazine eliminates acute myeloid leukemia cells by perturbing subcellular localization of FLT3-ITD and KIT-D816V. Nat. Commun. 2020, 11, 4147. [Google Scholar] [CrossRef]
- Onishi, M.; Nosaka, T.; Misawa, K.; Mui, A.L.; Gorman, D.; McMahon, M.; Miyajima, A.; Kitamura, T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol. Cell Biol. 1998, 18, 3871–3879. [Google Scholar] [CrossRef]
- Schuringa, J.J.; Chung, K.Y.; Morrone, G.; Moore, M.A. Constitutive activation of STAT5A promotes human hematopoietic stem cell self-renewal and erythroid differentiation. J. Exp. Med. 2004, 200, 623–635. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, 739–743. [Google Scholar] [CrossRef]
- Albers, C.; Leischner, H.; Verbeek, M.; Yu, C.; Illert, A.L.; Peschel, C.; von Bubnoff, N.; Duyster, J. The secondary FLT3-ITD F691L mutation induces resistance to AC220 in FLT3-ITD+ AML but retains in vitro sensitivity to PKC412 and Sunitinib. Leukemia 2013, 27, 1416–1418. [Google Scholar] [CrossRef]
- Fujiwara, R.; Taniguchi, Y.; Rai, S.; Iwata, Y.; Fujii, A.; Fujimoto, K.; Kumode, T.; Serizawa, K.; Morita, Y.; Espinoza, J.L.; et al. Chlorpromazine cooperatively induces apoptosis with tyrosine kinase inhibitors in EGFR-mutated lung cancer cell lines and restores the sensitivity to gefitinib in T790M-harboring resistant cells. Biochem. Biophys. Res. Commun. 2022, 626, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xi, H.; Liao, M.; Zhang, Y.; Ma, H.; Wu, M.; Xue, Q.; Sun, H.; Zhang, Y.; Xia, Y. Repurposed antipsychotic chlorpromazine inhibits colorectal cancer and pulmonary metastasis by inducing G2/M cell cycle arrest, apoptosis, and autophagy. Cancer Chemother. Pharmacol. 2022, 89, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Jhou, A.J.; Chang, H.C.; Hung, C.C.; Lin, H.C.; Lee, Y.C.; Liu, W.T.; Han, K.F.; Lai, Y.W.; Lin, M.Y.; Lee, C.H. Chlorpromazine, an antipsychotic agent, induces G2/M phase arrest and apoptosis via regulation of the PI3K/AKT/mTOR-mediated autophagy pathways in human oral cancer. Biochem. Pharmacol. 2021, 184, 114403. [Google Scholar] [CrossRef]
- Li, N.; Rao, L.; Zhao, X.; Shen, J.; Su, D.; Ma, G.; Sun, S.; Ma, Q.; Zhang, L.; Dong, C.; et al. Chlorpromazine affects autophagy in association with altered Rag GTPase-mTORC1-TFEB signaling. Front. Cell Dev. Biol. 2023, 11, 1266198. [Google Scholar] [CrossRef]
- Udrea, A.M.; Staicu, A.; Smarandache, A.; Andrei, I.R.; Badea, M.A.; Avram, S.; Pascu, M.L.; Pirvulescu, R.A.; Balas, M. Enhancement of chlorpromazine efficacy in breast cancer treatment by 266 nm laser irradiation. Sci. Rep. 2024, 14, 30329. [Google Scholar] [CrossRef]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J. Exp. Clin. Cancer Res. 2021, 40, 347. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Bebawy, M.; Morris, M.B.; Roufogalis, B.D. Selective modulation of P-glycoprotein-mediated drug resistance. Br. J. Cancer 2001, 85, 1998–2003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii-Hanamoto, A.; Tanaka, H.; Fujimoto, K.; Haeno, T.; Miyake, Y.; Fujiwara, R.; Kumode, T.; Serizawa, K.; Morita, Y.; Hanamoto, H.; et al. Antipsychotic Chlorpromazine Suppresses STAT5 Signaling, Overcomes Resistance Mediated by the Gatekeeper Mutation FLT3-ITD/F691L, and Synergizes with Quizartinib in FLT3-ITD-Positive Cells. Curr. Issues Mol. Biol. 2025, 47, 797. https://doi.org/10.3390/cimb47100797
Fujii-Hanamoto A, Tanaka H, Fujimoto K, Haeno T, Miyake Y, Fujiwara R, Kumode T, Serizawa K, Morita Y, Hanamoto H, et al. Antipsychotic Chlorpromazine Suppresses STAT5 Signaling, Overcomes Resistance Mediated by the Gatekeeper Mutation FLT3-ITD/F691L, and Synergizes with Quizartinib in FLT3-ITD-Positive Cells. Current Issues in Molecular Biology. 2025; 47(10):797. https://doi.org/10.3390/cimb47100797
Chicago/Turabian StyleFujii-Hanamoto, Aki, Hirokazu Tanaka, Ko Fujimoto, Takahiro Haeno, Yoshiaki Miyake, Ryosuke Fujiwara, Takahiro Kumode, Kentaro Serizawa, Yasuyoshi Morita, Hitoshi Hanamoto, and et al. 2025. "Antipsychotic Chlorpromazine Suppresses STAT5 Signaling, Overcomes Resistance Mediated by the Gatekeeper Mutation FLT3-ITD/F691L, and Synergizes with Quizartinib in FLT3-ITD-Positive Cells" Current Issues in Molecular Biology 47, no. 10: 797. https://doi.org/10.3390/cimb47100797
APA StyleFujii-Hanamoto, A., Tanaka, H., Fujimoto, K., Haeno, T., Miyake, Y., Fujiwara, R., Kumode, T., Serizawa, K., Morita, Y., Hanamoto, H., Rai, S., & Matsumura, I. (2025). Antipsychotic Chlorpromazine Suppresses STAT5 Signaling, Overcomes Resistance Mediated by the Gatekeeper Mutation FLT3-ITD/F691L, and Synergizes with Quizartinib in FLT3-ITD-Positive Cells. Current Issues in Molecular Biology, 47(10), 797. https://doi.org/10.3390/cimb47100797





