4-Coumarate CoA Ligase Family in Soybean Responds to Heterodera glycines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Nematode
2.2. Lignin Dyeing
2.3. Quantitative RT-PCR
2.4. Vector Constructs and Plant Transformation
2.5. Statistical Analysis
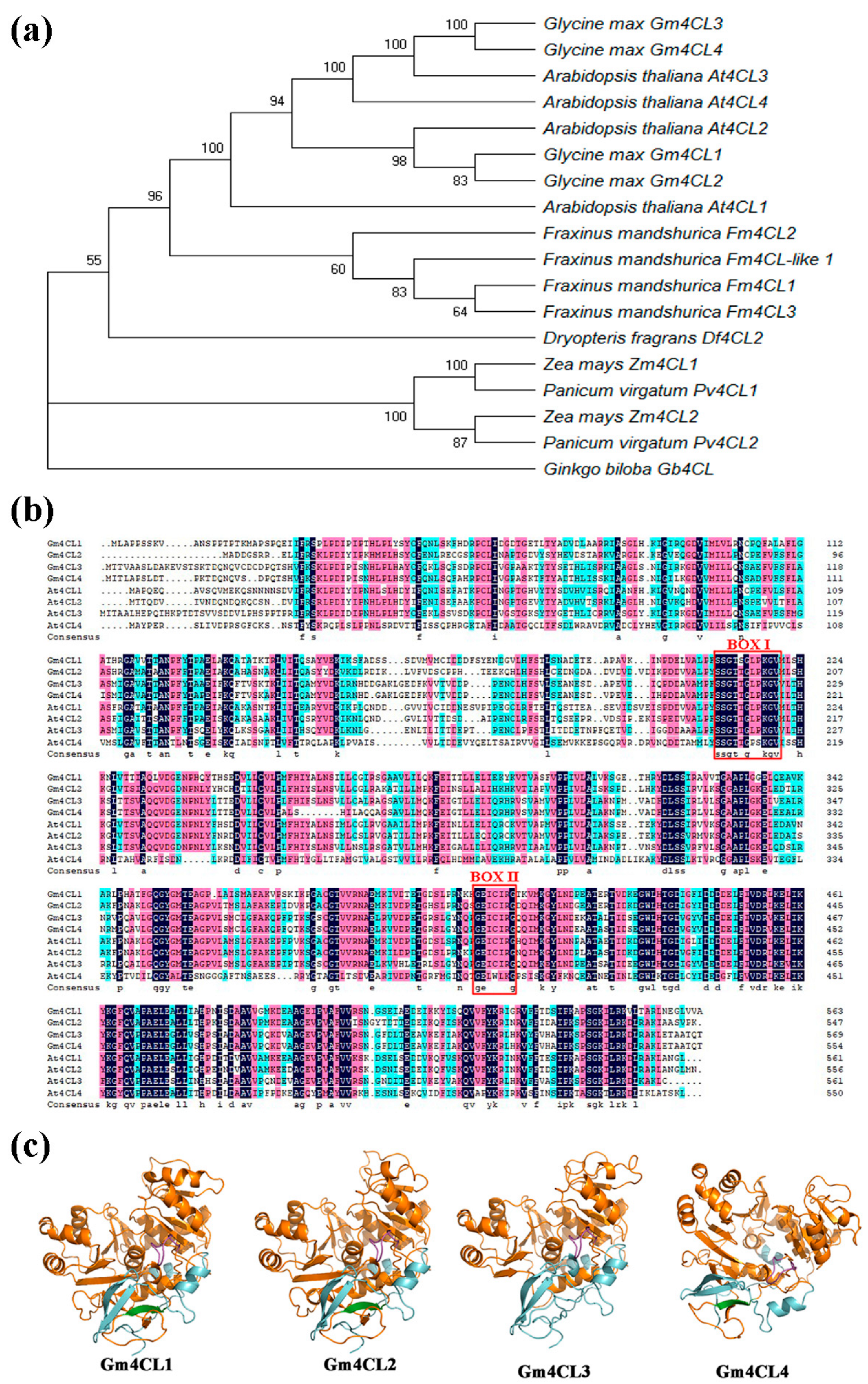
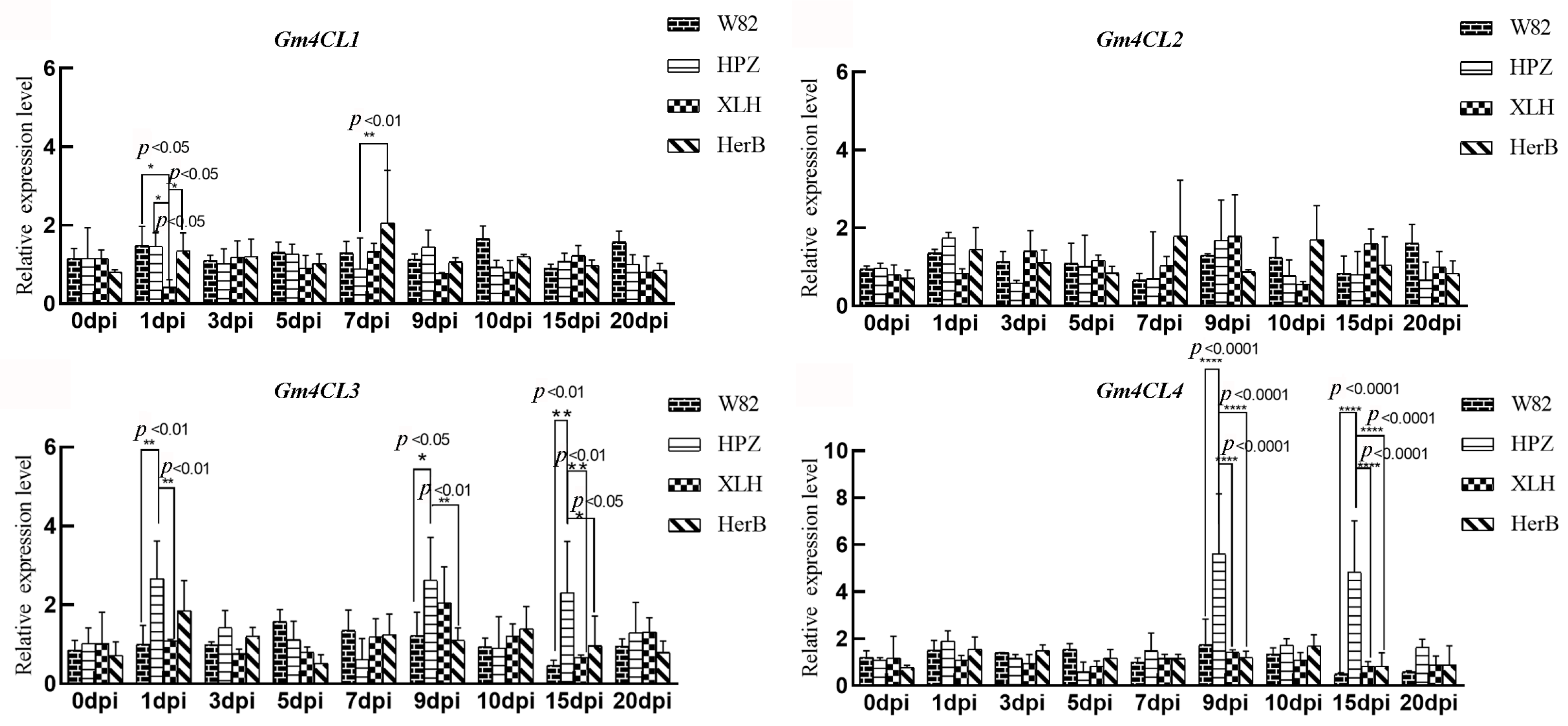
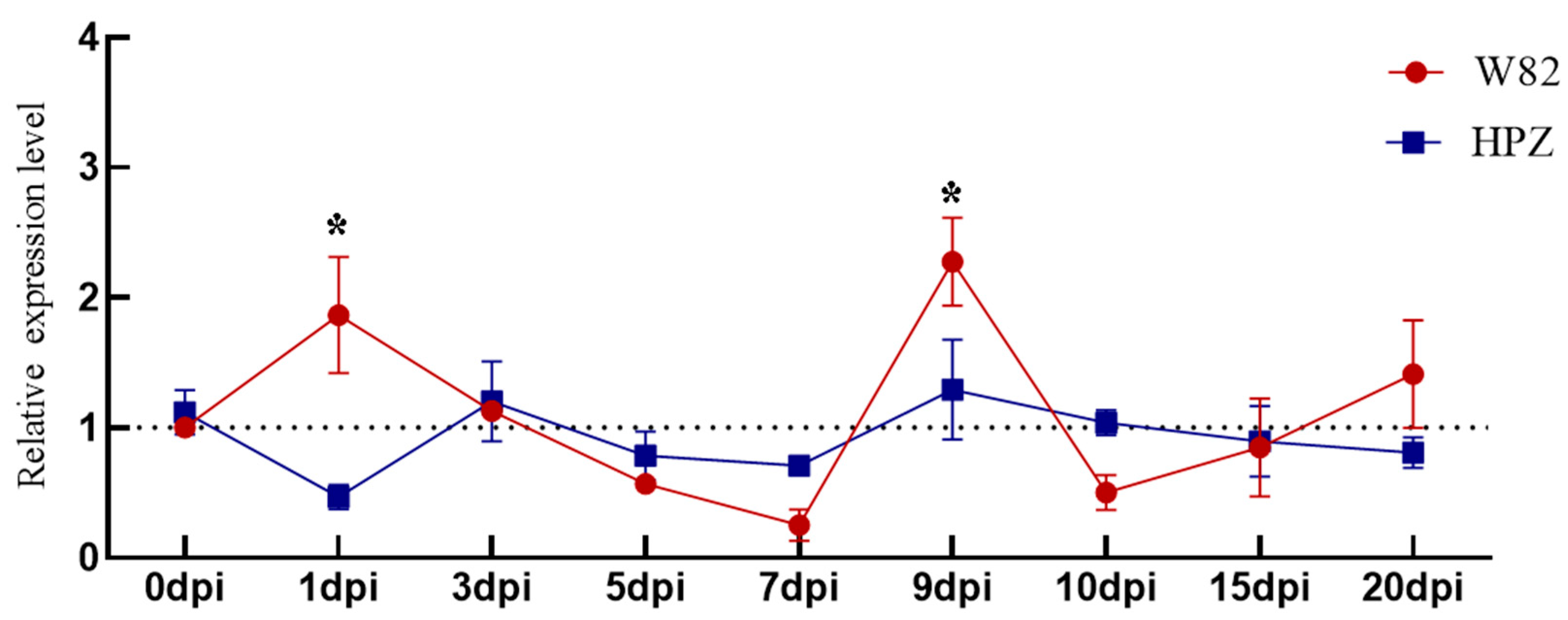
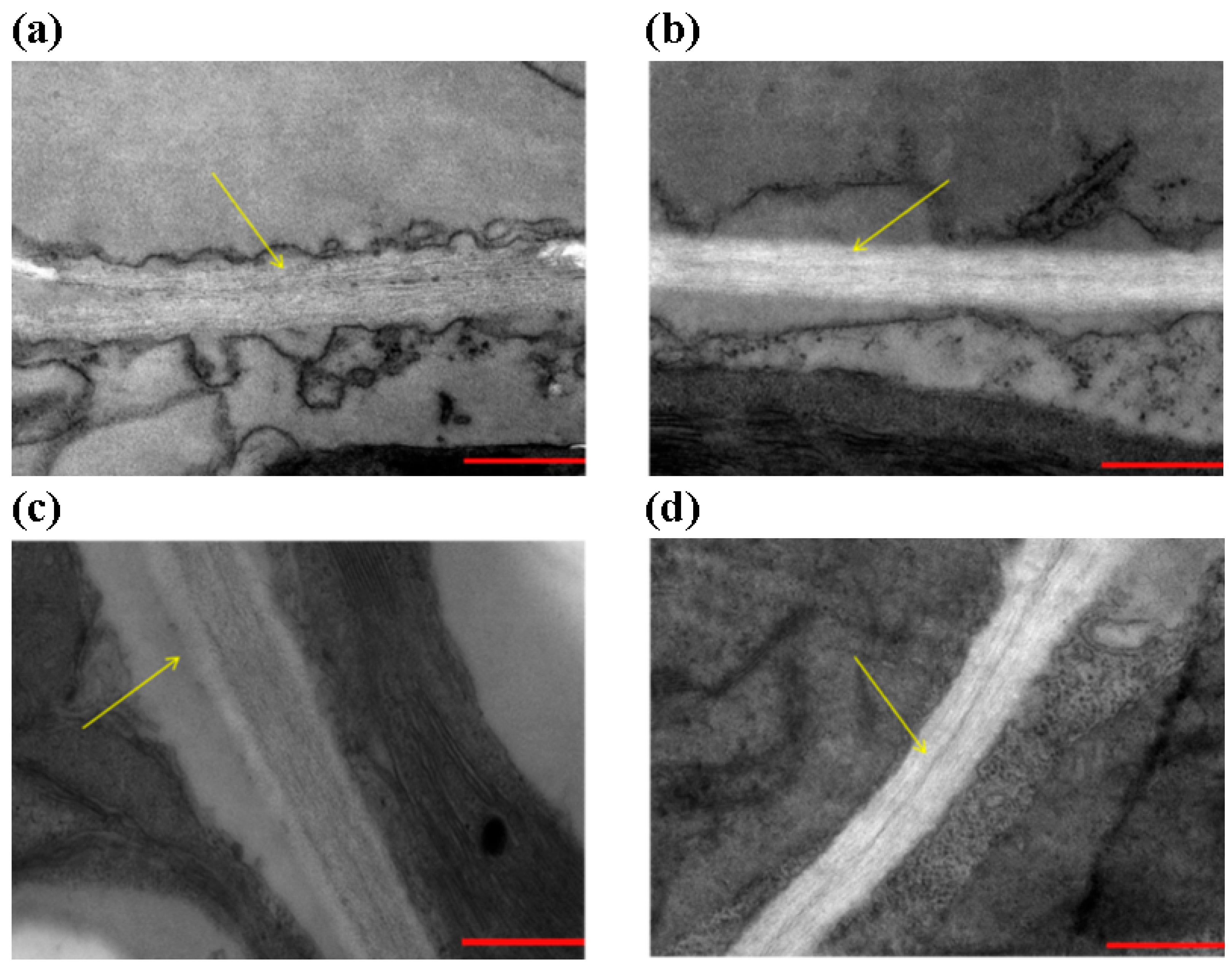
3. Results
3.1. Lignin Response in Roots of SCN-Resistant and SCN-Susceptible Soybean Varieties
3.2. Bioinformatics Analysis of 4CL Family Genes
3.3. Response of the Gm4CL Gene Family to SCN Stress
3.4. Expression of 4CL-Downstream Lignin-Peroxidase (POD) Genes Under SCN Stress
3.5. Over-Expression of Gm4CL3 Promotes Cell-Wall Thickening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, C.A.; Lilley, C.J.; McCarthy, J.; Atkinson, H.J.; Urwin, P.E. Plant-parasitic nematodes respond to root exudate signals with host-specific gene expression patterns. PLoS Pathog. 2019, 15, e1007503. [Google Scholar] [CrossRef]
- Wieczorek, K.; Golecki, B.; Gerdes, L.; Heinen, P.; Szakasits, D.; Durachko, D.M.; Cosgrove, D.J.; Kreil, D.P.; Puzio, P.S.; Bohlmann, H.; et al. Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2006, 48, 98–112. [Google Scholar] [CrossRef]
- Riggs, R.D.; Kim, K.S.; Gipson, I. Ultrastructural changes in peking soybeans infected with heterodera glycines. Phytopathology 1973, 63, 76–84. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.J.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- An, C.; Sheng, L.; Du, X.; Wang, Y.; Zhang, Y.; Song, A.; Jiang, J.; Guan, Z.; Fang, W.; Chen, F.; et al. Overexpression of CmMYB15 provides chrysanthemum resistance to aphids by regulating the biosynthesis of lignin. Hortic. Res. 2019, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.C.; Bonine, C.A.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Li, X.; Ma, K.; Zhan, Y.; Zeng, F. Molecular cloning and functional analysis of 4-Coumarate:CoA ligase 4(4CL-like 1)from Fraxinus mandshurica and its role in abiotic stress tolerance and cell wall synthesis. BMC Plant Biol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Goswami, S.K.; Gujjar, R.S.; Kumar, R.; Kumar, R.; Lal, M.K.; Kumari, M. Mechanistic insights on lignin-mediated plant defense against pathogen infection. Plant Physiol. Biochem. PPB 2025, 228, 110224. [Google Scholar] [CrossRef] [PubMed]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef]
- Zhang, C.; Zang, Y.; Liu, P.; Zheng, Z.; Ouyang, J. Characterization, functional analysis and application of 4-Coumarate: CoA ligase genes from Populus trichocarpa. J. Biotechnol. 2019, 302, 92–100. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.I.; Pysh, L.; Chapple, C. Four Isoforms of Arabidopsis 4-Coumarate:CoA Ligase Have Overlapping yet Distinct Roles in Phenylpropanoid Metabolism. Plant Physiol. 2015, 169, 2409–2421. [Google Scholar]
- Lindermayr, C.; Möllers, B.; Fliegmann, J.; Uhlmann, A.; Lottspeich, F.; Meimberg, H.; Ebel, J. Divergent members of a soybean (Glycine max L.) 4-coumarate:coenzyme A ligase gene family. Eur. J. Biochem. 2002, 269, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, Z.; Liu, Y.; Li, Y.; Su, K.; Bai, Z.; Guo, S.; Hu, Z.; Zhang, Z.; Bao, Y.; et al. Mutation of 4-coumarate: Coenzyme A ligase 1 gene affects lignin biosynthesis and increases the cell wall digestibility in maize brown midrib5 mutants. Biotechnol. Biofuels 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Yoo, C.G.; Flanagan, A.; Pu, Y.; Debnath, S.; Ge, Y.; Ragauskas, A.J.; Wang, Z.Y. Defined tetra-allelic gene disruption of the 4-coumarate:coenzyme A ligase 1 (Pv4CL1) gene by CRISPR/Cas9 in switchgrass results in lignin reduction and improved sugar release. Biotechnol. Biofuels 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Chang, Y.; Li, B.; Shao, S.L.; Zhen-Zhu, Z. Functional analysis of 4-coumarate: CoA ligase from Dryopteris fragrans in transgenic tobacco enhances lignin and flavonoids. Genet. Mol. Biol. 2020, 43, e20180355. [Google Scholar] [CrossRef]
- Jin, Z.; Wungsintaweekul, J.; Kim, S.H.; Kim, J.H.; Shin, Y.; Ro, D.K.; Kim, S.U. 4-Coumarate:coenzyme A ligase isoform 3 from Piper nigrum (Pn4CL3) catalyzes the CoA thioester formation of 3,4-methylenedioxycinnamic and piperic acids. Biochem. J. 2020, 477, 61–74. [Google Scholar] [CrossRef]
- Stuible, H.; Büttner, D.; Ehlting, J.; Hahlbrock, K.; Kombrink, E. Mutational analysis of 4-coumarate:CoA ligase identifies functionally important amino acids and verifies its close relationship to other adenylate-forming enzymes. FEBS Lett. 2000, 467, 117–122. [Google Scholar] [CrossRef]
- Holbein, J.; Franke, R.B.; Marhavý, P.; Fujita, S.; Górecka, M.; Sobczak, M.; Geldner, N.; Schreiber, L.; Grundler, F.M.W.; Siddique, S. Root endodermal barrier system contributes to defence against plant-parasitic cyst and root-knot nematodes. Plant J. Cell Mol. Biol. 2019, 100, 221–236. [Google Scholar] [CrossRef]
- Hamberger, B.; Hahlbrock, K. The 4-coumarate:CoA ligase gene family in Arabidopsis thaliana comprises one rare, sinapate-activating and three commonly occurring isoenzymes. Proc. Natl. Acad. Sci. USA 2004, 101, 2209–2214. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Ma, J.; Yuan, M.; Zhan, Y.; Li, Y.; Li, H.; Teng, W.; Zhao, X.; Han, Y. Genome-wide identification and expression profiling of 4-coumarate:coenzyme A ligase genes influencing soybean isoflavones at the seedling stage. Crop Pasture Sci. 2024, 75, CP23147. [Google Scholar] [CrossRef]
- Edens, R.M.; Anand, S.C.; Bolla, R.I. Enzymes of the Phenylpropanoid Pathway in Soybean Infected with Meloidogyne incognita or Heterodera glycines. J. Nematol. 1995, 27, 292–303. [Google Scholar] [PubMed]
- Bohlmann, H.; Sobczak, M. The plant cell wall in the feeding sites of cyst nematodes. Front. Plant Sci. 2014, 5, 89. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, S.; Zhang, S.; Fan, F.; Yang, C.; Duan, Y.; Chen, Q.; Liu, C. 4-Coumarate CoA Ligase Family in Soybean Responds to Heterodera glycines. Curr. Issues Mol. Biol. 2025, 47, 795. https://doi.org/10.3390/cimb47100795
Wang H, Liu S, Zhang S, Fan F, Yang C, Duan Y, Chen Q, Liu C. 4-Coumarate CoA Ligase Family in Soybean Responds to Heterodera glycines. Current Issues in Molecular Biology. 2025; 47(10):795. https://doi.org/10.3390/cimb47100795
Chicago/Turabian StyleWang, Hui, Shumei Liu, Shunbin Zhang, Fengjiao Fan, Chuanwen Yang, Yuxi Duan, Qiumin Chen, and Chen Liu. 2025. "4-Coumarate CoA Ligase Family in Soybean Responds to Heterodera glycines" Current Issues in Molecular Biology 47, no. 10: 795. https://doi.org/10.3390/cimb47100795
APA StyleWang, H., Liu, S., Zhang, S., Fan, F., Yang, C., Duan, Y., Chen, Q., & Liu, C. (2025). 4-Coumarate CoA Ligase Family in Soybean Responds to Heterodera glycines. Current Issues in Molecular Biology, 47(10), 795. https://doi.org/10.3390/cimb47100795





