Switching from a High-Fat to a Regular Chow Diet Improves Obesity Progression in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Feeding of Mice
2.2. Routine Measurements
2.3. Serum Indicator Testing
2.3.1. Tissue Staining Experiments
2.3.2. Microbial Sequencing
2.3.3. Statistics
3. Results
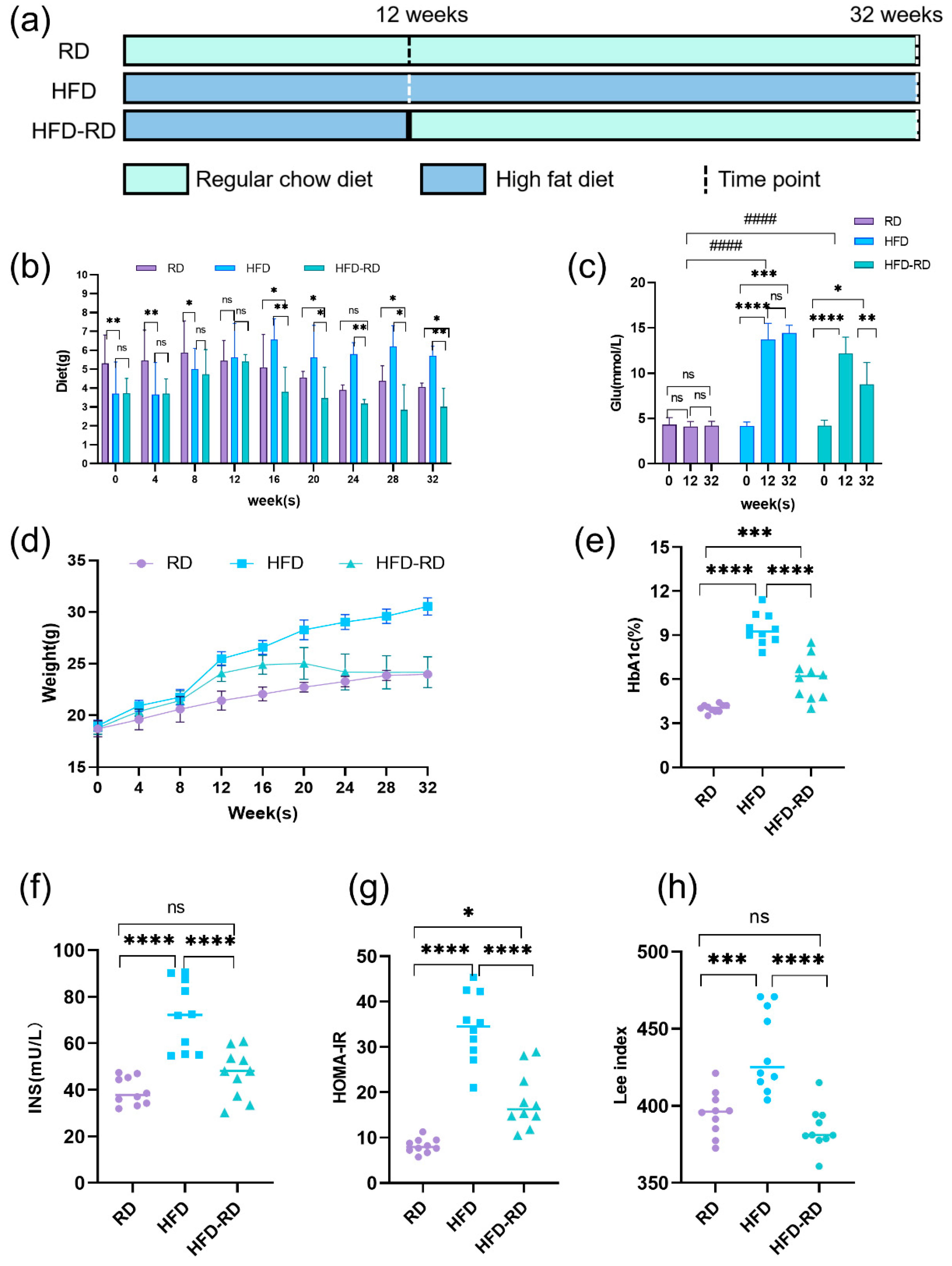
3.1. RD Improves Obesity Symptoms in Mice
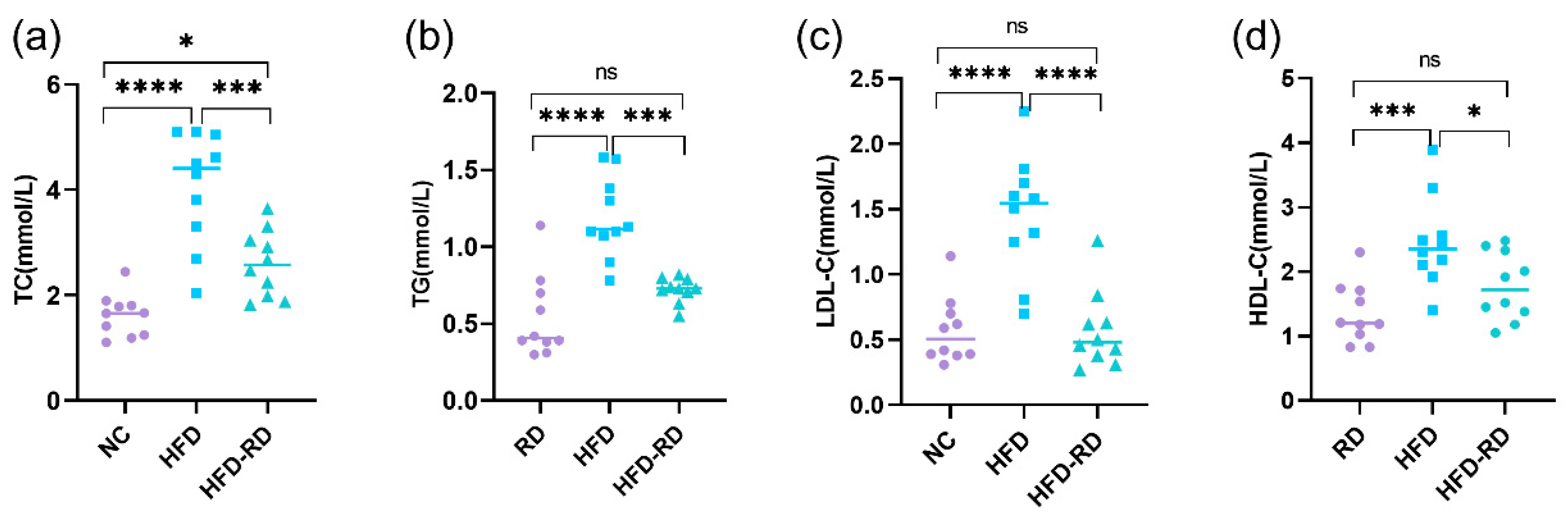
3.2. RD Improves NAFLD in Obese Mice
3.3. Reducing Dietary Fat Improves Low-Inflammatory Status in Obese Mice
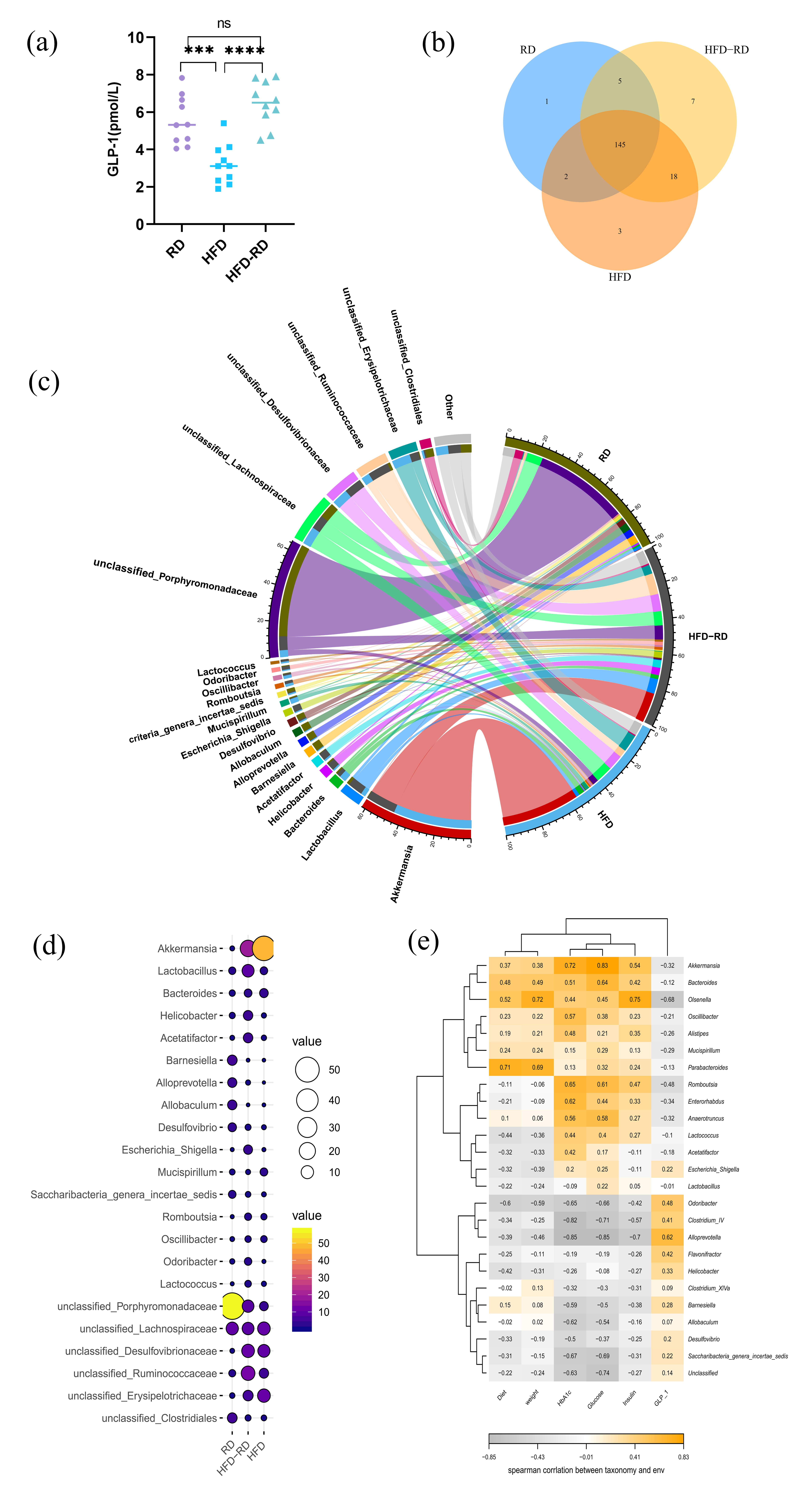
3.4. Effect of RD on the Intestinal Microbiota of Obese Mice
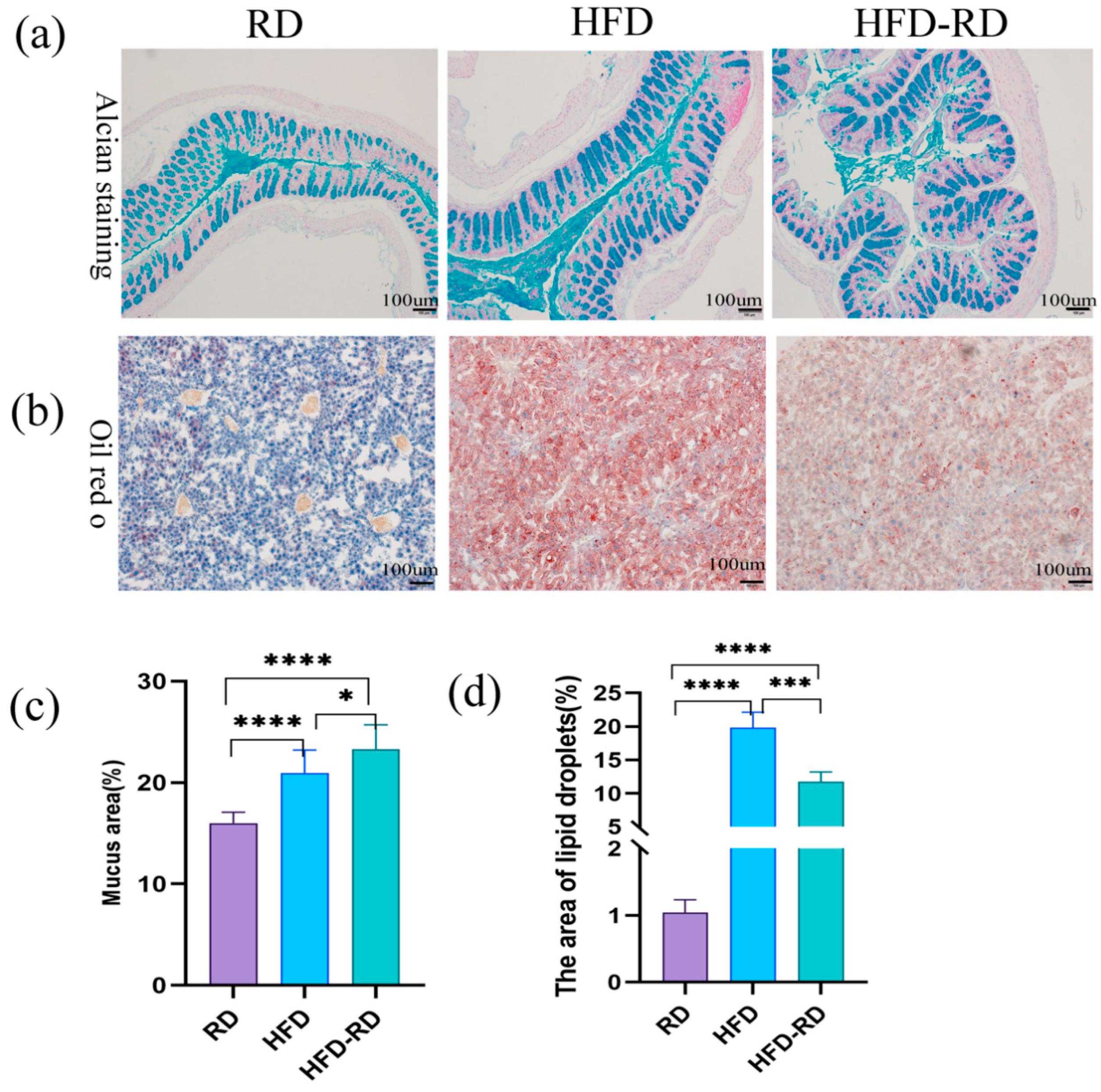
3.5. Effects of RD on Histology in Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef]
- Nikolaus, C.J.; Hebert, L.E.; Zamora-Kapoor, A.; Sinclair, K.I. Risk of Food Insecurity in Young Adulthood and Longitudinal Changes in Cardiometabolic Health: Evidence from the National Longitudinal Study of Adolescent to Adult Health. J. Nutr. 2022, 152, 1944–1952. [Google Scholar] [CrossRef]
- Hardy, O.T.; Perugini, R.A.; Nicoloro, S.M.; Gallagher-Dorval, K.; Puri, V.; Straubhaar, J.; Czech, M.P. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg. Obes. Relat. Dis. 2011, 7, 60–67. [Google Scholar] [CrossRef]
- Coelho, O.G.L.; Cândido, F.G.; Alfenas, R.C.G. Dietary fat and gut microbiota: Mechanisms involved in obesity control. Crit. Rev. Food Sci. Nutr. 2019, 59, 3045–3053. [Google Scholar] [CrossRef]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef]
- Zou, Z.-Y.; Hu, Y.-R.; Ma, H.; Wang, Y.-Z.; He, K.; Xia, S.; Wu, H.; Xue, D.-F.; Li, X.-G.; Ye, X.-L. Coptisine attenuates obesity-related inflammation through LPS/TLR-4-mediated signaling pathway in Syrian golden hamsters. Fitoterapia 2015, 105, 139–146. [Google Scholar] [CrossRef]
- Yang, T.; Zhou, W.; Xu, W.; Ran, L.; Yan, Y.; Lu, L.; Mi, J.; Zeng, X.; Cao, Y. Modulation of gut microbiota and hypoglycemic/hypolipidemic activity of flavonoids from the fruits of Lycium barbarum on high-fat diet/streptozotocin-induced type 2 diabetic mice. Food Funct. 2022, 13, 11169–11184. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Armstrong, N.J.; McClung, H.L.; Player, R.A.; Rood, J.C.; Racicot, K.; Soares, J.W.; Montain, S.J. A diet of U.S. military food rations alters gut microbiota composition and does not increase intestinal permeability. J. Nutr. Biochem. 2019, 72, 108217. [Google Scholar] [CrossRef]
- Wen, X.; Feng, X.; Xin, F.; An, R.; Huang, H.; Mao, L.; Liu, P.; Zhang, J.; Huang, H.; Liu, X.; et al. vulgatus ameliorates high-fat diet-induced obesity through modulating intestinal serotonin synthesis and lipid absorption in mice. Gut Microbes 2024, 16, 2423040. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Oneca, M.; Urtasun, R.; Pajares, M.J.; Goñi, S.; Riezu-Boj, J.I.; Milagro, F.I.; Ayo, J.; Encio, I.J.; Barajas, M.; et al. Pediococcus acidilactici pA1c® Improves the Beneficial Effects of Metformin Treatment in Type 2 Diabetes by Controlling Glycaemia and Modulating Intestinal Microbiota. Pharmaceutics 2023, 15, 1203. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Machado, M.; Morais, R.M.; Calhau, C.; Pintado, M. Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria. Foods 2023, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, X.; Lin, D.; Li, T.; Zhang, N.; Huo, Y.; Zhu, P.; Guo, F.; Huang, F. Conjugated linoleic acid alleviates glycolipid metabolic disorders by modulating intestinal microbiota and short-chain fatty acids in obese rats. Food Funct. 2023, 14, 1685–1698. [Google Scholar] [CrossRef]
- Liu, J.; Ding, H.; Yan, C.; He, Z.; Zhu, H.; Ma, K.Y. Effect of tea catechins on gut microbiota in high fat diet-induced obese mice. J. Sci. Food Agric. 2023, 103, 2436–2445. [Google Scholar] [CrossRef]
- Fotschki, B.; Cholewińska, E.; Ognik, K.; Sójka, M.; Milala, J.; Fotschki, J.; Wiczkowski, W.; Juśkiewicz, J. Dose-Related Regulatory Effect of Raspberry Polyphenolic Extract on Cecal Microbiota Activity, Lipid Metabolism and Inflammation in Rats Fed a Diet Rich in Saturated Fats. Nutrients 2023, 15, 354. [Google Scholar] [CrossRef]
- Mantoku, A.; Chatani, M.; Aono, K.; Inohaya, K.; Kudo, A. Osteoblast and osteoclast behaviors in the turnover of attachment bones during medaka tooth replacement. Dev. Biol. 2016, 409, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jia, Y.; Yan, H.; Shen, Q.; Bian, W.; Zhao, D.; Xu, Y.; Jin, Y.; Yang, M. Prdm16-Mediated Browning is Involved in Resistance to Diet-Induced and Monosodium Glutamate-Induced Obesity. Diabetes Metab. Syndr. Obes. 2021, 14, 4351–4360. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef]
- Boye, A.; Barku, V.Y.A.; Acheampong, D.O.; Ofori, E.G. Abrus precatorius Leaf Extract Reverses Alloxan/Nicotinamide-Induced Diabetes Mellitus in Rats through Hormonal (Insulin, GLP-1, and Glucagon) and Enzymatic (α-Amylase/α-Glucosidase) Modulation. Biomed. Res. Int. 2021, 2021, 9920826. [Google Scholar] [CrossRef]
- Vogeser, M.; König, D.; Frey, I.; Predel, H.-G.; Parhofer, K.G.; Berg, A. Fasting serum insulin and the homeostasis model of insulin resistance (HOMA-IR) in the monitoring of lifestyle interventions in obese persons. Clin. Biochem. 2007, 40, 964–968. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Wang, Y.; Huang, J.; Yang, X.; Ma, J.; Liu, Z.; Wang, F.; Tang, X. Modified Gegen Qinlian decoction ameliorates DSS-induced chronic colitis in mice by restoring the intestinal mucus barrier and inhibiting the activation of γδT17 cells. Phytomedicine 2023, 111, 154660. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Y.H.; Gao, Y.; Li, Z.H.; Gu, W.J.; Zhang, C.G. A histological study of mouse tissues and water loss following lyophilization. Biotech. Histochem. 2020, 95, 325–332. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, W.; Xin, Y.; Bai, J.; Peng, H.; Fu, L.; Liu, J.; Li, L.; Ma, Y.; Jiang, H. Antidiabetic and Antiobesity Effects of Artemether in db/db Mice. Biomed. Res. Int. 2018, 2018, 8639523. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, X.; Fang, X.; Guo, A.; Li, E. Inhibitory effects of fermented Ougan (Citrus reticulata cv. Suavissima) juice on high-fat diet-induced obesity associated with white adipose tissue browning and gut microbiota modulation in mice. Food Funct. 2021, 12, 9300–9314. [Google Scholar] [CrossRef]
- Drucker, D.J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef] [PubMed]
- Song, E.-J.; Shin, N.R.; Jeon, S.; Nam, Y.-D.; Kim, H. Impact of the herbal medicine, Ephedra sinica stapf, on gut microbiota and body weight in a diet-induced obesity model. Front. Pharmacol. 2022, 13, 1042833. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Y.; Tan, S.; Wang, J. Effects of Banana Resistant Starch on the Biochemical Indexes and Intestinal Flora of Obese Rats Induced by a High-Fat Diet and Their Correlation Analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef]
- Pugliese, G.; Muscogiuri, G.; Barrea, L.; Laudisio, D.; Savastano, S.; Colao, A. Irritable bowel syndrome: A new therapeutic target when treating obesity? Hormones 2019, 18, 395–399. [Google Scholar] [CrossRef]
- Chanda, D.; De, D. Meta-analysis reveals obesity associated gut microbial alteration patterns and reproducible contributors of functional shift. Gut. Microbes. 2024, 16, 2304900. [Google Scholar] [CrossRef]
- Zhu, M.; Ouyang, J.; Zhou, F.; Zhao, C.; Zhu, W.; Liu, C.; Huang, P.; Li, J.; Tang, J.; Zhang, Z.; et al. Polysaccharides from Fu brick tea ameliorate obesity by modulating gut microbiota and gut microbiota-related short chain fatty acid and amino acid metabolism. J. Nutr. Biochem. 2023, 118, 109356. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 2018, 28, 415–431.e4. [Google Scholar] [CrossRef]
- Minderis, P.; Fokin, A.; Dirmontas, M.; Ratkevicius, A. Hypocaloric Low-Carbohydrate and Low-Fat Diets with Fixed Protein Lead to Similar Health Outcomes in Obese Mice. Obesity 2020, 28, 1494–1502. [Google Scholar] [CrossRef]
- Li, R.; Svenson, K.L.; Donahue, L.R.; Peters, L.L.; Churchill, G.A. Relationships of dietary fat, body composition, and bone mineral density in inbred mouse strain panels. Physiol. Genom. 2008, 33, 26–32. [Google Scholar] [CrossRef]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2020, 31, 878–881. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yuan, C.-S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, 56672. [Google Scholar]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef]
- Münzberg, H.; Qualls-Creekmore, E.; Yu, S.; Morrison, C.D.; Berthoud, H.-R. Hedonics Act in Unison with the Homeostatic System to Unconsciously Control Body Weight. Front. Nutr. 2016, 3, 6. [Google Scholar] [CrossRef]
- de Wouters d’Oplinter, A.; Rastelli, M.; Van Hul, M.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hu, S.; Yang, D.; Wang, L.; Togo, J.; Wu, Y.; Li, B.; Li, M.; Wang, G.; Zhang, X.; et al. The hedonic overdrive model best explains high-fat diet-induced obesity in C57BL/6 mice. Obesity 2024, 32, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef]
- Edwards-Hampton, S.A.; Ard, J. The latest evidence and clinical guidelines for use of meal replacements in very-low-calorie diets or low-calorie diets for the treatment of obesity. Diabetes Obes. Metab. 2024, 26 (Suppl. 4), 28–38. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Almiron-Roig, E.; Marmonier, C.; Lluch, A. Dietary energy density and body weight: Is there a relationship? Nutr. Rev. 2004, 62, 403–413. [Google Scholar] [CrossRef]
- Song, S.W.; Bae, Y.J.; Lee, D.T. Effects of caloric restriction with varying energy density and aerobic exercise on weight change and satiety in young female adults. Nutr. Res. Pract. 2010, 4, 414–420. [Google Scholar] [CrossRef][Green Version]
- Hao, L.; Scott, S.; Abbasi, M.; Zu, Y.; Khan, M.S.H.; Yang, Y.; Wu, D.; Zhao, L.; Wang, S. Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice. J. Pharmacol. Exp. Ther. 2019, 369, 419–427. [Google Scholar] [CrossRef]
- Brunelli, D.T.; Boldrini, V.O.; Bonfante, I.L.P.; Duft, R.G.; Mateus, K.; Costa, L.; Chacon-Mikahil, M.P.T.; Teixeira, A.M.; Farias, A.S.; Cavaglieri, C.R. Obesity Increases Gene Expression of Markers Associated With Immunosenescence in Obese Middle-Aged Individuals. Front. Immunol. 2021, 12, 806400. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Durrer, C.; Neudorf, H.; Bammert, T.D.; Botezelli, J.D.; Johnson, J.D.; DeSouza, C.A.; Little, J.P. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: A randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1210–R1219. [Google Scholar] [CrossRef]
- Lule, K.O.; Akarsu, E.; Sayiner, Z.A.; Lule, N.O.; Balci, S.O.; Demirel, C.; Bozdag, Z.; Korkmaz, M.; Yilmaz, I. The effects of metformin, pioglitazone, exenatide and exercise on fatty liver in obese diabetic rats: The role of IRS-1 and SOCS-3 molecules. Inflammopharmacology 2022, 30, 243–250. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gao, W.; Yang, H.; Zeng, R.; Yin, Z. Adipocyte enhancer binding protein 1 knockdown alleviates osteoarthritis through inhibiting NF-κB signaling pathway-mediated inflammation and extracellular matrix degradation. J. Cell Commun. Signal. 2024, 18, e12022. [Google Scholar] [CrossRef]
- Gissler, M.C.; Anto-Michel, N.; Pennig, J.; Scherrer, P.; Li, X.; Marchini, T.; Pfeiffer, K.; Härdtner, C.; Abogunloko, T.; Mwinyella, T.; et al. Genetic Deficiency of TRAF5 Promotes Adipose Tissue Inflammation and Aggravates Diet-Induced Obesity in Mice. Arter. Thromb. Vasc. Biol. 2021, 41, 2563–2574. [Google Scholar] [CrossRef]
- Albouery, M.; Bretin, A.; Buteau, B.; Grégoire, S.; Martine, L.; Gambert, S.; Bron, A.M.; Acar, N.; Chassaing, B.; Bringer, M.-A. Soluble Fiber Inulin Consumption Limits Alterations of the Gut Microbiota and Hepatic Fatty Acid Metabolism Caused by High-Fat Diet. Nutrients 2021, 13, 1037. [Google Scholar] [CrossRef]
- Rosas-Villegas, A.; Sánchez-Tapia, M.; Avila-Nava, A.; Ramírez, V.; Tovar, A.R.; Torres, N. Differential Effect of Sucrose and Fructose in Combination with a High Fat Diet on Intestinal Microbiota and Kidney Oxidative Stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, X.; Huangfu, B.; Hu, Y.; Xu, J.; Gao, R.; Huang, K.; He, X. Sulforaphane Ameliorates Nonalcoholic Fatty Liver Disease Induced by High-Fat and High-Fructose Diet via LPS/TLR4 in the Gut-Liver Axis. Nutrients 2023, 15, 743. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, C.; Gao, J.; Xu, C.; Wu, X.; Xu, T.; Cui, C.; Wei, H.; Peng, J.; et al. Functional Fiber Reduces Mice Obesity by Regulating Intestinal Microbiota. Nutrients 2022, 14, 2676. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, L.; Zhang, H.; Yang, Y.; Jiang, L.; Wu, D.; Shu, H.; Zhang, H.; Xie, L.; Zhou, K.; et al. Dietary fiber alleviates alcoholic liver injury via Bacteroides acidifaciens and subsequent ammonia detoxification. Cell Host Microbe 2024, 32, 1331–1346.e6. [Google Scholar] [CrossRef]
- Moon, H.; Lee, K.; Ha, J.-H.; Kim, N.Y.; Shin, H.R.; Cho, T.J.; Oh, N.S.; Park, J.; Tang, J.; Kim, J.K.; et al. Momoridica charantia and fermented Momoridica charantia with Leuconostoc mesenteroides MKSR change intestinal microbial diversity indices and compositions in high-fat and high-cholesterol diet-fed C57BL/6 male mice. Front. Vet. Sci. 2024, 11, 1496067. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1880221. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.-S.; Kim, T.-Y.; Lee, S.-H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.-N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity—An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef] [PubMed]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia muciniphila: Key player in metabolic and gastrointestinal disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1927633. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Wang, W.; Fan, Z.; Yan, Q.; Pan, T.; Luo, J.; Wei, Y.; Li, B.; Fang, Z.; Lu, W. Gut microbiota determines the fate of dietary fiber-targeted interventions in host health. Gut Microbes 2024, 16, 2416915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Y.; Nan, L.; Zhu, Q.; Wang, S.; Xie, Y.; Dong, X.; Cao, C.; Lin, X.; Lu, Y.; et al. Impact of structurally diverse polysaccharides on colonic mucin O-glycosylation and gut microbiota. NPJ Biofilms Microbiomes 2023, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Sun, G.; Duan, J.; Luo, C.; Yangji, C.; Zhong, R.; Chen, L.; Zhu, Y.; Wangdui, B.; Zhang, H. Alterations in gut microbiota improve SCFA production and fiber utilization in Tibetan pigs fed alfalfa diet. Front. Microbiol. 2022, 13, 969524. [Google Scholar] [CrossRef]
- He, X.; Li, W.; Chen, Y.; Lei, L.; Li, F.; Zhao, J.; Zeng, K.; Ming, J. Dietary fiber of Tartary buckwheat bran modified by steam explosion alleviates hyperglycemia and modulates gut microbiota in db/db mice. Food Res. Int. 2022, 157, 111386. [Google Scholar] [CrossRef] [PubMed]

| Composition | High-Fat Diet (HFD), Grams | Regular Chow Diet (RD), Grams |
|---|---|---|
| Protein | 203.00 | 180.00 |
| Carbohydrate | 197.80 | 176.60 |
| Fiber | 50.00 | 50.00 |
| Fat | 270.00 | 40.00 |
| Mineral | 50.00 | 25.22 |
| Vitamin | 3.00 | 1.34312 |
| Dye | 0.05 | / |
| Total calories | 60 kcal% fat | 4.9 kcal% fat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, F.; Wang, X.; Wang, S.; Ding, L. Switching from a High-Fat to a Regular Chow Diet Improves Obesity Progression in Mice. Curr. Issues Mol. Biol. 2025, 47, 791. https://doi.org/10.3390/cimb47100791
Wang Y, Chen F, Wang X, Wang S, Ding L. Switching from a High-Fat to a Regular Chow Diet Improves Obesity Progression in Mice. Current Issues in Molecular Biology. 2025; 47(10):791. https://doi.org/10.3390/cimb47100791
Chicago/Turabian StyleWang, Yuying, Fenglin Chen, Xiaozhong Wang, Shiwan Wang, and Lei Ding. 2025. "Switching from a High-Fat to a Regular Chow Diet Improves Obesity Progression in Mice" Current Issues in Molecular Biology 47, no. 10: 791. https://doi.org/10.3390/cimb47100791
APA StyleWang, Y., Chen, F., Wang, X., Wang, S., & Ding, L. (2025). Switching from a High-Fat to a Regular Chow Diet Improves Obesity Progression in Mice. Current Issues in Molecular Biology, 47(10), 791. https://doi.org/10.3390/cimb47100791






