The Time of Blood Collection Does Not Alter the Composition of Leucocyte-Poor Platelet-Rich Plasma: A Quantitative Analysis of Platelets and Key Regenerative Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. PRP Preparation
2.4. Hematological Analysis
2.5. Protein Analysis
2.6. Statistical Analysis
3. Results
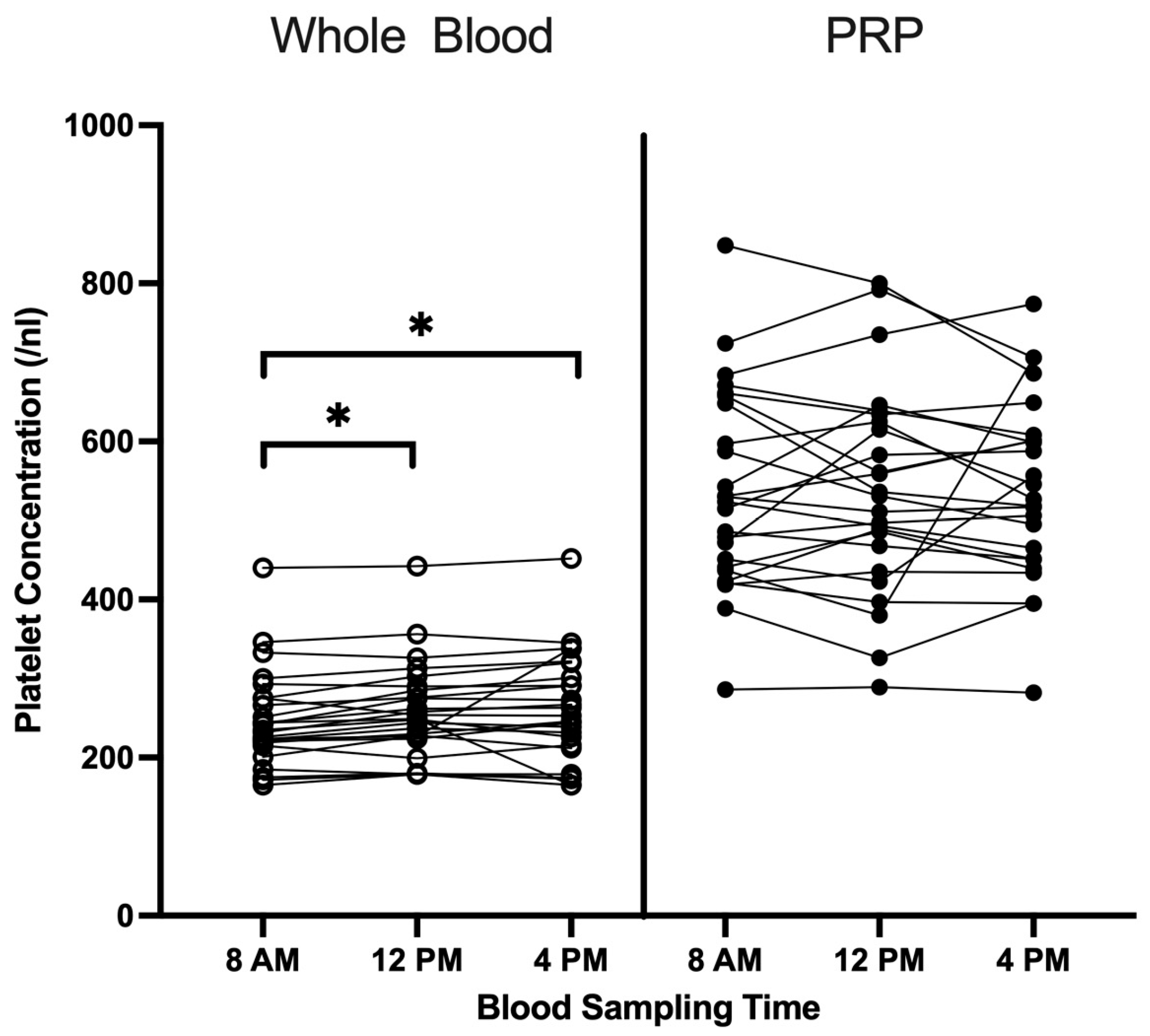
3.1. Cell Profile in Whole Blood and LP-PRP Across Blood Collection Times
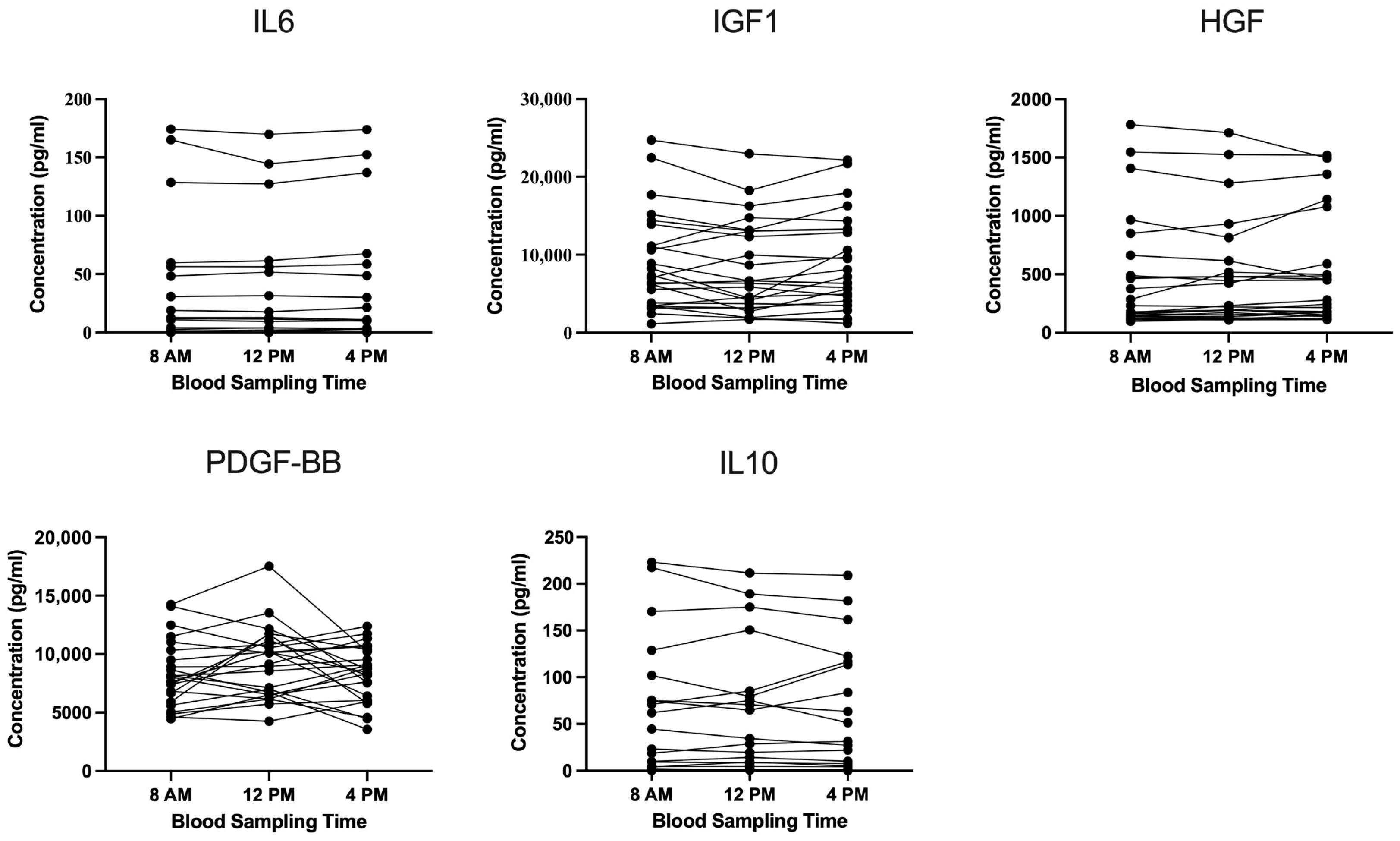
3.2. Cytokine and Growth-Factor Analysis in LP-PRP Across Blood Collection Times
3.3. Analysis of Data Variability in LP-PRP Based on Blood Collection Times
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| CV | Coefficient of Variation |
| DMARD | Disease-Modifying Anti-Rheumatic Drug |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HGF | Hepatocyte Growth Factor |
| IGF | Insulin-like Growth Factor |
| IL | Interleukin |
| IQR | Interquartile Range |
| LP-PRP | Leukocyte-Poor Platelet-Rich Plasma |
| mL | milliliter |
| nL | nanoliter |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| OA | Osteoarthritis |
| PDGF | Platelet-Derived Growth Factor |
| pg | picogram |
| PRP | Platelet-Rich Plasma |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| TGF | Transforming Growth Factor |
References
- Tischer, T.; Bode, G.; Buhs, M.; Marquass, B.; Nehrer, S.; Vogt, S.; Zinser, W.; Angele, P.; Spahn, G.; Welsch, G.H.; et al. Platelet-rich plasma (PRP) as therapy for cartilage, tendon and muscle damage—German working group position statement. J. Exp. Orthop. 2020, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Cerza, F.; Carnì, S.; Carcangiu, A.; Di Vavo, I.; Schiavilla, V.; Pecora, A.; De Biasi, G.; Ciuffreda, M. Comparison Between Hyaluronic Acid and Platelet-Rich Plasma, Intra-articular Infiltration in the Treatment of Gonarthrosis. Am. J. Sports Med. 2012, 40, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Laver, L.; Filardo, G.; Sanchez, M.; Magalon, J.; Tischer, T.; Abat, F.; Bastos, R.; Cugat, R.; Iosifidis, M.; Kocaoglu, B.; et al. The use of injectable orthobiologics for knee osteoarthritis: A European ESSKA-ORBIT consensus. Part 1-Blood-derived products (platelet-rich plasma). Knee Surg. Sports Traumatol. Arthrosc. 2024, 32, 783–797. [Google Scholar] [CrossRef]
- Eppley, B.L.; Woodell, J.E.; Higgins, J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast. Reconstr. Surg. 2004, 114, 1502–1508. [Google Scholar] [CrossRef]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef]
- Miroshnychenko, O.; Chalkley, R.J.; Leib, R.D.; Everts, P.A.; Dragoo, J.L. Proteomic analysis of platelet-rich and platelet-poor plasma. Regen. Ther. 2020, 15, 226–235. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral. Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Secrets of platelet exocytosis—What do we really know about platelet secretion mechanisms? Br. J. Haematol. 2013, 165, 204–216. [Google Scholar] [CrossRef]
- Le, A.D.; Enweze, L.; DeBaun, M.R.; Dragoo, J.L. Platelet-rich plasma. Clin. Sports Med. 2019, 38, 17–44. [Google Scholar] [CrossRef]
- Mazzocca, A.D.; McCarthy, M.B.; Chowaniec, D.M.; Cote, M.P.; Romeo, A.A.; Bradley, J.P.; Arciero, R.A.; Beitzel, K. Platelet-rich plasma differs according to preparation method and human variability. J. Bone Jt. Surg. Am. 2012, 94, 308–316. [Google Scholar] [CrossRef]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.B.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2018, 46, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, T.; Sugaya, H.; Gosho, M.; Aoto, K.; Kanamori, A.; Yamazaki, M. Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J. Exp. Orthop. 2019, 6, 4. [Google Scholar] [CrossRef]
- Budkowska, M.; Lebiecka, A.; Marcinowska, Z.; Woźniak, J.; Jastrzębska, M.; Dołęgowska, B. The circadian rhythm of selected parameters of the hemostasis system in healthy people. Thromb. Res. 2019, 182, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bremner, W.F.; Sothern, R.B.; Kanabrocki, E.L.; Ryan, M.; McCormick, J.B.; Dawson, S.; Connors, E.S.; Rothschild, R.; Third, J.L.; Vahed, S.; et al. Relation between circadian patterns in levels of circulating lipoprotein (a), fibrinogen, platelets, and related lipid variables in men. Am. Heart J. 2000, 139, 164–173. [Google Scholar] [CrossRef]
- Jovičić, A.; Mandić, S. Circadian variations of platelet aggregability and fibrinolytic activity in healthy subjects. Thromb. Res. 1991, 62, 65–74. [Google Scholar] [CrossRef]
- Tofler, G.H.; Brezinski, D.; Schafer, A.I.; Czeisler, C.A.; Rutherford, J.D.; Willich, S.N.; Gleason, R.E.; Williams, G.H.; Muller, J.E. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N. Engl. J. Med. 1987, 316, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Aoto, K.; Kanamori, A.; Yoshioka, T.; Uemura, K.; Sakane, M.; Yamazaki, M. Circadian variation of growth factor levels in platelet-rich plasma. Clin. J. Sport. Med. 2014, 24, 509–512. [Google Scholar] [CrossRef]
- Wiegertjes, R.; van de Loo, F.A.J.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 9, 2681–2694. [Google Scholar] [CrossRef]
- Ellis, I.; Schnabel, L.V.; Berglund, A.K. Defining the Profile: Characterizing Cytokines in Tendon Injury to Improve Clinical Therapy. J. Immunol. Regen. Med. 2022, 16, 100059. [Google Scholar] [CrossRef]
- Bikle, D.D.; Tahimic, C.; Chang, W.; Wang, Y.; Philippou, A.; Barton, E.R. Role of IGF-I signaling in muscle bone interactions. Bone 2015, 80, 79–88. [Google Scholar] [CrossRef]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Semin. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, S.; Merolle, L.; Marraccini, C.; Quartieri, E.; Mori, D.; Schiroli, D.; Berni, P.; Iotti, B.; Di Bartolomeo, E.; Baricchi, R.; et al. Apheresis Platelet Rich-Plasma for Regenerative Medicine: An In Vitro Study on Osteogenic Potential. Int. J. Mol. Sci. 2021, 22, 8764. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.J.; Gray, B.; Wallis, J.A.; Taylor, N.F.; Kemp, J.L.; Hunter, D.J.; Barton, C.J. Recommendations for the management of hip and knee osteoarthritis: A systematic review of clinical practice guidelines. Osteoarthr. Cartil. 2023, 31, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
| Total Study Population | |
|---|---|
| Number of patients, n | 25 |
| Sex, n; % | |
| Female | 13; 52% |
| Male | 12; 48% |
| Age in years, mean ± SD; IQR | 31.44 ± 3.54; 5.00 |
| BMI 1 (kg/m2), mean ± SD; IQR | 22.81 ± 2.31; 3.24 |
| Blood cell concentration (whole blood), mean ± SD; IQR | |
| Leucocytes (/nL) | 6.18 ± 1.34; 2.13 |
| Erythrocytes (×103/nL) | 4.85± 0.35; 0.48 |
| Platelets (/nL) | 255.60 ± 61.72; 68.67 |
| Time | 8 a.m. | 12 p.m. | 4 p.m. | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | |||
| Whole blood 1 | Platelets (/nL) | 248.08 | 60.97 | 257.72 | 60.70 | 261.00 | 68.98 | 0.008 |
| Erythrocytes (×103/nL) | 4.87 | 0.39 | 4.83 | 0.37 | 4.84 | 0.35 | 0.089 | |
| Leukocytes (/nL) | 5.49 | 1.29 | 6.30 | 1.50 | 6.76 | 1.57 | <0.001 | |
| Lymphocytes (%) | 33.10 | 7.14 | 32.72 | 7.65 | 34.03 | 7.45 | 0.468 | |
| Monocytes (%) | 5.64 | 1.40 | 4.85 | 1.25 | 5.21 | 1.09 | <0.001 | |
| Basophils (%) | 0.72 | 0.21 | 0.67 | 0.21 | 0.61 | 0.22 | 0.088 | |
| Eosinophils (%) | 3.36 | 1.90 | 2.69 | 2.00 | 2.84 | 1.99 | 0.001 | |
| Neutrophils (%) | 54.76 | 7.82 | 56.82 | 8.90 | 55.22 | 7.52 | 0.326 | |
| PRP 1 | Platelets (/nL) | 537.04 | 126.27 | 538.00 | 130.65 | 539.84 | 114.95 | 0.619 |
| PRP-to-whole blood platelet ratio 1 | 2.10 | 0.38 | 2.10 | 0.32 | 2.10 | 0.32 | 0.432 | |
| IL6 | IGF1 | HGF | PDGF-BB | IL10 | |
|---|---|---|---|---|---|
| 8 a.m. | 177.82 | 69.24 | 102.98 | 33.67 | 111.15 |
| 12 p.m. | 173.53 | 71.07 | 96.06 | 32.25 | 107.51 |
| 4 p.m. | 173.69 | 65.78 | 90.59 | 28.62 | 106.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platzer, H.; Bork, A.; Wellbrock, M.; Horsch, A.; Pourbozorg, G.; Gantz, S.; Sorbi, R.; Hagmann, S.; Bangert, Y.; Moradi, B. The Time of Blood Collection Does Not Alter the Composition of Leucocyte-Poor Platelet-Rich Plasma: A Quantitative Analysis of Platelets and Key Regenerative Proteins. Curr. Issues Mol. Biol. 2025, 47, 788. https://doi.org/10.3390/cimb47100788
Platzer H, Bork A, Wellbrock M, Horsch A, Pourbozorg G, Gantz S, Sorbi R, Hagmann S, Bangert Y, Moradi B. The Time of Blood Collection Does Not Alter the Composition of Leucocyte-Poor Platelet-Rich Plasma: A Quantitative Analysis of Platelets and Key Regenerative Proteins. Current Issues in Molecular Biology. 2025; 47(10):788. https://doi.org/10.3390/cimb47100788
Chicago/Turabian StylePlatzer, Hadrian, Alena Bork, Malte Wellbrock, Axel Horsch, Ghazal Pourbozorg, Simone Gantz, Reza Sorbi, Sébastien Hagmann, Yannic Bangert, and Babak Moradi. 2025. "The Time of Blood Collection Does Not Alter the Composition of Leucocyte-Poor Platelet-Rich Plasma: A Quantitative Analysis of Platelets and Key Regenerative Proteins" Current Issues in Molecular Biology 47, no. 10: 788. https://doi.org/10.3390/cimb47100788
APA StylePlatzer, H., Bork, A., Wellbrock, M., Horsch, A., Pourbozorg, G., Gantz, S., Sorbi, R., Hagmann, S., Bangert, Y., & Moradi, B. (2025). The Time of Blood Collection Does Not Alter the Composition of Leucocyte-Poor Platelet-Rich Plasma: A Quantitative Analysis of Platelets and Key Regenerative Proteins. Current Issues in Molecular Biology, 47(10), 788. https://doi.org/10.3390/cimb47100788






