Notoginsenoside R1 Attenuates H/R Injury in H9c2 Cells by Maintaining Mitochondrial Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Hypoxia/Reoxygenation (H/R) Model Establishment and Drug Administration
2.2. Cell Viability Assay
2.3. Lactate Dehydrogenase (LDH) Detection
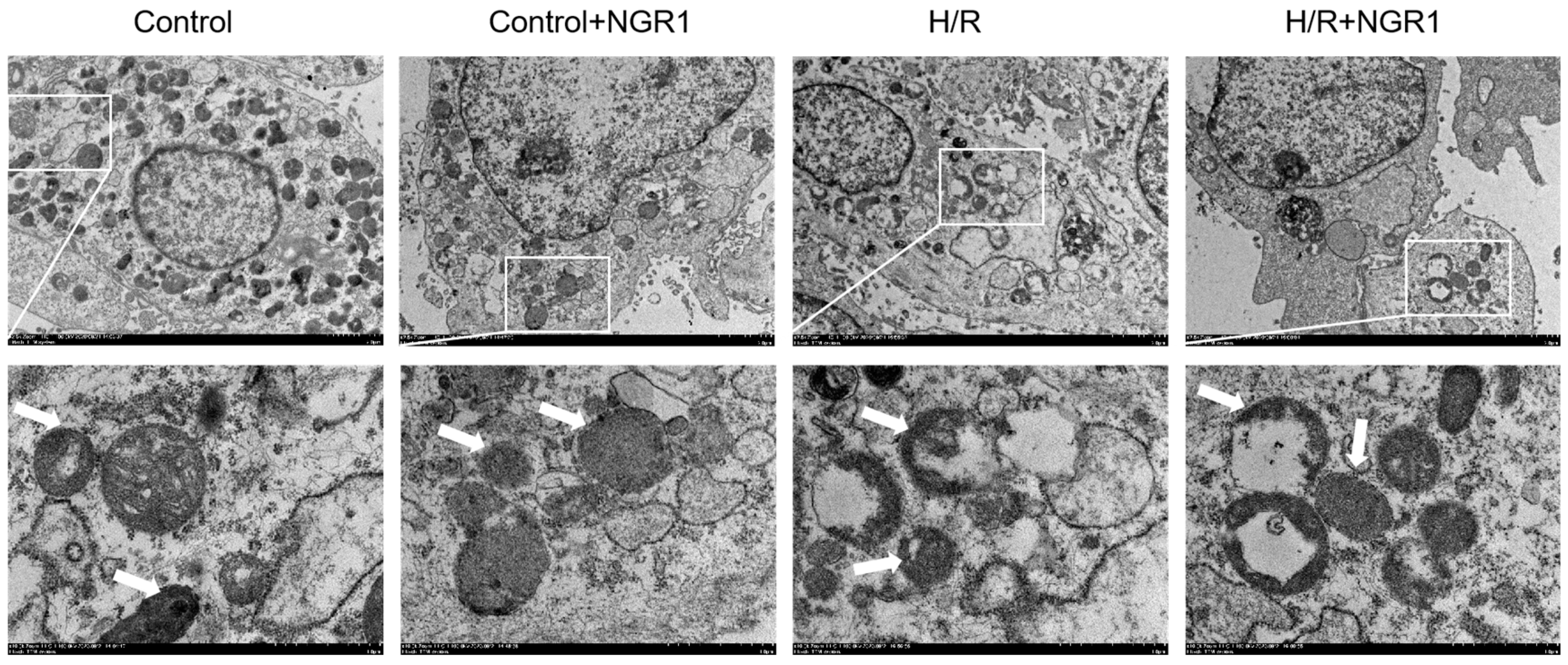
2.4. Observation of Mitochondrial Ultrastructure
2.5. Detection of mPTP Opening
2.6. Mitochondrial Membrane Potential Measurement
2.7. Mitochondrial Reactive Oxygen Species (MitoROS) Level Measurement
2.8. Mitochondrial Respiratory Chain Complexes Activity Assay
2.9. Cellular ATP Level Measurement
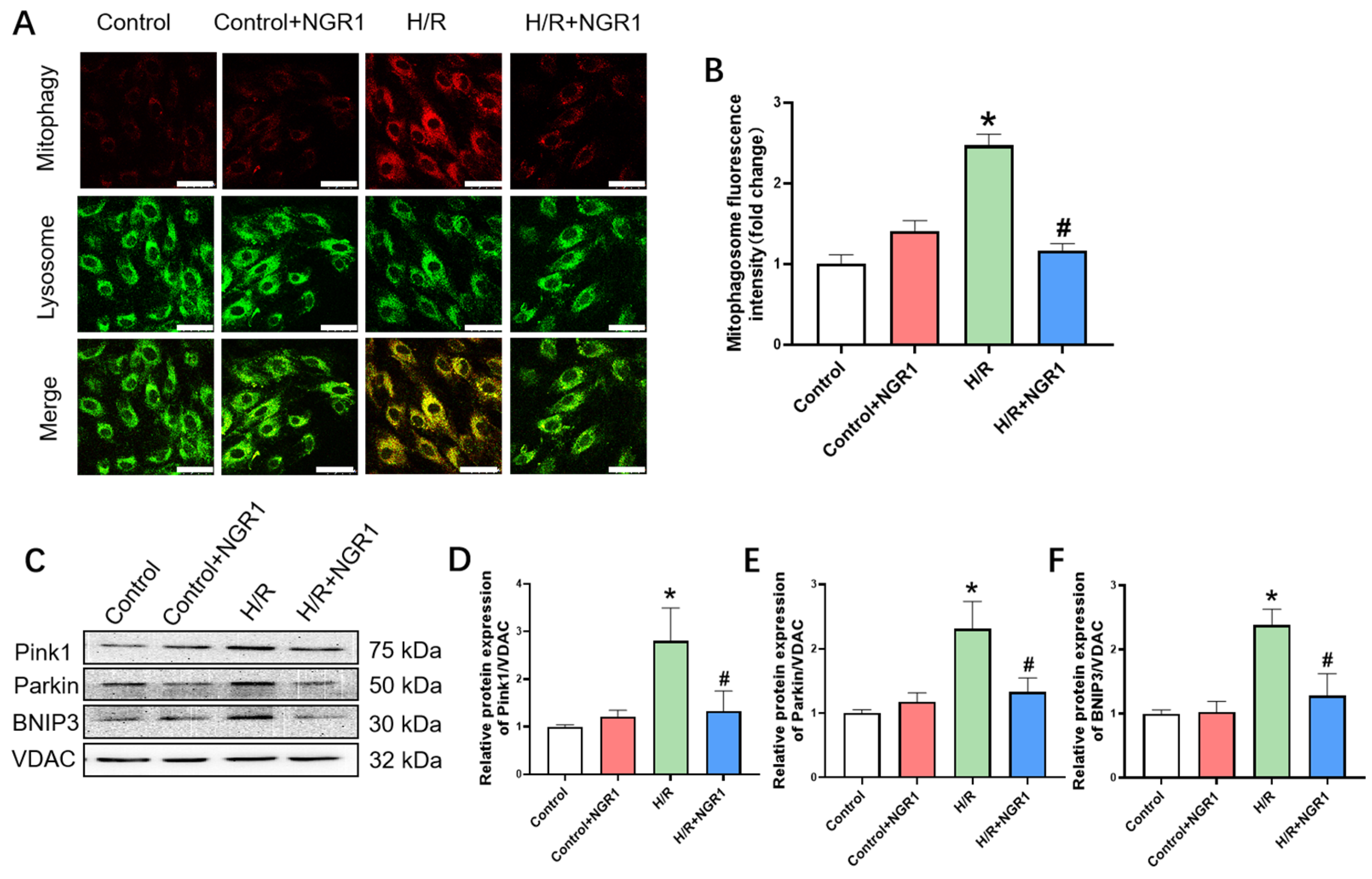
2.10. Mitophagy Measurement
2.11. Western Blotting of Mitochondrial-Associated Proteins
2.12. Statistical Analysis
3. Results
3.1. Cell Damage and Survival
3.2. Mitochondrial Structural Damage
3.3. Mitochondrial Functional Damage
3.4. Mitochondrial Dynamics and Mitochondrial Biogenesis
3.5. Mitophagy
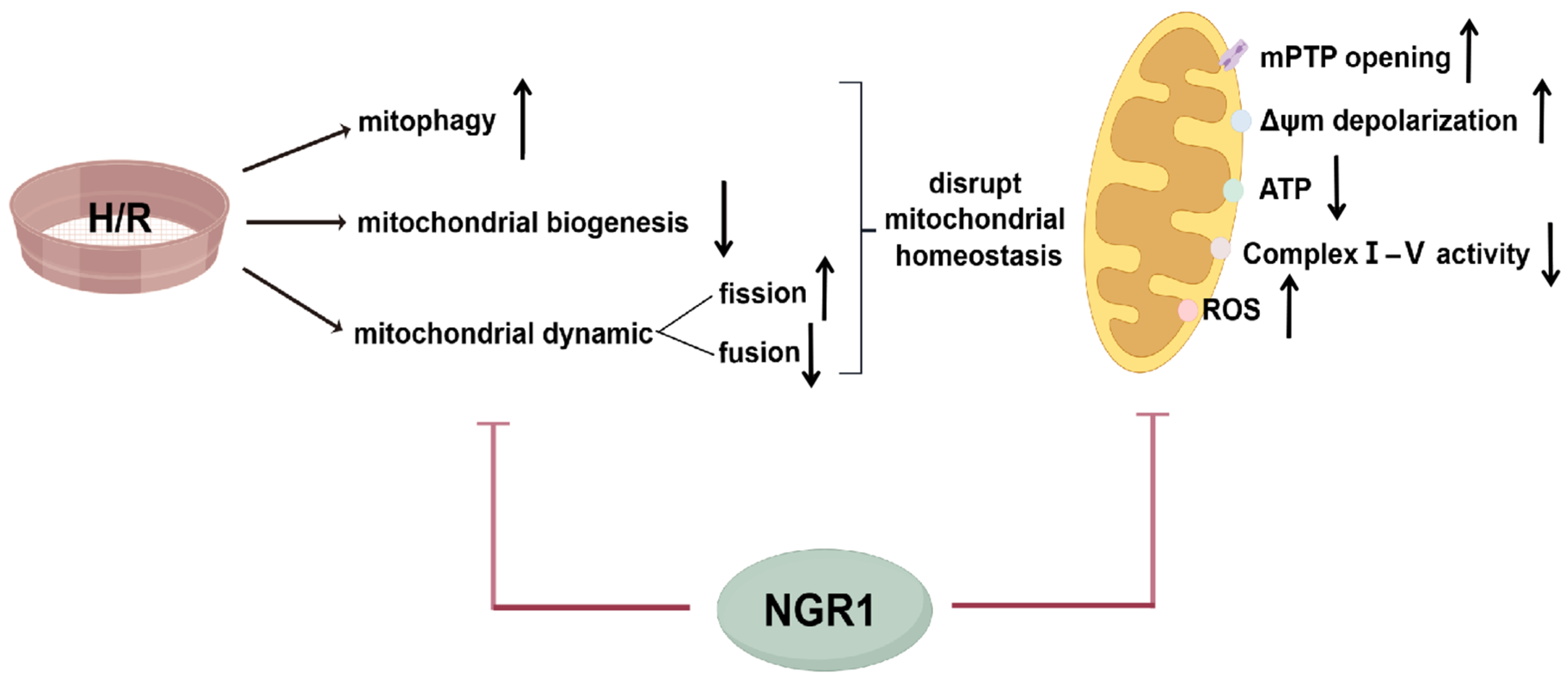
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, J.; Han, L.; Liu, Y.; Li, K.; Wu, Y. A bibliometric study related to the treatment of myocardial ischemia-reperfusion Injury. J. Cardiothorac. Surg. 2024, 19, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Li, W.; Wu, J.; Hu, X.; Tang, T.; Liu, X. Cardiac-targeted and ROS-responsive liposomes containing puerarin for attenuating myocardial ischemia-reperfusion injury. Nanomedicine 2024, 19, 2335–2355. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, Z.; Zhang, Y.; Yang, M.; Wang, C.; Xu, J.; Zhu, Y.; Mi, Y.; Jiang, J.; Sun, Z. Ferroptosis and myocardial ischemia-reperfusion: Mechanistic insights and new therapeutic perspectives. Front. Pharmacol. 2024, 15, 1482986. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xing, J.; Su, X.; Li, X.; Yan, X.; Zhao, J.; Tao, H. IL-38 attenuates myocardial ischemia-reperfusion injury by inhibiting macrophage inflammation. Immun. Inflamm. Dis. 2023, 11, e898. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef]
- Bkaily, G.; Homeostasis, D.J.C. Transporters, and Blockers in Health and Diseases of the Cardiovascular System. Int. J. Mol. Sci. 2023, 24, 8803. [Google Scholar] [CrossRef]
- Trinchese, G.; Cimmino, F.; Catapano, A.; Cavaliere, G.; Mollica, M.P. Mitochondria: The gatekeepers between metabolism and immunity. Front. Immunol. 2024, 15, 1334006. [Google Scholar]
- Popoiu, T.A.; Maack, C.; Bertero, E. Mitochondrial calcium signaling and redox homeostasis in cardiac health and disease. Front. Mol. Med. 2023, 3, 1235188. [Google Scholar] [CrossRef] [PubMed]
- Rudokas, M.W.; McKay, M.; Toksoy, Z.; Eisen, J.N.; Bogner, M.; Young, L.H.; Akar, F.G. Mitochondrial network remodeling of the diabetic heart: Implications to ischemia related cardiac dysfunction. Cardiovasc. Diabetol. 2024, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Tang, Q.; Pan, Z.; Yin, Y.; Zhang, L.; Wen, K. Sphingosine-1-phosphate attenuates hypoxia/reoxygenation-induced cardiomyocyte injury via a mitochondrial pathway. Biochem. Biophys. Res. Commun. 2019, 510, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Zhang, M.; Zhou, L.; Jin, S.; Cheng, H.; Li, X.; Shi, Y.; Xiang, T.; Zhang, Z.; Liu, Z.; et al. Mitochondria transplantation alleviates cardiomyocytes apoptosis through inhibiting AMPKα-mTOR mediated excessive autophagy. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23655. [Google Scholar] [CrossRef]
- Yang, P.-X.; Fan, X.-X.; Liu, M.-X.; Zhang, X.-Z.; Cao, L.; Wang, Z.-Z.; Tian, J.-Z.; Zhang, Y.-W.; Xiao, W. Longxuetongluo Capsule alleviate ischemia/reperfusion induced cardiomy ocyte apoptosis through modulating oxidative stress and mitochondrial dysfunction. Phytomedicine 2024, 134, 155993. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, H.; Song, J.; Wang, T.; Dong, Y.; Zhan, A.; Li, Y.; Liang, G. AMPK Activation Alleviates Myocardial Ischemia-Reperfusion Injury by Regulating Drp1-Mediated Mitochondrial Dynamics. Front. Pharmacol. 2022, 13, 862204. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Zhang, Y.; Ren, J. Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging. Oxid. Med. Cell Longev. 2019, 2019, 9825061. [Google Scholar] [CrossRef]
- Vasileiou, P.V.S.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.I.; Chronopoulos, E.; Passias, P.G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial Homeostasis and Cellular Senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef]
- Su, Z.; Li, P.; Ding, W.; Gao, Y. Urolithin A improves myocardial ischemia-reperfusion injury by attenua ting oxidative stress and ferroptosis through Nrf2 pathway. Int. Immunopharmacol. 2024, 143, 113394. [Google Scholar] [CrossRef] [PubMed]
- Mui, D.; Zhang, Y. Mitochondrial scenario: Roles of mitochondrial dynamics in acute myocardial ischemia/reperfusion injury. J. Recept. Signal Transduct. Res. 2021, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Liu, Y.; Guo, H.; Zhang, Z.; Chen, Q.; Hao, G.; Zhang, C.; Zhang, Y. Prolylcarboxypeptidase Mitigates Myocardial Ischemia/Reperfusion Injury by Stabilizing Mitophagy. Front. Cell Dev. Biol. 2020, 8, 584933. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, G.; Luo, Y.; Wang, M.; Chen, R.; Zhang, J.; Ai, Q.; Xing, N.; Sun, X. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci. Rep. 2016, 6, 21730. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Li, W.; Chen, R.; Li, K.; Cao, Y.; Chen, G.; Jiang, L. Exploring the molecular mechanism of notoginsenoside R1 in sepsis-induced cardiomyopathy based on network pharmacology and experiments validation. Front. Pharmacol. 2023, 14, 1101240. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Lv, S. Notoginsenoside-R1 ameliorates palmitic acid-induced insulin resistance and oxidative stress in HUVEC via Nrf2/ARE pathway. Food Sci. Nutr. 2023, 11, 7791–7802. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, J.; Baldini, M.; Lei, H.; Li, L.; Luo, S.; Wu, J.; Liu, X.; Shan, D.; Xie, Y.; et al. Integrating network pharmacology and experimental models to identify notoginsenoside R1 ameliorates atherosclerosis by inhibiting macrophage NLRP3 inflammasome activation. J. Nat. Med. 2024, 78, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, Z.; Chen, J.; Tian, X.; Zhang, R.; Liang, Q.; Liu, Z.; Cheng, Y. Notoginsenoside R1 attenuates ischemic heart failure by modulating MDM2/beta arrestin2-mediated beta2-adrenergic receptor ubiquitination. Biomed. Pharmacother. 2024, 177, 117004. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Hou, G.; Cao, H.; Zhao, Y.; Yang, B. Notoginsenoside R1 protects oxygen and glucose deprivation-induced injury by upregulation of miR-21 in cardiomyocytes. J. Cell Biochem. 2019, 120, 9181–9192. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-J.; Shi, H.-Q.; Ren, F.-F.; Zhao, X.-S.; Chen, Q.-Y.; Wang, D.-J.; Wu, L.-P.; Chu, M.-P.; Lai, T.-F.; Li, L. Notoginsenoside R1 protects against myocardial ischemia/reperfusion in jury in mice via suppressing TAK1-JNK/p38 signaling. Acta Pharmacol. Sin. 2023, 44, 1366–1379. [Google Scholar] [CrossRef]
- Feng, M.G.; Xiang, L.H.; Li, Y.; Bai, R.R.; Feng, Z.M.; Zhao, Z.G.; Dou, Z.Y.; Zhao, W.H.; Guo, H.; Lv, Y.; et al. Existing Forms of Notoginsenoside R(1) in Rats and Their Potential Bioactivities. J. Agric. Food Chem. 2024, 72, 27248–27264. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Xu, Y.W.; Mou, F.F.; Shan, X.L.; Wang, Q.L.; Liu, B.N.; Ning, K.; Liu, J.J.; Wang, Y.C.; et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 2022, 54, 102384. [Google Scholar] [CrossRef]
- Li, X.Q.; Huang, T.Y. Notoginsenoside R1 alleviates high glucose-induced inflammation and oxidative stress in HUVECs via upregulating miR-147a. Kaohsiung J. Med. Sci. 2021, 37, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Zhuang, Q.; Guo, F.; Fu, L.; Dong, Y.; Xie, S.; Ding, X.; Hu, S.; Zhou, X.D.; Jiang, Y.; Zhou, H.; et al. 1-Deoxynojirimycin promotes cardiac function and rescues mitochondrial cristae in mitochondrial hypertrophic cardiomyopathy. J. Clin. Investig. 2023, 133, e164660. [Google Scholar] [CrossRef] [PubMed]
- Sileikyte, J.; Forte, M. The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxid. Med. Cell Longev. 2019, 2019, 3403075. [Google Scholar] [CrossRef]
- Xia, T.; Wang, Y.N.; Zhou, C.X.; Wu, L.M.; Liu, Y.; Zeng, Q.H.; Zhang, X.L.; Yao, J.H.; Wang, M.; Fang, J.P. Ginsenoside Rh2 and Rg3 inhibit cell proliferation and induce apoptosis by increasing mitochondrial reactive oxygen species in human leukemia Jurkat cells. Mol. Med. Rep. 2017, 15, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, C.S.; Yan, L.; Cui, Y.C.; Liu, Y.Y.; Mu, H.N.; He, K.; Hu, B.H.; Chang, X.; Sun, K.; et al. Ginsenoside Rg1 Ameliorates Rat Myocardial Ischemia-Reperfusion Injury by Modulating Energy Metabolism Pathways. Front. Physiol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Mendoza, A.; Patel, P.; Robichaux, D.; Ramirez, D.; Karch, J. Inhibition of the mPTP and Lipid Peroxidation Is Additively Protective Against I/R Injury. Circ. Res. 2024, 134, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Seidlmayer, L.K.; Gomez-Garcia, M.R.; Shiba, T.; Porter, G.A., Jr.; Pavlov, E.V.; Bers, D.M.; Dedkova, E.N. Dual role of inorganic polyphosphate in cardiac myocytes: The importance of polyP chain length for energy metabolism and mPTP activation. Arch. Biochem. Biophys. 2019, 662, 177–189. [Google Scholar] [CrossRef]
- Guan, S.; Zhao, L.; Peng, R. Mitochondrial Respiratory Chain Supercomplexes: From Structure to Function. Int. J. Mol. Sci. 2022, 23, 13880. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Murphy, E.; Liu, J.C. Mitochondrial Permeability Transition Pore and Calcium Handling. Methods Mol. Biol. 2018, 1782, 187–196. [Google Scholar]
- Sorge, M.; Savore, G.; Gallo, A.; Acquarone, D.; Sbroggio, M.; Velasco, S.; Zamporlini, F.; Femmino, S.; Moiso, E.; Morciano, G.; et al. An intrinsic mechanism of metabolic tuning promotes cardiac resilience to stress. EMBO Mol. Med. 2024, 16, 2450–2484. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, X.; Shi, Z.; Bai, X.; Xia, Y.; Zheng, Y.; Xu, D.; Chen, F.; You, Y.; Fang, J.; et al. KDM3A Senses Oxygen Availability to Regulate PGC-1α-Mediated Mitochondrial Biogenesis. Mol. Cell 2019, 76, 885–895.e7. [Google Scholar] [CrossRef]
- Cardanho-Ramos, C.; Morais, V.A. Mitochondrial Biogenesis in Neurons: How and Where. Int. J. Mol. Sci. 2021, 22, 13059. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Shang, S.; Liu, L.; Sun, C.; Du, W. Irisin improves ROS-induced mitohormesis imbalance in H9c2 cells. Mol. Med. Rep. 2024, 30, 240. [Google Scholar] [CrossRef]
- Kang, J.W.; Choi, H.S.; Lee, S.M. Resolvin D1 attenuates liver ischaemia/reperfusion injury through modulating thioredoxin 2-mediated mitochondrial quality control. Br. J. Pharmacol. 2018, 175, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, S.; Xing, Y.; Pan, L.; Zhao, R.; Zhao, Y.; Liu, L.; Wu, M. Novel Insights and Current Evidence for Mechanisms of Atherosclerosis: Mitochondrial Dynamics as a Potential Therapeutic Target. Front. Cell Dev. Biol. 2021, 9, 673839. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Z.; Wang, L.; Jiang, T.; Dong, D.; Sun, M. The PINK1/Parkin signaling pathway-mediated mitophagy: A forgotten protagonist in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2024, 209, 107466. [Google Scholar] [CrossRef]
- Lampert, M.A.; Orogo, A.M.; Najor, R.H.; Hammerling, B.C.; Leon, L.J.; Wang, B.J.; Kim, T.; Sussman, M.A.; Gustafsson, B. Å BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 2019, 15, 1182–1198. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, P.; Hu, T.; Ning, K.; Bao, Y. Maintaining Mitochondrial Homeostasis is an Important Mechanism of Notoginsenoside R1 in Alleviating Hypoxia/Reoxygenation Injury in H9c2 Cells. In Proceedings of the International Conference of Physiological Sciences Proceedings, Qingdao, China, 22–25 August 2024. [Google Scholar]
- Han, J.Y.; Li, Q.; Ma, Z.Z.; Fan, J.Y. Effects and mechanisms of compound Chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. Pharmacol. Ther. 2017, 177, 146–173. [Google Scholar] [CrossRef]
- Delvoye, F.; Loyau, S.; Labreuche, J.; Taylor, G.; Maier, B.; Piotin, M.; Blanc, R.; Escalard, S.; Di Meglio, L.; Maacha, M.B.; et al. Intravenous abciximab as a rescue therapy for immediate reocclusion after successful mechanical thrombectomy in acute ischemic stroke patients. Platelets 2022, 33, 285–290. [Google Scholar] [CrossRef]




| Observations | H/R | NGR1 | ||
|---|---|---|---|---|
| Cell damage and viability | Cell viability | OD value | ↓ | ↑ |
| Cell damage | LDH | ↑ | ↓ | |
| Mitochondrial structure and function | Structure | Swelling, etc. | ↑ | ↓ |
| mPTP | Green fluorescence intensity | ↓ | ↑ | |
| Δψm | JC-1 aggregates/monomers | ↓ | ↑ | |
| Energy synthesis | ATP | ↓ | ↑ | |
| ETC complexes I-V | Activities | ↓ | ↑ | |
| Oxidative stress | ROS | ↑ | ↓ | |
| Mechanisms of mitochondrial homeostasis | Mitochondrial Biogenesis | PGC-1α, Nrf1, and Nrf2 | ↓ | ↑ |
| Mitochondrial Dynamics | Opa1, Mfn1, and Mfn2 | ↓ | ↑ | |
| Drp1 and Fis1 | ↑ | ↓ | ||
| Mitophagy | Fluorescence intensities | ↑ | ↓ | |
| Pink1, Parkin, and BNIP3 | ↑ | ↓ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, P.; Hu, T.; Ning, K.; Bao, Y. Notoginsenoside R1 Attenuates H/R Injury in H9c2 Cells by Maintaining Mitochondrial Homeostasis. Curr. Issues Mol. Biol. 2025, 47, 44. https://doi.org/10.3390/cimb47010044
Xu Y, Wang P, Hu T, Ning K, Bao Y. Notoginsenoside R1 Attenuates H/R Injury in H9c2 Cells by Maintaining Mitochondrial Homeostasis. Current Issues in Molecular Biology. 2025; 47(1):44. https://doi.org/10.3390/cimb47010044
Chicago/Turabian StyleXu, Yuanbo, Piao Wang, Ting Hu, Ke Ning, and Yimin Bao. 2025. "Notoginsenoside R1 Attenuates H/R Injury in H9c2 Cells by Maintaining Mitochondrial Homeostasis" Current Issues in Molecular Biology 47, no. 1: 44. https://doi.org/10.3390/cimb47010044
APA StyleXu, Y., Wang, P., Hu, T., Ning, K., & Bao, Y. (2025). Notoginsenoside R1 Attenuates H/R Injury in H9c2 Cells by Maintaining Mitochondrial Homeostasis. Current Issues in Molecular Biology, 47(1), 44. https://doi.org/10.3390/cimb47010044






