Serum hsa-miR-22-3p, hsa-miR-885-5p, Lipase-to-Amylase Ratio, C-Reactive Protein, CA19-9, and Neutrophil-to-Lymphocyte Ratio as Prognostic Factors in Advanced Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Specimen Collection and Handling for miRNA Isolation
2.3. Isolation of miRNA
2.4. Reverse Transcription of miRNA
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Age, BMI, and Sex
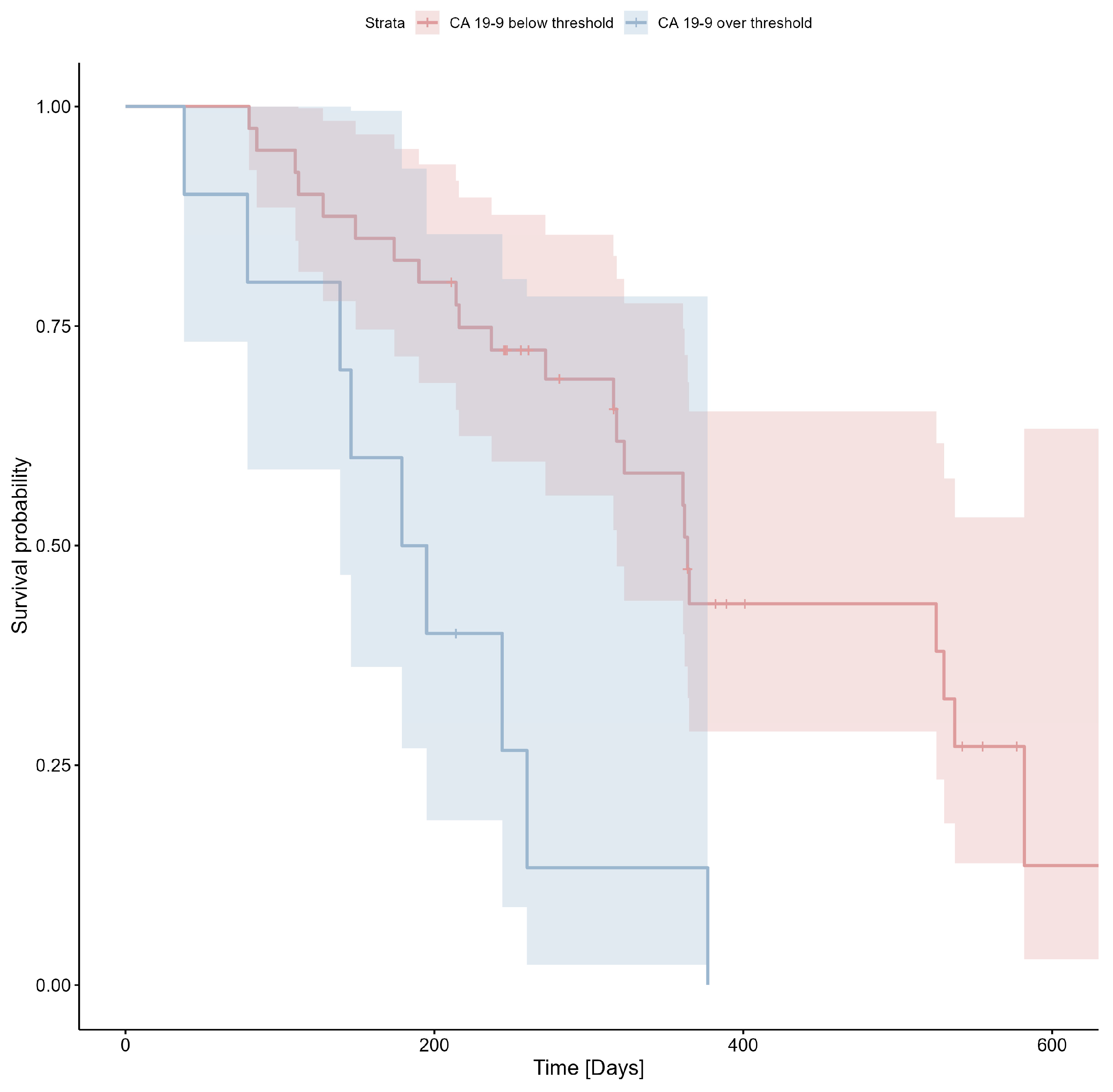
3.2. CA19-9
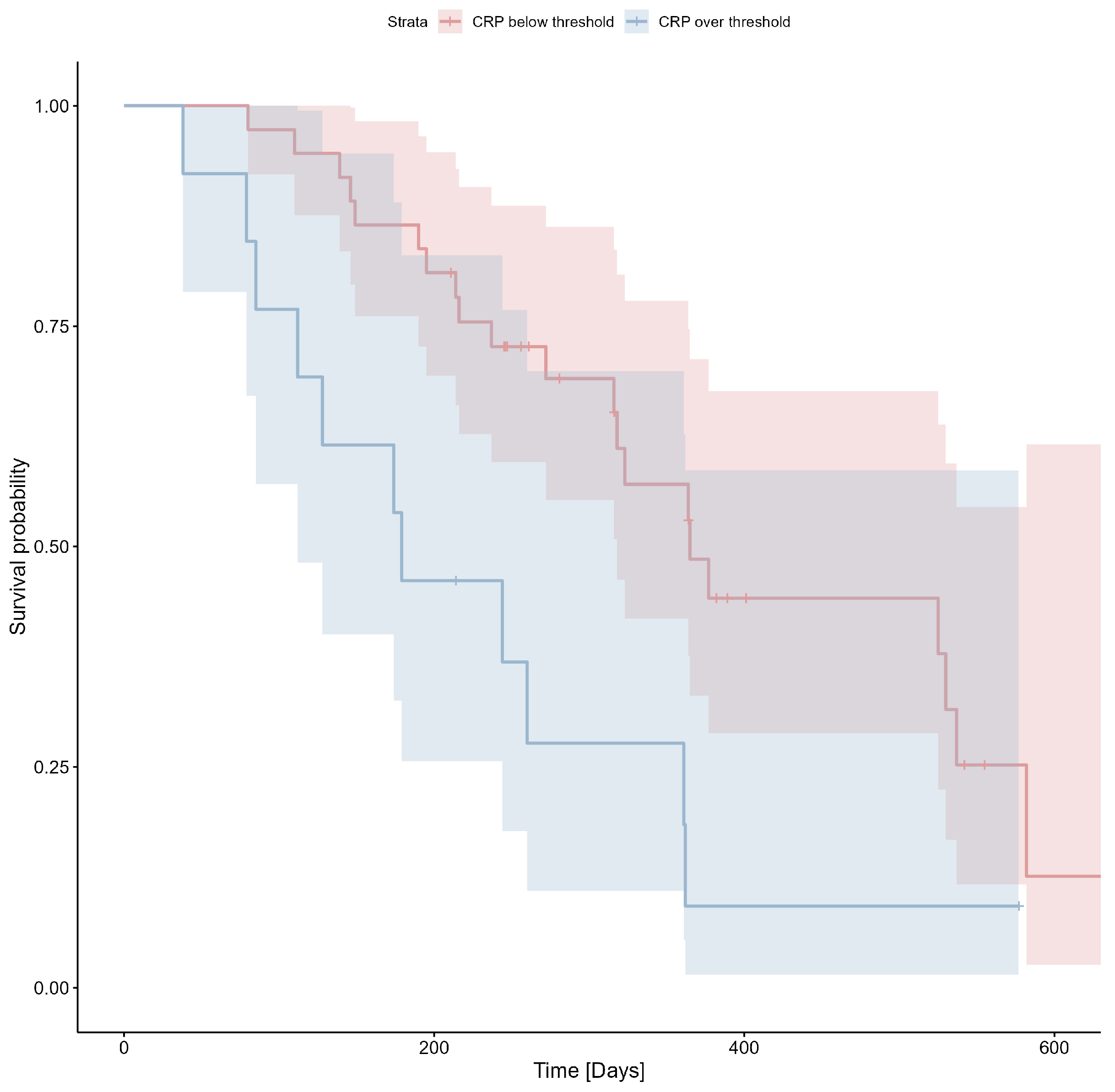
3.3. CRP
3.4. Albumin
3.5. Serum Lipase and Amylase, LAR
3.6. NLR, PLR, and LMR
3.7. hsa-miR-22-3p
3.8. hsa-miR-885-5p
3.9. Multivariate Analysis
4. Discussion
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Rachagani, S.; Macha, M.A.; Heimann, N.; Seshacharyulu, P.; Haridas, D.; Chugh, S.; Batra, S.K. Clinical implications of miRNAs in the pathogenesis, diagnosis and therapy of pancreatic cancer. Adv. Drug Deliv. Rev. 2015, 81, 16–33. [Google Scholar] [CrossRef]
- Ghaneh, P.; Costello, E.; Neoptolemos, J.P. Biology and management of pancreatic cancer. Gut 2007, 56, 1134. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Cancer Stat Facts: Pancreatic Cancer. NCI Website. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 4 October 2022).
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, M.; Bhattacharya, A.; Sarafbidabad, M.; Syuhada Sazali, E.; Krishna Ghoshal, S.; Satgunam, M.; Singh, R.; Rezaei Ardani, M.; Missaoui, N.; Kahri, H.; et al. CA19-9 and CEA biosensors in pancreatic cancer. Clin. Chim. Acta 2024, 554, 117788. [Google Scholar] [CrossRef]
- Bonazzi, V.F.; Aoude, L.G.; Brosda, S.; Bradford, J.J.; Lonie, J.M.; Loffler, K.A.; Gartside, M.G.; Patel, K.; Mukhopadhyay, P.; Keane, C.; et al. C-reactive protein is a prognostic biomarker in pancreatic ductal adenocarcinoma patients. Asia-Pac. J. Clin. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ekka, N.M.; Kujur, A.D.; Guria, R.; Mundu, M.; Mishra, B.; Sekhar, S.; Kumar, A.; Prakash, J.; Birua, H. Serum Lipase Amylase Ratio as an Indicator to Differentiate Alcoholic From Non-alcoholic Acute Pancreatitis: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e35618. [Google Scholar] [CrossRef]
- Stotz, M.; Barth, D.A.; Riedl, J.M.; Asamer, E.; Klocker, E.V.; Kornprat, P.; Hutterer, G.C.; Prinz, F.; Lackner, K.; Stöger, H.; et al. The Lipase/Amylase Ratio (LAR) in Peripheral Blood Might Represent a Novel Prognostic Marker in Patients with Surgically Resectable Pancreatic Cancer. Cancers 2020, 12, 1798. [Google Scholar] [CrossRef]
- Shusterman, M.; Jou, E.; Kaubisch, A.; Chuy, J.W.; Rajdev, L.; Aparo, S.; Tang, J.; Ohri, N.; Negassa, A.; Goel, S. The Neutrophil-to-Lymphocyte Ratio is a Prognostic Biomarker in an Ethnically Diverse Patient Population with Advanced Pancreatic Cancer. J. Gastrointest. Cancer 2020, 51, 868–876. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, S.; Fathy, A.H.; Qian, H.; Zhao, Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: A comprehensive meta-analysis of 17 cohort studies. OncoTargets Ther. 2018, 11, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- O’Neill, R.S.; Stoita, A. Biomarkers in the diagnosis of pancreatic cancer: Are we closer to finding the golden ticket? World J. Gastroenterol. 2021, 27, 4045. [Google Scholar] [CrossRef] [PubMed]
- Abue, M.; Yokoyama, M.; Shibuya, R.; Tamai, K.; Yamaguchi, K.; Sato, I.; Tanaka, N.; Hamada, S.; Shimosegawa, T.; Sugamura, K.; et al. Circulating miR-483-3p and miR-21 is highly expressed in plasma of pancreatic cancer. Int. J. Oncol. 2015, 46, 539. [Google Scholar] [CrossRef] [PubMed]
- Alemar, B.; Izetti, P.; Gregório, C.; Macedo, G.S.; Castro, M.A.A.; Osvaldt, A.B.; Matte, U.; Ashton-Prolla, P. miRNA-21 and miRNA-34a Are Potential Minimally Invasive Biomarkers for the Diagnosis of Pancreatic Ductal Adenocarcinoma. Pancreas 2016, 45, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Stroese, A.J.; Ullerich, H.; Koehler, G.; Raetzel, V.; Senninger, N.; Dhayat, S.A. Circulating microRNA-99 family as liquid biopsy marker in pancreatic adenocarcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2377–2390. [Google Scholar] [CrossRef]
- Vieira, N.F.; Serafini, L.N.; Novais, P.C.; Neto, F.S.L.; De Assis Cirino, M.L.; Kemp, R.; Ardengh, J.C.; Saggioro, F.P.; Gaspar, A.F.; Sankarankutty, A.K.; et al. The role of circulating miRNAs and CA19-9 in pancreatic cancer diagnosis. Oncotarget 2021, 12, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shao, C.; Li, A.; Zhang, X.; Guo, X.; Li, J. Diagnostic Value of Plasma miR-181b, miR-196a, and miR-210 Combination in Pancreatic Cancer. Gastroenterol. Res. Pract. 2020, 2020, 6073150. [Google Scholar] [CrossRef]
- Slater, E.P.; Strauch, K.; Rospleszcz, S.; Ramaswamy, A.; Esposito, I.; Klöppel, G.; Matthäi, E.; Heeger, K.; Fendrich, V.; Langer, P.; et al. MicroRNA-196a and -196b as Potential Biomarkers for the Early Detection of Familial Pancreatic Cancer. Transl. Oncol. 2014, 7, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Karasek, P.; Gablo, N.; Hlavsa, J.; Kiss, I.; Vychytilova-Faltejskova, P.; Hermanova, M.; Kala, Z.; Slaby, O.; Prochazka, V. Pre-operative Plasma miR-21-5p Is a Sensitive Biomarker and Independent Prognostic Factor in Patients with Pancreatic Ductal Adenocarcinoma Undergoing Surgical Resection. Cancer Genom. Proteom. 2018, 15, 321–327. [Google Scholar] [CrossRef]
- Cote, G.A.; Gore, A.J.; McElyea, S.D.; Heathers, L.E.; Xu, H.; Sherman, S.; Korc, M. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. Am. J. Gastroenterol. 2014, 109, 1942–1952. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, X.; Zhang, Y.; Zhu, X.; Liu, H. Systematic Review and Meta-Analysis of Diagnostic Accuracy of miRNAs in Patients with Pancreatic Cancer. Dis. Markers 2018, 2018, 6292396. [Google Scholar] [CrossRef]
- Ali, S.; Dubaybo, H.; Brand, R.E.; Sarkar, F.H. Differential Expression of MicroRNAs in Tissues and Plasma Co-exists as a Biomarker for Pancreatic Cancer. J. Cancer Sci. Ther. 2015, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhuang, L.; Zhang, J.; Fan, J.; Luo, J.; Chen, H.; Wang, K.; Liu, L.; Chen, Z.; Meng, Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol. Oncol. 2013, 7, 334–345. [Google Scholar] [CrossRef]
- Wnuk, J.; Strzelczyk, J.K.; Gisterek, I. Clinical Value of Circulating miRNA in Diagnosis, Prognosis, Screening and Monitoring Therapy of Pancreatic Ductal Adenocarcinoma-A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 5113. [Google Scholar] [CrossRef] [PubMed]
- Franklin, O.; Jonsson, P.; Billing, O.; Lundberg, E.; Öhlund, D.; Nyström, H.; Lundin, C.; Antti, H.; Sund, M. Plasma Micro-RNA Alterations Appear Late in Pancreatic Cancer. Ann. Surg. 2018, 267, 775. [Google Scholar] [CrossRef]
- Ganepola, G.A.; Rutledge, J.R.; Suman, P.; Yiengpruksawan, A.; Chang, D.H. Novel blood-based microRNA biomarker panel for early diagnosis of pancreatic cancer. World J. Gastrointest. Oncol. 2014, 6, 22–33. [Google Scholar] [CrossRef]
- Hussein, N.A.E.M.; Kholy, Z.A.E.; Anwar, M.M.; Ahmad, M.A.; Ahmad, S.M. Plasma miR-22-3p, miR-642b-3p and miR-885-5p as diagnostic biomarkers for pancreatic cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yu, D.; Wei, N.; Fu, H.; Cai, T.; Huang, Y.; Wu, C.; Zheng, X.; Du, Q.; Lin, D.; et al. An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples. FEBS J. 2010, 277, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Yagi, S.; Ito, T.; Lowenstein, C.J. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS ONE 2011, 6, e20291. [Google Scholar] [CrossRef]
- Lui, W.O.; Pourmand, N.; Patterson, B.K.; Fire, A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007, 67, 6031–6043. [Google Scholar] [CrossRef]
- Dong, H.X.; Wang, R.; Jin, X.Y.; Zeng, J.; Pan, J. LncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via inhibiting hsa-mir-22-3p. J. Cell. Physiol. 2018, 233, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Wang, C.Q.; Li, X.T.; Yang, H.; Zhou, J.; Song, Y.J. MiR-22-3p Suppresses Cell Migration and Invasion by Targeting PLAGL2 in Breast Cancer. J. Coll. Physicians Surg. Pak. 2021, 31, 937–940. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Z.; Fang, L. miR-22-3p/PGC1 β Suppresses Breast Cancer Cell Tumorigenesis via PPAR γ. PPAR Res. 2021, 2021, 6661828. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, H.; Zeng, S.; Li, L. miR-885-5p Inhibits Invasion and Metastasis in Gastric Cancer by Targeting Malic Enzyme 1. DNA Cell Biol. 2021, 40, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, X.; Luo, H.; Hua, Q.; Zhang, F. CircPTPRM accelerates malignancy of papillary thyroid cancer via miR-885-5p/DNMT3A axis. J. Clin. Lab. Anal. 2022, 36, e24688. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, M.Y.; Su, X. MiR-885-5p promotes gastric cancer proliferation and invasion through regulating YPEL1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7913–7919. [Google Scholar] [CrossRef] [PubMed]
- Lixin, S.; Wei, S.; Haibin, S.; Qingfu, L.; Tiemin, P. miR-885-5p inhibits proliferation and metastasis by targeting IGF2BP1 and GALNT3 in human intrahepatic cholangiocarcinoma. Mol. Carcinog. 2020, 59, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Wang, Q.; Wang, H. Identification of miR-885-5p as a Tumor Biomarker: Regulation of Cellular Function in Cervical Cancer. Gynecol. Obstet. Investig. 2021, 86, 525–532. [Google Scholar] [CrossRef]
- Papadoniou, N.; Kosmas, C.; Gennatas, K.; Polyzos, A.; Mouratidou, D.; Skopelitis, E.; Tzivras, M.; Sougioultzis, S.; Papastratis, G.; Karatzas, G.; et al. Prognostic Factors in Patients with Locally Advanced (Unresectable) or Metastatic Pancreatic Adenocarcinoma: A Retrospective Analysis. Anticancer Res. 2008, 28, 543–549. [Google Scholar]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Long, F.; Jaiswar, M.; Yang, L.; Wang, C.; Zhou, Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis. Sci. Rep. 2015, 5, 11026. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tao, L.; Lu, M.; Xiu, D. Prognostic role of platelet to lymphocyte ratio in pancreatic cancers: A meta-analysis including 3028 patients. Medicine 2018, 97, e9616. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.J.; Ma, J.Y.; Hu, G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis. Clin. Chim. Acta 2018, 481, 142–146. [Google Scholar] [CrossRef]
- Szkandera, J.; Stotz, M.; Absenger, G.; Stojakovic, T.; Samonigg, H.; Kornprat, P.; Schaberl-Moser, R.; Alzoughbi, W.; Lackner, C.; Ress, A.L.; et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br. J. Cancer 2013, 110, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, A.M.; Mustonen, H.; Haglund, C.; Seppänen, H. Changes in CRP and CA19-9 during Preoperative Oncological Therapy Predict Postoperative Survival in Pancreatic Ductal Adenocarcinoma. Oncology 2021, 99, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Maisey, N.R.; Norman, A.R.; Hill, A.; Massey, A.; Oates, J.; Cunningham, D. CA19-9 as a prognostic factor in inoperable pancreatic cancer: The implication for clinical trials. Br. J. Cancer 2005, 93, 740. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, J.M.; Schipper, M.; Zalupski, M.M.; Lawrence, T.S.; Abrams, R.; Francis, I.R.; Khan, G.; Leslie, W.; Ben-Josef, E. Prognostic significance of carbohydrate antigen 19-9 in unresectable locally advanced pancreatic cancer treated with dose-escalated intensity modulated radiation therapy and concurrent full-dose gemcitabine: Analysis of a prospective phase 1/2 dose escalation study. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 96–101. [Google Scholar] [CrossRef]
- Bauer, T.M.; El-Rayes, B.F.; Li, X.; Hammad, N.; Philip, P.A.; Shields, A.F.; Zalupski, M.M.; Bekaii-Saab, T. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: A pooled analysis of 6 prospective trials. Cancer 2013, 119, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Hammad, N.; Heilbrun, L.K.; Philip, P.A.; Shields, A.F.; Zalupski, M.M.; Venkatramanamoorthy, R.; El-Rayes, B.F. CA19-9 as a predictor of tumor response and survival in patients with advanced pancreatic cancer treated with gemcitabine based chemotherapy. Asia-Pac. J. Clin. Oncol. 2010, 6, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cetin, S.; Dede, I. Prognostic value of the neutrophil-to-lymphocyte ratio and carbohydrate antigen 19-9 in estimating survival in patients with metastatic pancreatic cancer. J. Cancer Res. Ther. 2020, 16, 909–916. [Google Scholar] [CrossRef]
- García-Herrera, J.S.; Muñoz-Montaño, W.R.; López-Basave, H.N.; Morales-Vásquez, F.; Castillo-Morales, C.; Rivera-Mogollán, L.G.; Hernández-Castañeda, K.F. Combination of neutrophil-to-lymphocyte ratio and serum CA 19-9 as a prognostic factor in pancreatic cancer. J. Gastrointest. Oncol. 2024, 15, 1805–1819. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J.; Du, Y.; Li, Z.; Ren, Y.; Gu, J.; Wang, X.; Gong, Y.; Wang, W.; Kong, X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int. J. Cancer 2012, 131, 683–691. [Google Scholar] [CrossRef]
- Boland, J.W.; Allgar, V.; Boland, E.G.; Kaasa, S.; Hjermstad, M.J.; Johnson, M.J. Predictors and trajectory of performance status in patients with advanced cancer: A secondary data analysis of the international European Palliative Care Cancer Symptom study. Palliat. Med. 2018, 33, 206. [Google Scholar] [CrossRef] [PubMed]
- Amano, K.; Maeda, I.; Morita, T.; Baba, M.; Miura, T.; Hama, T.; Mori, I.; Nakajima, N.; Nishi, T.; Sakurai, H.; et al. C-reactive protein, symptoms and activity of daily living in patients with advanced cancer receiving palliative care. J. Cachexia Sarcopenia Muscle 2017, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Keshavarz-Fathi, M.; Baracos, V.; Arends, J.; Mahmoudi, M.; Rezaei, N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018, 127, 91–104. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Wang, S.; Wang, Y.; Wu, Y.; Hu, R. Prognostic significance of preoperative lymphocytes, albumin, and neutrophils (LANR) index in resectable pancreatic ductal adenocarcinoma. BMC Cancer 2024, 24, 568. [Google Scholar] [CrossRef]
- Hameed, A.M.; Lam, V.W.T.; Pleass, H.C. Significant elevations of serum lipase not caused by pancreatitis: A systematic review. HPB 2014, 17, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, R.; Caklili, O.T.; Tekin, S.; Hacisahinogullari, H.; Tanrikulu, S.; Koc, M.S.; DInccag, N. Comparison of amylase and lipase levels of patients with Type 2 diabetes under different treatment modalities. Biomark. Med. 2022, 16, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Okuda, T.; Sakagami, J.; Harada, T.; Ohara, T.; Taniguchi, M.; Sakai, H.; Oka, K.; Hara, T.; Tsuji, T.; et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci. Rep. 2020, 10, 18758. [Google Scholar] [CrossRef] [PubMed]
- Pointer, D.T.; Roife, D.; Powers, B.D.; Murimwa, G.; Elessawy, S.; Thompson, Z.J.; Schell, M.J.; Hodul, P.J.; Pimiento, J.M.; Fleming, J.B.; et al. Neutrophil to lymphocyte ratio, not platelet to lymphocyte or lymphocyte to monocyte ratio, is predictive of patient survival after resection of early-stage pancreatic ductal adenocarcinoma. BMC Cancer 2020, 20, 750. [Google Scholar] [CrossRef]
- Domagała-Haduch, M.; Wnuk, J.; Michalecki, Ł.; Gisterek, I. Neutrophil-to-lymphocyte ratio (NLR) as a prognostic factor in patients during palliative treatment of pancreatic ductal adeoncarcinoma with FOLFIRINOX regimen. Biul. Pol. Tow. Onkol. Nowotw. 2023, 8, 83–86. [Google Scholar]
- Li, W.; Chen, Y.; Wang, X.; Shi, Y.; Dai, G.; Li, X. Pretreatment platelet to lymphocyte ratio is predictive of overall survival in metastatic pancreatic ductal adenocarcinoma. Transl. Cancer Res. 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fang, Y.; Mo, Z.; Lin, Y.; Ji, C.; Jian, Z. Prognostic value of lymphocyte to monocyte ratio in pancreatic cancer: A systematic review and meta-analysis including 3338 patients. World J. Surg. Oncol. 2020, 18, 186. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Valihrach, L.; Androvic, P.; Kubista, M. Circulating miRNA analysis for cancer diagnostics and therapy. Mol. Asp. Med. 2020, 72, 100825. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuan, Y.; Cho, J.H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA Spectrum between Serum and Plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef] [PubMed]
- Ameling, S.; Kacprowski, T.; Chilukoti, R.K.; Malsch, C.; Liebscher, V.; Suhre, K.; Pietzner, M.; Friedrich, N.; Homuth, G.; Hammer, E.; et al. Associations of circulating plasma microRNAs with age, body mass index and sex in a population-based study. BMC Med. Genom. 2015, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- De Boer, H.C.; Van Solingen, C.; Prins, J.; Duijs, J.M.G.J.; Huisman, M.V.; Rabelink, T.J.; Van Zonneveld, A.J. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur. Heart J. 2013, 34, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Halushka, M.K. RNA Biology Toward the promise of microRNAs-Enhancing reproducibility and rigor in microRNA research. RNA Biol. 2016, 13, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tong, Y.; Zhong, A.; Wang, Y.; Lu, R.; Guo, L. Identification of Serum microRNA-25 as a novel biomarker for pancreatic cancer. Medicine 2020, 99, e23863. [Google Scholar] [CrossRef]
- Akamatsu, M.; Makino, N.; Ikeda, Y.; Matsuda, A.; Ito, M.; Kakizaki, Y.; Saito, Y.; Ishizawa, T.; Kobayashi, T.; Furukawa, T.; et al. Specific MAPK-Associated MicroRNAs in Serum Differentiate Pancreatic Cancer from Autoimmune Pancreatitis. PLoS ONE 2016, 11, e0158669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, L.; Xiao, Y.; Zhu, J.; Li, Z. Circulating microRNA-182 in plasma and its potential diagnostic and prognostic value for pancreatic cancer. Med. Oncol. 2014, 31, 225. [Google Scholar] [CrossRef] [PubMed]
- Michael Traeger, M.; Rehkaemper, J.; Ullerich, H.; Steinestel, K.; Wardelmann, E.; Senninger, N.; Abdallah Dhayat, S. The ambiguous role of microRNA-205 and its clinical potential in pancreatic ductal adenocarcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, Z.; Wang, T.; Huang, Z.; Zhu, W.; Miao, Y. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis. Gene 2018, 673, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, J.W.; Wang, L.; Liu, H.; Yan, Y.; Hu, S.Y. MicroRNA-221-3p is up-regulated and serves as a potential biomarker in pancreatic cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 482–487. [Google Scholar] [CrossRef]
- Shi, W.; Lu, Y.; Gong, R.; Sun, J.J.; Liu, G. Serum miR-629 is a novel molecular marker for diagnosis and the prognosis of pancreatic cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5187–5193. [Google Scholar] [CrossRef]
- Lin, M.S.; Chen, W.C.; Huang, J.X.; Gao, H.J.; Sheng, H.H. Aberrant expression of microRNAs in serum may identify individuals with pancreatic cancer. Int. J. Clin. Exp. Med. 2014, 7, 5226–5234. [Google Scholar] [PubMed]
- Hua, Y.; Chen, H.; Wang, L.; Wang, F.; Wang, P.; Ning, Z.; Li, Y.; Liu, L.; Chen, Z.; Meng, Z. Low serum miR-373 predicts poor prognosis in patients with pancreatic cancer. Cancer Biomark. 2017, 20, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Diagnostic performance for declined microRNA-133a in pancreatic cancer. J. Cell. Biochem. 2019, 121, 3882–3886. [Google Scholar] [CrossRef] [PubMed]
- Guraya, S. Prognostic significance of circulating microRNA-21 expression in esophageal, pancreatic and colorectal cancers; a systematic review and meta-analysis. Int. J. Surg. 2018, 60, 41–47. [Google Scholar] [CrossRef]
- Wald, P.; Liu, X.S.; Pettit, C.; Dillhoff, M.; Manilchuk, A.; Schmidt, C.; Wuthrick, E.; Chen, W.; Williams, T.M. Prognostic value of microRNA expression levels in pancreatic adenocarcinoma: A review of the literature. Oncotarget 2017, 8, 73345. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Lieberman, J.A.; Lockwood, C.M. Variability in, variability out: Best practice recommendations to standardize pre-analytical variables in the detection of circulating and tissue microRNAs. Clin. Chem. Lab. Med. 2017, 55, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kowdley, K.V. Method for microRNA isolation from clinical serum samples. Anal. Biochem. 2012, 431, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Duy, J.; Koehler, J.W.; Honko, A.N.; Minogue, T.D. Optimized microRNA purification from TRIzol-treated plasma. BMC Genom. 2015, 16, 95. [Google Scholar] [CrossRef]

| Patient Characteristics | General | Male | Female | p-Value |
|---|---|---|---|---|
| Number of participants | 50 | 22 (44%) | 28 (56%) | |
| Age [years] | Median: 67.5 [years] IQR60–72.0 | Median: 67.5 [years] IQR 63–70 | Median: 68 [years] IQR60–72.5 | Mann–Whitney U test p > 0.05 |
| BMI [kg/m2] | Median: 23.19 [kg/m2] IQR 21.2–25 | Median: 23.74 [kg/m2] IQR 21.46–24.85 | Median: 21.91 [kg/m2] IQR 18.99–26.06 | Mann–Whitney U test p > 0.05 |
| T stage n (% of patient population) | T2 = 6 (12%) T3 = 10 (20%) T4 = 34 (68%) | T2 = 2 (9.09%) T3 = 5 (22.73%) T4 = 15 (68.18%) | T2 = 4 (14.29%) T3 = 5 (17.86%) T4 = 19(67.85%) | Mann–Whitney U test p > 0.05 |
| N stage n (% of patient population) | N0 = 19 (38%) N1 = 21 (42%) N2 = 6 (12%) N3 = 2 (4%) lack of data = 2 (4%) | N0 = 10 (45.45%) N1 = 10 (45.45%) N2 = 1 (4.54%) N3 = 1 (4.54%) | N0 = 9 (32.14%) N1 = 11 (39.29%) N2 = 5 (17.86) N3 = 1 (3.57%) lack of data = 2 (7.14%) | Mann–Whitney U test p > 0.05 |
| M stage n (% of patients population) | M0 = 24 (48%) M1 = 26 (52%) | M0 = 9 (40.91%) M1 = 13 (59.09%) | M0 = 15 (53.57%) M1 = 13 (46.43%) | Yate’s test p > 0.05 |
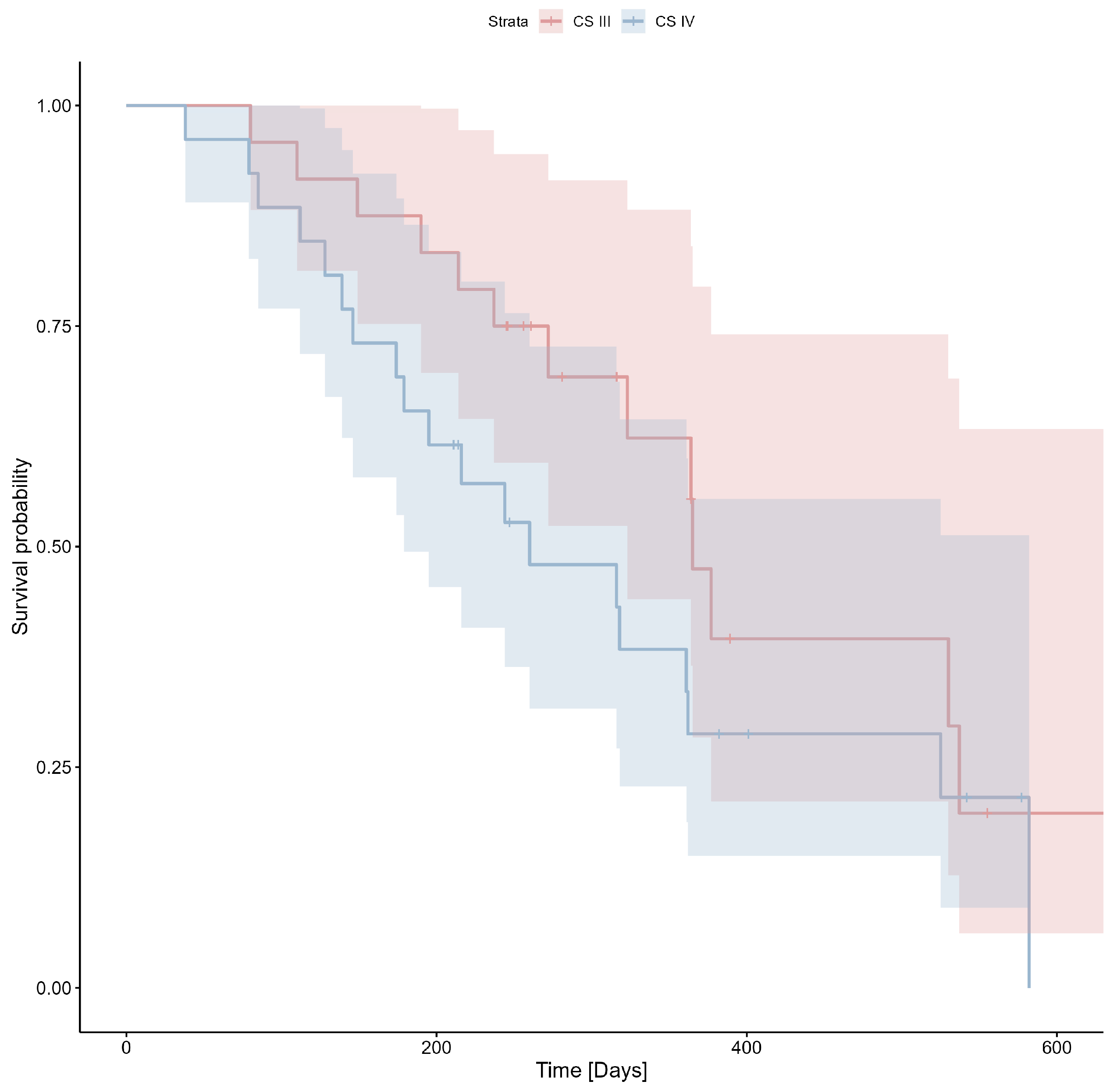
| CS n (% of patiennt population) | III = 24 (48%) IV = 26(52%) | III = 9 (40.91%) IV = 13 (59.09%) | III = 15 (53.57%) IV = 13 (46.43%) | Yate’s test p > 0.05 |
| ECOG n (% of patient population) | 0 = 11 (22%) 1 = 26 (52%) 2 = 10 (20%) 3 = 3 (6%) | 0 = 5(22.73%) 1 = 13 (59.09%) 2 = 3 (13.64%) 3 = 1 (4.54%) | 0 = 6 (21.43%) 1 = 13 (46.43%) 2 = 7 (25%) 3 = 2 (7.14%) | Mann–Whitney U test p > 0.05 |
| History of cholecystectomy | Yes [n = 9] (18%) No [n = 41] (82%) | Yes [n = 3] (13.64%) No [n = 19] (86.36%) | Yes [n = 6] (21.43%) No [n = 22] (78.57%) | Yate’s test p > 0.05 |
| History of palliative pancreatic bypass surgery | Yes [n = 4] (8%) No [n = 46] (92%) | Yes [n = 1] (4.55%) No [n = 21] (95.46%) | Yes [n = 3] (10.71%) No [n = 25] (89.29%) | Yate’s test p > 0.05 |
| History of biliary stent placement | Yes [n = 24] (48%) No [n = 26] (52%) | Yes [n = 7] (31.82%) No [n = 15] 68.18%) | Yes [n = 17] (39.29%) No [n = 11] (60.71%) | Yate’s test p > 0.05 (0.08) |
| History of pulmonary disease | Yes [n = 4] (8%) No [n = 46] (92%) | Yes [n = 2] (9.09%) No [n = 20] (90.91%) | Yes [n = 2] (7.14%) No [n = 46] (92.86%) | Yate’s test p > 0.05 |
| History of diabetes | Yes [n = 19] (38%) No [n = 31] (62%) | Yes [n = 10] (45.45%) No [n = 12] (54.55%) | Yes [n = 9] (32.14%) No [n = 19] (67.86%) | Yate’s test p > 0.05 |
| History of cardiovascular disease | Yes [n = 37] (74%) No [n = 13] (26%) | Yes [n = 15] (68.18%) No [n = 7] (31.82%) | Yes [n = 22] (78.57%) No [n = 6] (21.43%) | Yate’s test p > 0.05 |
| History of anticoagulant medication intake | Yes [n = 10] (20%) No [n = 40] (80%) | Yes [n = 8] (36.36%) No [n = 14] (63.64%) | Yes [n = 2] (7.14%) No [n = 26] (92.86%) | Yate’s test p = 0.027 |
| History of hypotensive medications | Yes [n = 32] (64%) No [n = 18] (36%) | Yes [n = 11] (50%) No [n = 11] (50%) | Yes [n = 21] (25%) No [n = 7] (75%) | Yate’s test p > 0.05 (0.08) |
| History of oral hypoglycemic medications | Yes [n = 13] (26%) No [n = 37] (74%) | Yes [n = 8] (36.36%) No [n = 14] (63.64%) | Yes [n = 5] (17.86%) No [n = 23] (82.14%) | Yate’s test p > 0.05 |
| History of alcohol usage | Yes [n = 9] (18%) No [n = 41] (82%) | Yes [n = 4] (18.18%) No [n = 18] (81.82%) | Yes [n = 5] (17.86%) No [n = 23] (82.14%) | Yate’s test p > 0.05 |
| History of smoking | Yes [n = 21] (42%) No [n = 29] (58%) | Yes [n = 9] (40.91%) No [n = 13] (59.09%) | Yes [n = 12] (42.86%) No [n = 16] (57.14%) | Yate’s test p > 0.05 |
| Systemic therapy administered | FOLFIRINOX = 29 (58%) GCB = 11 (22%) nab-PXL + GCB = 6 (12%) haven’t started = 4 (8%) | FOLFIRINOX = 14 (63.64%) GCB = 4 (18.18%) nab-PXL + GCB = 3 (13.64%) haven’t started = 1 (4.54%) | FOLFIRINOX = 15 (53.56%) GCB = 7 (25%) nab-PXL + GCB = 3 (10.72%) haven’t started = 3 (10.72%) |
| Biomarker | Biomarker Serum Levels (U/L) n = 49 | Biomarker Serum Levels CS III (U/L) n = 23 | Biomarker Serum Levels CS IV (U/L) n = 26 | p-Value |
|---|---|---|---|---|
| Lipase | Median: 32.6 IQR: 18.6–48.7 | Median: 36.8 IQR: 19.6–54.6 | Median: 26.9 IQR: 15.0–44.3 | Mann–Whitney U test p > 0.05 |
| Amylase | Median: 41.9 IQR: 31.5–75.5 | Median: 44.9 IQR: 35.7–73.8 | Median: 41.4 IQR: 26.5–76.2 | Mann–Whitney U test p > 0.05 |
| Parameter | General n = 50 | CS III n = 24 | CS IV n = 26 | p-Value |
|---|---|---|---|---|
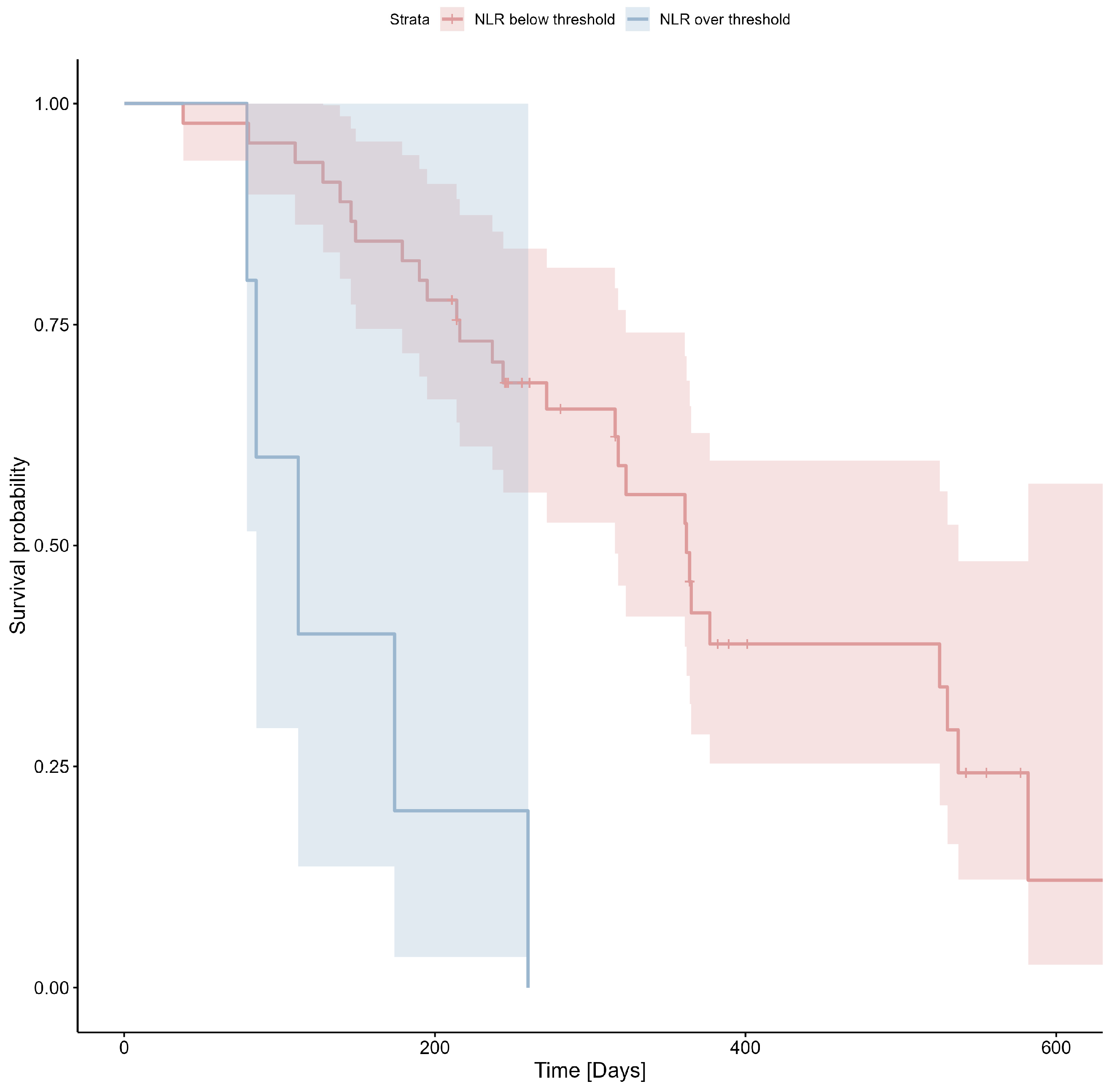
| Neutrophil-to-lymphocyte ratio (NLR) | Median: 2.71 IQR: 2.12–4.25 | Median: 2.46 IQR: 2.05–3.15 | Median: 3.69 IQR: 2.26–5.83 | Mann–Whitney U test p = 0.043 |
| Platelet-to-lymphocyte ratio (PLR) | Median: 132.66 IQR: 97.26–171.55 | Median: 129.85 IQR: 97.26–161.68 | Median: 155.68 IQR: 110.63–233.82 | Mann–Whitney U test p > 0.05 |
| Lymphocyte-to-monocyte ratio (LMR) | Median: 2.94 IQR: 1.81–3.81 | Median: 3.42 IQR: 2.11–3.85 | Median: 2.36 IQR: 1.29–3.59 | Mann–Whitney U test p > 0.05 |
| Sample | General Expression Levels | Sex | Clinical Stage | ||
|---|---|---|---|---|---|
| Serum hsa-miR-885 (n = 29) | Median: 0.030 IQR: 0.009–0.523 | Male (n = 14) | Female (n = 15) | CS III (n = 14) | CS IV (n = 15) |
| Median: 0.013 IQR: 0.0003–0.045 | Median: 0.05 IQR: 0.011–0.735 | Median: 0.013 IQR: 0.0003–0.523 | Median: 0.045 IQR: 0.012–0.735 | ||
| Mann–Whitney U test p > 0.05 | Mann–Whitney U test p > 0.05 | ||||
| Plasma hsa-miR-885 (n = 27) | Median: 0.082 IQR: 0.002–0.305 | Male (n = 12) | Female (n = 15) | CS III (n = 15) | CS IV (n = 12) |
| Median: 0.037 IQR: 0.003–0.261 | Median: 0.051 IQR: 0.011–0.735 | Median: 0.019 IQR: 0.0007–0.142 | Median: 0.256 IQR: 0.03–1.271 | ||
| Mann–Whitney U test p > 0.05 | Mann–Whitney U test p > 0.05 (0.053) | ||||
| Serum hsa-miR-22-3p (n = 20) | Median: 0.373 IQR: 0.087–1.05 | Male (n = 7) | Female (n = 13) | CS III (n = 13) | CS IV (n = 7) |
| Median: 0.793 IQR: 0.246–3.203 | Median: 0.292 IQR: 0.0232–0.694 | Median: 0.421 IQR: 0.023–1.086 | Median: 0.329 IQR: 0.151–0.793 | ||
| Mann–Whitney U test p > 0.05 | Mann–Whitney U test p > 0.05 | ||||
| Plasma hsa-miR-22-3p (n = 16) | Median: 0.258 IQR: 0.111–2.025 | Male (n = 8) | Female (n = 8) | CS III (n = 8) | CS IV (n = 8) |
| Median: 0.255 IQR: 0.109–2.1 | Median: 0.362 IQR: 0.111–2.025 | Median: 0.392 IQR: 0.193–2.476 | Median: 0.190 IQR: 0.063–1.65 | ||
| Mann–Whitney U test p > 0.05 | Mann–Whitney U test p > 0.05 | ||||
| Variables | Beta Coefficient | Standard Error | p-Value | Hazard Ratio | Confidence Interval (95%) |
|---|---|---|---|---|---|
| Age | 0.0674 | 0.0266 | 0.011 | 1.0696 | 1.0158–1.1264 |
| Clinical stage | 0.2393 | 0.4003 | p > 0.05 | 1.0228 | 0.4295–2.4363 |
| CA19-9 | 0.0001 | 0.0001 | 0.024 | 1.0002 | 1.0001–1.0003 |
| CRP | 0.0087 | 0.0033 | 0.008 | 1.0087 | 1.0022–1.0153 |
| NLR | 0.1382 | 0.053 | 0.022 | 1.1482 | 1.0354–1.2734 |
| Overall model fit | Chi-square: 227,996, p = 0.0004 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnuk, J.; Hudy, D.; Strzelczyk, J.K.; Michalecki, Ł.; Dybek, K.; Gisterek-Grocholska, I. Serum hsa-miR-22-3p, hsa-miR-885-5p, Lipase-to-Amylase Ratio, C-Reactive Protein, CA19-9, and Neutrophil-to-Lymphocyte Ratio as Prognostic Factors in Advanced Pancreatic Ductal Adenocarcinoma. Curr. Issues Mol. Biol. 2025, 47, 27. https://doi.org/10.3390/cimb47010027
Wnuk J, Hudy D, Strzelczyk JK, Michalecki Ł, Dybek K, Gisterek-Grocholska I. Serum hsa-miR-22-3p, hsa-miR-885-5p, Lipase-to-Amylase Ratio, C-Reactive Protein, CA19-9, and Neutrophil-to-Lymphocyte Ratio as Prognostic Factors in Advanced Pancreatic Ductal Adenocarcinoma. Current Issues in Molecular Biology. 2025; 47(1):27. https://doi.org/10.3390/cimb47010027
Chicago/Turabian StyleWnuk, Jakub, Dorota Hudy, Joanna Katarzyna Strzelczyk, Łukasz Michalecki, Kamil Dybek, and Iwona Gisterek-Grocholska. 2025. "Serum hsa-miR-22-3p, hsa-miR-885-5p, Lipase-to-Amylase Ratio, C-Reactive Protein, CA19-9, and Neutrophil-to-Lymphocyte Ratio as Prognostic Factors in Advanced Pancreatic Ductal Adenocarcinoma" Current Issues in Molecular Biology 47, no. 1: 27. https://doi.org/10.3390/cimb47010027
APA StyleWnuk, J., Hudy, D., Strzelczyk, J. K., Michalecki, Ł., Dybek, K., & Gisterek-Grocholska, I. (2025). Serum hsa-miR-22-3p, hsa-miR-885-5p, Lipase-to-Amylase Ratio, C-Reactive Protein, CA19-9, and Neutrophil-to-Lymphocyte Ratio as Prognostic Factors in Advanced Pancreatic Ductal Adenocarcinoma. Current Issues in Molecular Biology, 47(1), 27. https://doi.org/10.3390/cimb47010027







