Molecular Findings Before Vision Loss in the Streptozotocin-Induced Rat Model of Diabetic Retinopathy
Abstract
1. Introduction
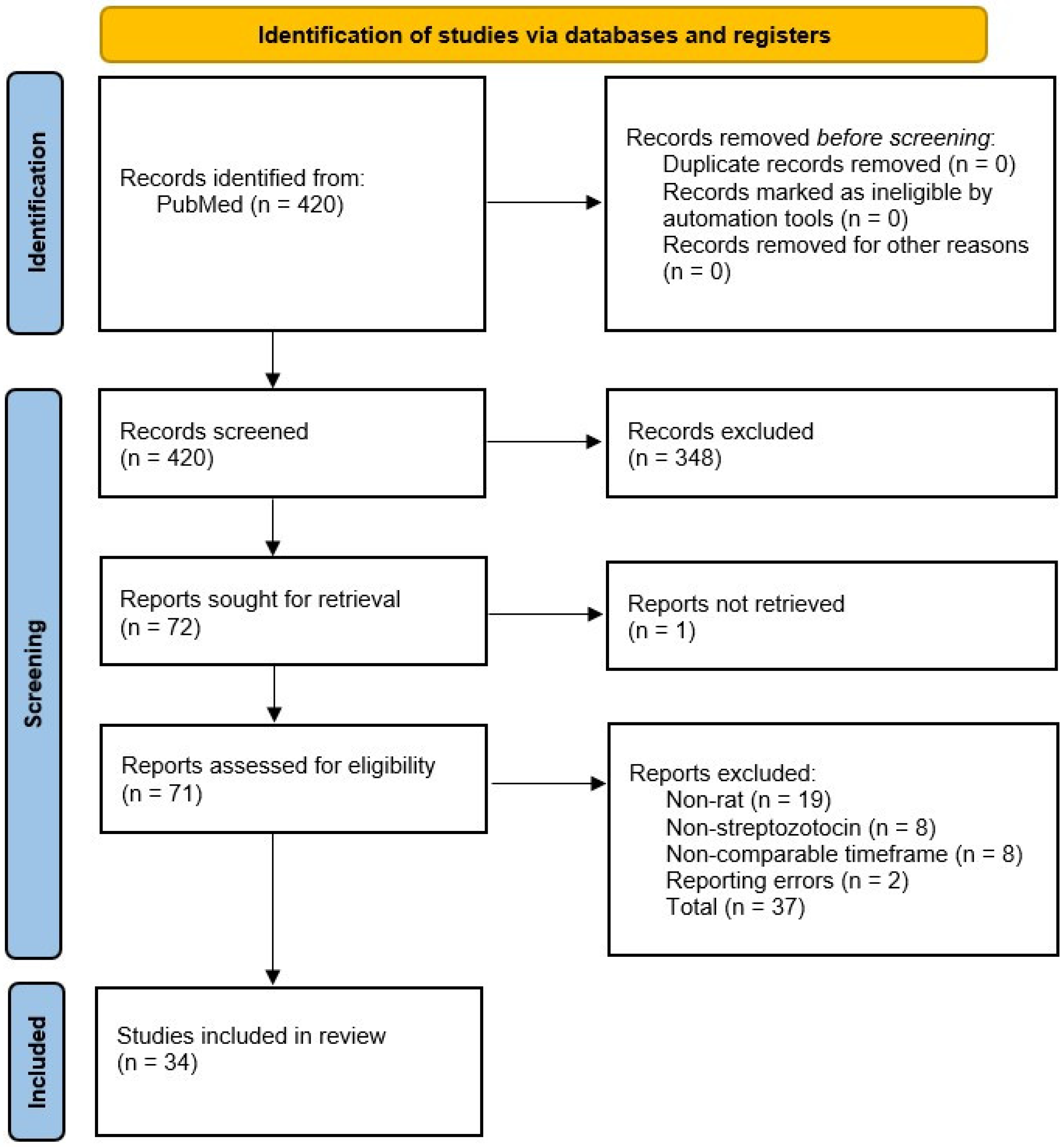
2. Search Strategy
3. Chemically Induced Diabetic Rat Model
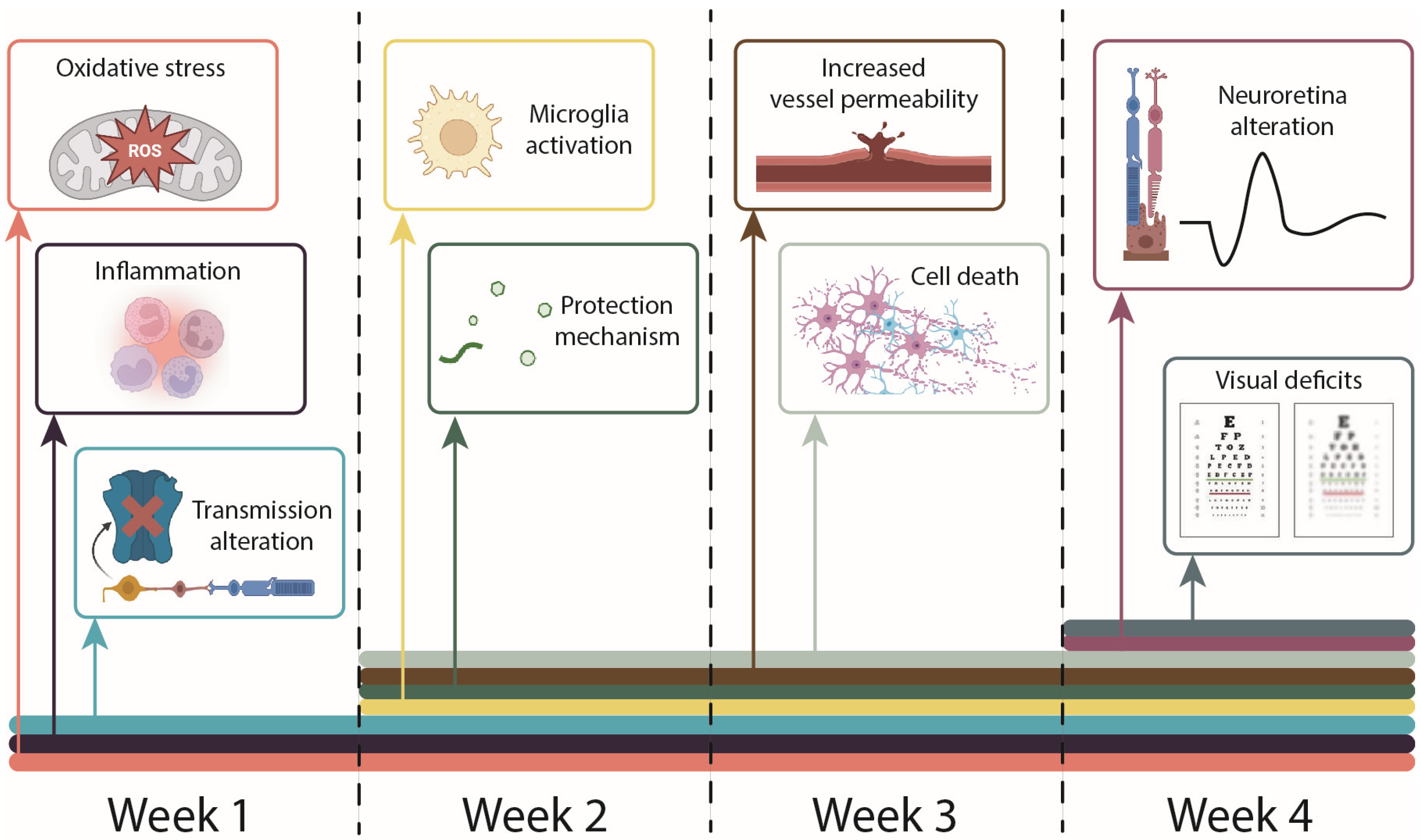
4. First Week of Hyperglycemia—Oxidative Stress and Microglia Activation
| Findings | Author, Year | |
|---|---|---|
| Week 1 | ||
| Oxidative stress | ||
| ↑ miR-365 ↑ miR-221 ↓ TIMP3 | Wang, 2018 [6] | |
| ↓ Nrf2 ↓ Keap1 | Albert-Garay, 2019 [7] | |
| Inflammation | ||
| ↑ TNFα | Wang, 2018 [6]; Puglia, 2020 [9] | |
| ↑ Il-1β | Wang, 2018 [6] | |
| Neurotransmission alteration | ||
| ↓ Glycine receptor a2, a4 subunits ↑ Glycine receptor b subunit | Morales-Calixto, 2019 [36] | |
| Week 2 | ||
| Protection mechanism | ||
| ↑ EPO | Gu, 2019 [41] | |
| Oxidative stress | ||
| ↑ miR-365 ↑ miR-221 ↓ TIMP3 | Wang, 2018 [6]; | |
| ↑ Nitrotyrosine cells in the INL, GCL, RPE | Dionysopoulou, 2023 [37] | |
| Inflammation | ||
| ↑ TNFα | Dionysopoulou, 2023 [37]; Özay, 2020 [40]; Wang, 2018 [6] | |
| ↑ B1 receptor | Hachana, 2018 [38] | |
| ↑ INF-gamma | Özay, 2020 [40] | |
| ↑ c-myc | Zhang, 2019 [61] | |
| ↑ Il-1β | Wang, 2018 [6] | |
| Microglia activation | ||
| ↑ Iba-1 | Dionysopoulou, 2023 [37] | |
| ↑ Amoeboid morphology | Hachana, 2018 [38] | |
| ↑ iCAM-1 | Shi, 2021 [8] | |
| Vessel permeability | ||
| ↑ VEGF | Dionysopoulou, 2023 [37] | |
| ↑ VEGF-A ↑ VEGFR-2 | Hachana, 2018 [38] | |
| ↑ Phosphorylated VE-cadherin | Liu, 2020 [39] | |
| ↑ Evans Blue extravasation | Hachana, 2018 [38] | |
| Cell death | ||
| ↑ MMP-2, MMP-9 | Özay, 2020 [40] | |
| ↓ PERG | Dionysopoulou, 2023 [37] | |
| ↓ GCL and IPL thickness | Dionysopoulou, 2023 [37] | |
| Week 3 | ||
| Protection mechanism | ||
| ↑ HO-1 | Giunta, 2023 [42] | |
| Oxidative stress | ||
| ↓ Nrf2 | Albert-Garay, 2021 [7] | |
| ↑ G-6-P ↑ Glycogen ↑ Lactate | Ramírez-Pérez, 2020 [43] | |
| Inflammation | ||
| ↑ COX-2 ↑ iNOS | Giunta, 2023 [42] | |
| Week 4 | ||
| Protection mechanism | ||
| ↑ EPO ↑ EPOR | Giunta, 2023 [42] | |
| Oxidative stress | ||
| ↓ SOD ↑ iPF2a | Fathalipour, 2019 [45] | |
| ↑ ROS ↑ Nrf2 ↑ HO-1 | Canovai, 2022 [14] | |
| ↓ Nrf2, NQO1, HO-q ↑ Keap1 ↑ MDA ↓ SOD ↓ CAT ↓ GPx | Shi, 2020 [12] | |
| Inflammation | ||
| ↑ IL-1β | Bai, 2021 [44]; Zhang, 2019 [61]; Ibán-Arias, 2018 [54] | |
| ↑ Il-18 | Bai, 2021 [44] | |
| ↑ Il-6 | Canovai, 2022 [14]; Clapp, 2019 [56]; Zhang, 2019 [61] | |
| ↑ HIF-1a ↑ ANGPTL4 | Yang, 2019 [48] | |
| ↑ NFκB | Canovai, 2022 [14]; Shi, 2020 [12] | |
| ↑ SOX9 | Li, 2023 [47] | |
| ↓ FKN | Jiang, 2022 [51] | |
| ↑ TNFα | Shi, 2020 [12]; Ibán-Arias, 2018 [54]; Zhang, 2019 [61] | |
| ↑ MMP-2 ↓ IL-10 ↓ TIMP-1 | Shi, 2020 [12] | |
| ↑ c-myc | Zhang, 2019 [61] | |
| ↓ Iba-1 | Shi, 2021 [8] | |
| Microglia activation | ||
| ↑ SOX9 ↑ TXNIP | Li, 2023 [47] | |
| ↑ GFAP | Li, 2023 [47]; Canovai, 2022 [14]; Zhang, 2018 [53]; Ibán-Arias, 2018 [54]; Gu, 2019 [41] | |
| Vessel permeability | ||
| ↑ HIF-1α | Canovai, 2022 [14]; Yang, 2019 [48]; Gu, 2019 [41] | |
| ↑ VEGF | Zhang, 2018 [53]; Canovai, 2022 [14]; Gu, 2019 [41]; Clapp, 2019 [56] | |
| ↑ Evans Blue extravasation | Canovai, 2022 [14]; Clapp, 2019 [56] | |
| ↑ ANGPTL4 | Yang, 2019 [48] | |
| ↑ Vessel formation in IPL | Shi, 2020 [12] | |
| ↓ miR29a ↓ miR-29b | Zhang, 2018 [53] | |
| ↓ MEG3 | He, 2021 [55] | |
| ↑ Dilated tortuous vessels ↑ hemorrhage | Fu, 2021 [11] | |
| Cell death | ||
| ↓ RGCs | Fathalipour, 2019 [45] | |
| ↑ Condensed nuclei in GCL | Shi, 2020 [12] | |
| ↓ Glutamine synthase | Zhang, 2018 [53]; Gu, 2019 [41] | |
| ↓ GLAST | Gu, 2019 [41] | |
| ↓ β-III tubulin | Ma, 2018 [10] | |
| ↓ Cell viability | Bai, 2021 [44] | |
| ↑ TUNEL-positive cells | Bai, 2021 [44]; Ma, 2018 [10]; Ibán-Arias, 2018 [54] | |
| ↓ TH protein | Ma, 2018 [10] | |
| ↑ Caspase 3 | Canovai, 2022 [14]; Ma, 2018 [10] | |
| ↑ ONL cell death | Jiang, 2022 [51] | |
| ↑ p75NTR | Ibán-Arias, 2018 [45] | |
| ↓ ONL, INL thickness | Bai, 2021 [44] | |
| ↑ Degenerate capillaries | Bai, 2021 [44] | |
| ↓ Retinal thickness | Fathalipour, 2019 [45]; Li, 2023 [43]; Fu, 2021 [11] | |
| No MMP level alteration | Şahin, 2021 [57] | |
| Neuroretinal alteration | ||
| ↓ Electroretinogram a-wave, b-wave amplitude | Naderi, 2019 [13]; Canovai, 2022 [14] | |
| ↓ CNTF protein | Ma, 2018 [10] | |
| ↑ p-ERK | Fathalipour, 2019 [45]; Ibán-Arias, 2018 [45] | |
| ↓ p-AKT | Fathalipour, 2019 [36] | |
| ↓ Uptake of [18F]FP-(+)-DTBZ ↓ VMAT2 | Li, 2020 [49] | |
| ↓ NFL-, bNOS-, and TH-IRs | Ibán-Arias, 2019 [50]; Ibán-Arias, 2018 [45] | |
| Visual function deficits | ||
| ↓ Spatial frequency thresholds ↓ Contrast sensitivity | Allen, 2018 [15] |
5. Second Week of Hyperglycemia—Increased Vessel Permeability
6. Third Week of Hyperglycemia—Progression of Redox and Inflammatory Imbalances
7. Fourth Week of Hyperglycemia—Visual Deficits
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.K.S.; de Clerck, E.; Polivka, J., Jr.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sabanayagam, C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: From Epidemiology to Artificial Intelligence. Ophthalmologica 2020, 243, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Mounirou, B.A.M.; Adam, N.D.; Yakoura, A.K.H.; Aminou, M.S.M.; Liu, Y.T.; Tan, L.Y. Diabetic Retinopathy: An Overview of Treatments. Indian J. Endocrinol. Metab. 2022, 26, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, J.; Yazdanyar, A. Animal models of diabetic retinopathy. Ann. Transl. Med. 2021, 9, 1272. [Google Scholar] [CrossRef]
- Aung, M.H.; Park, H.N.; Han, M.K.; Obertone, T.S.; Abey, J.; Aseem, F.; Thule, P.M.; Iuvone, P.M.; Pardue, M.T. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J. Neurosci. 2014, 34, 726–736. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Chen, X.; Yang, Y.; Wang, F.; Li, W.; Awuti, M.; Sun, Y.; Lian, C.; Li, Z.; et al. miR-365 promotes diabetic retinopathy through inhibiting Timp3 and increasing oxidative stress. Exp. Eye Res. 2018, 168, 89–99. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Sanchez-Chavez, G.; Salceda, R. Retinal Nrf2 expression in normal and early streptozotocin-diabetic rats. Neurochem. Int. 2021, 145, 105007. [Google Scholar] [CrossRef]
- Shi, F.J.; Xie, H.; Zhang, C.Y.; Qin, H.F.; Zeng, X.W.; Lou, H.; Zhang, L.; Xu, G.T.; Zhang, J.F.; Xu, G.X. Is Iba-1 protein expression a sensitive marker for microglia activation in experimental diabetic retinopathy? Int. J. Ophthalmol. 2021, 14, 200–208. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Ostacolo, C.; Maria Sommella, E.; Campiglia, P.; Carbone, C.; Drago, F.; Pignatello, R.; Bucolo, C. Ocular Formulation Based on Palmitoylethanolamide-Loaded Nanostructured Lipid Carriers: Technological and Pharmacological Profile. Nanomaterials 2020, 10, 287. [Google Scholar] [CrossRef]
- Ma, M.; Xu, Y.; Xiong, S.; Zhang, J.; Gu, Q.; Ke, B.; Xu, X. Involvement of ciliary neurotrophic factor in early diabetic retinal neuropathy in streptozotocin-induced diabetic rats. Eye 2018, 32, 1463–1471. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, X.; He, G.H.; Chen, S.; Gu, Z.H.; Zhang, Y.L.; Li, L.Y. Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging. Int. J. Ophthalmol. 2021, 14, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Cheng, Y.; Dong, X.; Zhang, M.; Pei, C.; Zhang, M. Effects of rhaponticin on retinal oxidative stress and inflammation in diabetes through NRF2/HO-1/NF-kappaB signalling. J. Biochem. Mol. Toxicol. 2020, 34, e22568. [Google Scholar] [CrossRef] [PubMed]
- Naderi, A.; Zahed, R.; Aghajanpour, L.; Amoli, F.A.; Lashay, A. Long term features of diabetic retinopathy in streptozotocin-induced diabetic Wistar rats. Exp. Eye Res. 2019, 184, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Canovai, A.; Amato, R.; Melecchi, A.; Dal Monte, M.; Rusciano, D.; Bagnoli, P.; Cammalleri, M. Preventive Efficacy of an Antioxidant Compound on Blood Retinal Barrier Breakdown and Visual Dysfunction in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 2021, 12, 811818. [Google Scholar] [CrossRef]
- Allen, R.S.; Hanif, A.M.; Gogniat, M.A.; Prall, B.C.; Haider, R.; Aung, M.H.; Prunty, M.C.; Mees, L.M.; Coulter, M.M.; Motz, C.T.; et al. TrkB signalling pathway mediates the protective effects of exercise in the diabetic rat retina. Eur. J. Neurosci. 2018, 47, 1254–1265. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar] [CrossRef]
- Martin-Carro, B.; Donate-Correa, J.; Fernandez-Villabrille, S.; Martin-Virgala, J.; Panizo, S.; Carrillo-Lopez, N.; Martinez-Arias, L.; Navarro-Gonzalez, J.F.; Naves-Diaz, M.; Fernandez-Martin, J.L.; et al. Experimental Models to Study Diabetes Mellitus and Its Complications: Limitations and New Opportunities. Int. J. Mol. Sci. 2023, 24, 10309. [Google Scholar] [CrossRef]
- Yokoi, N.; Misako, N.; Masanori, F.; He-Yao, W.; Toshiko, H.; Susumu, S.; Kajuro, K. Establishment and Characterization of the Komeda Diebetes-prone Rat as a Segregation Inbred Strain. Exp. Anim. 2003, 52, 295–301. [Google Scholar] [CrossRef]
- Singh, R.; Gholipourmalekabadi, M.; Shafikhani, S.H. Animal models for type 1 and type 2 diabetes: Advantages and limitations. Front. Endocrinol. 2024, 15, 1359685. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental animal models for diabetes and its related complications-a review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussaniy, H.A.; Alburghaif, A.H.; Naji, M.A. Leptin hormone and its effectiveness in reproduction, metabolism, immunity, diabetes, hopes and ambitions. J. Med. Life 2021, 14, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.; Mitrea, D.R.; Florea, A.; Chis, I.C.; Suciu, S.; David, L.; Moldovan, B.E.; Muresan, L.E.; Lenghel, M.; Ungur, R.A.; et al. Effects of Gold Nanoparticles Functionalized with Bioactive Compounds from Cornus mas Fruit on Aorta Ultrastructural and Biochemical Changes in Rats on a Hyperlipid Diet-A Preliminary Study. Antioxidants 2022, 11, 1343. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Garcia-Ayuso, D.; Ortin-Martinez, A.; Jimenez-Lopez, M.; Galindo-Romero, C.; Cuenca, N.; Pinilla, I.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas-Perez, M.P. Changes in the photoreceptor mosaic of P23H-1 rats during retinal degeneration: Implications for rod-cone dependent survival. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5888–5900. [Google Scholar] [CrossRef]
- Huber, G.; Heynen, S.; Imsand, C.; vom Hagen, F.; Muehlfriedel, R.; Tanimoto, N.; Feng, Y.; Hammes, H.P.; Grimm, C.; Peichl, L.; et al. Novel rodent models for macular research. PLoS ONE 2010, 5, e13403. [Google Scholar] [CrossRef]
- Plevkova, J.; Brozmanova, M.; Harsanyiova, J.; Sterusky, M.; Honetschlager, J.; Buday, T. Various aspects of sex and gender bias in biomedical research. Physiol. Res. 2020, 69, S367–S378. [Google Scholar] [CrossRef]
- Korpole, N.R.; Kurada, P.; Korpole, M.R. Gender Difference in Ocular Diseases, Risk Factors and Management with Specific Reference to Role of Sex Steroid Hormones. J. Midlife Health 2022, 13, 20–25. [Google Scholar] [CrossRef]
- Hao, M.; Li, Y.; Lin, W.; Xu, Q.; Shao, N.; Zhang, Y.; Kuang, H. Estrogen prevents high-glucose-induced damage of retinal ganglion cells via mitochondrial pathway. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 83–90. [Google Scholar] [CrossRef]
- Yousefi, H.; Komaki, A.; Shahidi, S.; Habibi, P.; Sadeghian, R.; Ahmadiasl, N.; Daghigh, F. Diabetic neovascularization defects in the retina are improved by genistein supplementation in the ovariectomized rat. Inflammopharmacology 2021, 29, 1579–1586. [Google Scholar] [CrossRef]
- Yamashita, H.; Sugihara, K.; Yamada, C.; Tsutsumi, S.; Iwaki, Y. Effect of estrogen on electroretinographic responses in streptozotocin-induced diabetic female rats. Exp. Eye Res. 2010, 90, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Schmidl, D.; Schmetterer, L.; Garhofer, G.; Popa-Cherecheanu, A. Gender differences in ocular blood flow. Curr. Eye Res. 2015, 40, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Siebert, E.; Raja, V.; Mehrotra, C.; Richards, J.; Khan, J.; Graham, D.F. Determinants of progression of diabetic retinopathy in pregnancy. Diabetes Res. Clin. Pract. 2024, 214, 111784. [Google Scholar] [CrossRef] [PubMed]
- Jingi, A.M.; Tankeu, A.T.; Ateba, N.A.; Noubiap, J.J. Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: The synergistic hypothesis. BMC Endocr. Disord. 2017, 17, 63. [Google Scholar] [CrossRef]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef]
- Morales-Calixto, E.; Velazquez-Flores, M.A.; Sanchez-Chavez, G.; Ruiz Esparza-Garrido, R.; Salceda, R. Glycine receptor is differentially expressed in the rat retina at early stages of streptozotocin-induced diabetes. Neurosci. Lett. 2019, 712, 134506. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Wikstrom, P.; Bucolo, C.; Romano, G.L.; Micale, V.; Svensson, R.; Spyridakos, D.; Mastrodimou, N.; Georgakis, S.; Verginis, P.; et al. Topically Administered NOX4 Inhibitor, GLX7013114, Is Efficacious in Treating the Early Pathological Events of Diabetic Retinopathy. Diabetes 2023, 72, 638–652. [Google Scholar] [CrossRef]
- Hachana, S.; Bhat, M.; Senecal, J.; Huppe-Gourgues, F.; Couture, R.; Vaucher, E. Expression, distribution and function of kinin B(1) receptor in the rat diabetic retina. Br. J. Pharmacol. 2018, 175, 968–983. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Zhang, C.; Xie, H.; Yang, Q.; Li, W.; Tian, H.; Lu, L.; Xu, J.Y.; Xu, G.; et al. Erythropoietin maintains VE-cadherin expression and barrier function in experimental diabetic retinopathy via inhibiting VEGF/VEGFR2/Src signaling pathway. Life Sci. 2020, 259, 118273. [Google Scholar] [CrossRef]
- Ozay, Y.; Ozek, D.; Yildirim, F.; Yildirim, Z. The effect of diabetes on vitreous levels of adiponectin and inflammatory cytokines in experimental rat model. Adv. Clin. Exp. Med. 2020, 29, 449–452. [Google Scholar] [CrossRef]
- Gu, L.; Xu, H.; Zhang, C.; Yang, Q.; Zhang, L.; Zhang, J. Time-dependent changes in hypoxia- and gliosis-related factors in experimental diabetic retinopathy. Eye 2019, 33, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Romano, G.L.; Rossi, S.; Eandi, C.M.; Pricoco, E.; D’Agata, V. Drug-Repurposing Strategy for Dimethyl Fumarate. Pharmaceuticals 2023, 16, 974. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Perez, G.; Sanchez-Chavez, G.; Salceda, R. Mitochondrial bound hexokinase type I in normal and streptozotocin diabetic rat retina. Mitochondrion 2020, 52, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yang, F.; Wang, R.; Yan, Q. Ghrelin Ameliorates Diabetic Retinal Injury: Potential Therapeutic Avenues for Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2021, 2021, 8043299. [Google Scholar] [CrossRef]
- Fathalipour, M.; Eghtedari, M.; Borges, F.; Silva, T.; Moosavi, F.; Firuzi, O.; Mirkhani, H. Caffeic Acid Alkyl Amide Derivatives Ameliorate Oxidative Stress and Modulate ERK1/2 and AKT Signaling Pathways in a Rat Model of Diabetic Retinopathy. Chem. Biodivers. 2019, 16, e1900405. [Google Scholar] [CrossRef]
- Kida, T.; Oku, H.; Horie, T.; Osuka, S.; Fukumoto, M.; Ikeda, T. Protein kinase C-mediated insulin receptor phosphorylation in diabetic rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1427–1434. [Google Scholar] [CrossRef]
- Li, S.; Ouyang, G.; Yuan, L.; Wu, X.; Zhang, L. SRY-box transcription factor 9 modulates Müller cell gliosis in diabetic retinopathy by upregulating TXNIP transcription. Exp. Anim. 2023, 72, 302–313. [Google Scholar] [CrossRef]
- Yang, X.; Cao, J.; Du, Y.; Gong, Q.; Cheng, Y.; Su, G. Angiopoietin-Like Protein 4 (ANGPTL4) Induces Retinal Pigment Epithelial Barrier Breakdown by Activating Signal Transducer and Activator of Transcription 3 (STAT3): Evidence from ARPE-19 Cells Under Hypoxic Condition and Diabetic Rats. Med. Sci. Monit. 2019, 25, 6742–6754. [Google Scholar] [CrossRef]
- Li, J.; Chen, P.; Bao, Y.; Sun, Y.; He, J.; Liu, X. PET Imaging of Vesicular Monoamine Transporter 2 in Early Diabetic Retinopathy Using [(18)F]FP-(+)-DTBZ. Mol. Imaging Biol. 2020, 22, 1161–1169. [Google Scholar] [CrossRef]
- Iban-Arias, R.; Lisa, S.; Poulaki, S.; Mastrodimou, N.; Charalampopoulos, I.; Gravanis, A.; Thermos, K. Effect of topical administration of the microneurotrophin BNN27 in the diabetic rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2429–2436. [Google Scholar] [CrossRef]
- Jiang, M.; Xie, H.; Zhang, C.; Wang, T.; Tian, H.; Lu, L.; Xu, J.Y.; Xu, G.T.; Liu, L.; Zhang, J. Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J. Cell. Mol. Med. 2022, 26, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Han, J.S.; Park, C.K. Neuroprotective Effects of Nicotinamide (Vitamin B(3)) on Neurodegeneration in Diabetic Rat Retinas. Nutrients 2022, 14, 1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, L.; Chen, J.; Lin, S.; Cai, D.; Chen, C.; Chen, Z. Downregulation of MicroRNA 29a/b exacerbated diabetic retinopathy by impairing the function of Muller cells via Forkhead box protein O4. Diabetes Vasc. Dis. Res. 2018, 15, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ibán-Arias, R.; Lisa, S.; Mastrodimou, N.; Kokona, D.; Koulakis, E.; Iordanidou, P.; Kouvarakis, A.; Fothiadaki, M.; Papadogkonaki, S.; Sotiriou, A.; et al. The Synthetic Microneurotrophin BNN27 Affects Retinal Function in Rats With Streptozotocin-Induced Diabetes. Diabetes 2018, 67, 321–333. [Google Scholar] [CrossRef]
- He, Y.; Dan, Y.; Gao, X.; Huang, L.; Lv, H.; Chen, J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E598–E608. [Google Scholar] [CrossRef]
- Clapp, C.; Diaz-Lezama, N.; Adan-Castro, E.; Ramirez-Hernandez, G.; Moreno-Carranza, B.; Sarti, A.C.; Falzoni, S.; Solini, A.; Di Virgilio, F. Pharmacological blockade of the P2X7 receptor reverses retinal damage in a rat model of type 1 diabetes. Acta Diabetol. 2019, 56, 1031–1036. [Google Scholar] [CrossRef]
- Sahin, A.; Kaya, S.; Baylan, M. The effects of caffeic acid phenethyl ester on retina in a diabetic rat model. Cutan. Ocul. Toxicol. 2021, 40, 268–273. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Wu, L.; Wang, Q.; Chen, J.; Zhang, S.; Chen, Z. C-myc contributes to the release of Muller cells-derived proinflammatory cytokines by regulating lncRNA MIAT/XNIP pathway. Int. J. Biochem. Cell Biol. 2019, 114, 105574. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Banerjee, K.; Mondal, L.K. The Amelioration of Detrimental Biochemical Anomalies by Supplementing B, C, and E Vitamins in Subjects with Type 2 Diabetes Mellitus May Reduce the Rate of Development of Diabetic Retinopathy. J. Diabetes Res. 2022, 2022, 3886710. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Pinazo-Duran, M.D.; Garcia-Medina, M.; Zanon-Moreno, V.; Pons-Vazquez, S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; Jackson, G.R.; Quillen, D.A.; Klein, R.; Liao, J.; Gardner, T.W. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in mild to moderate nonproliferative diabetic retinopathy: A randomized proof-of-concept clinical trial. JAMA Ophthalmol. 2014, 132, 1137–1142. [Google Scholar] [CrossRef]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef]
- Biswal, M.R.; Wang, Z.; Paulson, R.J.; Uddin, R.R.; Tong, Y.; Zhu, P.; Li, H.; Lewin, A.S. Erythropoietin Gene Therapy Delays Retinal Degeneration Resulting from Oxidative Stress in the Retinal Pigment Epithelium. Antioxidants 2021, 10, 842. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, F.L.; Wang, G.H. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Gavard, J.; Gutkind, J.S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006, 8, 1223–1234. [Google Scholar] [CrossRef]
- Aragon-Sanabria, V.; Pohler, S.E.; Eswar, V.J.; Bierowski, M.; Gomez, E.W.; Dong, C. VE-Cadherin Disassembly and Cell Contractility in the Endothelium are Necessary for Barrier Disruption Induced by Tumor Cells. Sci. Rep. 2017, 7, 45835. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Aveleira, C.A.; Leal, E.C.; Simoes, N.F.; Fernandes, C.R.; Meirinhos, R.I.; Baptista, F.I.; Ambrosio, A.F. Heme oxygenase-1 protects retinal endothelial cells against high glucose- and oxidative/nitrosative stress-induced toxicity. PLoS ONE 2012, 7, e42428. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Simo-Servat, O.; Bogdanov, P.; Hernandez, C. Diabetic Retinopathy: Role of Neurodegeneration and Therapeutic Perspectives. Asia Pac. J. Ophthalmol. 2022, 11, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [CrossRef]
- Lawry, J. Detection of apoptosis by the TUNEL assay. Methods Mol. Med. 2004, 88, 183–190. [Google Scholar] [CrossRef]
- Do Rhee, K.; Wang, Y.; Ten Hoeve, J.; Stiles, L.; Nguyen, T.T.T.; Zhang, X.; Vergnes, L.; Reue, K.; Shirihai, O.; Bok, D.; et al. Ciliary neurotrophic factor-mediated neuroprotection involves enhanced glycolysis and anabolism in degenerating mouse retinas. Nat. Commun. 2022, 13, 7037. [Google Scholar] [CrossRef]
- Prokosch, V.; Brockhaus, K.; Anders, F.; Liu, H.; Mercieca, K.; Gericke, A.; Melkonyan, H.; Thanos, S. Elevated intraocular pressure induces neuron-specific beta-III-tubulin expression in non-neuronal vascular cells. Acta Ophthalmol. 2020, 98, e617–e630. [Google Scholar] [CrossRef]
- Ishikawa, M. Abnormalities in Glutamate Metabolism and Excitotoxicity in the Retinal Diseases. Scientifica 2013, 2013, 528940. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.; Oh, S.B.; Kim, H.Y.; Kim, C.; Kim, T.Y.; Park, Y.H. Superoxide dismutase 3 prevents early stage diabetic retinopathy in streptozotocin-induced diabetic rat model. PLoS ONE 2022, 17, e0262396. [Google Scholar] [CrossRef]
| Rat Model | Sex | Age (Weeks) | Initial Weight (g) | STZ Scheme | Cut-Off Glycemia (mg/dL) | Time from Diagnosis (days) | Time from Diagnosis (weeks) | Final Glycemia (mg/dL) | Author, Year |
|---|---|---|---|---|---|---|---|---|---|
| Week 1 | |||||||||
| Sprague Dawley | male | NA | 150 | 1 dose ip 60 mg/kg | 250 | 7 | 1 | NA | Wang, 2018 [6] |
| Long Evans | female | 8 | 179 ± 7 | 1 dose ip 98/kg | 250 | 7 | 1 | 355 ± 14 | Albert-Garay, 2021 [7] |
| Sprague Dawley | male | NA | 175–200 | 1 dose iv 60 mg/kg | 250 | 10 | 1 | NA | Puglia, 2020 [9] |
| Sprague Dawley | male | NA | 120 | 1 dose ip 60 mg/kg | 300 | 7 | 1 | 544 ± 14 | Shi, 2021 [8] |
| Long Evans | female | NA | 180–200 | 1 dose ip 90 mg/kg | 250 | 7 | 1 | 382 ± 18 | Morales-Calixto, 2019 [36] |
| Week 2 | |||||||||
| Sprague Dawley | male, female | NA | 200–300 | 1 dose ip 70 mg/kg | 300 | 14 | 2 | NA | Dionysopoulou, 2023 [37] |
| Wistar albino | NA | 6–8 | 200–250 | 1 dose ip 65 mg/kg | 360 | 14 | 2 | 480 ± 34 | Hachana, 2018 [38] |
| Sprague Dawley | male | NA | 130–160 | 1 dose ip 60 mg/kg | 300 | 14 | 2 | NA | Liu, 2020 [39] |
| Wistar albino | male | 12–16 | 180–240 | 1 dose ip 45 mg/kg | 250 | 14 | 2 | NA | Özay, 2020 [40] |
| Sprague Dawley | male | NA | 180 | 1 dose ip 60 mg/kg | 250 | 14 | 2 | NA | Gu, 2019 [41] |
| Week 3 | |||||||||
| Sprague Dawley | male | NA | 200–250 | 1 dose ip 60 mg/kg | 250 | 21 | 3 | 312 ± 28 | Giunta, 2023 [42] |
| Long Evans | female | 8 | 170 ± 15 | 1 dose ip 98/kg | 250 | 20 | 3 | 483 ± 15 | Albert-Garay, 2021 [7] |
| Long Evans | NA | NA | 1 dose ip 98 mg/kg | 250 | 20 | 3 | NA | Ramírez-Pérez, 2020 [43] | |
| Long Evans | female | NA | 180–200 | 1 dose ip 90 mg/kg | 250 | 21 | 3 | 480 ± 15 | Morales-Calixto, 2019 [36] |
| Week 4 | |||||||||
| Wistar albino | male | NA | 270–300 | 1 dose ip 55 mg/kg | 300 | 28 | 4 | 454 ± 56 | Naderi, 2019 [13] |
| Wistar albino | male | NA | 220–280 | 1 dose ip 60 mg/kg | 300 | 28 | 4 | 324 ± 18 | Bai, 2021 [44] |
| Wistar albino | male | NA | 250–300 | 3 dose ip 65 mg/kg | 300 | 28 | 4 | NA | Ma, 2018 [10] |
| Sprague Dawley | male | NA | 200–225 | 1 dose ip 60 mg/kg | 300 | 28 | 4 | 494 ± 21 | Fathalipour, 2019 [45] |
| Wistar albino | male | 9 | NA | 1 dose ip 60 mg/kg | 250 | 28 | 4 | NA | Kida, 2019 [46] |
| Sprague Dawley | male | NA | NA | 1 dose ip 65 mg/kg | 360 | 28 | 4 | 396 ± 36 | Li, 2023 [47] |
| Sprague Dawley | male | 8 | 180–220 | 1 dose ip 65 mg/kg | 300 | 28 | 4 | 504 ± 18 | Yang, 2019 [48] |
| Sprague Dawley | male | 8 | 130–160 | 1 dose ip 65 mg/kg | 300 | 28 | 4 | 468 ± 36 | Li, 2020 [49] |
| Sprague Dawley | male, female | NA | 180–300 | 1 dose ip 70 mg/kg | 350 | 28 | 4 | NA | Ibán-Arias, 2019 [50] |
| Sprague Dawley | male | 8 | 200 | 1 dose ip 65 mg/kg | 250 | 30 | 4 | 550±10 | Canovai, 2020 [14] |
| Sprague Dawley | male | NA | 120–160 | 1 dose ip 60 mg/kg | 300 | 28 | 4 | 502 ± 21 | Jiang, 2022 [51] |
| Wistar albino | male | NA | 1 dose ip 55 mg/kg | 450 | 28 | 4 | 540 ± 36 | Shi, 2020 [12] | |
| Sprague Dawley | male | 7–8 | 200–300 | 1 dose ip 60 mg/kg | 350 | 28 | 4 | 590 | Jung, 2022 [52] |
| Sprague Dawley | male | 4–6 | 1 dose ip 60 mg/kg | 300 | 28 | 4 | NA | Zhang, 2018 [53] | |
| Long Evans | male | 9 | 325–350 | 1 dose ip 100 mg/kg | 250 | 28 | 4 | NA | Allen, 2018 [15] |
| Sprague Dawley | male, female | NA | 180–300 | 1 dose ip 70 mg/kg | 350 | 28 | 4 | NA | Ibán-Arias, 2018 [54] |
| Sprague Dawley | male | 6–7 | 260–360 | 1 dose ip 60 mg/kg | 300 | 30 | 4 | NA | He, 2021 [55] |
| Sprague Dawley | male | 6–8 | 180–220 | 1 dose ip 60 mg/kg | 300 | 30 | 4 | 400 ± 30 | Fu, 2021 [11] |
| Wistar albino | male | NA | 150–180 | 1 dose ip 60 mg/kg | 250 | 30 | 4 | NA | Clapp, 2019 [56] |
| Sprague Dawley | male | 28 | 250 ± 50 | 1 dose ip 35 mg/kg | 250 | 28 | 4 | NA | Şahin, 2021 [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, M.; Capraș, R.-D.; Paşcalău, R.; Filip, G.A. Molecular Findings Before Vision Loss in the Streptozotocin-Induced Rat Model of Diabetic Retinopathy. Curr. Issues Mol. Biol. 2025, 47, 28. https://doi.org/10.3390/cimb47010028
Moldovan M, Capraș R-D, Paşcalău R, Filip GA. Molecular Findings Before Vision Loss in the Streptozotocin-Induced Rat Model of Diabetic Retinopathy. Current Issues in Molecular Biology. 2025; 47(1):28. https://doi.org/10.3390/cimb47010028
Chicago/Turabian StyleMoldovan, Mădălina, Roxana-Denisa Capraș, Raluca Paşcalău, and Gabriela Adriana Filip. 2025. "Molecular Findings Before Vision Loss in the Streptozotocin-Induced Rat Model of Diabetic Retinopathy" Current Issues in Molecular Biology 47, no. 1: 28. https://doi.org/10.3390/cimb47010028
APA StyleMoldovan, M., Capraș, R.-D., Paşcalău, R., & Filip, G. A. (2025). Molecular Findings Before Vision Loss in the Streptozotocin-Induced Rat Model of Diabetic Retinopathy. Current Issues in Molecular Biology, 47(1), 28. https://doi.org/10.3390/cimb47010028








