Plant Antimicrobial Peptides and Their Main Families and Roles: A Review of the Literature
Abstract
1. Introduction
1.1. Antimicrobial Peptides: Brief History and Mechanism of Action
1.2. Classification and Structure of AMPs
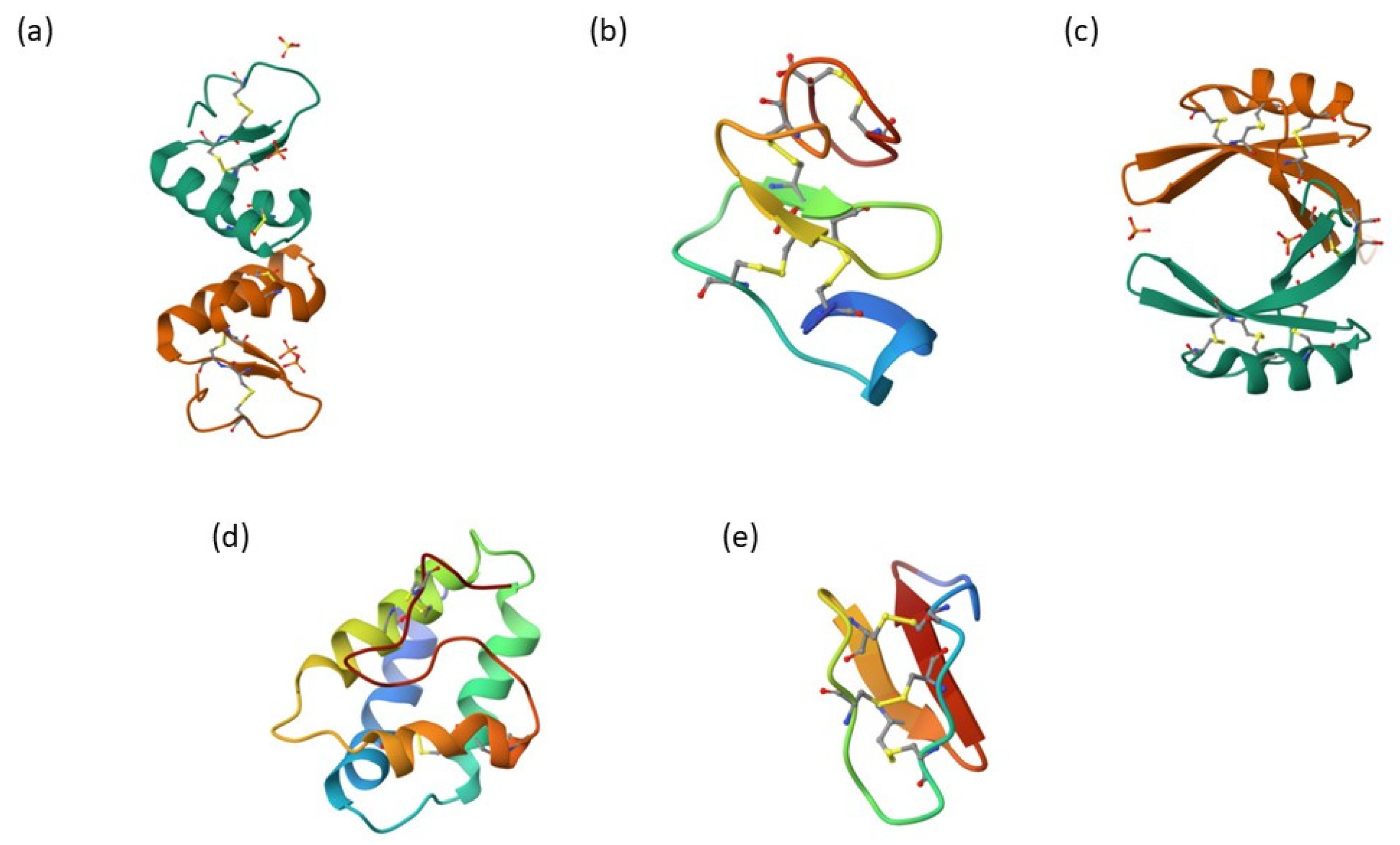
2. Plant Antimicrobial Peptides
2.1. Thionins
2.2. Heveins
2.3. Defensins
2.4. Lipid Transfer Proteins
2.5. Cyclotides
3. AMPs from the Solanaceae Family
4. Toxicity and Potential Applications of Plant AMPs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fry, D.E. Antimicrobial peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef]
- Luong, H.X.; Thanh, T.T.; Tran, T.H. Antimicrobial peptides—Advances in development of therapeutic applications. Life Sci. 2020, 260, 118407. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Balls, A.K.; Hale, W.S.; Harris, T.H. A crystalline protein obtained from a lipoprotein of wheat flour. Cereal Chem. 1942, 19, 279–288. [Google Scholar]
- Ohtani, S.; Okada, T.; Yoshizumi, H.; Kagamiyama, H. Complete primary structures of two subunits of purothionin A, a lethal protein for brewer’s yeast from wheat flour. J. Biochem. 1977, 82, 753–767. [Google Scholar] [CrossRef]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef]
- Stec, B. Plant thionins—The structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms: My perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A. Defensins of grasses: A systematic review. Biomolecules 2020, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Shelenkov, A.A.; Andreev, Y.A.; Odintsova, T.I. Hevein-Like Antimicrobial Peptides of Plants. Biochemistry 2017, 82, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Qin, Y.; Qin, Z.D.; Chen, J.; Cai, C.G.; Li, L. From antimicrobial to anticancer peptides: The transformation of peptides. Recent Pat. Anti-Canc. Drug Discov. 2019, 14, 70–84. [Google Scholar] [CrossRef]
- Zhong, G.; Cheng, J.; Liang, Z.C.; Xu, L.; Lou, W.; Bao, C.; Ong, Z.Y.; Dong, H.; Yang, Y.Y.; Fan, W. Short synthetic β-sheet antimicrobial peptides for the treatment of multidrug-resistant Pseudomonas aeruginosa burn wound infections. Adv. Heal. Mater. 2017, 6, 1601134. [Google Scholar] [CrossRef]
- Bobone, S.; Stella, L. Selectivity of antimicrobial peptides: A complex interplay of multiple equilibria. Adv. Exp. Med. Biol. 2019, 1117, 175–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Wang, G., Ed.; CABI Publishing: Wallingford, UK, 2010; 240p. [Google Scholar]
- Lima, P.G.; Oliveira, J.T.A.; Amaral, J.L.; Freitas, C.D.T.; Souza, P.F.N. Synthetic antimicrobial peptides: Characteristics, design, and potential as alternative molecules to overcome microbial resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, J.; Han, J.; Gao, L.; Liu, H.; Lu, Z.; Zhao, H.; Bie, X. Insights into the antimicrobial activity and cytotoxicity of engineered α-helical peptide amphiphiles. J. Med. Chem. 2016, 59, 10946–10962. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Hirano, M.; Saito, C.; Yokoo, H.; Goto, C.; Kawano, R.; Misawa, T.; Demizu, Y. Development of antimicrobial stapled peptides based on magainin 2 sequence. Molecules 2021, 26, 444. [Google Scholar] [CrossRef] [PubMed]
- Manzo, G.; Hind, C.K.; Ferguson, P.M.; Amison, R.T.; Hodgson-Casson, A.C.; Ciazynska, K.A.; Weller, B.J.; Clarke, M.; Lam, C.; Man, R.C. A pleurocidin analogue with greater conformational flexibility, enhanced antimicrobial potency and in vivo therapeutic efficacy. Commun. Biol. 2020, 3, 697. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, D.; Wang, C.; Shan, A.; Bi, C.; Li, Y.; Gan, W. Hybridization with insect cecropin a (1–8) improve the stability and selectivity of naturally occurring peptides. Int. J. Mol. Sci. 2020, 21, 1470. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Mishra, A.K.; Choi, J.; Moon, E.; Baek, K.H. Tryptophan-rich and proline-rich antimicrobial peptides. Molecules 2018, 23, 815. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Lind, T.K.; Lone, A.; Gerelli, Y.; Hansen, P.R.; Jenssen, H.; Cárdenas, M.; Lund, R. A biophysical study of the interactions between the antimicrobial peptide indolicidin and lipid model systems. Biochim. Biophys. Acta—Biomembr. 2019, 1861, 1355–1364. [Google Scholar] [CrossRef]
- Shruti, S.R.; Rajasekaran, R. Identification of therapeutic peptide scaffold from tritrpticin family for urinary tract infections using in silico techniques. J. Biomol. Struct. Dyn. 2020, 38, 4407–4417. [Google Scholar] [CrossRef] [PubMed]
- Boparai, J.K.; Sharma, P.K. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Sinha, R.; Shukla, P. Antimicrobial peptides: Recent insights on biotechnological interventions and future perspectives. Protein Pept. Lett. 2019, 26, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, A.B.; Martínez Ceron, M.C.; Romero, S.M.; Cascone, O.; Camperi, S.A.; Giudicessi, S.L. Péptidos antimicrobianos de plantas. Rev. Farm. 2018, 160, 28–46. [Google Scholar]
- Santos-Silva, C.A.D.; Zupin, L.; Oliveira-Lima, M.; Vilela, L.M.B.; Bezerra-Neto, J.P.; Ferreira-Neto, J.R.; Oliveira-Silva, R.L.D.; Pires, C.D.J.; Aburjaile, F.F.; Oliveira, M.F.D. Plant antimicrobial peptides: State of the art, in silico prediction and perspectives in the omics Era. Bioinform. Biol. Insights 2020, 14, 1177932220952739. [Google Scholar] [CrossRef]
- Iwai, T.; Kaku, H.; Honkura, R.; Nakamura, S.; Ochiai, H.; Sasaki, T.; Ohashi, Y. Enhanced resistance to seed-transmitted bacterial diseases in transgenic rice plants overproducing an oat cell-wall-bound thionin. Mol. Plant Microbe. Interact. 2002, 15, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Loeza-Angeles, H.; Sagrero-Cisneros, E.; Lara-Zárate, L.; Villagómez-Gómez, E.; López-Meza, J.E.; Ochoa-Zarzosa, A. Thionin Thi2.1 from Arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity. Biotechnol. Lett. 2008, 30, 1713–1719. [Google Scholar] [CrossRef]
- Ochoa-Zarzosa, A.; Loeza-Angeles, H.; Sagrero-Cisneros, E.; Villagómez-Gómez, E.; Lara-Zárate, L.; López-Meza, J.E. Antibacterial activity of thionin Thi2.1 from Arabidopsis thaliana expressed by bovine endothelial cells against Staphylococcus aureus isolates from bovine mastitis. Vet. Microbiol. 2008, 127, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, N.; Tanaka, T.; Shimamura, T.; Mitsukawa, N.; Hori, E.; Koda, K.; Otani, M.; Hirai, M.; Nakamura, K. Transgenic sweet potato expressing thionin from barley gives resistance to black rot disease caused by Ceratocystis fimbriata in leaves and storage roots. Plant Cell Rep. 2012, 31, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Kul’ko, A.B.; Kisl, O.V.; Sadykova, V.S.; Mikhailov, V.F.; Vasilieva, I.M.; Shulenina, L.V.; Zasukhina, G.D.; Rogozhin, E.A. Investigation of thionins from black cumin (Nigella sativa L.) seeds showing cytotoxic, regulatory and antifungal activity. Antibiot. Khimioter. 2016, 61, 8–16. [Google Scholar]
- Melo, F.R.; Rigden, D.J.; Franco, O.L.; Mello, L.V.; Ary, M.B.; Grossi de Sá, M.F.; Bloch, C., Jr. Inhibition of trypsin by cowpea thionin: Characterization, molecular modeling, and docking. Proteins 2002, 48, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, E.; Hammad, I.; Bakry, A. Production of transgenic Allium cepa by nanoparticles to resist Aspergillus niger infection. Mol. Biol. Rep. 2022, 49, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Martinez, C.M.; Morris, J.A.; Hedley, P.E.; Bos, J.I.B. Barley transcriptome analyses upon interaction with different aphid species identify thionins contributing to resistance. Plant Cell Environ. 2017, 40, 2628–2643. [Google Scholar] [CrossRef] [PubMed]
- Vila-Perelló, M.; Sánchez-Vallet, A.; García-Olmedo, F.; Molina, A.; Andreu, D. Synthetic and structural studies on Pyrularia pubera thionin: A single-residue mutation enhances activity against Gram-negative bacteria. FEBS Lett. 2003, 536, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Osório e Castro, V.R.; Vernon, L.P. Stimulation of prothrombinase activity by the nonapeptide Thr-Trp-Ala-Arg-Asn-Ser-Tyr-Asn-Val, a segment of a plant thionin. Peptides 2003, 24, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Gullbo, J.; Lindholm, P.; Larsson, R.; Thunberg, E.; Samuelsson, G.; Bohlin, L.; Claeson, P. Ligatoxin B, a new cytotoxic protein with a novel helix-turn-helix DNA-binding domain from the mistletoe Phoradendron liga. Biochem. J. 2002, 366 Pt 2, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Gullbo, J.; Lindholm, P.; Ek, B.; Thunberg, E.; Samuelsson, G.; Larsson, R.; Bohlin, L.; Claeson, P. Small, novel proteins from the mistletoe Phoradendron tomentosum exhibit highly selective cytotoxicity to human breast cancer cells. Cell. Mol. Life Sci. 2003, 60, 165–175. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Vassilieva, I.M.; Korostyleva, T.V.; Utkina, L.L.; Slavokhotova, A.A.; Rogozhin, E.A.; Shiyan, A.N.; Pukhalskii, V.A.; Zasukhina, G.D. Antimutagenic activity of wheat β-purothionin Tk-AMP-BP. Russ. J. Genet. 2011, 47, 1128–1131. [Google Scholar] [CrossRef]
- Hao, G.; Bakker, M.G.; Kim, H.S. Enhanced resistance to Fusarium graminearum in transgenic Arabidopsis plants expressing a modified plant thionin. Phytopathology 2020, 110, 1056–1066. [Google Scholar] [CrossRef]
- Barashkova, A.S.; Sadykova, V.S.; Salo, V.A.; Zavriev, S.K.; Rogozhin, E.A. Nigellothionins from black cumin (Nigella sativa L.) seeds demonstrate strong antifungal and cytotoxic activity. Antibiotics 2021, 10, 166. [Google Scholar] [CrossRef]
- Fujimura, M.; Ideguchi, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of novel antimicrobial peptides, Tu-AMP 1 and Tu-AMP 2, from bulbs of tulip (Tulipa gesneriana L.). Biosci. Biotechnol. Biochem. 2004, 68, 571–577. [Google Scholar] [CrossRef]
- Ji, H.; Gheysen, G.; Ullah, C.; Verbeek, R.; Shang, C.; De Vleesschauwer, D.; Höfte, M.; Kyndt, T. The role of thionins in rice defence against root pathogens. Mol. Plant Pathol. 2015, 16, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, K.P.B.; Van Damme, E.J.M.; Peumans, W.J.; Coosemans, J. Ee-CBP, a hevein-type antimicrobial peptide from bark of the spindle tree (Euonymus europaeus L.). Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2002, 67, 327–331. [Google Scholar] [PubMed]
- Huang, R.H.; Xiang, Y.; Liu, X.Z.; Zhang, Y.; Hu, Z.; Wang, D.C. Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv. FEBS Lett. 2002, 521, 87–90. [Google Scholar] [CrossRef]
- Kanokwiroon, K.; Teanpaisan, R.; Wititsuwannakul, D.; Hooper, A.B.; Wititsuwannakul, R. Antimicrobial activity of a protein purified from the latex of Hevea brasiliensis on oral microorganisms. Mycoses 2008, 51, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, Y.; Pan, Y.H.; Zhang, C.H.; Yuan, W.X. A hevein-like protein and a class I chitinase with antifungal activity from leaves of the paper mulberry. Biomed. Chromatogr. 2011, 25, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A.; Slezina, M.P.; Slavokhotova, A.A.; Istomina, E.A.; Korostyleva, T.V.; Smirnov, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. A novel antifungal peptide from leaves of the weed Stellaria media L. Biochimie 2015, 116, 125–132. [Google Scholar] [CrossRef]
- Koo, J.C.; Chun, H.J.; Park, H.C.; Kim, M.C.; Koo, Y.D.; Koo, S.C.; Ok, H.M.; Park, S.J.; Lee, S.H.; Yun, D.J.; et al. Over-expression of a seed specific hevein-like antimicrobial peptide from Pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants. Plant Mol. Biol. 2002, 50, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Kiba, A.; Saitoh, H.; Nishihara, M.; Omiya, K.; Yamamura, S. C-terminal domain of a hevein-like protein from Wasabia japonica has potent antimicrobial activity. Plant Cell Physiol. 2003, 44, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.C.; Lee, S.Y.; Chun, H.J.; Cheong, Y.H.; Choi, J.S.; Kawabata, S.; Miyagi, M.; Tsunasawa, S.; Ha, K.S.; Bae, D.W.; et al. Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity. Biochim. Biophys. Acta 1998, 1382, 80–90. [Google Scholar] [CrossRef]
- Lee, O.S.; Lee, B.; Park, N.; Koo, J.C.; Kim, Y.H.; Prasad, D.T.; Karigar, C.; Chun, H.J.; Jeong, B.R.; Kim, D.H.; et al. Pn-AMPs, the hevein-like proteins from Pharbitis nil confers disease resistance against phytopathogenic fungi in tomato, Lycopersicum esculentum. Phytochemistry 2003, 62, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Renaud, V.; Sanier, C.; Cambillau, L.; Arokiaraj, P.; Jones, H.; Ruengsri, N.; Chrestin, H.; Montoro, P. Molecular characterization of new members of the Hevea brasiliensis hevein multigene family and analysis of their promoter region in rice. Biochim. Biophys. Acta 2005, 1727, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Shukurov, R.R.; Voblikova, V.D.; Nikonorova, A.K.; Komakhin, R.A.; Komakhina, V.V.; Egorov, T.A.; Grishin, E.V.; Odintsova, T.I. Transformation of tobacco and Arabidopsis plants with Stellaria media genes encoding novel hevein-like peptides increases their resistance to fungal pathogens. Transgenic Res. 2012, 21, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Beliaev, D.V.; Yuorieva, N.O.; Tereshonok, D.V.; Tashlieva, I.I.; Derevyagina, M.K.; Meleshin, A.A.; Rogozhin, E.A.; Kozlov, S.A. High resistance of potato to early blight is achieved by expression of the Pro-SmAMP1 gene for hevein-like antimicrobial peptides from common chickweed (Stellaria media). Plants 2021, 10, 1395. [Google Scholar] [CrossRef]
- Dos Santos, I.S.; de Carvalho, A.O.; de Souza-Filho, G.A.; do Nascimento, V.V.; Machado, O.L.T.; Gomes, V.M. Purification of a defensin isolated from Vigna unguiculata seeds, its functional expression in Escherichia coli, and assessment of its insect alpha-amylase inhibitory activity. Protein Expr. Purif. 2010, 71, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, P.B.; Lay, F.T.; Murad, A.M.; Anderson, M.A.; Franco, O.L. Novel insights on the mechanism of action of alpha-amylase inhibitors from the plant defensin family. Proteins 2008, 73, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Games, P.D.; dos Santos, I.S.; Mello, E.O.; Diz, M.S.S.; Carvalho, A.O.; de Souza-Filho, G.A.; Da Cunha, M.; Vasconcelos, I.M.; Ferreira, B.D.S.; Gomes, V.M. Isolation, characterization and cloning of a cDNA encoding a new antifungal defensin from Phaseolus vulgaris L. seeds. Peptides 2008, 29, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, J.; Wu, F.; Li, X.; Teng, M.; Gong, W. cDNA cloning, functional expression and antifungal activities of a dimeric plant defensin SPE10 from Pachyrrhizus erosus seeds. Plant Mol. Biol. 2005, 57, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shiau, Y.S.; Horng, S.B.; Chen, C.S.; Huang, P.T.; Lin, C.; Hsueh, Y.C.; Lou, K.L. Structural analysis of the unique insecticidal activity of novel mungbean defensin VrD1 reveals possibility of homoplasy evolution between plant defensins and scorpion neurotoxins. J. Mol. Recognit. 2006, 19, 441–450. [Google Scholar] [CrossRef]
- Cabral, K.M.S.; Almeida, M.S.; Valente, A.P.; Almeida, F.C.L.; Kurtenbach, E. Production of the active antifungal Pisum sativum defensin 1 (Psd1) in Pichia pastoris: Overcoming the inefficiency of the STE13 protease. Protein Expr. Purif. 2003, 31, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Olli, S.; Kirti, P.B. Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenum-graecum L. J. Biochem. Mol. Biol. 2006, 39, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, N.A.; Shah, D.; Abbas, D.; Madkour, M. Stable integration and expression of a plant defensin in tomato confers resistance to fusarium wilt. GM Crops 2010, 1, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, Y.M.; McKenna, J.A.; McGinness, B.S.; Hinch, J.; Poon, S.; Connelly, A.A.; Anderson, M.A.; Heath, R.L. Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J. Exp. Bot. 2014, 65, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Sagaram, U.S.; Pandurangi, R.; Kaur, J.; Smith, T.J.; Shah, D.M. Structure-activity determinants in antifungal plant defensins MsDef1and MtDef4 with different modes of action against Fusarium graminearum. PLoS ONE 2011, 6, e18550. [Google Scholar] [CrossRef]
- Kader, J.C. Proteins and the intracellular exchange of lipids. I. Stimulation of phospholipid exchange between mitochondria and microsomal fractions by proteins isolated from potato tuber. Biochim. Biophys. Acta 1975, 380, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K. Lipid transfer proteins rectify inter-organelle flux and accurately deliver lipids at membrane contact sites. J. Lipid Res. 2018, 59, 1341–1366. [Google Scholar] [CrossRef]
- Wong, L.H.; Čopič, A.; Levine, T.P. Advances on the transfer of lipids by lipid transfer proteins. Trends Biochem. Sci. 2017, 42, 516–530. [Google Scholar] [CrossRef]
- de Oliveira Carvalho, A.; Gomes, V.M. Role of plant lipid transfer proteins in plant cell physiology—A concise review. Peptides 2007, 28, 1144–1153. [Google Scholar] [CrossRef]
- Kjellberg, M.A.; Backman, A.P.E.; Möuts, A.; Mattjus, P. Purification and validation of lipid transfer proteins. Methods Mol. Biol. 2017, 1609, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bard, G.C.V.; Zottich, U.; Souza, T.A.M.; Ribeiro, S.F.F.; Dias, G.B.; Pireda, S.; Da Cunha, M.; Rodrigues, R.; Pereira, L.S.; Machado, O.L.T.; et al. Purification, biochemical characterization, and antimicrobial activity of a new lipid transfer protein from Coffea canephora seeds. Genet. Mol. Res. 2016, 15, 1–16. [Google Scholar] [CrossRef]
- Maximiano, M.R.; Franco, O.L. Biotechnological applications of versatile plant lipid transfer proteins (LTPs). Peptides 2021, 140, 170531. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.O.W.; Kim, W.; Hwang, B.K. Three pathogen-inducible genes encoding lipid transfer protein from pepper are differentially activated by pathogens, abiotic, and environmental stresses. Plant Cell. Environ. 2003, 26, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Park, C.J.; Shin, R.; Park, J.M.; Lee, G.J.; You, J.S.; Paek, K.H. Induction of pepper cDNA encoding a lipid transfer protein during the resistance response to tobacco mosaic virus. Plant Mol. Biol. 2002, 48, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Chen, J.; Li, N.; Lin, Y.; Sun, C.; Cao, K. Resistance function of rice lipid transfer protein LTP110. J. Biochem. Mol. Biol. 2003, 36, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wu, J.H.; Ng, T.B.; Ye, X.Y.; Rao, P.F. A non-specific lipid transfer protein with antifungal and antibacterial activities from the mung bean. Peptides 2004, 25, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, H.; Chu, Z.; Ji, C.; Xu, Y.; Cao, W.; Zhou, S.; Song, Y.; Liu, H.; Zhu, C. A nonspecific lipid transfer protein, StLTP10, mediates resistance to Phytophthora infestans in potato. Mol. Plant Pathol. 2021, 22, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Li, X.; She, R.; Pei, Y. Isolation and characterization of a novel thermostable non-specific lipid transfer protein-like antimicrobial protein from motherwort (Leonurus japonicus Houtt) seeds. Peptides 2006, 27, 3122–3128. [Google Scholar] [CrossRef]
- Lin, P.; Xia, L.; Ng, T.B. First isolation of an antifungal lipid transfer peptide from seeds of a Brassica species. Peptides 2007, 28, 1514–1519. [Google Scholar] [CrossRef]
- Gonorazky, A.G.; Regente, M.C.; de la Canal, L. Stress induction and antimicrobial properties of a lipid transfer protein in germinating sunflower seeds. J. Plant Physiol. 2005, 162, 618–624. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Tian, L.; Su, M.; Ho, W.-S.; Sun, S.S.M.; Chung, H.Y.; Wong, H.N.C.; Ooi, V.E.C. Isolation, characterization, molecular cloning and modeling of a new lipid transfer protein with antiviral and antiproliferative activities from Narcissus tazetta. Peptides 2008, 29, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Oshchepkova, Y.I.; Veshkurova, O.N.; Rogozhin, E.A.; Musolyamov, A.K.; Smirnov, A.N.; Odintsova, T.I.; Egorov, T.A.; Grishin, E.V.; Salikhov, S.I. Isolation of the lipid-transporting protein Ns-LTP1 from seeds of the garden fennel flower (Nigella sativa). Russ. J. Bioorg. Chem. 2009, 35, 315–319. [Google Scholar] [CrossRef]
- Zottich, U.; Da Cunha, M.; Carvalho, A.O.; Dias, G.B.; Silva, N.C.M.; Santos, I.S.; Nascimento, V.V.; Miguel, E.C.; Machado, O.L.T.; Gomes, V.M. Purification, biochemical characterization and antifungal activity of a new lipid transfer protein (LTP) from Coffea canephora seeds with α-amylase inhibitor properties. Biochim. Biophys. Acta—Gen. Subjects 2011, 1810, 375–383. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.E.; Darwish, N.I.; Garcia-Sanchez, J.; Tyagi, N.; Trick, H.N.; McCormick, S.; Dill-Macky, R.; Tumer, N.E. A lipid transfer protein has antifungal and antioxidant activity and suppresses Fusarium head blight disease and DON accumulation in transgenic wheat. Phytopathology 2021, 111, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kirubakaran, S.I.; Begum, S.M.; Ulaganathan, K.; Sakthivel, N. Characterization of a new antifungal lipid transfer protein from wheat. Plant Physiol. Biochem. 2008, 46, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.N.; Mineev, K.S.; Finkina, E.I.; Arseniev, A.S.; Ovchinnikova, T.V. A novel lipid transfer protein from the dill Anethum graveolens L.: Isolation, structure, heterologous expression, and functional characteristics. J. Pept. Sci. 2016, 22, 59–66. [Google Scholar] [CrossRef]
- Roy-Barman, S.; Sautter, C.; Chattoo, B.B. Expression of the lipid transfer protein Ace-AMP1 in transgenic wheat enhances antifungal activity and defense responses. Transgenic Res. 2006, 15, 435–446. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Zhao, S.; Su, J.; Li, M.; Ma, L.; Yu, X.; Wang, X. Wheat Apoplast-Localized Lipid Transfer Protein TaLTP3 Enhances Defense Responses Against Puccinia triticina. Front. Plant Sci. 2021, 12, 771806. [Google Scholar] [CrossRef]
- Goransson, U.; Svangard, E.; Claeson, P.; Bohlin, L. Novel strategies for isolation and characterization of cyclotides: The discovery of bioactive macrocyclic plant polypeptides in the Violaceae. Curr. Protein Pept. Sci. 2004, 5, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.; Göransson, U.; Johansson, S.; Claeson, P.; Gullbo, J.; Larsson, R.; Bohlin, L.; Backlund, A. Cyclotides: A novel type of cytotoxic agents. Mol. Cancer Ther. 2002, 1, 365–369. [Google Scholar]
- Du, Q.; Huang, Y.H.; Bajpai, A.; Frosig-Jorgensen, M.; Zhao, G.; Craik, D.J. Evaluation of the in vivo aphrodisiac activity of a cyclotide extract from Hybanthus enneaspermus. J. Nat. Prod. 2020, 83, 3736–3743. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Chan, L.Y.; Gilding, E.K.; Henriques, S.T.; Condon, N.D.; Ravipati, A.S.; Kaas, Q.; Huang, Y.-H.; Craik, D.J. Discovery and mechanistic studies of cytotoxic cyclotides from the medicinal herb Hybanthus enneaspermus. J. Biol. Chem. 2020, 295, 10911–10925. [Google Scholar] [CrossRef] [PubMed]
- Niyomploy, P.; Chan, L.Y.; Harvey, P.J.; Poth, A.G.; Colgrave, M.L.; Craik, D.J. Discovery and characterization of cyclotides from Rinorea species. J. Nat. Prod. 2018, 81, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.E.F.; Najas, J.Z.G.; Magalhães, L.G.; Bobey, A.F.; Mendonça, J.N.; Lopes, N.P.; Leme, F.M.; Teixeira, S.P.; Trovó, M.; Andricopulo, A.D.; et al. Inhibition of breast cancer cell migration by cyclotides isolated from Pombalia calceolaria. J. Nat. Prod. 2018, 81, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, C.; Muratspahić, E.; Tomašević, N.; Münch, J.; Gruber, C.W. In vitro inhibition of HIV-1 by cyclotide-enriched extracts of Viola tricolor. Front. Pharmacol. 2022, 13, 888961. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Colgrave, M.L.; Daly, N.L.; Rosengren, K.J.; Gustafson, K.R.; Craik, D.J. Isolation and characterization of novel cyclotides from Viola hederaceae: Solution structure and anti-HIV activity of vhl-1, a leaf-specific expressed cyclotide. J. Biol. Chem. 2005, 280, 22395–22405. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.L.; Colgrave, M.L.; Gustafson, K.R.; Ireland, D.C.; Goransson, U.; Craik, D.J. Anti-HIV cyclotides from the Chinese medicinal herb Viola yedoensis. J. Nat. Prod. 2008, 71, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dayani, L.; Dinani, M.S.; Aliomrani, M.; Hashempour, H.; Varshosaz, J.; Taheri, A. Immunomodulatory effects of cyclotides isolated from Viola odorata in an experimental autoimmune encephalomyelitis animal model of multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 64, 103958. [Google Scholar] [CrossRef]
- Nworu, C.S.; Ejikeme, T.I.; Ezike, A.C.; Ndu, O.; Akunne, T.C.; Onyeto, C.A.; Okpalanduka, P.; Akah, P.A. Anti-plasmodial and anti-inflammatory activities of cyclotide-rich extract and fraction of Oldenlandia affinis (R. & S.) DC (Rubiaceae). Afr. Health Sci. 2017, 17, 827–843. [Google Scholar]
- Gattringer, J.; Ndogo, O.E.; Retzl, B.; Ebermann, C.; Gruber, C.W.; Hellinger, R. Cyclotides Isolated from Violet Plants of Cameroon Are Inhibitors of Human Prolyl Oligopeptidase. Front. Pharmacol. 2021, 12, 707596. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.A.; Barriga, A.; Albericio, F.; Romero, M.S.; Guzmán, F. Identification of Peptides in flowers of Sambucus nigra with antimicrobial activity against aquaculture pathogens. Molecules 2018, 23, 1033. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Park, S.; Burman, R.; Göransson, U. Bactericidal activity of cyclotides where phosphatidylethanolamine-lipid selectivity determines antimicrobial spectra. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1986–2000. [Google Scholar] [CrossRef]
- Zarrabi, M.; Dalirfardouei, R.; Sepehrizade, Z.; Kermanshahi, R.K. Comparison of the antimicrobial effects of semipurified cyclotides from Iranian Viola odorata against some of plant and human pathogenic bacteria. J. Appl. Microbiol. 2013, 115, 367–375. [Google Scholar] [CrossRef]
- Rajendran, S.; Slazak, B.; Mohotti, S.; Muhammad, T.; Strömstedt, A.A.; Kapusta, M.; Wilmowicz, E.; Göransson, U.; Hettiarachchi, C.M.; Gunasekera, S. Screening for cyclotides in sri lankan medicinal plants: Discovery, characterization, and bioactivity screening of cyclotides from Geophila repens. J. Nat. Prod. 2023, 86, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.; West, J.; Waine, C.; Craik, D.; Anderson, M. Biosynthesis and insecticidal properties of plant cyclotides: The cyclic knotted proteins from Oldenlandia affinis. Proc. Natl. Acad. Sci. USA 2001, 98, 10614–10619. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.V.; Rosengren, K.J.; Daly, N.L.; Plan, M.; Stevens, J.; Scanlon, M.J.; Waine, C.; Norman, D.G.; Anderson, M.A.; Craik, D.J. Isolation, solution structure, and insecticidal activity of kalata B2, a circular protein with a twist: Do Möbius strips exist in nature? Biochemistry 2005, 44, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Jackson, M.A.; Huang, Y.H.; Yap, K.; Du, Q.; Chau, T.C.Y.; Craik, D.J.; Gilding, E.K. Nematicidal activity of cyclotides: Toxicity against Caenorhabditis elegans. J. Nat. Prod. 2023, 86, 1222–1229. [Google Scholar] [CrossRef]
- Almaghrabi, B.; Ali, M.A.; Zahoor, A.; Shah, K.H.; Bohlmann, H. Arabidopsis thionin-like genes are involved in resistance against the beet-cyst nematode (Heterodera schachtii). Plant. Physiol. Biochem. 2019, 140, 55–67. [Google Scholar] [CrossRef]
- Colilla, F.J.; Rocher, A.; Mendez, E. γ-Purothionins: Amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm. FEBS Lett. 1990, 270, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Yamuna, S.; Kumar, V.; Subramaniam, D.; Kumaravel, K. Antimicrobial peptides from plants and their mode of action. Am. Int. J. Res. Sci. Technol. Eng. Math. 2019, 105, 265–269. [Google Scholar]
- Höng, K.; Austerlitz, T.; Bohlmann, T.; Bohlmann, H. The thionin family of antimicrobial peptides. PLoS ONE 2021, 16, e0254549. [Google Scholar] [CrossRef] [PubMed]
- Charity, J.A.; Hughes, P.; Anderson, M.A.; Bittisnich, D.J.; Whitecross, M.; Higgins, T.J.V. Pest and disease protection conferred by expression of barley β-hordothionin and Nicotiana alata proteinase inhibitor genes in transgenic tobacco. Funct. Plant Biol. 2005, 32, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.L.; Prasad, V.; Sanjaya; Chen, K.H.; Liu, P.C.; Chan, M.T.; Cheng, C.P. Transgenic tomato plants expressing an Arabidopsis thionin (Thi2.1) driven by fruit-inactive promoter battle against phytopathogenic attack. Planta 2005, 221, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Carvalho, A.O.; Rodrigues, R.; Trindade, F.G.; Da Cunha, M.; Gomes, V.M. Thionin-like peptide from Capsicum annuum fruits: Mechanism of action and synergism with fluconazole against Candida species. BMC Microbiol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Oard, S.V.; Enright, F.M. Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi. Plant Cell Rep. 2006, 25, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F. Plant antimicrobial peptides: An overview about classification, toxicity and clinical applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Tan, W.L.; Kini, S.G.; Xiao, T.; Serra, A.; Sze, S.K.; Tam, J.P. Vaccatides: Antifungal Glutamine-Rich Hevein-Like Peptides from Vaccaria hispanica. Front. Plant. Sci. 2017, 8, 1100. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.P.; Salles, H.O.; de Oliveira, H.D.; de Souza, B.B.P.; de Lima Cardozo Filho, J.; Sifuentes, D.N.; Prates, M.V.; Junior, C.B.; Bemquerer, M.P.; do Egito, A.S. Mo-HLPs: New flocculating agents identified from Moringa oleifera seeds belong to the hevein-like peptide family. J. Proteom. 2020, 217, 103692. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Mokshina, N. Using FIBexDB for In-Depth analysis of flax lectin gene expression in response to Fusarium oxysporum infection. Plants 2022, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Poon, S.; McKenna, J.A.; Connelly, A.A.; Barbeta, B.L.; McGinness, B.S.; Fox, J.L.; Daly, N.L.; Craik, D.J.; Heath, R.L.; et al. The C-terminal propeptide of a plant defensin confers cytoprotective and subcellular targeting functions. BMC Plant Biol. 2014, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Aguieiras, M.C.L.; Resende, L.M.; Souza, T.A.M.; Nagano, C.S.; Chaves, R.P.; Taveira, G.B.; Carvalho, A.O.; Rodrigues, R.; Gomes, V.M.; Mello, E.O. Potent Anti-Candida fraction isolated from Capsicum chinense Fruits contains an antimicrobial peptide that is similar to plant defensin and is able to inhibit the activity of different α-amylase enzymes. Probiotics Antimicrob. Proteins 2021, 13, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.M.; de Oliveira Mello, É.; de Lima Aguieiras, M.C.; Nagano, C.S.; Chaves, R.P.; Taveira, G.B.; da Silva, M.S.; de Oliveira Carvalho, A.; Gomes, V.M. Inhibition of serine protease, α-amylase and growth of phytopathogenic fungi by antimicrobial peptides from Capsicum chinense fruits. Probiotics Antimicrob. Proteins 2021, 15, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.L.; Lião, L.M.; Alves, E.S.F.; Migliolo, L.; Dias, S.C.; Franco, O.L. A structural perspective of plant antimicrobial peptides. Biochem. J. 2018, 475, 3359–3375. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, V.; Bukhteeva, I.; Kit, O.Y.; Nesmelova, I.V. Plant defensins from a structural perspective. Int. J. Mol. Sci. 2020, 21, 5307. [Google Scholar] [CrossRef] [PubMed]
- Skalska, J.; Andrade, V.M.; Cena, G.L.; Harvey, P.J.; Gaspar, D.; Mello, É.O.; Henriques, S.T.; Valle, J.; Gomes, V.M.; Conceição, K.; et al. Synthesis, structure, and activity of the antifungal plant defensin PvD1. J. Med. Chem. 2020, 63, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Skalska, J.; Oliveira, F.D.; Figueira, T.N.; Mello, É.O.; Gomes, V.M.; McNaughton-Smith, G.; Castanho, M.A.R.B.; Gaspar, D. Plant defensin PvD1 modulates the membrane composition of breast tumour-derived exosomes. Nanoscale 2019, 11, 23366–23381. [Google Scholar] [CrossRef]
- Ishaq, N.; Bilal, M.; Iqbal, H.M.N. Medicinal potentialities of plant defensins: A review with applied perspectives. Medicines 2019, 6, 29. [Google Scholar] [CrossRef]
- Jiménez-Alcántar, P.; López-Gómez, R.; López-Meza, J.E.; Ochoa-Zarzosa, A. PaDef (Persea americana var. Drymifolia), a plant antimicrobial peptide, triggers apoptosis, and induces global epigenetic modifications on histone 3 in an acute lymphoid leukemia cell line. Front. Mol. Biosci. 2022, 9, 801816. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; François, I.E.J.A.; Takemoto, J.Y.; Ferket, K.K.A.; Meert, E.M.K.; Cammue, B.P.A. DmAMP1, an antifungal plant defensin from dahlia (Dahlia merckii), interacts with sphingolipids from Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2003, 226, 169–173. [Google Scholar] [CrossRef]
- Turrini, A.; Sbrana, C.; Pitto, L.; Ruffini Castiglione, M.; Giorgetti, L.; Briganti, R.; Bracci, T.; Evangelista, M.; Nuti, M.P.; Giovannetti, M. The antifungal Dm-AMP1 protein from Dahlia merckii expressed in Solanum melongena is released in root exudates and differentially affects pathogenic fungi and mycorrhizal symbiosis. New Phytol. 2004, 163, 393–403. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Velivelli, S.; Shah, D.M.; Samac, D.A. Plant defensin peptides have antifungal and antibacterial activity against human and plant pathogens. Phytopathology 2019, 109, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lay, F.T.; Brugliera, F.; Anderson, M.A. Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 2003, 131, 1283–1293. [Google Scholar] [CrossRef]
- Van Der Weerden, N.L.; Lay, F.T.; Anderson, M.A. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J. Biol. Chem. 2008, 283, 14445–14452. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of a novel type of antimicrobial peptides, Fa-AMP1 and Fa-AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench.). Biosci. Biotechnol. Biochem. 2003, 67, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Spelbrink, R.G.; Dilmac, N.; Allen, A.; Smith, T.J.; Shah, D.M.; Hockerman, G.H. Differential antifungal and calcium channel-blocking activity among structurally related plant defensins. Plant Physiol. 2004, 135, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Hsu, M.P.; Tan, C.H.; Sung, H.Y.; Kuo, C.G.; Fan, M.J.; Chen, H.M.; Chen, S.; Chen, C.S. Cloning and characterization of a plant defensin VaD1 from azuki bean. J. Agric. Food Chem. 2005, 53, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B. Limenin, a defensin-like peptide with multiple exploitable activities from shelf beans. J. Pept. Sci. 2006, 12, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Warnecke, D.C.; François, I.E.J.A.; Leipelt, M.; Heinz, E.; Ott, C.; Zähringer, U.; Thomma, B.P.H.J.; Ferket, K.K.A.; Cammue, B.P.A. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 2004, 279, 3900–3905. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; François, I.E.J.A.; Meert, E.M.K.; Li, Q.T.; Cammue, B.P.A.; Thevissen, K. The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J. Mol. Microbiol. Biotechnol. 2007, 13, 243–247. [Google Scholar] [CrossRef]
- Thevissen, K.; de Mello Tavares, P.; Xu, D.; Blankenship, J.; Vandenbosch, D.; Idkowiak-Baldys, J.; Govaert, G.; Bink, A.; Rozental, S.; de Groot, P.W.J.; et al. The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 2012, 84, 166–180. [Google Scholar] [CrossRef]
- Vriens, K.; Cools, T.L.; Harvey, P.J.; Craik, D.J.; Braem, A.; Vleugels, J.; De Coninck, B.; Cammue, B.P.A.; Thevissen, K. The radish defensins RsAFP1 and RsAFP2 act synergistically with caspofungin against Candida albicans biofilms. Peptides 2016, 75, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Park, H.C.; Kang, Y.H.; Chun, H.J.; Koo, J.C.; Cheong, Y.H.; Kim, C.Y.; Kim, M.C.; Chung, W.S.; Kim, J.C.; Yoo, J.H.; et al. Characterization of a stamen-specific cDNA encoding a novel plant defensin in Chinese cabbage. Plant Mol. Biol. 2002, 50, 59–69. [Google Scholar] [CrossRef]
- Kanzaki, H.; Nirasawa, S.; Saitoh, H.; Ito, M.; Nishihara, M.; Terauchi, R.; Nakamura, I. Overexpression of the wasabi defensin gene confers enhanced resistance to blast fungus (Magnaporthe grisea) in transgenic rice. Theor. Appl. Genet. 2002, 105, 809. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Okamoto, T.; Tsuda, S.; Imai, R. A novel plant defensin-like gene of winter wheat is specifically induced during cold acclimation. Biochem. Biophys. Res. Commun. 2002, 298, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Chen, G.H.; Hsu, H.C.; Li, S.S.; Chen, C.S. Cloning and functional expression of a mungbean defensin VrD1 in Pichia pastoris. J. Agric. Food Chem. 2004, 52, 2256–2261. [Google Scholar] [CrossRef] [PubMed]
- da Silva Gebara, R.; Taveira, G.B.; de Azevedo dos Santos, L.; Calixto, S.D.; Simão, T.L.B.V.; Lassounskaia, E.; Muzitano, M.F.; Ferreira, A.T.; Perales, J.; Rodrigues, R.; et al. Identification and characterization of two defensins from Capsicum annuum fruits that exhibit antimicrobial activity. Probiotics Antimicrob. Proteins 2020, 12, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Mello, É.O.; Carvalho, A.O.; Regente, M.; Pinedo, M.; de La Canal, L.; Rodrigues, R.; Gomes, V.M. Antimicrobial activity and mechanism of action of a thionin-like peptide from Capsicum annuum fruits and combinatorial treatment with fluconazole against Fusarium solani. Biopolymers 2017, 108, e23008. [Google Scholar] [CrossRef]
- Taveira, G.B.; Mello, É.O.; Souza, S.B.; Monteiro, R.M.; Ramos, A.C.; Carvalho, A.O.; Rodrigues, R.; Okorokov, L.A.; Gomes, V.M. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of caspases and extracellular H+ flux. Biosci. Rep. 2018, 38, BSR20180119. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; Carmona-Gutierrez, D.; Lefevre, S.; Govaert, G.; François, I.E.J.A.; Madeo, F.; Santos, R.; Cammue, B.P.A.; Thevissen, K. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida albicans. FEBS Lett. 2009, 583, 2513–2516. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Lin, C.Y.; Kuan, C.C.; Sung, H.Y.; Chen, C.S. A novel defensin encoded by a mungbean cDNA exhibits insecticidal activity against bruchid. J. Agric. Food Chem. 2002, 50, 7258–7263. [Google Scholar] [CrossRef] [PubMed]
- de Veer, S.J.; Kan, M.W.; Craik, D.J. Cyclotides: From Structure to Function. Chem. Rev. 2019, 119, 12375–12421. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, J.; Craik, D.J. Discovery, structure, function, and applications of cyclotides: Circular proteins from plants. J. Exp. Bot. 2016, 67, 4801–4812. [Google Scholar] [CrossRef]
- Grain, L. Isolation of oxytocic peptides from Oldenlandia affinis by solvent extraction of tetraphenylborate complexes and chromatography on sephadex LH-20. Lloydia 1973, 36, 207–208. [Google Scholar] [PubMed]
- Slazak, B.; Haugmo, T.; Badyra, B.; Göransson, U. The life cycle of cyclotides: Biosynthesis and turnover in plant cells. Plant Cell Rep. 2020, 39, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yang, Y.; Uche, F.I.; Hart, S.R.; Li, W.W.; Yuan, C. Coupling Plant-Derived Cyclotides to Metal Surfaces: An Antibacterial and Antibiofilm Study. Int. J. Mol. Sci. 2018, 19, 793. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Du, Q.; Craik, D.J. Cyclotides: Disulfide-rich peptide toxins in plants. Toxicon 2019, 172, 33–44. [Google Scholar] [CrossRef]
- Oguis, G.K.; Gilding, E.K.; Jackson, M.A.; Craik, D.J. Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant With Applications in Agriculture and Medicine. Front. Plant Sci. 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Smithies, B.J.; Durek, T.; Craik, D.J. Synthesis and Protein Engineering Applications of Cyclotides. Aust. J. Chem. 2016, 70, 152–161. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Craik, D.J. Cyclotide Structure and Function: The Role of Membrane Binding and Permeation. Biochemistry 2017, 56, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, Z.; Aslam, N.; Zafar, F.; Humma, R.; Jamil, A. Isolation of novel Cyclotide encoding genes from some Solanaceae species and evolutionary link to other families. Pak. J. Agric. Sci. 2021, 58, 169–177. [Google Scholar]
- Mammari, N.; Krier, Y.; Albert, Q.; Devocelle, M.; Varbanov, M.; on behalf of the OEMONOM. Plant-derived antimicrobial peptides as potential antiviral agents in systemic viral infections. Pharmaceuticals 2021, 14, 774. [Google Scholar] [CrossRef]
- Afroz, M.; Akter, S.; Ahmed, A.; Rouf, R.; Shilpi, J.A.; Tiralongo, E.; Hossain, M.A.; Jahan, R.; Rahmatullah, M. Ethnobotany and antimicrobial peptides from plants of the Solanaceae family: An update and future prospects. Front. Pharmacol. 2020, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef] [PubMed]
- Almasia, N.I.; Nahirñak, V.; Hopp, H.E.; Vazquez-Rovere, C. Potato Snakin-1: An antimicrobial player of the trade-off between host defense and development. Plant Cell Rep. 2020, 39, 839–849. [Google Scholar] [CrossRef]
- Slezina, M.P.; Istomina, E.A.; Kulakovskaya, E.V.; Abashina, T.N.; Odintsova, T.I. Synthetic oligopeptides mimicking γ-core regions of cysteine-rich peptides of Solanum lycopersicum possess antimicrobial activity against human and plant pathogens. Curr. Issues Mol. Biol. 2021, 43, 1226–1242. [Google Scholar] [CrossRef]
- Maracahipes, Á.C.; Taveira, G.B.; Mello, E.O.; Carvalho, A.O.; Rodrigues, R.; Perales, J.; Teixeira-Ferreira, A.; Silva, M.S.; Rocha, G.L.; Fernandes, K.V.S.; et al. Biochemical analysis of antimicrobial peptides in two different Capsicum genotypes after fruit infection by Colletotrichum gloeosporioides. Biosci. Rep. 2019, 39, BSR20181889. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.P.; Ribeiro, S.F.F.; Carvalho, A.O.; Vasconcelos, I.M.; Rodrigues, R.; Da Cunha, M.; Gomes, V.M. Isolation and partial characterization of a novel lipid transfer protein (LTP) and antifungal activity of peptides from chilli pepper seeds. Protein Pept. Lett. 2010, 17, 311–318. [Google Scholar] [CrossRef]
- Diz, M.S.S.; Carvalho, A.O.; Rodrigues, R.; Neves-Ferreira, A.G.C.; Da Cunha, M.; Alves, E.W.; Okorokova-Façanha, A.L.; Oliveira, M.A.; Perales, J.; Machado, O.L.T.; et al. Antimicrobial peptides from chilli pepper seeds causes yeast plasma membrane permeabilization and inhibits the acidification of the medium by yeast cells. Biochim. Biophys. Acta—Gen. Subjects 2006, 1760, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Dos Santos, L.; Taveira, G.B.; da Silva, M.S.; da Silva Gebara, R.; da Silva Pereira, L.; Perales, J.; Teixeira-Ferreira, A.; Mello, E.O.; Carvalho, A.O.; Rodrigues, R.; et al. Antimicrobial peptides from Capsicum chinense fruits: Agronomic alternatives against phytopathogenic fungi. Biosci. Rep. 2020, 40, BSR20200950. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Gebara, R.S.; Taveira, G.B.; Carvalho, A.d.O.; Rodrigues, R.; Mello, E.O.; Nagano, C.S.; Chaves, R.P.; Gomes, V.M. Anti-Candida potential of peptides from immature and ripe fruits of Capsicum chinense Jacq. Probiotics Antimicrob. Proteins 2022, 15, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.D.S.; Souza, T.A.M.; Walter, R.; Sudré, C.P.; Dos Santos, L.D.A.; Maracahipes, Á.C.; Taveira, G.B.; Carvalho, V.S.; Nagano, C.S.; Chaves, R.P.; et al. Identification of enzyme inhibitors and antimicrobial activities from Capsicum annuum L. protein extracts against Colletotrichum scovillei. Hortic. Environ. Biotechnol. 2021, 62, 493–506. [Google Scholar] [CrossRef]
- Ribeiro, S.F.F.; Carvalho, A.O.; Da Cunha, M.; Rodrigues, R.; Cruz, L.P.; Melo, V.M.M.; Vasconcelos, I.M.; Melo, E.J.T.; Gomes, V.M. Isolation and characterization of novel peptides from chilli pepper seeds: Antimicrobial activities against pathogenic yeasts. Toxicon 2007, 50, 600–611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.S.; Gomes, V.M.; Taveira, G.B.; de Azevedo dos Santos, L.; Maracahipes, Á.C.; Rodrigues, R.; de Oliveira Carvalho, A.; Fernandes, K.V.S.; Oliveira, A.E.A. Bifunctional inhibitors from Capsicum chinense seeds with antimicrobial activity and specific mechanism of action against phytopathogenic fungi. Protein Pept. Lett. 2021, 28, 149–163. [Google Scholar] [CrossRef]
- da Silva, M.S.; de Azevedo dos Santos, L.; Taveira, G.B.; Nagano, C.S.; Chaves, R.P.; de Oliveira Carvalho, A.; Rodrigues, R.; Gomes, V.M. Trypsin/α-amylase inhibitors from Capsicum chinense seeds: Characterization and antifungal activity against fungi of agronomic importance. Protein Pept. Lett. 2023, 30, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Vieira Bard, G.C.; Nascimento, V.V.; Oliveira, A.E.A.; Rodrigues, R.; Da Cunha, M.; Dias, G.B.; Vasconcelos, I.M.; Carvalho, A.O.; Gomes, V.M. Vicilin-like peptides from Capsicum baccatum L. seeds are α-amylase inhibitors and exhibit antifungal activity against important yeasts in medical mycology. Biopolymers 2014, 102, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Vieira Bard, G.C.; Nascimento, V.V.; Ribeiro, S.F.F.; Rodrigues, R.; Perales, J.; Teixeira-Ferreira, A.; Carvalho, A.O.; Fernandes, K.V.S.; Gomes, V.M. Characterization of peptides from Capsicum annuum hybrid seeds with inhibitory activity against α-amylase, serine proteinases and fungi. Protein J. 2015, 34, 122–129. [Google Scholar] [CrossRef]
- Games, P.D.; da Silva, E.Q.G.; Barbosa, M.O.; Almeida-Souza, H.O.; Fontes, P.P.; de Magalhães, M.J., Jr.; Pereira, P.R.G.; Prates, M.V.; Franco, G.R.; Faria-Campos, A.; et al. Computer aided identification of a Hevein-like antimicrobial peptide of bell pepper leaves for biotechnological use. BMC Genom. 2016, 17 (Suppl. 12), 999. [Google Scholar] [CrossRef]
- Flores-Alvarez, L.J.; Jiménez-Alcántar, P.; Ochoa-Zarzosa, A.; López-Meza, J.E. The Antimicrobial Peptide γ-Thionin from Habanero Chile (Capsicum chinense) induces caspase-independent apoptosis on human K562 Chronic myeloid leukemia cells and regulates epigenetic marks. Molecules 2023, 28, 3661. [Google Scholar] [CrossRef]
- Falcón-Ruiz, E.A.; López-Meza, J.E.; Ochoa-Zarzosa, A. The plant defensins PaDef and γ-thionin inhibit the endothelial cell response to VEGF. Peptides 2023, 165, 171008. [Google Scholar] [CrossRef] [PubMed]
- Cherene, M.B.; Taveira, G.B.; Almeida-Silva, F.; da Silva, M.S.; Cavaco, M.C.; da Silva-Ferreira, A.T.; Perales, J.E.A.; Carvalho, A.O.; Venâncio, T.M.; Motta, O.V.; et al. Structural and biochemical characterization of three antimicrobial peptides from Capsicum annuum L. var. annuum leaves for anti-Candida use. Probiotics Antimicrob. Proteins 2023, 16, 1270–1287. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.S.; Taveira, G.B.; Souza, T.A.M.; Carvalho, A.O.; Carvalho, V.S.; Rodrigues, R.; Fernandes, K.V.S.; Mello, E.O.; Gomes, V.M. Biotive protease inhibitor from leaves of Capsicum annuum L.: Potential use as an antifungal molecule against Colletotrichum scovillei. JSFA Rep. 2023, 3, 248–257. [Google Scholar] [CrossRef]
- Aldayel, M.F. The synergistic effect of Capsicum aqueous extract (Capsicum annuum) and chitosan against multidrug-resistant bacteria. J. King Saud. Univ.—Science 2023, 35, 102438. [Google Scholar] [CrossRef]
- Cotabarren, J.; Ozón, B.; Claver, S.; Geier, F.; Rossotti, M.; Garcia-Pardo, J.; Obregón, W.D. A multifunctional trypsin protease inhibitor from yellow bell pepper seeds: Uncovering its dual antifungal and hypoglycemic properties. Pharmaceutics 2023, 15, 781. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.N.; Cândido, E.S.; Franco, O.L. Computer-Aided Design of Antimicrobial Peptides: Are We Generating Effective Drug Candidates? Front. Microbiol. 2020, 10, 3097. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.B.; Seo, J. Antimicrobial peptides under clinical investigation. Pept Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.J.A.; Kvansakul, M.; Lay, F.T.; Phan, T.K.; Hulett, M.D. Defensin–lipid interactions in membrane targeting: Mechanisms of action and opportunities for the development of antimicrobial and anticancer therapeutics. Biochem. Soc. Trans. 2022, 50, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P.S. Peptide Toxicity Prediction. In Computational Peptidology; Methods in Molecular Biology; Zhou, P., Huang, J., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1268, pp. 143–157. Available online: http://link.springer.com/10.1007/978-1-4939-2285-7_7 (accessed on 18 December 2024).
- Wei, D.; Zhang, X. Biosynthesis, bioactivity, biotoxicity and applications of antimicrobial peptides for human health. Biosaf. Health 2022, 4, 118–134. [Google Scholar] [CrossRef]






| Thionins | Plant | Biological Activity | Main Target | Reference |
|---|---|---|---|---|
| Asthi1 | Oryza sativa L. | Antibacterial | Burkholderia plantarii | [35] |
| Thionin Thi2.1 | Arabidopsis thaliana L. | Antibacterial, antifungal, and cytotoxic | Staphylococcus aureus, Escherichia coli, Candida albicans; various mammalian cell lines | [36] |
| Thionin Thi2.1 (transgenic) | Lycopersicon esculentum Mill. | Antimicrobial | Ralstonia solanacearum and Fusarium oxysporum f. sp. lycopersici | [37] |
| Barley thionin | Ipomoea batatas (L.) Lam. | Antifungal | Ceratocystis fimbriata | [38] |
| NsW1 and NsW2 | Nigella sativa L. | Antifungal and Cytotoxic | Aspergillus ochraceus and A. fumigatus; RD, Jukart cell lines | [39] |
| Cp-thionin | Vigna unguiculata L. | Trypsin inhibitor | Trypsin | [40] |
| Thionin | Nicotiana tabacum | Antifungal and antibacterial | Botrytis cinerea, Ralstonia solanacearum, Helicoverpa armigera | [41] |
| Thionin | Allium cepa | Antifungal | Aspergillus niger | [42] |
| Barley thionin | Nicotiana benthamiana Domin. | Antimicrobial and insecticidal | Myzus persicae | [43] |
| Pp-TH | Pyrularia pubera M. | Antifungal and antibacterial activities anticoagulant | R. meliloti, X. campestris pv. translucens, and X. campestris pv. campestris, C. michiganensis; Pseudonebularia cucumerina, F. oxysporum, and B. cinerea | [44,45] |
| Ligatoxin B | Phoradendron liga (Gill.) Eichl | Cytotoxic | Human lymphoma cell line U-937-GTB and the primary multidrug-resistant renal adenocarcinoma cell line ACHN | [46] |
| Phoratoxins C-F | Phoradendron tomentosum (DC.) Engelm. ex A.Gray | Antitumoral | Solid tumor and hematological tumor | [47] |
| β-purothionin | Triticum kiharae | Antimutagenic | Human RD cells; DNA protection from cadmium chloride | [48] |
| Thionin Thi2.1 | Arabidopsis thaliana L. | Antibacterial, antifungal, and cytotoxic | Staphylococcus aureus, Escherichia coli, Candida albicans; various mammalian cell lines | [36] |
| Thionin-like AMPs | Arabidopsis thaliana L. | Nematocidal | Heterodera schachtii | [36] |
| Mthionin | Arabidopsis thaliana L. | Antifungal | Fusarium graminearum | [49] |
| PsoTHI1.7 | Papaver somniferum L. | Antifungal | Fusarium oxysporum f. sp. matthiolae and Botrytis cinerea | [50] |
| Tu-MP 1 (a) and Tu-MP 2 (b) | Tulipa gesneriana L. | Antifungal and antibacterial | P. carotovora, A. radiobacter, A. rhizogenes, C. michiganensis, and C. flaccumfaciens; F. oxysporum and G. candidum | [51] |
| Thionin | Oryza sativa L. | Nematocidal | Meloidogyne graminicola, Pythium graminicola | [52] |
| Heveins | Plant | Biological Activity | Main Target | Reference |
| Ee-CBP | Euonymus europaeus L. | Antifungal | Botrytis cinerea | [53] |
| Hevein-like AMPs | Eucommia ulmoides Oliv. | Antifungal | Phytophthora infestans, Aculops lycopersicu, | [54] |
| Verticillium dahliae, Gibberella zeae, | ||||
| Alternaria nicotianae, Fusarium moniliforme, | ||||
| Fusarium oxysporum, Corythucha gossypii | ||||
| Hb-AMP1 | Hevea brasiliensis Muell. Arg. | Antifungal and antibacterial | Candida albicans, Candida tropicalis, Candida krusei, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Aggregatibacter actinomycetemcomitans | [55] |
| Hevein-like AMPs | Broussonetia papyrifera (L.) L’Hér. ex Vent., Morus papyrifera L. | Antifungal | Trichoderma viride | [56] |
| Sm-AMP-X | Stellaria media L. | Antifungal and antibacterial | Fusarium solani, Alternaria alternata, Bipolaris sorokiniana, Botrytis cinerea, Aspergillus niger, Escherichia coli, P. carotovora, Clavibacter michiganensis pv. michiganensis, Agrobacterium rhizogenes, Bacillus subtilis, Pseudomonas syringae pv. tomato | [57] |
| Pn-AMP1 and Pn-AMP2 | Pharbitis nil L. | Antifungal | Candida albicans, Candida tropicalis, Candida krusei, Phytophthora capsici | [58] |
| Hevein-like genes | Hevea brasiliensis Muell. Arg. | Antifungal | Magnaporthe grisea | [59] |
| Wj-AMP | Wasabia japonica L. | Antifungal and antibacterial | Botrytis cinerea, Pseudomonas cichorii, Pseudomonas glumae, Pseudomonas plantaii, Agrobacterium tumefaciens, Escherichia coli | [60] |
| Sm-AMP | Stellaria media L. | Antifungal | Bipolaris sorokiniana, Thielaviopsis basicola | [61] |
| Pro-SmAMP1 | Stellaria media L. | Antifungal | Alternaria spp. | [62] |
| Hevein-like genes | Linum usitatissimum L. | Antifungal | Fusarium oxysporum | [63] |
| Hevein-like peptides | Moringa oleifera | Coagulation/ flocculation activities | Highly efficient potential of peptides | [64] |
| Defensins | Plant | Biological Activity | Main Target | Reference |
| PaDef1 | Persea americana var. drymifolia Mill. | Anticancer | Jurkat lineage of acute lymphocytic leukemia cells | [65] |
| DmDef1 | Dahlia merckii Lehm. | Antifungal | Saccharomyces cerevisiae | [66] |
| DmDef2, DmDef3 | Dahlia merckii Lehm. | Antifungal | Botrytis cinerea, Verticillium albo-atrum | [67] |
| MtDef1, MtDef2 | Medicago truncatula Gaertn. | Antibacterial and antifungal | Escherichia coli, Pseudomonas syringae pv. syringae, Sinorhizobium meliloti, Xanthomonas alfalfae subsp. alfalfae, Phytophthora medicaginis, Fusarium solani | [68] |
| NaDef1, NaDef2 | Nicotiana alata Link and Otto | Antifungal and antibacterial | Fusarium oxysporum, Botrytis cinerea | [69] |
| FeDef1, FeDef2 | Fagopyrum esculentum Moench. | Antibacterial and antifungal | Clavibacter michiganensis, Curtobacterium flaccumfaciens, P. carotovora, Agrobacterium spp., Fusarium oxysporum, Geotrichum candidum | [70] |
| MsDef1 | Medicago sativa L. | Antifungal | Fusarium graminearum | [71] |
| VaDef1, VaDef2 | Vigna angularis (Willd.) Ohwi and H. Ohashi | Antibacterial and antifungal | Fusarium oxysporum, Fusarium sp. pisi, Trichophyton rubrum, Staphylococcus epidermidis, Bacillus cereus, Xanthomonas campestris pv. vesicatoria | [72] |
| PlDef1 | Phaseolus limensis L. | Antifungal and antibacterial | Botrytis cinerea, Fusarium oxysporum, Mycosphaerella arachidicola | [73] |
| RsAFP2 | Raphanus sativus | Antifungal | Candida albicans, Pichia pastoris | [74] |
| BcDef1 | Brassica campestris L. ssp. pekinensis | Antifungal | Alternaria solani, Neurospora crassa, Phytophthora parasitica, Fusarium oxysporum | [75] |
| OsDef1 | Oryza sativa cv. Sasanishiki | Antifungal | Magnaporthe grisea | [76] |
| TaDef1 | Triticum aestivum L. | Antibacterial | Pseudomonas cichorii | [77] |
| VrDef1 | Vigna radiata (L.) Wilczek | Insecticidal and antifungal | Callosobruchus chinensis larvae, Fusarium oxysporum, Pyricularia oryzae, Rhizoctonia solani, Trichophyton rubrum | [78] |
| VuDef1 | Vigna unguiculata | Enzymatic inhibitor | Acanthoscelides obtectus, Zabrotes subfasciatus | [79] |
| PvDef1 | Phaseolus vulgaris cv. Pérola | Antifungal and antibacterial | Candida albicans, Candida parapsilosis, Candida tropicalis, Candida guilliermondii, Kluyveromyces marxianus, Saccharomyces cerevisiae, Fusarium oxysporum, Fusarium solani, Fusarium lateritium, Rhizoctonia solani | [80] |
| PeDef1 | Pachyrhizus erosus (L.) Urb. | Antifungal | Fusarium oxysporum f. sp. vasinfectum, Verticillium dahliae, Aspergillus flavus, Penicillium spp., Colletotrichum gloeosporioides, Botrytis cinerea, Bipolaris maydis, Aspergillus niger, Fusarium oxysporum f. sp. lycopersici, Rhizopus stolonifer | [81] |
| LmDef1 | Lepidium meyenii | Antimicrobial | Phytophthora infestans | [82] |
| PsDef1 | Pisum sativum | Antifungal | Aspergillus niger | [83] |
| TfDef1 | Trigonella foenum-graecum | Antifungal | Rhizoctonia solani, Phaeoisariopsis personata | [84] |
| MsDef2 | Medicago sativa L. | Antifungal | Fusarium oxysporum f. sp. lycopersici | [85] |
| NaDef3 | Nicotiana alata | Antifungal | Fusarium oxysporum, Verticillium dahliae | [86] |
| RsAFP2 | Raphanus sativus | Antifungal | Specific amino acid region affecting activity (RsAFP2) | [87] |
| MsDef1, MtDef4 | Medicago sativa, Medicago truncatula | Antifungal | Specific amino acid region affecting activity (MsDef1, MtDef4) | [88] |
| LTPs | Plant | Biological Activity | Main Target | Reference |
| LTP | Coffea canephora L. | Antifungal and antibacterial | Colletotrichum lindemuthianum, Colletotrichum gloeosporioides, Fusarium solani, Fusarium lateritium, Colletotrichum sp., Xanthomonas euvesicatoria | [80] |
| LTP-like AMPs | Capsicum annuum L. cv. Bugang | Antibacterial and antiviral | Tobacco mosaic virus (TMV), Xanthomonas campestris pv. vesicatoria | [82,83] |
| LTP | Oryza sativa | Antimicrobial | Pyricularia oryzae, Xanthomonas oryzae | [84] |
| nsLTP AMPs | Phaseolus mungo | Antifungal and antibacterial | Fusarium solani, Fusarium oxysporum, Pythium aphanidermatum, Sclerotium rolfsii, Gram-positive bacteria Staphylococcus aureus | [85] |
| LTP | Potato plants | Antimicrobial | Phytophthora infestans | [86] |
| nsLTP-like AMPs | Leonurus japonicus Houtt. | Antifungal and antibacterial | Fusarium oxysporum, Alternaria brassicae, Bipolaris maydis, Rhizoctonia cerealis, Saccharomyces cerevisiae, Bacillus subtilis | [87] |
| LTP-like AMPs | Brassica campestris L. | Antifungal | Fusarium oxysporum, Mycosphaerella arachidicola | [88] |
| LTP | Helianthus annuus | Antifungal | Fusarium solani f. sp. eumartii, Alternaria alternata | [89] |
| LTP-like AMPs | Narcissus tazetta var. chinensis L. | Antiviral | Respiratory syncytial virus (RSV), Influenza A (H1N1) virus | [90] |
| LTP | Nigella sativa L. | Antifungal | Pythium debaryanum, oomycetes Phytophthora infestans | [91] |
| LTP-like AMPs | Coffea canephora | Antifungal | Candida albicans, Candida tropicalis, inhibition of α-amylase in mammals | [92] |
| nsLTP-like AMPs | Transgenic wheat | Antifungal | Fusarium graminearum | [93] |
| LTP-like AMPs | Wheat | Antifungal | Alternaria sp., Rhizoctonia solani, Curvularia lunata, Bipolaris oryzae, Cylindrocladium scoparium, Botrytis cinerea, Sarocladium oryzae | [94] |
| LTP-like AMPs | Anethum graveolens L. | Antifungal | Weak antifungal activity, inhibition of spore germination, delay in hyphae elongation of Aspergillus niger | [95] |
| LTP | Allium cepa L. | Antifungal | Blumeria graminis f. sp. tritici, Neovossia indica | [96] |
| LTP | Triticum aestivum | Antifungal | Puccinia triticina Erikss. | [97] |
| Cyclotides | Plant | Biological Activity | Main Target | Reference |
| kalata B1, kalata B2, cycloviolacin O2 | Viola arvensis Murr., Viola odorata L. | Antitumor and cytotoxic effects | Human tumor cell lines | [98,99] |
| hylin C, hylin D | Hybanthus enneaspermus (L.) F. Muell. | Anticancer, aphrodisiac properties | Erectile function in male rats, membrane blistering and cell necrosis | [100,101] |
| rin A, rin B | Rinorea spp. | Cytotoxicity | Pathogenic bacteria and possibly cancer cells | [102] |
| pom A, pom B | Pombalia calceolaria | Anticancer | Human tumor cell lines | [103] |
| varv A, varv F | Viola tricolor | Inhibitory activity against HIV | Human immunodeficiency virus (HIV) | [104] |
| vhl-1 | Viola hederacea | Inhibitory activity against HIV | Human immunodeficiency virus (HIV) | [105] |
| viy A | Viola yedoensis Makino | Anti-HIV | Human immunodeficiency virus (HIV) | [106] |
| Cyclotides | Viola odorata | Anti-inflammatory, reduces demyelination | Inflammation, neurological deficits in experimental autoimmune encephalomyelitis model | [107] |
| Cyclotides | Oldenlandia affinis | Anti-inflammatory, antiplasmodial | Plasmodium berghei, acute and chronic inflammation in rodent models | [108] |
| Cyclotides | Allexis spp. | Protease inhibitor | Inhibition of human prolyl oligopeptidase activity | [109] |
| SNC1 and SNC2 | Sambucus nigra | Antibacterial | Aeromonas salmonicida, Vibrio ordalii, Vibrio anguillaru, Flavobacterium psychrophilum, E. coli | [110] |
| cycloviolacin O2 | Viola tricolor | Antibacterial | Pseudomonas aeruginosa and Staphylococcus aureus, Escherichia coli and Bacillus subtilis. | [111] |
| Cyclotides | Viola odorata | Antibacterial | Staphylococcus aureus | [112] |
| Cyclotides | Geophila repens | Antibacterial and cytotoxic activity | Escherichia coli, U-937 human lymphoma cell line | [113] |
| Kalata B1, Kalata B2, OlaC1, OlaC2 | Oldenlandia affinis | Insecticidal | Moth larvae Helicoverpa armigera | [114,115] |
| Cyclotides | O. affinis, Clitoria ternatea, Viola odorata, Hybanthus enneaspermus | Nematocidal effects | Caenorhabditis elegans juvenile | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, S.S.S.; Cherene, M.B.; Taveira, G.B.; de Oliveira Mello, É.; de Oliveira Carvalho, A.; Gomes, V.M. Plant Antimicrobial Peptides and Their Main Families and Roles: A Review of the Literature. Curr. Issues Mol. Biol. 2025, 47, 1. https://doi.org/10.3390/cimb47010001
de Oliveira SSS, Cherene MB, Taveira GB, de Oliveira Mello É, de Oliveira Carvalho A, Gomes VM. Plant Antimicrobial Peptides and Their Main Families and Roles: A Review of the Literature. Current Issues in Molecular Biology. 2025; 47(1):1. https://doi.org/10.3390/cimb47010001
Chicago/Turabian Stylede Oliveira, Samuel Salomão Silva, Milena Bellei Cherene, Gabriel Bonan Taveira, Érica de Oliveira Mello, André de Oliveira Carvalho, and Valdirene Moreira Gomes. 2025. "Plant Antimicrobial Peptides and Their Main Families and Roles: A Review of the Literature" Current Issues in Molecular Biology 47, no. 1: 1. https://doi.org/10.3390/cimb47010001
APA Stylede Oliveira, S. S. S., Cherene, M. B., Taveira, G. B., de Oliveira Mello, É., de Oliveira Carvalho, A., & Gomes, V. M. (2025). Plant Antimicrobial Peptides and Their Main Families and Roles: A Review of the Literature. Current Issues in Molecular Biology, 47(1), 1. https://doi.org/10.3390/cimb47010001






