Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Agents
2.2. Animals and Ethical Approval
2.3. Mucuna pruriens (L.) DC. and L-DOPA with Dose Verification
2.4. Parameters to Validate PD Induction and Treatment
2.4.1. Behavioral Assessment
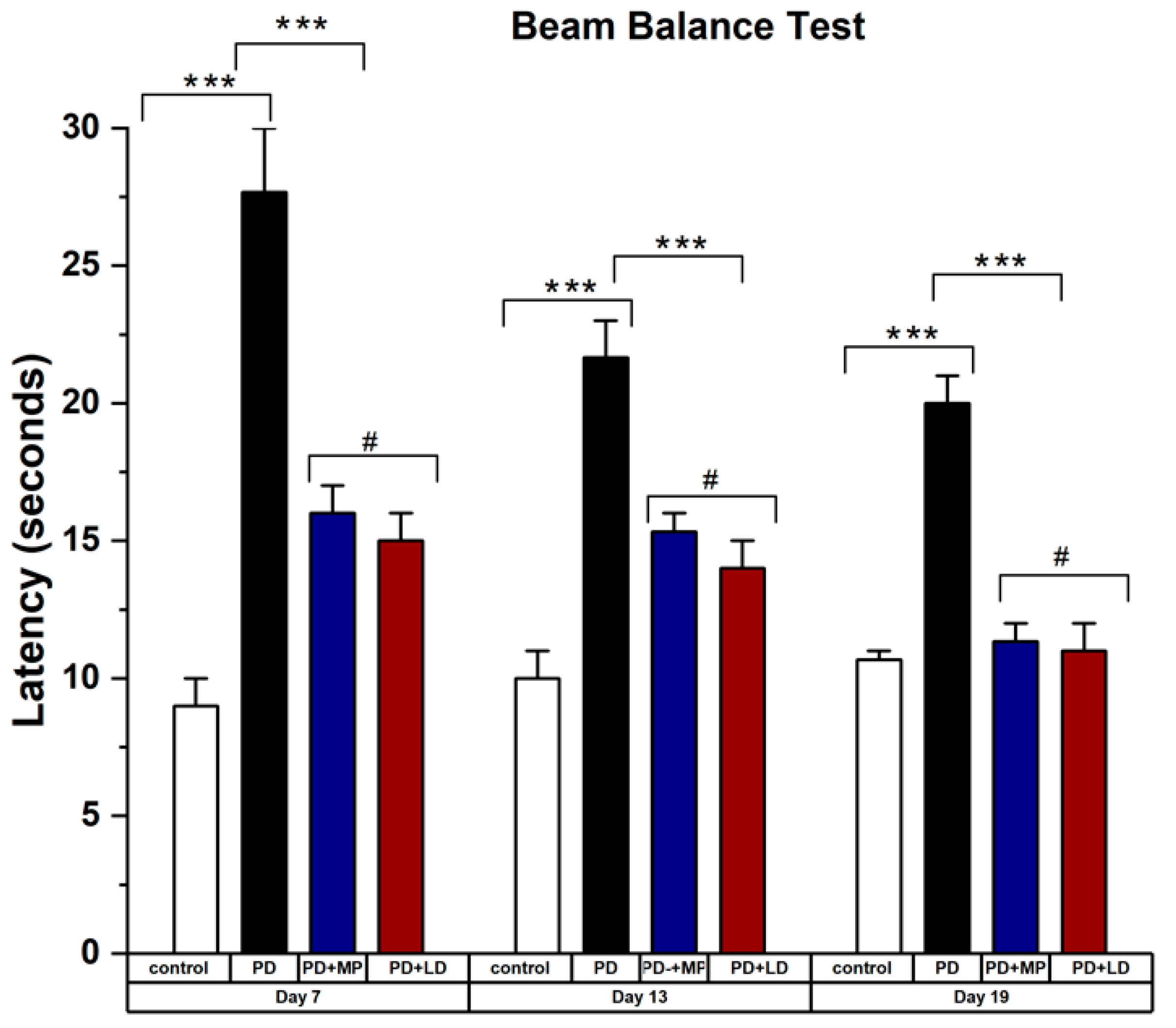
Beam Balance Test
Olfactory Test/Buried Pellet Test
2.4.2. Enzyme-Linked Immunosorbent Assay
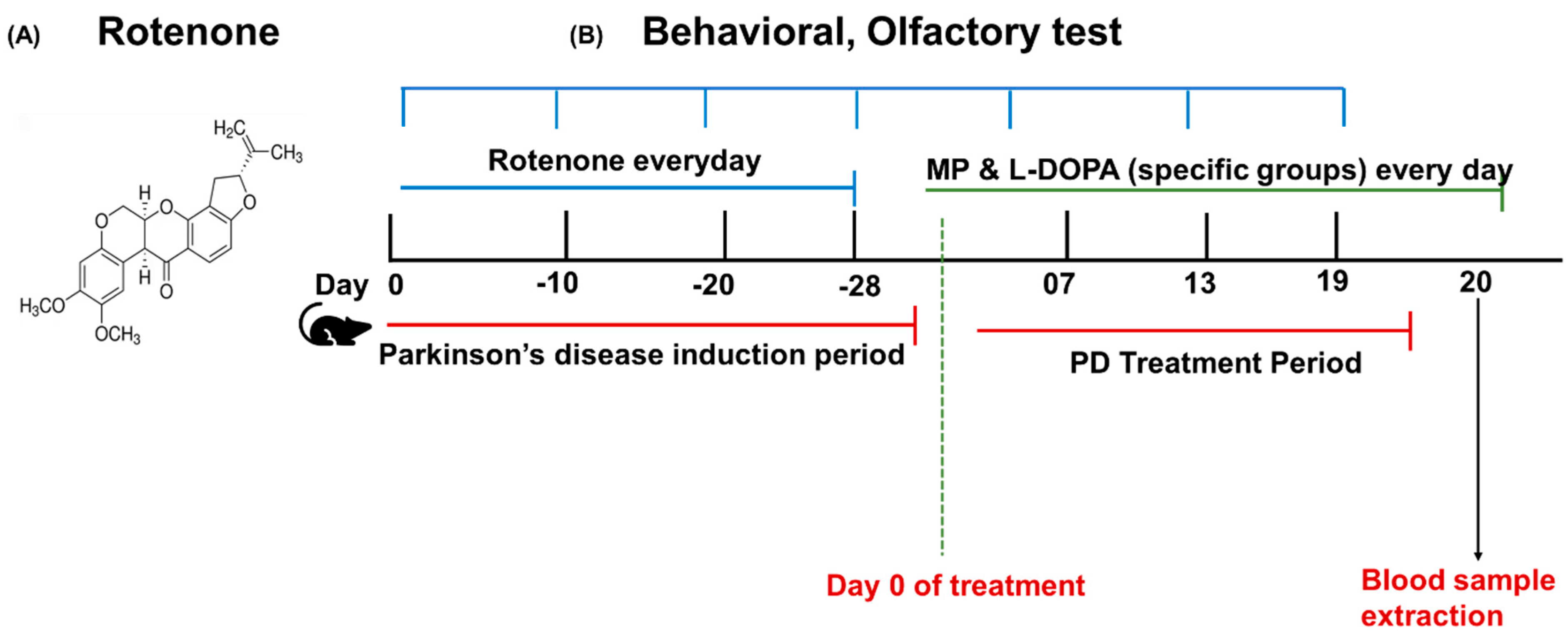
2.5. Rotenone Model Induction and Treatment Plan
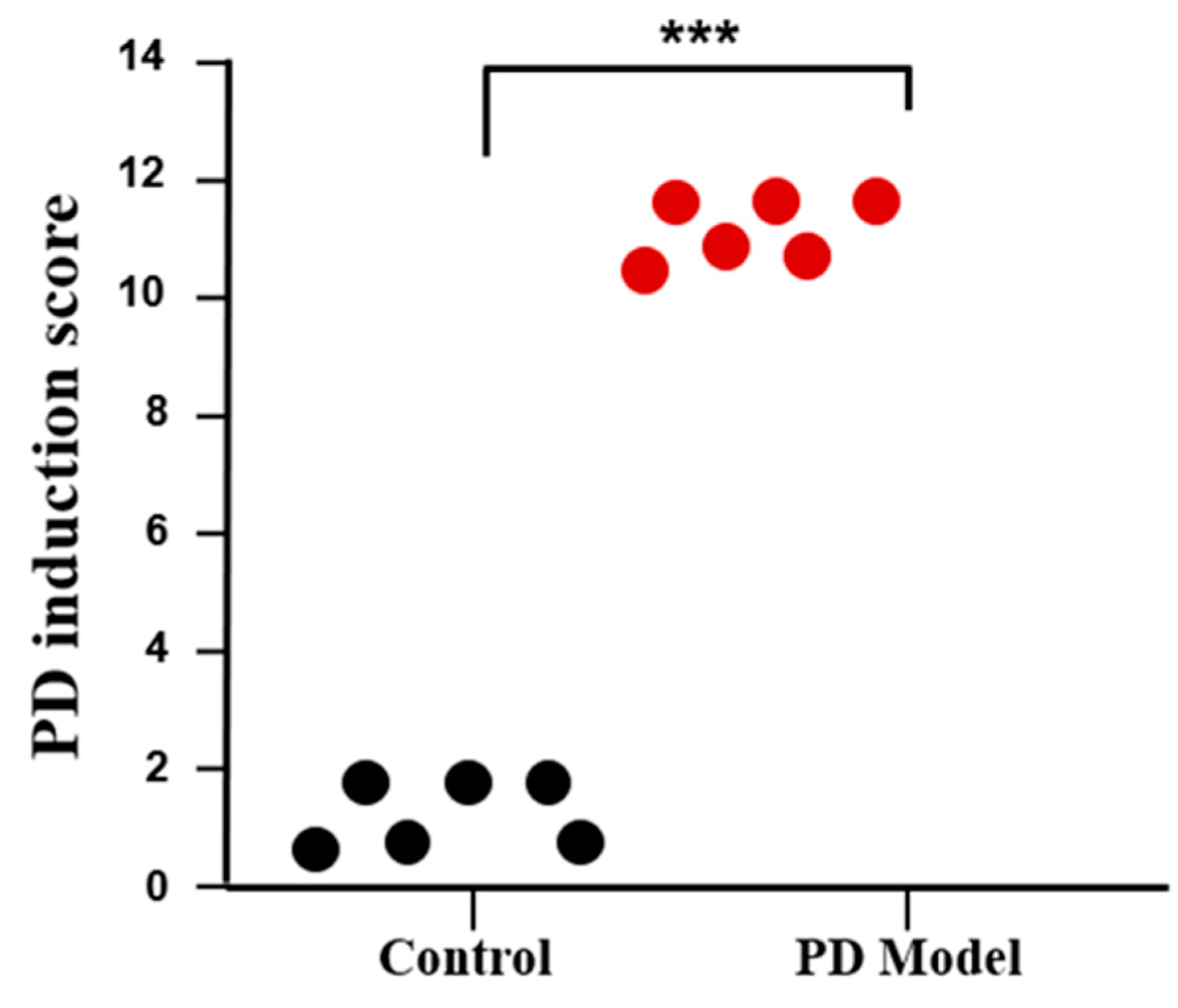
2.6. PD Induction Score
2.7. Experimental Design
2.8. Statistical Analysis
3. Results
3.1. Rotenone-Induced Parkinson’s Disease (PD) Symptoms in Mice
3.2. Comparative Study of MP and L-DOPA in Improving the Symptoms of Parkinson’s Disease
3.2.1. Effects of MP and Improving the Physical Symptoms of PD in Comparison to L-DOPA
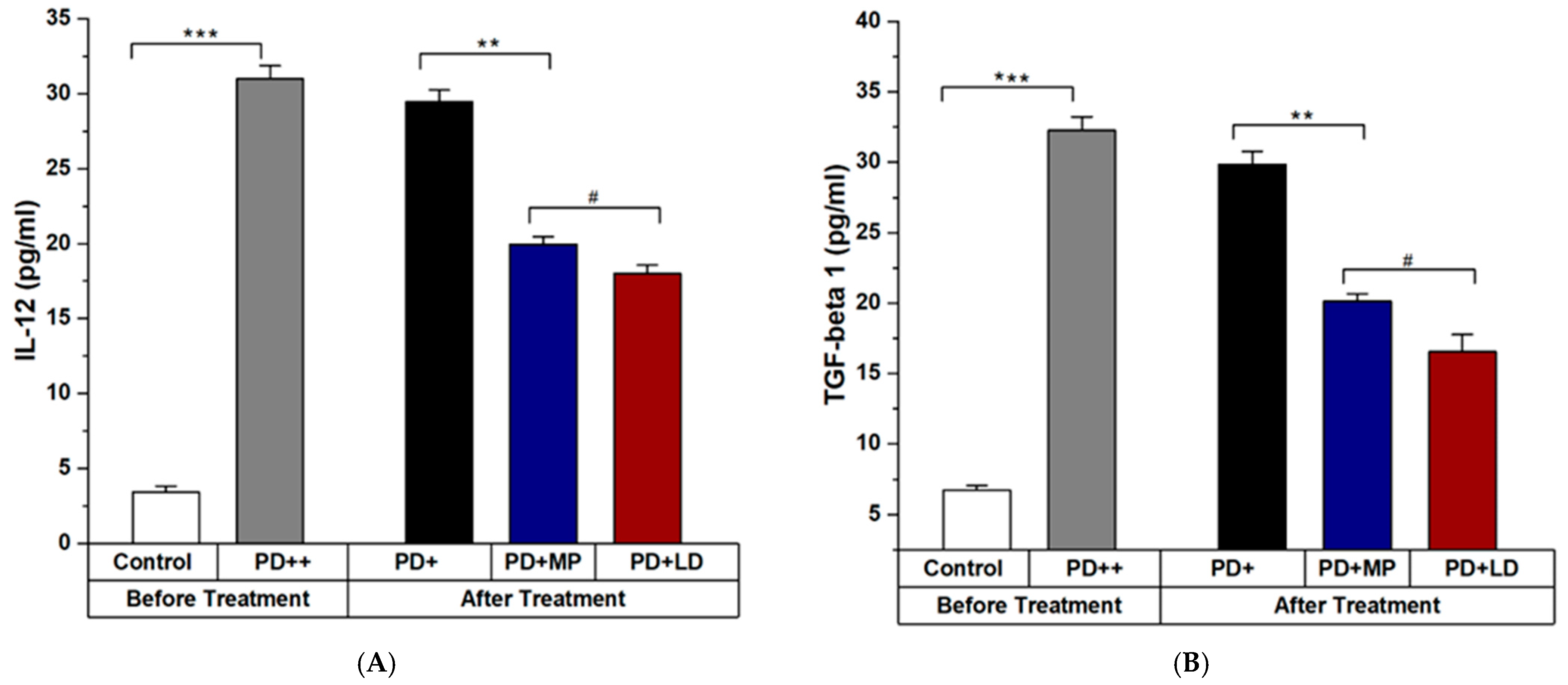
3.2.2. Effects of MP in Improving the Inflammation of PD in Comparison to L-DOPA
4. Discussion
Future Work and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- DeMaagd, G.; Philip, A. Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm. Ther. 2015, 40, 504. [Google Scholar]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Lee, H.M.; Koh, S.-B. Many faces of Parkinson’s disease: Non-motor symptoms of Parkinson’s disease. J. Mov. Disord. 2015, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S.; Hartmann, A. Neuroinflammatory processes in Parkinson’s disease. Park. Relat. Disord. 2005, 11, S9–S15. [Google Scholar] [CrossRef]
- Ma, K.; Xiong, N.; Shen, Y.; Han, C.; Liu, L.; Zhang, G.; Wang, L.; Guo, S.; Guo, X.; Xia, Y. Weight loss and malnutrition in patients with Parkinson’s disease: Current knowledge and future prospects. Front. Aging Neurosci. 2018, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Chang, S.-C.; Lee, J. Significant roles of neuroinflammation in Parkinson’s disease: Therapeutic targets for PD prevention. Arch. Pharmacal Res. 2019, 42, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Cytokines in Parkinson’s disease. Adv. Res. Neurodegener. 2000, 7, 143–151. [Google Scholar]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef]
- Deets, K.A.; Vance, R.E. Inflammasomes and adaptive immune responses. Nat. Immunol. 2021, 22, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Borsche, M.; König, I.R.; Delcambre, S.; Petrucci, S.; Balck, A.; Brüggemann, N.; Zimprich, A.; Wasner, K.; Pereira, S.L.; Avenali, M. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 2020, 143, 3041–3051. [Google Scholar] [CrossRef]
- Arena, G.; Sharma, K.; Agyeah, G.; Krüger, R.; Grünewald, A.; Fitzgerald, J. Neurodegeneration and neuroinflammation in Parkinson’s disease: A self-sustained loop. Curr. Neurol. Neurosci. Rep. 2022, 22, 427–440. [Google Scholar] [CrossRef]
- Brodacki, B.; Staszewski, J.; Toczyłowska, B.; Kozłowska, E.; Drela, N.; Chalimoniuk, M.; Stępien, A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFα, and INFγ concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 2008, 441, 158–162. [Google Scholar] [CrossRef]
- Alharthy, K.M.; Althurwi, H.N.; Albaqami, F.F.; Altharawi, A.; Alzarea, S.I.; Al-Abbasi, F.A.; Nadeem, M.S.; Kazmi, I. Barbigerone potentially alleviates rotenone-activated Parkinson’s disease in a rodent model by reducing oxidative stress and neuroinflammatory cytokines. ACS Omega 2023, 8, 4608–4615. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in Parkinson’s disease. In Parkinson’s Disease and Related Disorders; Springer: Vienna, Austria, 2006; pp. 373–381. [Google Scholar]
- Gao, H.-M.; Liu, B.; Zhang, W.; Hong, J.-S. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003, 17, 1–22. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, S. Do studies in humans better depict Th17 cells? Blood J. Am. Soc. Hematol. 2009, 114, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Wyss-Coray, T. A role for TGF-β signaling in neurodegeneration: Evidence from genetically engineered models. Curr. Alzheimer Res. 2006, 3, 505–513. [Google Scholar] [CrossRef]
- Samantasinghar, A.; Ahmed, F.; Rahim, C.S.A.; Kim, K.H.; Kim, S.; Choi, K.H. Artificial intelligence-assisted repurposing of lubiprostone alleviates tubulointerstitial fibrosis. Transl. Res. 2023, 262, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the Context of an Inflammatory Cytokine Milieu Supports De Novo Differentiation of IL-17-Producing T Cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Karampetsou, M.; Vekrellis, K.; Melachroinou, K. The promise of the TGF-β superfamily as a therapeutic target for Parkinson’s disease. Neurobiol. Dis. 2022, 171, 105805. [Google Scholar] [CrossRef] [PubMed]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming growth factor-β induces development of the TH17 lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Evans, A.; Manson, A.; Patsalos, P.; Ratnaraj, N.; Watt, H.; Timmermann, L.; Van der Giessen, R.; Lees, A. Mucuna pruriens in Parkinson’s disease: A double blind clinical and pharmacological study. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1672–1677. [Google Scholar] [CrossRef]
- Sakata, M.; Miyamoto, K.; Koh, J.; Nagashima, Y.; Kondo, T.; Ito, H. Japanese Mucuna pruriens (Hasshou beans) Showed Fast-acting and Long-lasting Effects in Parkinson’s Disease. Intern. Med. 2024, 3171-23. [Google Scholar] [CrossRef]
- Mahajani, S.; Doshi, V.; Parikh, K.; Manyam, B. Bioavailability of l-DOPA from HP-200—A Formulation of Seed Powder of Mucuna pruriens (Bak): A Pharmacokinetic and Pharmacodynamic Study. Phytother. Res. 1996, 10, 254–256. [Google Scholar] [CrossRef]
- Cannon, J.R.; Tapias, V.; Na, H.M.; Honick, A.S.; Drolet, R.E.; Greenamyre, J.T. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol. Dis. 2009, 34, 279–290. [Google Scholar] [CrossRef]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of Parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Greene, J.G.; Miller, G.W. Behavioral phenotyping of mouse models of Parkinson’s disease. Behav. Brain Res. 2010, 211, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.; Mo, M.; Song, C.; Wang, X.; Chen, S.; Liu, Y. Minocycline protects against rotenone-induced neurotoxicity correlating with upregulation of Nurr1 in a Parkinson’s disease rat model. BioMed Res. Int. 2019, 2019, 6843265. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Beneficial effect of vitamin E in rotenone induced model of PD: Behavioural, neurochemical and biochemical study. Exp. Neurobiol. 2013, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Thakur, P.; Nehru, B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease. Neuroscience 2013, 231, 420–431. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaigham, S.B.; Paeng, D.-G. Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice. Curr. Issues Mol. Biol. 2024, 46, 9234-9244. https://doi.org/10.3390/cimb46080545
Zaigham SB, Paeng D-G. Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice. Current Issues in Molecular Biology. 2024; 46(8):9234-9244. https://doi.org/10.3390/cimb46080545
Chicago/Turabian StyleZaigham, Sheher Bano, and Dong-Guk Paeng. 2024. "Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice" Current Issues in Molecular Biology 46, no. 8: 9234-9244. https://doi.org/10.3390/cimb46080545
APA StyleZaigham, S. B., & Paeng, D.-G. (2024). Effects of Mucuna pruriens (L.) DC. and Levodopa in Improving Parkinson’s Disease in Rotenone Intoxicated Mice. Current Issues in Molecular Biology, 46(8), 9234-9244. https://doi.org/10.3390/cimb46080545






