IL-17A Cytokine-Regulated Glut1 Expression in Placenta Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and IL-17A Cytokine Treatment
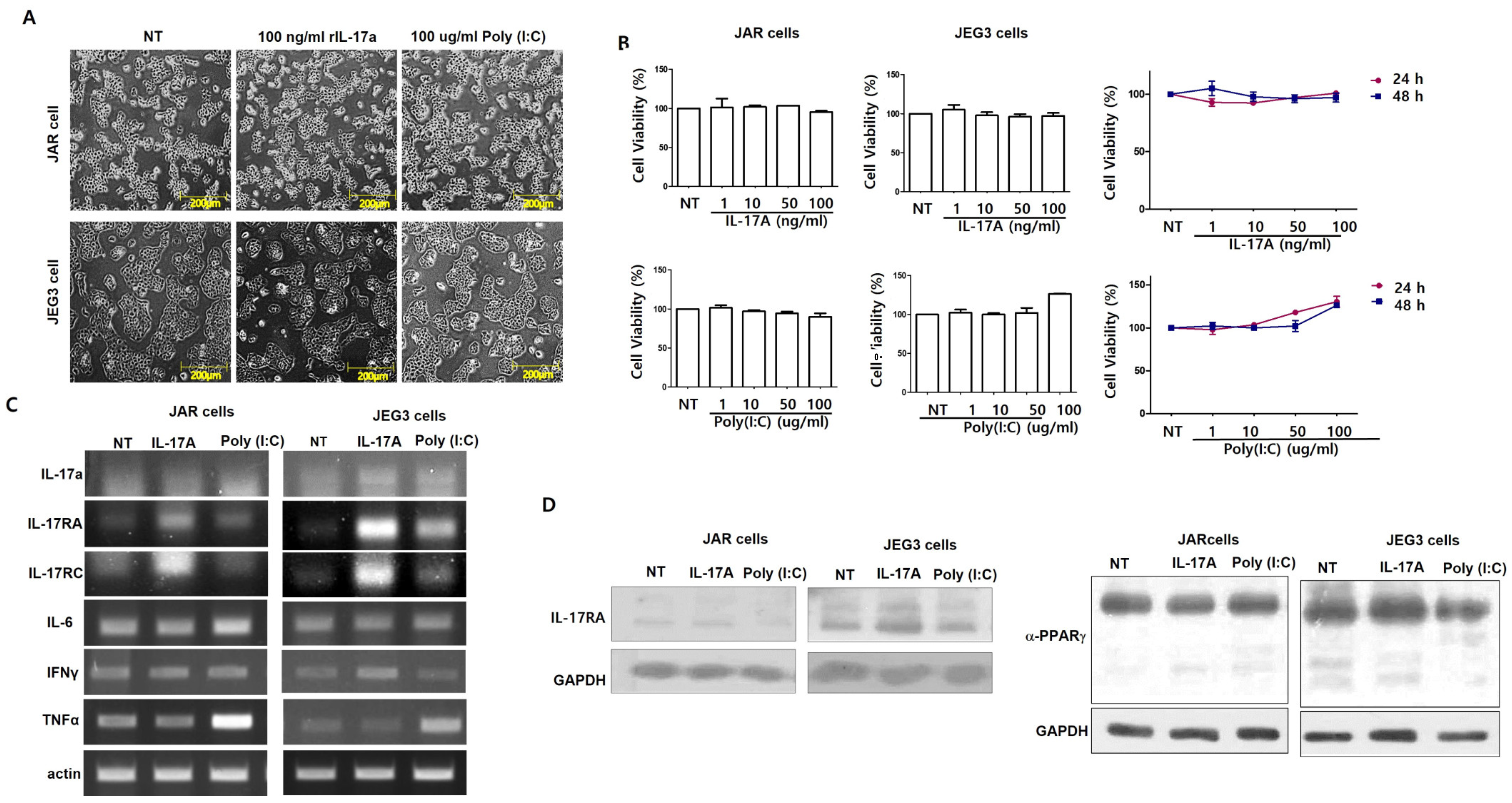
2.2. MTT Assay and Cell Proliferation Assay
2.3. Reverse Transcriptase PCR (RT-PCR)
2.4. Western Blotting
2.5. Maternal Immune Activation (MIA)
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Robertson, S.A.; Seamark, R.F.; Guilbert, L.J.; Wegmann, T.G. The Role of Cytokines in Gestation. Crit. Rev. Immunol. 1994, 14, 239–292. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Wong, H.; Hoeffer, C. Maternal IL-17A in autism. Exp. Neurol. 2018, 299, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, D.C.; Hogg, J.P.; Scott, J.; Wallace, K.; Herse, F.; Moseley, J.; Wallukat, G.; Dechend, R.; LaMarca, B. Administration of inter-leukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 2013, 62, 1068–1073. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediat. Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef]
- Ito, M.; Nakashima, A.; Hidaka, T.; Okabe, M.; Bac, N.D.; Ina, S.; Yoneda, S.; Shiozaki, A.; Sumi, S.; Tsuneyama, K.; et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J. Reprod. Immunol. 2010, 84, 75–85. [Google Scholar] [CrossRef]
- Nelson, D.M.; Myatt, L. The human placenta in health and disease. Obstet. Gynecol. Clin. 2020, 47, XV–XVIII. [Google Scholar] [CrossRef]
- Kalhan, S.C.; D’Angelo, L.J.; Savin, S.M.; Adam, P.A. Glucose production in pregnant women at term gestation: Sources of glucose for human fetus. J. Clin. Investig. 1979, 63, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Castrejon, M.; Powell, T.L. Placental Nutrient Transport in Gestational Diabetic Pregnancies. Front. Endocrinol. 2017, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal–Fetal Nutrient Transport in Pregnancy Pathologies: The Role of the Placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef] [PubMed]
- Al-Bishri, W.M. Glucose transporter 1 deficiency, AMP-activated protein kinase activation and immune dysregulation in autism spectrum disorder: Novel biomarker sources for clinical diagnosis. Saudi J. Biol. Sci. 2023, 30, 103849. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.S.; Morrison, J.P.; Southwell, M.F.; Foley, J.F.; Bolon, B.; Elmore, S.A. Histology atlas of the developing prenatal and postnatal mouse central nervous system, with emphasis on prenatal days E7. 5 to E18. 5. Toxicol. Pathol. 2017, 45, 705–744. [Google Scholar] [CrossRef] [PubMed]
- Pragallapati, S.; Manyam, R. Glucose transporter 1 in health and disease. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 443. [Google Scholar] [CrossRef]
- Illsley, N.P.; Baumann, M.U. Human placental glucose transport in fetoplacental growth and metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165359. [Google Scholar] [CrossRef]
- Tashev, S.A.; Parsons, D.; Hillman, C.; Harris, S.; Lofthouse, E.M.; Goggin, P.; Chatelet, D.S.; Cleal, J.K.; Smyth, N.; Palaiologou, H.; et al. Folding of the syncytiotrophoblast basal plasma membrane increases the surface area available for exchange in human placenta. Placenta 2021, 117, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Karteris Zachariades, E.; Foster, H.; Goumenou, A.; Thomas, P.; Rand-Weaver, M.; Karteris, E. Expression of membrane and nuclear progesterone receptors in two human placental choriocarcinoma cell lines (JEG-3 and BeWo): Effects of syncytialization. Int. J. Mol. Med. 2011, 27, 767–774. [Google Scholar] [CrossRef][Green Version]
- Drwal, E.; Rak, A.; Gregoraszczuk, E. Co-culture of JEG-3, BeWo and syncBeWo cell lines with adrenal H295R cell line: An al-ternative model for examining endocrine and metabolic properties of the fetoplacental unit. Cytotechnology 2018, 70, 285–297. [Google Scholar] [CrossRef]
- Gaither, K.; Quraishi, A.N.; Illsley, N.P. Diabetes alters the expression and activity of the human placental GLUT1 glucose trans-porter. J. Clin. Endocrinol. Metab. 1999, 84, 695–701. [Google Scholar] [PubMed]
- Takagi, H.; King, G.L.; Aiello, L.P. Hypoxia upregulates glucose transport activity through an adenosine-mediated increase of GLUT1 expression in retinal capillary endothelial cells. Diabetes 1998, 47, 1480–1488. [Google Scholar] [CrossRef]
- Das, U.G.; Sadiq, H.F.; Soares, M.J.; Hay, W.W., Jr.; Devaskar, S.U. Time-dependent physiological regulation of rodent and ovine pla-cental glucose transporter (GLUT-1) protein. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 274, R339–R347. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. In-terleukin-17–producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreac-tivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef]
- Hou, S.; Jiao, Y.; Yuan, Q.; Zhai, J.; Tian, T.; Sun, K.; Chen, Z.; Wu, Z.; Zhang, J. S100A4 protects mice from high-fat diet-induced obesity and inflammation. Mod. Pathol. 2018, 98, 1025–1038. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Ma, S.; Liu, S.; Chen, Z.; Mo, Z.; You, Z. Interleukin-17A Differentially Induces Inflammatory and Metabolic Gene Expression in the Adipose Tissues of Lean and Obese Mice. Int. J. Mol. Sci. 2016, 17, 522. [Google Scholar] [CrossRef]
- Zhu, X.W.; Mulcahy, L.A.; Mohammed, R.A.A.; Lee, A.H.S.; Franks, H.A.; Kilpatrick, L.; Yilmazer, A.; Paish, E.C.; Ellis, I.O.; Patel, P.M.; et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008, 10, R95. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Tian, Z.; Wei, H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell. Mol. Immunol. 2014, 11, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P.L.; Leone, G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genes 2007, 45, 129–134. [Google Scholar] [CrossRef]


| Forward | Reverse | |
|---|---|---|
| IL-17a | TAATGGCCCTGAGGAATGGC | AGGAAGCCTGAGTCTAGGGG |
| IL-17RA | TGCCCTGTGGGTGTACTGGT | GGAGGCAGGCCATCGGTGTA |
| IL-17RC | CTGCCCTTGTGCAGTTTGG | CAGATTCGTACCTCACTCCCTA |
| IL-6 | TACCCCCAGGAGAAGATTCC | AGTGCCTCTTTGCTGCTTTC |
| IFNγ | AACTGTCGCCAGCAGCTAAA | TTGGCCCCTGAGATAAAGCC |
| TNFα | GACAAGCCTGTAGCCCATGT | GGAGGTTGACCTTGGTCTGG |
| SLC2A1 | GCTTATGGGCTTCTCCAAACT | GGTGACACCTCTCCCACATAC |
| SLC2A3 | GATCGGCTCTTTCCAGTTTG | CAATCATGCCACCAACAGAG |
| SLC7A8 | CCAGTGTGTTGGCCATGATC | TGCAACCGTTACCCCATAGAA |
| ACTIN | CAAGAGATGGCCACGGCTGCT | TCCTTCTGCATCCTGTCGGCA |
| Forward | Reverse | |
|---|---|---|
| IL-17RA | AGATGCCAGGATCCTGTACC | CACAGTCACAGCGTGTCTCA |
| TLR3 | CCTCCAACTGTCTACCAGTTCC | GCCTGGCTAAGTTATTGTGG |
| VEGFα | GCCTTGTTCAGAGCGGAGAA | TGTCAACGGTGACGATGATG |
| ZO-1 | AAAACGCTCTACAGGCTCCC | GGCCTGGGCTGGATCATAAC |
| SLC1A3 | GAGGCAGCTCCCAGGTTTA | GATTACTCCCCCGCAGCCTA |
| SLC2A1 | GTGACAAGACACCCGAGGAG | CTGTCGGTTCGGAAGAGGTC |
| SLC2A3 | TCTCCTAAGTCACCGAGCCA | GCTACCTCAAACACACCCGA |
| SLC6A4 | TGGTTTGTGCTCATCGTGGT | GCATTTCCTTCACGTCGCTG |
| SLC7A5 | CTGGGTGAGACAGTTGGGTC | GCCATTCCAGTAGACACCCC |
| SLC7A8 | GGGTGTCTACTGGCAACACA | CGGGTCCTTGACAGGAGTAG |
| SLC38A1 | TCTGACTTCGGTGACACTGC | TGCATCCTCCTCTCCCATGA |
| SLC38A4 | GCGGCCCTCTTTGGTTATCT | AGCGATCCCGAAATGCTTCA |
| TUBULIN | GAAGGACAGGAATGGGAACA | AGGTGTCTGGGAAGCTGAGA |
| Transporter | Function | Gene_Dpi |
|---|---|---|
| SLC1A3 | Excitatory amino acid transporter, high-affinity glutamate transporter family | 0.577 |
| SLC6A4 | Neurotransmitter serotonin from synaptic spaces | 0.808 |
| SLC6A8 | Neurotransmitter transporter, creatines | 0.846 |
| SLC9A9 | Sodium/proton exchanger, cation homeostasis | 0.577 |
| SLC25A12 | Calcium-binding mitochondrial carrier protein | 0.462 |
| SLC40A1 | Involved in iron export from duodenal epithelial cells | 0.731 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kim, H. IL-17A Cytokine-Regulated Glut1 Expression in Placenta Cells. Curr. Issues Mol. Biol. 2024, 46, 7386-7394. https://doi.org/10.3390/cimb46070438
Lee JY, Kim H. IL-17A Cytokine-Regulated Glut1 Expression in Placenta Cells. Current Issues in Molecular Biology. 2024; 46(7):7386-7394. https://doi.org/10.3390/cimb46070438
Chicago/Turabian StyleLee, Jeong Yeon, and Hyunju Kim. 2024. "IL-17A Cytokine-Regulated Glut1 Expression in Placenta Cells" Current Issues in Molecular Biology 46, no. 7: 7386-7394. https://doi.org/10.3390/cimb46070438
APA StyleLee, J. Y., & Kim, H. (2024). IL-17A Cytokine-Regulated Glut1 Expression in Placenta Cells. Current Issues in Molecular Biology, 46(7), 7386-7394. https://doi.org/10.3390/cimb46070438





