Protective Effect of Red Light-Emitting Diode against UV-B Radiation-Induced Skin Damage in SKH:HR-2 Hairless Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Animals
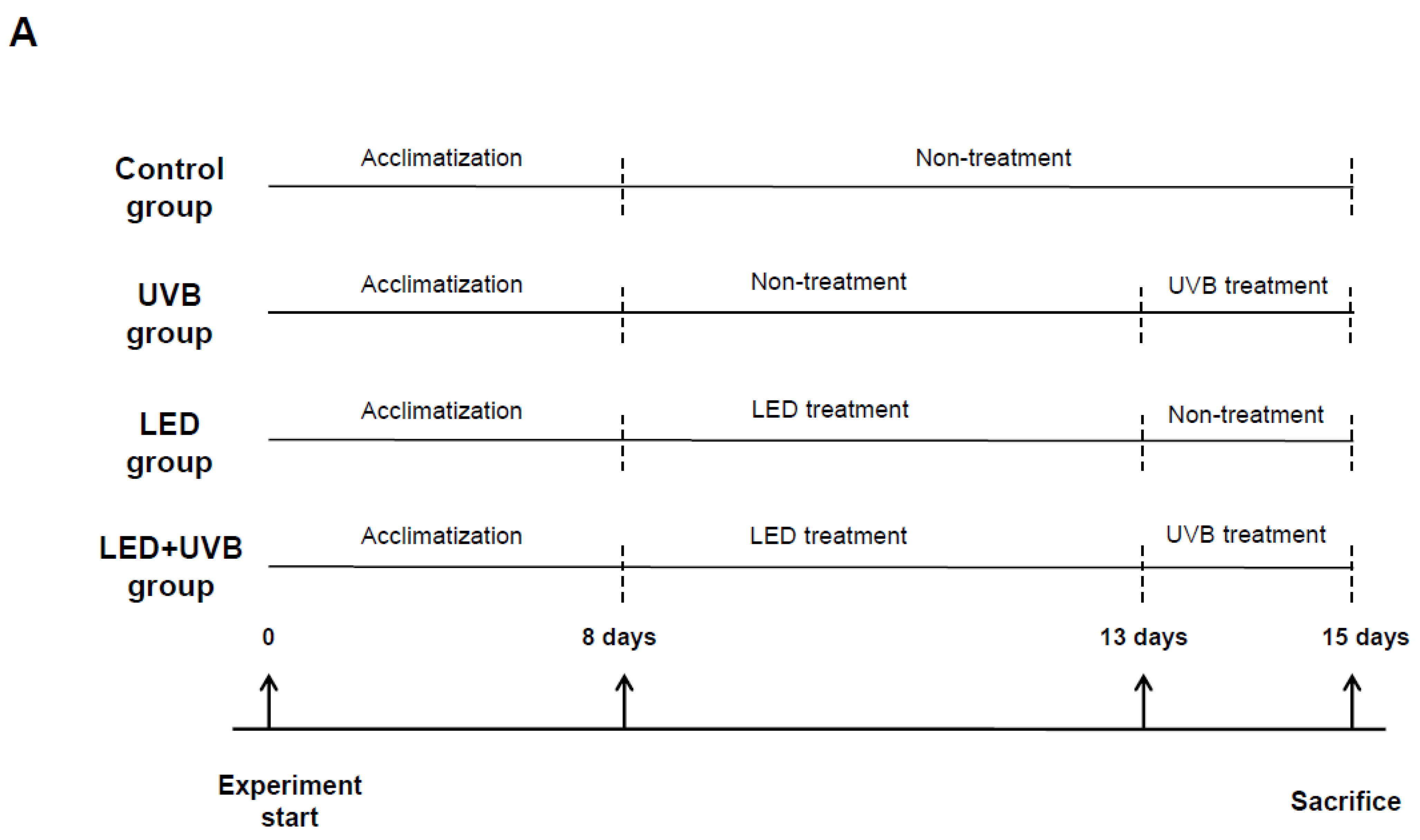
2.2. Experiment Design and Treatment
2.3. Hematoxylin and Eosin (H&E), Masson’s Trichrome, and Elastic Connective Tissue Staining
2.4. Western Blot Analysis
2.5. Statistical Analysis
3. Results
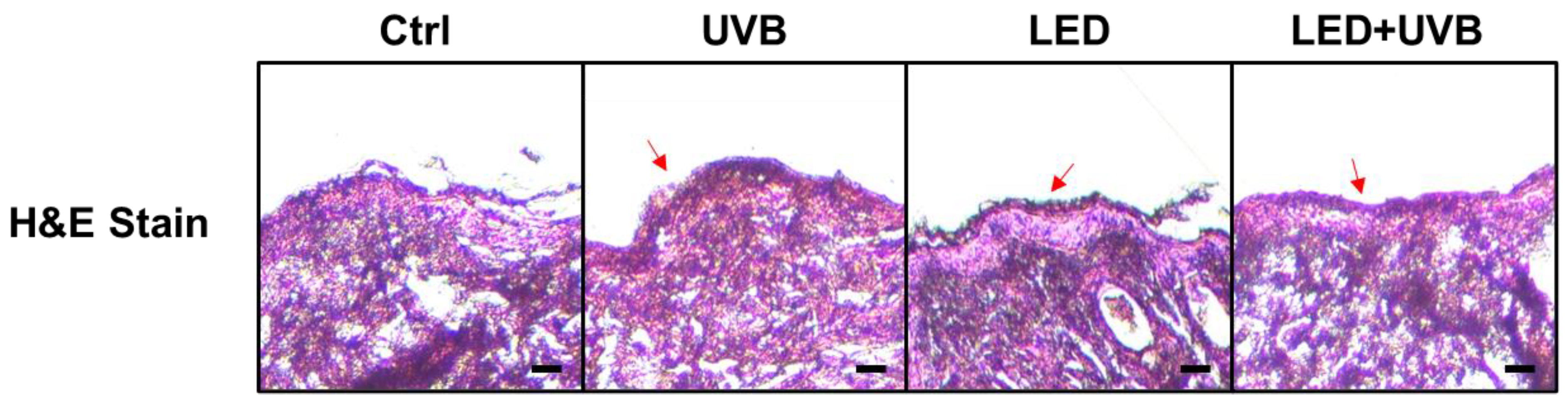
3.1. Histopathological Changes by LED Treatment in UVB-Irradiated Hairless Mice
3.2. Changes in Claudin-1 Expression on Dorsal Skin by LED Treatment in UVB-Irradiated Hairless Mice
3.3. Changes in Nrf-2 and HO-1 Expression in the Dorsal Skin by LED Treatment in UVB-Irradiated Hairless Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Sarandy, M.M.; Gonçalves, R.V.; Valacchi, G. Cutaneous Redox Senescence. Biomedicines 2024, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin senescence: Mechanisms and impact on whole-body aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [PubMed]
- Nanz, L.; Keim, U.; Katalinic, A.; Meyer, T.; Garbe, C.; Leiter, U. Epidemiology of Keratinocyte Skin Cancer with a Focus on Cutaneous Squamous Cell Carcinoma. Cancers 2024, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, C.; Jiang, G. Research progress on skin photoaging and oxidative stress. Postep. Dermatol. Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Z.; Xu, J. Application of adipose-derived stem cells in photoaging: Basic science and literature review. Stem Cell Res. Ther. 2020, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef]
- Pedić, L.; Pondeljak, N.; Šitum, M. Recent information on photoaging mechanisms and the preventive role of topical sunscreen products. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L.; Tomić, L.; Parać, E.; Pedić, L.; Lazić-Mosler, E. Key Factors in the Complex and Coordinated Network of Skin Keratinization: Their Significance and Involvement in Common Skin Conditions. Int. J. Mol. Sci. 2023, 25, 236. [Google Scholar] [CrossRef] [PubMed]
- Couturaud, V.; Le Fur, M.; Pelletier, M.; Granotier, F. Reverse skin aging signs by red light photobiomodulation. Skin Res. Technol. 2023, 29, e13391. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu Kassim, N.H.; Czernuszka, J.; Rahman, M.T. Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin. Skin Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: A comprehensive review. Heliyon 2023, 9, e13580. [Google Scholar] [CrossRef] [PubMed]
- Bäsler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 2016, 242, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Ballinger, E.; Choung, S.Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs. 2022, 20, 308. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Zheng, S.; Fang, M.; Kim, M.; Bellere, A.D.; Jeong, J.; Yi, T.H. Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials. Molecules 2023, 28, 1407. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E. Photobiomodulation: A review of the molecular evidence for low level light therapy. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1050–1060. [Google Scholar] [CrossRef]
- Rocha Mota, L.; Motta, L.J.; Duarte, I.D.S.; Horliana, A.C.R.T.; Silva, D.F.T.D.; Pavani, C. Efficacy of phototherapy to treat facial ageing when using a red versus an amber LED: A protocol for a randomised controlled trial. BMJ Open 2018, 8, e021419. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Austin, E.; Masub, N.; Kurtti, A.; George, C.; Jagdeo, J. Home-based devices in dermatology: A systematic review of safety and efficacy. Arch. Dermatol. Res. 2022, 314, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Yoon, K.; Lee, D.H.; Lee, Y.S.; Chung, J.H.; Park, G. Effects of 20-hydroxyecdysone on UVB-induced photoaging in hairless mice. Biomed. Pharmacother. 2023, 164, 114899. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Yoon, K.N.; Chung, J.H.; Lee, Y.S.; Lee, D.H.; Park, G. Chronic Ultraviolet Irradiation to the Skin Dysregulates Adrenal Medulla and Dopamine Metabolism In Vivo. Antioxidants 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, L.; Wang, X.; Li, X.; Xiong, W.; Zhang, X.; Zhang, Y.; Han, L.; Cao, K.; Chen, X.; et al. UVB irradiation differential regulate miRNAs expression in skin photoaging. An. Bras. Dermatol. 2022, 97, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Bae, I.Y. White-spotted flower chafer (Protaetia brevitarsis) ameliorates inflammatory responses in LPS-stimulated RAW 264.7 macrophages. J. Insects Food Feed 2023, 9, 1037–1046. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The role of cellular senescence in skin aging and age-related skin pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Yang, J.W.; Fan, G.B.; Tan, F.; Kong, H.M.; Liu, Q.; Zou, Y.; Tan, Y.M. The role and safety of UVA and UVB in UV-induced skin erythema. Front. Med. 2023, 10, 1163697. [Google Scholar] [CrossRef]
- Hajialiasgary Najafabadi, A.; Soheilifar, M.H.; Masoudi-Khoram, N. Exosomes in skin photoaging: Biological functions and therapeutic opportunity. Cell Commun. Signal. 2024, 22, 32. [Google Scholar] [CrossRef]
- Bai, G.L.; Wang, P.; Huang, X.; Wang, Z.Y.; Cao, D.; Liu, C.; Liu, Y.Y.; Li, R.L.; Chen, A.J. Rapamycin Protects Skin Fibroblasts From UVA-Induced Photoaging by Inhibition of p53 and Phosphorylated HSP27. Front. Cell Dev. Biol. 2021, 9, 633331. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Amin, S.; Russell, B.A.; Phelps, R.; Kellett, N.; Reilly, L.A. Combined 633-nm and 830-nm led treatment of photoaging skin. J. Drugs Dermatol. 2006, 5, 748–753. [Google Scholar]
- Glass, G.E. Photobiomodulation: The Clinical Applications of Low-Level Light Therapy. Aesthet. Surg. J. 2021, 41, 723–738. [Google Scholar] [CrossRef]
- Huang, A.; Nguyen, J.K.; Ho, D.; Jagdeo, J. Light Emitting Diode Phototherapy for Skin Aging. J. Drugs Dermatol. 2020, 19, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sorbellini, E.; Rucco, M.; Rinaldi, F. Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: An update. Lasers Med. Sci. 2018, 33, 1431–1439. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthet. Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef] [PubMed]
- Trębacz, H.; Barzycka, A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef]
- Kim, M.S.; Chun, K.E.; Lee, D.K.; Song, S.H. Evaluation of the Efficacy of an Elastin-Inducing Composition Containing Amino Acids, Copper, and Hyaluronic Acid: Results of an Open Single-Center Clinical Trial Study. Cosmetics 2022, 9, 51. [Google Scholar] [CrossRef]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: Over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Meng, X.; Guo, Z. Elastin Structure, Synthesis, Regulatory Mechanism and Relationship with Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 596702. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Lee, S.G.; Ham, S.; Jung, I.; Suk, J.; Lee, J.H. Exploring the Safety and Efficacy of Organic Light-Emitting Diode in Skin Rejuvenation and Wound Healing. Yonsei Med. J. 2024, 65, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Champagne, A.M.; Muñoz-Garcia, A.; Shtayyeh, T.; Tieleman, B.I.; Hegemann, A.; Clement, M.E.; Williams, J.B. Lipid composition of the stratum corneum and cutaneous water loss in birds along an aridity gradient. J. Exp. Biol. 2012, 215, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Kim, H.S. Interaction between the microbiota and the skin barrier in aging skin: A comprehensive review. Front. Physiol. 2024, 15, 1322205. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; He, J.; Zhang, Y.; He, R.; Zhang, X. Comprehensive functional evaluation of a novel collagen for the skin protection in human fibroblasts and keratinocytes. Biosci. Biotechnol. Biochem. 2023, 87, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Barakat, M.; Chen, D.; Chen, L. Bicellular Tight Junctions and Wound Healing. Int. J. Mol. Sci. 2018, 19, 3862. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Shin, D.H.; Lee, D.; Kang, S.M.; Seok, J.H.; Kang, H.Y.; Jeung, E.B. Expression of claudins, occludin, junction adhesion molecule A and zona occludens 1 in canine organs. Mol. Med. Rep. 2016, 14, 3697–3703. [Google Scholar] [CrossRef]
- Chen, T.C.; Chang, S.W. Non-lethal exposure to short-wavelength light-emitting diodes modulates tight-junction structure in human corneal epithelial cells via cAMP-dependent signaling. J. Photochem. Photobiol. B 2024, 252, 112869. [Google Scholar] [CrossRef]
- de Paula-Silva, M.; Broering, M.F.; Scharf, P.; da Rocha, G.H.O.; Farsky, S.; Lino-Dos-Santos-Franco, A. Red light-emitting diode treatment improves tissue recovery in DSS-induced colitis in mice. J. Photochem. Photobiol. B 2020, 212, 112018. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [PubMed]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Seol, S.I.; Kang, I.S.; Lee, J.S.; Lee, J.K.; Kim, C. Taurine Chloramine-Mediated Nrf2 Activation and HO-1 Induction Confer Protective Effects in Astrocytes. Antioxidants 2024, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Galicia-Moreno, M.; Lucano-Landeros, S.; Monroy-Ramirez, H.C.; Silva-Gomez, J.; Gutierrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Roles of Nrf2 in Liver Diseases: Molecular, Pharmacological, and Epigenetic Aspects. Antioxidants 2020, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Shin, D.; Lee, S.; Huang, Y.H.; Lim, H.W.; Lee, Y.; Jang, K.; Cho, Y.; Park, S.J.; Kim, D.D.; Lim, C.J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018, 56, 176–182. [Google Scholar] [CrossRef]
- Yi, R.; Zhang, J.; Sun, P.; Qian, Y.; Zhao, X. Protective Effects of Kuding Tea (Ilex kudingcha C. J. Tseng) Polyphenols on UVB-Induced Skin Aging in SKH1 Hairless Mice. Molecules 2019, 24, 1016. [Google Scholar] [CrossRef] [PubMed]
- Orhan, C.; Gencoglu, H.; Tuzcu, M.; Sahin, N.; Ozercan, I.H.; Morde, A.A.; Padigaru, M.; Sahin, K. Allyl isothiocyanate attenuates LED light-induced retinal damage in rats: Exploration for the potential molecular mechanisms. Cutan. Ocul. Toxicol. 2021, 40, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Tsai, S.C.; Yu, M.C.; Lin, Y.F.; Chen, C.C.; Chang, P.C. Light-emitting diode irradiation promotes donor site wound healing of the free gingival graft. J. Periodontol. 2015, 86, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Guermonprez, C.; Peno-Mazzarino, L.; Lati, E.; Rousseaud, A.; Declercq, L.; Kerdine-Römer, S. Photobiomodulation Controls Keratinocytes Inflammatory Response through Nrf2 and Reduces Langerhans Cells Activation. Antioxidants 2023, 12, 766. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.-C.; Ahn, S.; Shin, K.-O.; Lee, J.B.; Hwang, H.-J.; Choi, Y.-J. Protective Effect of Red Light-Emitting Diode against UV-B Radiation-Induced Skin Damage in SKH:HR-2 Hairless Mice. Curr. Issues Mol. Biol. 2024, 46, 5655-5667. https://doi.org/10.3390/cimb46060338
Cho E-C, Ahn S, Shin K-O, Lee JB, Hwang H-J, Choi Y-J. Protective Effect of Red Light-Emitting Diode against UV-B Radiation-Induced Skin Damage in SKH:HR-2 Hairless Mice. Current Issues in Molecular Biology. 2024; 46(6):5655-5667. https://doi.org/10.3390/cimb46060338
Chicago/Turabian StyleCho, Eun-Chae, Surin Ahn, Kyung-Ok Shin, Joon Byeong Lee, Hyo-Jeong Hwang, and Yean-Jung Choi. 2024. "Protective Effect of Red Light-Emitting Diode against UV-B Radiation-Induced Skin Damage in SKH:HR-2 Hairless Mice" Current Issues in Molecular Biology 46, no. 6: 5655-5667. https://doi.org/10.3390/cimb46060338
APA StyleCho, E.-C., Ahn, S., Shin, K.-O., Lee, J. B., Hwang, H.-J., & Choi, Y.-J. (2024). Protective Effect of Red Light-Emitting Diode against UV-B Radiation-Induced Skin Damage in SKH:HR-2 Hairless Mice. Current Issues in Molecular Biology, 46(6), 5655-5667. https://doi.org/10.3390/cimb46060338





