Is the Hedgehog Pathway Involved in the Pathophysiology of Schizophrenia? A Systematic Review of Current Evidence of Neural Molecular Correlates and Perspectives on Drug Development
Abstract
1. Introduction
2. Methods
3. Results
3.1. Included Studies
3.2. Causes of Heterogeneity among Study Results
3.3. Causes of Heterogeneity among Study Results
4. Discussion
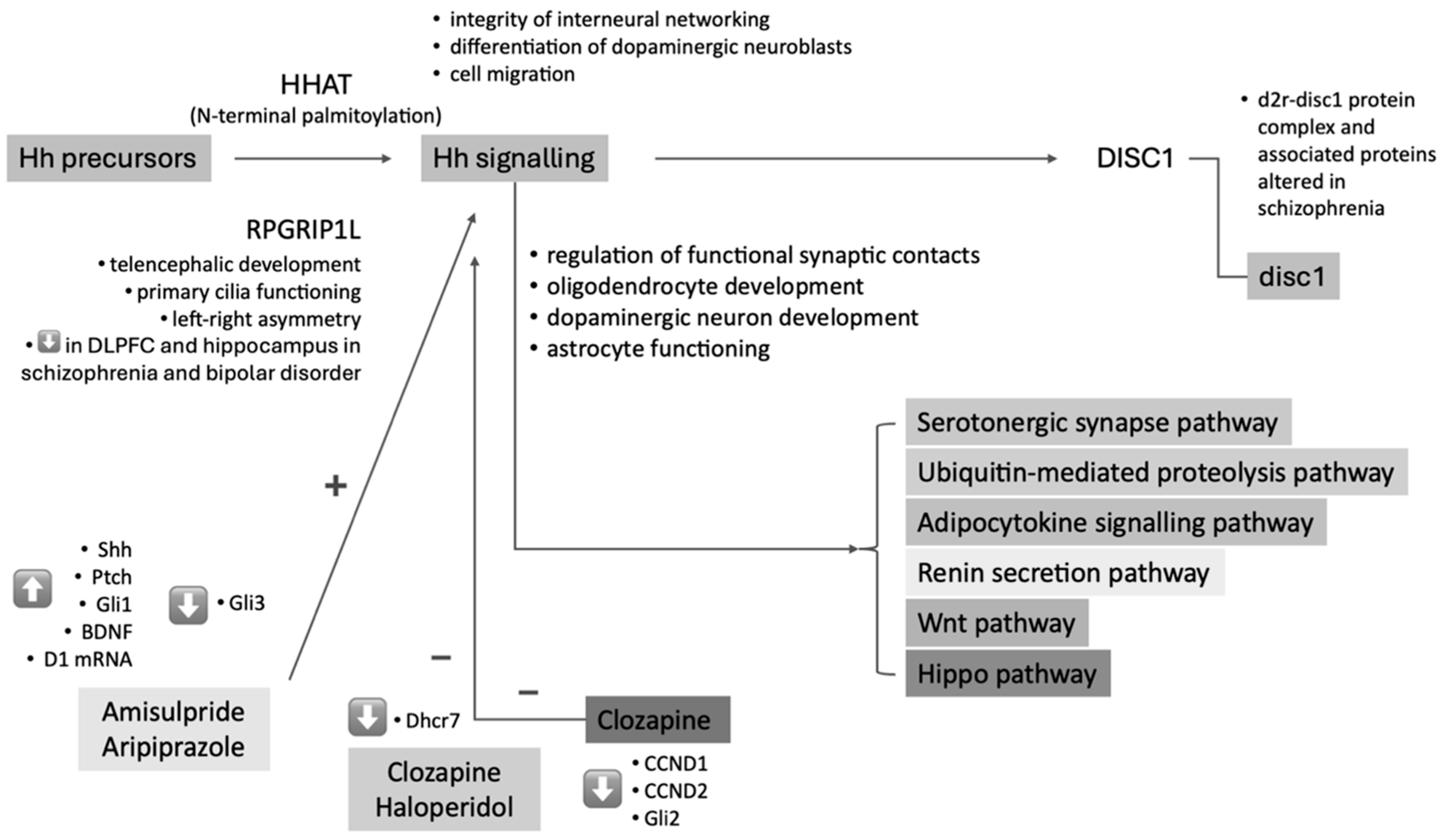
4.1. Hh Signalling and Schizophrenia Pathophysiology
4.2. Hh Pathway and Antipsychotics
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Karam, C.S.; Ballon, J.S.; Bivens, N.M.; Freyberg, Z.; Girgis, R.R.; Lizardi-Ortiz, J.E.; Markx, S.; Lieberman, J.A.; Javitch, J.A. Signaling Pathways in Schizophrenia: Emerging Targets and Therapeutic Strategies. Trends Pharmacol. Sci. 2010, 31, 381–390. [Google Scholar] [CrossRef]
- Marigo, V.; Davey, R.A.; Zuo, Y.; Cunningham, J.M.; Tabin, C.J. Biochemical Evidence That Patched Is the Hedgehog Receptor. Nature 1996, 384, 176–179. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, C.; Jiang, J. Hedgehog Regulates Smoothened Activity by Inducing a Conformational Switch. Nature 2007, 450, 252–258. [Google Scholar] [CrossRef]
- Deshpande, I.; Liang, J.; Hedeen, D.; Roberts, K.J.; Zhang, Y.; Ha, B.; Latorraca, N.R.; Faust, B.; Dror, R.O.; Beachy, P.A.; et al. Smoothened Stimulation by Membrane Sterols Drives Hedgehog Pathway Activity. Nature 2019, 571, 284–288. [Google Scholar] [CrossRef]
- Hui, C.-C.; Angers, S. Gli Proteins in Development and Disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 513–537. [Google Scholar] [CrossRef]
- Zhang, Y.; Beachy, P.A. Cellular and Molecular Mechanisms of Hedgehog Signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687. [Google Scholar] [CrossRef]
- Briscoe, J.; Thérond, P.P. The Mechanisms of Hedgehog Signalling and Its Roles in Development and Disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Shim, S.; Goyal, R.; Panoutsopoulos, A.A.; Balashova, O.A.; Lee, D.; Borodinsky, L.N. Calcium Dynamics at the Neural Cell Primary Cilium Regulate Hedgehog Signaling-Dependent Neurogenesis in the Embryonic Neural Tube. Proc. Natl. Acad. Sci. USA 2023, 120, e2220037120. [Google Scholar] [CrossRef]
- Panaccione, I.; Napoletano, F.; Forte, A.M.; Kotzalidis, G.D.; Del Casale, A.; Rapinesi, C.; Brugnoli, C.; Serata, D.; Caccia, F.; Cuomo, I.; et al. Neurodevelopment in Schizophrenia: The Role of the Wnt Pathways. Curr. Neuropharmacol. 2013, 11, 535–558. [Google Scholar] [CrossRef]
- Hoseth, E.Z.; Krull, F.; Dieset, I.; Mørch, R.H.; Hope, S.; Gardsjord, E.S.; Steen, N.E.; Melle, I.; Brattbakk, H.-R.; Steen, V.M.; et al. Exploring the Wnt Signaling Pathway in Schizophrenia and Bipolar Disorder. Transl. Psychiatry 2018, 8, 55. [Google Scholar] [CrossRef]
- Hoseth, E.Z.; Krull, F.; Dieset, I.; Mørch, R.H.; Hope, S.; Gardsjord, E.S.; Steen, N.E.; Melle, I.; Brattbakk, H.-R.; Steen, V.M.; et al. Attenuated Notch Signaling in Schizophrenia and Bipolar Disorder. Sci. Rep. 2018, 8, 5349. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.A.; Melka, M.G.; Gui, J.L.; Gallo, A.J.; O’Reilly, R.L.; Singh, S.M. Post-Zygotic Genomic Changes in Glutamate and Dopamine Pathway Genes May Explain Discordance of Monozygotic Twins for Schizophrenia. Clin. Transl. Med. 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Machold, R.; Hayashi, S.; Rutlin, M.; Muzumdar, M.D.; Nery, S.; Corbin, J.G.; Gritli-Linde, A.; Dellovade, T.; Porter, J.A.; Rubin, L.L.; et al. Sonic Hedgehog Is Required for Progenitor Cell Maintenance in Telencephalic Stem Cell Niches. Neuron 2003, 39, 937–950. [Google Scholar] [CrossRef]
- Palma, V.; Lim, D.A.; Dahmane, N.; Sánchez, P.; Brionne, T.C.; Herzberg, C.D.; Gitton, Y.; Carleton, A.; Alvarez-Buylla, A.; Ruiz i Altaba, A. Sonic Hedgehog Controls Stem Cell Behavior in the Postnatal and Adult Brain. Development 2005, 132, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Younsi, A.; Zheng, G.; Tail, M.; Harms, A.-K.; Roth, J.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Sonic Hedgehog Modulates the Inflammatory Response and Improves Functional Recovery after Spinal Cord Injury in a Thoracic Contusion-Compression Model. Eur. Spine J. 2021, 30, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Yousefifard, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Baikpour, M.; Safari, S.; Saadat, S.; Moghadas Jafari, A.; Asady, H.; Razavi Tousi, S.M.T.; Hosseini, M. Neural Stem/Progenitor Cell Transplantation for Spinal Cord Injury Treatment; A Systematic Review and Meta-Analysis. Neuroscience 2016, 322, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-D.; Yang, J.-L.; Hwang, W.-C.; Yang, D.-I. Emerging Roles of Sonic Hedgehog in Adult Neurological Diseases: Neurogenesis and Beyond. Int. J. Mol. Sci. 2018, 19, 2423. [Google Scholar] [CrossRef] [PubMed]
- Benallegue, N.; Kebir, H.; Kapoor, R.; Crockett, A.; Li, C.; Cheslow, L.; Abdel-Hakeem, M.S.; Gesualdi, J.; Miller, M.C.; Wherry, E.J.; et al. The Hedgehog Pathway Suppresses Neuropathogenesis in CD4 T Cell-Driven Inflammation. Brain 2021, 144, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Tail, M.; Zhang, H.; Zheng, G.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K.; Younsi, A. The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro. Cells 2022, 11, 736. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Abdelfattah, A.M.; Abuelezz, S.A.; Hendawy, N.; Negm, E.A.; Nawishy, S.A.E.K.; Khalil, A.M.M. Sonic Hedgehog Pathway as a New Target of Atypical Antipsychotics: Revisiting of Amisulpride and Aripiprazole Effects in a Rat Model of Schizophrenia. Life Sci. 2023, 316, 121366. [Google Scholar] [CrossRef]
- Betcheva, E.T.; Yosifova, A.G.; Mushiroda, T.; Kubo, M.; Takahashi, A.; Karachanak, S.K.; Zaharieva, I.T.; Hadjidekova, S.P.; Dimova, I.I.; Vazharova, R.V.; et al. Whole-Genome-Wide Association Study in the Bulgarian Population Reveals HHAT as Schizophrenia Susceptibility Gene. Psychiatr. Genet. 2013, 23, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.J.; Cunliffe, V.T.; Roy, S.; Wood, J.D. Sonic Hedgehog Functions Upstream of Disrupted-in-Schizophrenia 1 (Disc1): Implications for Mental Illness. Biol. Open 2015, 4, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Lauth, M.; Bergström, A.; Shimokawa, T.; Tostar, U.; Jin, Q.; Fendrich, V.; Guerra, C.; Barbacid, M.; Toftgård, R. DYRK1B-Dependent Autocrine-to-Paracrine Shift of Hedgehog Signaling by Mutant RAS. Nat. Struct. Mol. Biol. 2010, 17, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bousman, C.A.; Pantelis, C.; Skafidas, E.; Zhang, D.; Yue, W.; Everall, I.P. Pathway-Wide Association Study Identifies Five Shared Pathways Associated with Schizophrenia in Three Ancestral Distinct Populations. Transl. Psychiatry 2017, 7, e1037. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; Shimazawa, M.; Ohuchi, K.; Kuse, Y.; Nakamura, S.; Hara, H. Impaired Cerebellar Development in Mice Overexpressing V.G.F. Neurochem. Res. 2019, 44, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Panizzutti, B.; Bortolasci, C.C.; Spolding, B.; Kidnapillai, S.; Connor, T.; Richardson, M.F.; Truong, T.T.T.; Liu, Z.S.J.; Morris, G.; Gray, L.; et al. Transcriptional Modulation of the Hippo Signaling Pathway by Drugs Used to Treat Bipolar Disorder and Schizophrenia. Int. J. Mol. Sci. 2021, 22, 7164. [Google Scholar] [CrossRef] [PubMed]
- Reble, E.; Feng, Y.; Wigg, K.G.; Barr, C.L. DNA Variant in the RPGRIP1L Gene Influences Alternative Splicing. Mol. Neuropsychiatry 2020, 5, 97–106. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Jiang, Y.; Benz, T.L.; Long, S.B. Substrate and Product Complexes Reveal Mechanisms of Hedgehog Acylation by HHAT. Science 2021, 372, 1215–1219. [Google Scholar] [CrossRef]
- Chamoun, Z.; Mann, R.K.; Nellen, D.; von Kessler, D.P.; Bellotto, M.; Beachy, P.A.; Basler, K. Skinny Hedgehog, an Acyltransferase Required for Palmitoylation and Activity of the Hedgehog Signal. Science 2001, 293, 2080–2084. [Google Scholar] [CrossRef]
- Maynard, T.M.; Sikich, L.; Lieberman, J.A.; LaMantia, A.S. Neural Development, Cell-Cell Signaling, and the “Two-Hit” Hypothesis of Schizophrenia. Schizophr. Bull. 2001, 27, 457–476. [Google Scholar] [CrossRef]
- Hovatta, I.; Varilo, T.; Suvisaari, J.; Terwilliger, J.D.; Ollikainen, V.; Arajärvi, R.; Juvonen, H.; Kokko-Sahin, M.L.; Väisänen, L.; Mannila, H.; et al. A Genomewide Screen for Schizophrenia Genes in an Isolated Finnish Subpopulation, Suggesting Multiple Susceptibility Loci. Am. J. Hum. Genet. 1999, 65, 1114–1124. [Google Scholar] [CrossRef]
- Takeshita, M.; Yamada, K.; Hattori, E.; Iwayama, Y.; Toyota, T.; Iwata, Y.; Tsuchiya, K.J.; Sugihara, G.; Hashimoto, K.; Watanabe, H.; et al. Genetic Examination of the PLXNA2 Gene in Japanese and Chinese People with Schizophrenia. Schizophr. Res. 2008, 99, 359–364. [Google Scholar] [CrossRef]
- Betcheva, E.T.; Mushiroda, T.; Takahashi, A.; Kubo, M.; Karachanak, S.K.; Zaharieva, I.T.; Vazharova, R.V.; Dimova, I.I.; Milanova, V.K.; Tolev, T.; et al. Case-Control Association Study of 59 Candidate Genes Reveals the DRD2 SNP Rs6277 (C957T) as the Only Susceptibility Factor for Schizophrenia in the Bulgarian Population. J. Hum. Genet. 2009, 54, 98–107. [Google Scholar] [CrossRef]
- Andreu-Cervera, A.; Anselme, I.; Karam, A.; Laclef, C.; Catala, M.; Schneider-Maunoury, S. The Ciliopathy Gene Ftm/Rpgrip1l Controls Mouse Forebrain Patterning via Region-Specific Modulation of Hedgehog/Gli Signaling. J. Neurosci. 2019, 39, 2398–2415. [Google Scholar] [CrossRef]
- Wang, L.; De Solis, A.J.; Goffer, Y.; Birkenbach, K.E.; Engle, S.E.; Tanis, R.; Levenson, J.M.; Li, X.; Rausch, R.; Purohit, M.; et al. Ciliary Gene RPGRIP1L Is Required for Hypothalamic Arcuate Neuron Development. JCI Insight 2019, 4, e123337. [Google Scholar] [CrossRef]
- Andreu-Cervera, A.; Catala, M.; Schneider-Maunoury, S. Cilia, Ciliopathies and Hedgehog-Related Forebrain Developmental Disorders. Neurobiol. Dis. 2021, 150, 105236. [Google Scholar] [CrossRef]
- Pisanu, C.; Williams, M.J.; Ciuculete, D.M.; Olivo, G.; Del Zompo, M.; Squassina, A.; Schiöth, H.B. Evidence That Genes Involved in Hedgehog Signaling Are Associated with Both Bipolar Disorder and High BMI. Transl. Psychiatry 2019, 9, 315. [Google Scholar] [CrossRef]
- Sullivan, P.F. Questions about DISC1 as a Genetic Risk Factor for Schizophrenia. Mol. Psychiatry 2013, 18, 1050–1052. [Google Scholar] [CrossRef]
- Wang, J.; Su, P.; Yang, J.; Xu, L.; Yuan, A.; Li, C.; Zhang, T.; Dong, F.; Zhou, J.; Samsom, J.; et al. The D2R-DISC1 protein complex and associated proteins are altered in schizophrenia and normalised with antipsychotic treatment. J. Psychiatry Neurosci. 2022, 47, E134–E147. [Google Scholar] [CrossRef]
- Maniatis, T. A Ubiquitin Ligase Complex Essential for the NF-kappaB, Wnt/Wingless, and Hedgehog Signaling Pathways. Genes Dev. 1999, 13, 505–510. [Google Scholar] [CrossRef]
- Angot, E.; Loulier, K.; Nguyen-Ba-Charvet, K.T.; Gadeau, A.-P.; Ruat, M.; Traiffort, E. Chemoattractive Activity of Sonic Hedgehog in the Adult Subventricular Zone Modulates the Number of Neural Precursors Reaching the Olfactory Bulb. Stem Cells 2008, 26, 2311–2320. [Google Scholar] [CrossRef]
- Hor, C.H.H.; Tang, B.L. Sonic Hedgehog as a Chemoattractant for Adult NPCs. Cell Adhes. Migr. 2010, 4, 1–3. [Google Scholar] [CrossRef]
- Bernstein, H.-G.; Steiner, J.; Guest, P.C.; Dobrowolny, H.; Bogerts, B. Glial Cells as Key Players in Schizophrenia Pathology: Recent Insights and Concepts of Therapy. Schizophr. Res. 2015, 161, 4–18. [Google Scholar] [CrossRef]
- Takahashi, N.; Sakurai, T.; Davis, K.L.; Buxbaum, J.D. Linking Oligodendrocyte and Myelin Dysfunction to Neurocircuitry Abnormalities in Schizophrenia. Prog. Neurobiol. 2011, 93, 13–24. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Vogel, M.W. Desire, Disease, and the Origins of the Dopaminergic System. Schizophr. Bull. 2008, 34, 212–219. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Du, J.; Sun, J.; Baranova, A.; Zhang, F. BDNF Gene’s Role in Schizophrenia: From Risk Allele to Methylation Implications. Front. Psychiatry 2020, 11, 564277. [Google Scholar] [CrossRef]
- Rizos, E.N.; Siafakas, N.; Stefanis, N.; Douzenis, A.; Kontaxakis, V.; Laskos, E.; Kastania, A.; Zoumbourlis, V.; Lykouras, L. Association of Serum BDNF and Val66met Polymorphism of the Brain-Derived Neurotrophic Factor in a Sample of First Psychotic Episode Patients. Psychiatriki 2009, 20, 297–304. [Google Scholar]
- Ugbode, C.I.; Smith, I.; Whalley, B.J.; Hirst, W.D.; Rattray, M. Sonic Hedgehog Signalling Mediates Astrocyte Crosstalk with Neurons to Confer Neuroprotection. J. Neurochem. 2017, 142, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.A.; Fu, M.; Garcia, A.D.R. Sonic Hedgehog Signaling in Astrocytes. Cell. Mol. Life Sci. 2021, 78, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Notter, T. Astrocytes in Schizophrenia. Brain Neurosci. Adv. 2021, 5, 23982128211009148. [Google Scholar] [CrossRef]
- Memi, F.; Zecevic, N.; Radonjić, N. Multiple Roles of Sonic Hedgehog in the Developing Human Cortex Are Suggested by Its Widespread Distribution. Brain Struct. Funct. 2018, 223, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Healey, K.L.; Sepulveda-Orengo, M.T.; Reissner, K.J. Astroglial Correlates of Neuropsychiatric Disease: From Astrocytopathy to Astrogliosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 126–146. [Google Scholar] [CrossRef]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Sologova, S.S.; Mukhortova, P.; Levushkin, D.; Somasundaram, S.G.; Kirkland, C.E.; Bachurin, S.O.; Aliev, G. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front. Pharmacol. 2019, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Suhara, T.; Suzuki, K.; Kobayashi, K.; Inoue, O.; Terasaki, O.; Someya, Y.; Sassa, T.; Sudo, Y.; Matsushima, E.; et al. Decreased Prefrontal Dopamine D1 Receptors in Schizophrenia Revealed by PET. Nature 1997, 385, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ma, S.; Jia, C.; Su, Y.; Yang, S.; Zhou, K.; Liu, Y.; Cheng, J.; Lu, D.; Fan, L.; et al. Sonic Hedgehog Is a Regulator of Extracellular Glutamate Levels and Epilepsy. EMBO Rep. 2016, 17, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.; Kruse, M.S.; Forssberg, H.; Brismar, H.; Greengard, P.; Aperia, A. Selective Up-Regulation of Dopamine D1 Receptors in Dendritic Spines by NMDA Receptor Activation. Proc. Natl. Acad. Sci. USA 2002, 99, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T. The Glutamatergic Dysfunction Hypothesis for Schizophrenia. Harv. Rev. Psychiatry 1996, 3, 241–253. [Google Scholar] [CrossRef]
- Lauth, M.; Rohnalter, V.; Bergström, A.; Kooshesh, M.; Svenningsson, P.; Toftgård, R. Antipsychotic Drugs Regulate Hedgehog Signaling by Modulation of 7-Dehydrocholesterol Reductase Levels. Mol. Pharmacol. 2010, 78, 486–496. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Litingtung, Y.; Chiang, C. Cholesterol Modification Restricts the Spread of Shh Gradient in the Limb Bud. Proc. Natl. Acad. Sci. USA 2006, 103, 6548–6553. [Google Scholar] [CrossRef]
- Steen, V.M.; Skrede, S.; Polushina, T.; López, M.; Andreassen, O.A.; Fernø, J.; Hellard, S.L. Genetic Evidence for a Role of the SREBP Transcription System and Lipid Biosynthesis in Schizophrenia and Antipsychotic Treatment. Eur. Neuropsychopharmacol. 2017, 27, 589–598. [Google Scholar] [CrossRef]
- Ozcelik-Eroglu, E.; Ertugrul, A.; Oguz, K.K.; Has, A.C.; Karahan, S.; Yazici, M.K. Effect of Clozapine on White Matter Integrity in Patients with Schizophrenia: A Diffusion Tensor Imaging Study. Psychiatry Res. 2014, 223, 226–235. [Google Scholar] [CrossRef]
- Ding, M.; Wang, X. Antagonism between Hedgehog and Wnt signaling pathways regulates tumorigenicity. Oncol. Lett. 2017, 14, 6327–6333. [Google Scholar] [CrossRef]
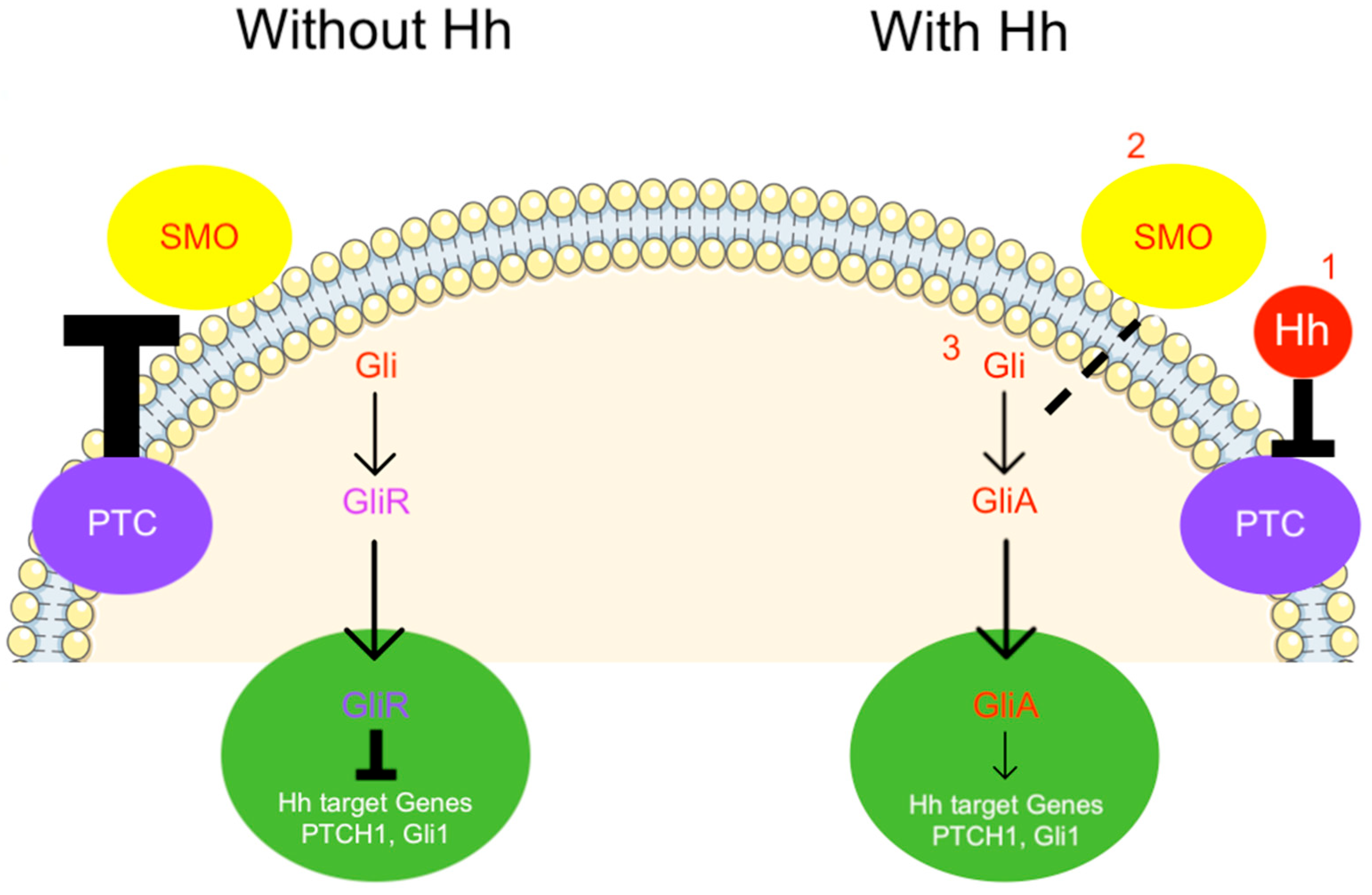

| Study | Type of Study | Sample | Main Results |
|---|---|---|---|
| Abdelfattah et al., 2023 [22] | Animal study | 60 male Wistar rats grouped in controls and socially isolated, treated with amisulpride or aripiprazole. | Social isolation-induced cognitive dysfunction alongside disorganised Shh-pathway protein levels and increased GFAP-stained astrocytes. Treatment reversed these effects, evidenced by elevated Shh, Ptch-1, Smo, GLI-1, dopamine-1 receptors, and BDNF, and decreased GLI-3 protein, GFAP immune reactivity in astrocytes, and inflammatory markers. |
| Betcheva et al., 2013 [23] | GWAS on 554,496 SNPs | 188 patients with schizophrenia and 376 controls | Significant association between schizophrenia and the intronic SNP rs7527939 in the HHAT gene. |
| Boyd et al., 2015 [24] | Animal study | Genetic and chemical modulation of Shh signalling on disc1 expression in the zebrafish embryo and double-labelling methods to characterise disc1-expressing cells in the hindbrain | The robust expression of disc1 in Olig2-positive midline progenitor cells is disrupted in Smo mutants, with Cyclopamine treatment mirroring the effects of disc1 knockdown on oligodendrocyte precursor cells specification by blocking disc1 expression. |
| Lauth et al., 2010 [25] | Animal study | NIH3T3 cells, ShhL2 cells, and Sufu (−/−) MEFs | Clozapine, chlorpromazine, haloperidol, and the antidepressant imipramine modulated DHCR7, influencing Hh signalling both in vitro and in vivo. |
| Liu et al., 2017 [26] | PWAS | 5033 patients with schizophrenia and 5332 controls across EA, AA and CH populations | Five pathways (serotonergic synapse, ubiquitin-mediated proteolysis, Hh, adipocytokine, and renin secretion) were associated with schizophrenia and shared among the three populations. Their respective SNP sets showed enrichment for SNPs with regulatory functions, indicating potential roles in genetic regulation across diverse populations. |
| Mizoguchi et al., 2019 [27] | Animal study | VGF-overexpressing mice | There were no changes in Shh signalling between Wild-type and VGF-overexpressing mice. |
| Panizzutti et al., 2021 [28] | Genome-wide mRNA expression study | NT2-N cells were treated with amisulpride, aripiprazole, clozapine, quetiapine, and risperidone. | Expression of genes in the Hh signalling pathway was decreased overall by clozapine and tended to be reduced by aripiprazole. Clozapine significantly reduced the expression of Gli2 and DCCN1 and showed a trend towards reducing the expression of CXCR4 and CCND1. |
| Reble et al., 2019 [29] | Post-mortem study | 7 patients with schizophrenia, 4 with bipolar disorder, and 79 controls | A common variant in RPGRIP1L, known as rs7203525 and involved in Hh signalling, has been identified to influence alternative splicing, increasing the inclusion of exon 20 of RPGRIP1L. A minigene assay combined with in vitro mutagenesis confirmed that this alternative splicing is directly linked to the alleles of this variant. The predominant RPGRIP1L isoform expressed in adult brains typically lacks exon 20. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Casale, A.; Modesti, M.N.; Gentile, G.; Guariglia, C.; Ferracuti, S.; Simmaco, M.; Borro, M. Is the Hedgehog Pathway Involved in the Pathophysiology of Schizophrenia? A Systematic Review of Current Evidence of Neural Molecular Correlates and Perspectives on Drug Development. Curr. Issues Mol. Biol. 2024, 46, 5322-5336. https://doi.org/10.3390/cimb46060318
Del Casale A, Modesti MN, Gentile G, Guariglia C, Ferracuti S, Simmaco M, Borro M. Is the Hedgehog Pathway Involved in the Pathophysiology of Schizophrenia? A Systematic Review of Current Evidence of Neural Molecular Correlates and Perspectives on Drug Development. Current Issues in Molecular Biology. 2024; 46(6):5322-5336. https://doi.org/10.3390/cimb46060318
Chicago/Turabian StyleDel Casale, Antonio, Martina Nicole Modesti, Giovanna Gentile, Cecilia Guariglia, Stefano Ferracuti, Maurizio Simmaco, and Marina Borro. 2024. "Is the Hedgehog Pathway Involved in the Pathophysiology of Schizophrenia? A Systematic Review of Current Evidence of Neural Molecular Correlates and Perspectives on Drug Development" Current Issues in Molecular Biology 46, no. 6: 5322-5336. https://doi.org/10.3390/cimb46060318
APA StyleDel Casale, A., Modesti, M. N., Gentile, G., Guariglia, C., Ferracuti, S., Simmaco, M., & Borro, M. (2024). Is the Hedgehog Pathway Involved in the Pathophysiology of Schizophrenia? A Systematic Review of Current Evidence of Neural Molecular Correlates and Perspectives on Drug Development. Current Issues in Molecular Biology, 46(6), 5322-5336. https://doi.org/10.3390/cimb46060318


_Kim.png)








