Identification of Key Hypolipidemic Components and Exploration of the Potential Mechanism of Total Flavonoids from Rosa sterilis Based on Network Pharmacology, Molecular Docking, and Zebrafish Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation for RS
2.2. Zebrafish Experimental Design
2.3. UPLC-Q-TOF-MS Analysis
2.4. Network Pharmacology Analysis of RS
2.5. Molecular Docking Analysis
2.6. Statistical Analyses
3. Results
3.1. Hypolipidemic Effects of RS
3.2. UPLC-Q-TOF-MS Analysis of RS
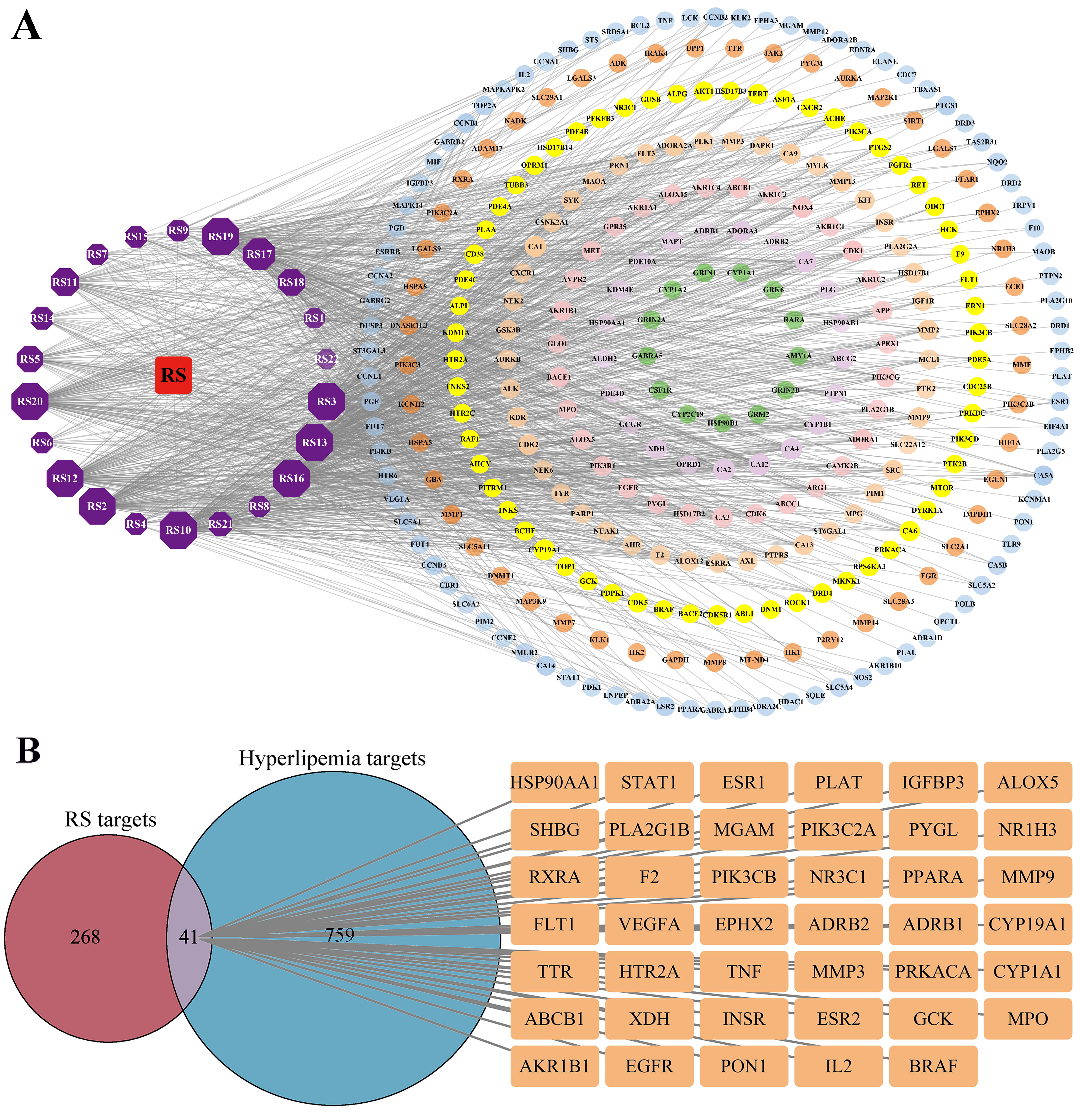
3.3. Identification of the Targets of RS
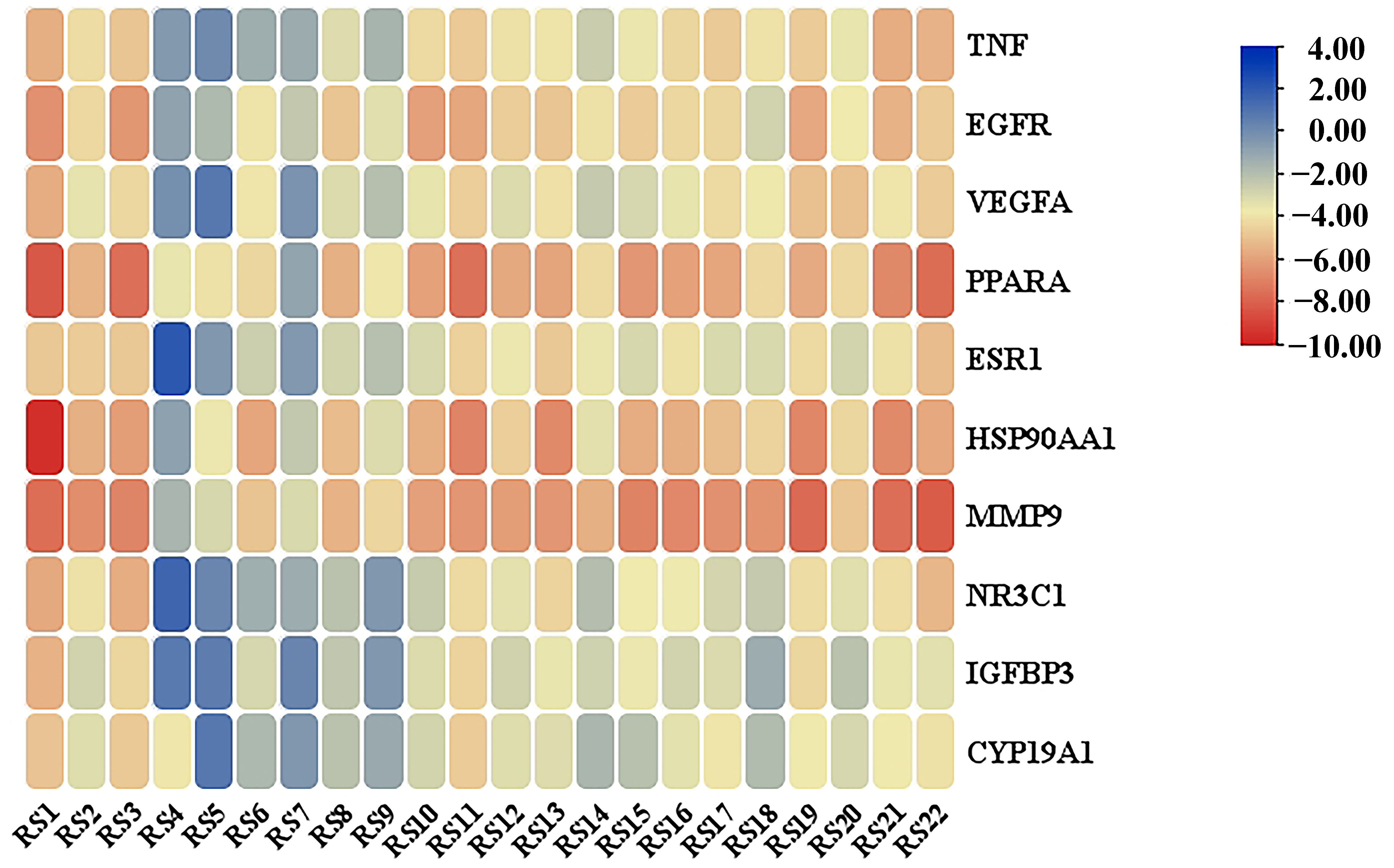
3.4. PPI Network Analysis
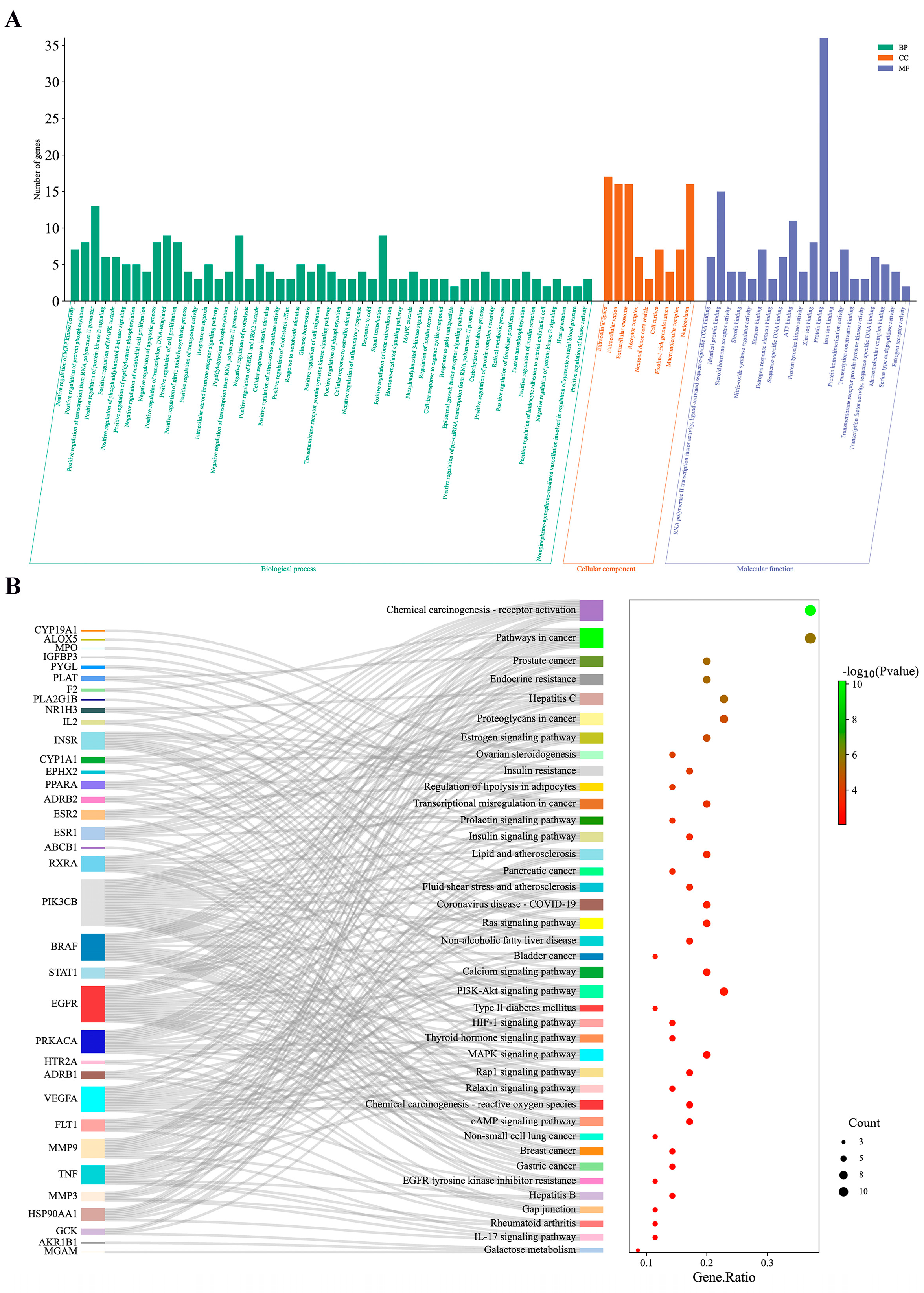
3.5. Enrichment Analysis of GO Functional and KEGG Pathway
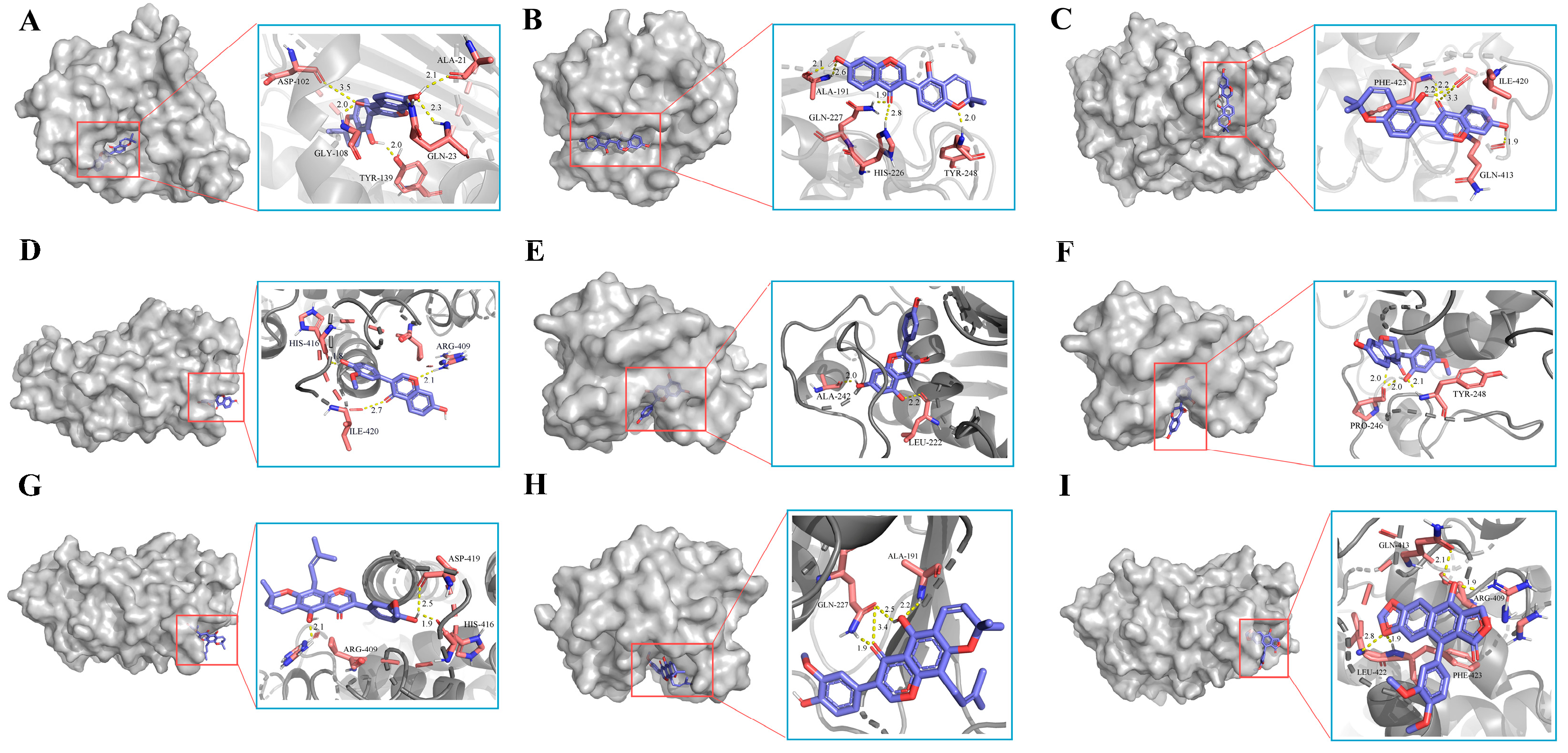
3.6. Molecular Docking Verification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: A scientific statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, C.M.; Cefalù, A.B.; Noto, D.; Averna, M.R. Role of nutraceuticals in hypolipidemic therapy. Front. Cardiovas. Med. 2015, 2, 22. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Xia, Y.; Xu, J.F.; Li, Q.Y.; Zhang, C.H.; Li, M.H. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol. 2020, 103, 304–320. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Zhang, X.; Yang, S.T. Nutraceuticals and functional foods in the management of hyperlipidemia. Crit. Rev. Food Sci. Nutr. 2014, 54, 1180–1201. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26, 17A–21A. [Google Scholar] [CrossRef]
- Zhang, T.T.; Jiang, J.G. Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert Opin. Investig. Drug. 2012, 21, 1625–1642. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Wu, P.H.; Han, S.C.H.; Wu, M.H. Beneficial effects of hydroalcoholic extract from Rosa roxburghii Tratt fruit on hyperlipidemia in high-fat-fed rats. Acta Cardiol. Sin. 2020, 36, 148–159. [Google Scholar] [CrossRef]
- Wen, X.P.; Pang, X.M.; Deng, X.X. Characterization of genetic relationships of Rosa roxburghii Tratt and its relatives using morphological traits, RAPD and AFLP markers. J. Hortic. Sci. Biotechnol. 2004, 79, 189–196. [Google Scholar] [CrossRef]
- He, J.Y.; Zhang, Y.H.; Ma, N.; Zhang, X.L.; Liu, M.H.; Fu, W.M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Du, L.J.; Hong, J.N.; Chen, Z.L.; Liu, H.J.; Li, S.S.; Xiao, X.; Yan, S.K. Molecular mechanism underlying the hypolipidemic effect of Shanmei capsule based on network pharmacology and molecular docking. Technol. Health Care 2021, 29, 239–256. [Google Scholar] [CrossRef]
- Barshir, R.; Fishilevich, S.; Iny-Stein, T.; Zelig, O.; Mazor, Y.; Guan-Golan, Y.; Safran, M.; Lancet, D. GeneCaRNA: A comprehensive gene-centric database of human non-coding RNAs in the GeneCards suite. J. Mol. Biol. 2021, 433, 166913. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Amberger, J.S.; Bocchini, C.; Scott, A.F.; Rasmussen, S.A. Online Mendelian inheritance in man (OMIM®): Victor McKusick’s magnum opus. Am. J. Med. Genet. A 2021, 185, 3259–3265. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Jiang, X.L.; Zhu, Y.; Ma, G.F.; Liu, P.; Chen, L.L. Qualitative and quantitative analysis of major components of Renshen-Yangrong Pill by UPLC-LTQ/Orbitrap/MS and UPLC-MS/MS. J. Pharmaceuti. Biomed. 2023, 227, 115276. [Google Scholar] [CrossRef] [PubMed]
- Dutschke, J.; Suchowski, M.; Pietsch, J. Simultaneous determination of selected catechins and pyrogallol in deer intoxications by HPLC-MS/MS. J. Chromatogr. B 2021, 1180, 122886. [Google Scholar] [CrossRef] [PubMed]
- Dermanović, M.; Jokić, A.M.; Stefanović, M. Quercetagetin 6, 7, 3′, 4′-tetramethyl ether: A new flavonol from Artemisia annua. Phytochemistry 1975, 14, 1873. [Google Scholar] [CrossRef]
- Qin, J.A.; Luo, J.Y.; Zhao, H.Z.; Zhang, S.S.; Zhang, X.G.; Yang, M.G. Determination and pharmacokinetics of chinensinaphthol methyl ether in rat urine by a sensitive and specific UFLC-ESI-MS/MS method. J. Chromatogr. B 2016, 1033–1034, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Quantitative variation of flavonoids and related compounds in Cosmos bipinnatus. Acta Soc. Bot. Pol. 1979, 48, 317–325. [Google Scholar] [CrossRef]
- Gao, M.Z.; Peng, X.W.; Tang, J.R.; Deng, J.; Wang, F.; Zhang, Y.J.; Zhao, P.; Kan, H.; Liu, Y. Anti-inflammatory effects of Camellia fascicularis polyphenols via attenuation of NF-κB and MAPK pathways in LPS-induced THP-1 macrophages. J. Inflamm. Res. 2022, 15, 851–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vareed, S.K.; Nair, M.G. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005, 76, 1465–1472. [Google Scholar] [CrossRef]
- Sanz, M.; Fernández de Simón, B.; Esteruelas, E.; Muñoz, A.M.; Cadahía, E.; Hernández, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef]
- Wong, K.C.; Law, M.C.; Wong, M.S.; Chan, T.H. Development of a UPLC-MS/MS bioanalytical method for the pharmacokinetic study of (−)-epiafzelechin, a flavan-3-ol with osteoprotective activity, in C57BL/6J mice. J. Chromatogr. B 2014, 967, 162–167. [Google Scholar] [CrossRef]
- Dai, Y.; Tu, F.J.; Yao, Z.H.; Ding, B.; Xu, W.; Qiu, X.H.; Yao, X.S. Rapid identification of chemical constituents in traditional Chinese medicine fufang preparation xianling gubao capsule by LC-linear ion trap/orbitrap mass spectrometry. Am. J. Chin. Med. 2013, 41, 1181–1198. [Google Scholar] [CrossRef]
- Li, S.M.; Sang, S.M.; Pan, M.H.; Lai, C.S.; Lo, C.Y.; Yang, C.S.; Ho, C.T. Anti-inflammatory property of the urinary metabolites of nobiletin in mouse. Bioorg. Med. Chem. Lett. 2007, 17, 5177–5181. [Google Scholar] [CrossRef]
- Jiang, L.H.; Shen, X.J.; Shoji, T.; Kanda, T.; Zhou, J.C.; Zhao, L.M. Characterization and activity of anthocyanins in Zijuan tea (Camellia sinensis var. kitamura). J. Agric. Food Chem. 2013, 61, 3306–3310. [Google Scholar] [CrossRef]
- Barnes, J.S.; Schug, K.A. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometry. Int. J. Mass Spectrom. 2011, 308, 71–80. [Google Scholar] [CrossRef]
- de Brito, E.S.; de Araújo, M.C.; Lin, L.Z.; Harnly, J. Determination of the flavonoid components of cashew apple (Anacardium occidentale) by LC-DAD-ESI/MS. Food Chem. 2007, 105, 1112–1118. [Google Scholar] [CrossRef]
- Wollenweber, E.; Seigler, D.S. Flavonoids from the exudate of Acacia neovernicosa. Phytochemistry 1982, 21, 1063–1066. [Google Scholar] [CrossRef]
- Prasain, J.K.; Jones, K.; Kirk, M.; Wilson, L.; Smith-Johnson, M.; Weaver, C.; Barnes, S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 4213–4218. [Google Scholar] [CrossRef]
- Li, S.M.; Wang, Z.Y.; Sang, S.M.; Huang, M.T.; Ho, C.T. Identification of nobiletin metabolites in mouse urine. Mol. Nutr. Food Res. 2006, 50, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, H.T.; Larsen, M.D.; Nielsen, M.W.; Adsersen, A.; Olsen, C.E.; Strasberg, D.; Smitt, U.W.; Jaroszewski, J.W. Methylenedioxy- and methoxyflavones from Melicope coodeana syn. Euodia simplex. Phytochemistry 2002, 60, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Blanchard, C.L. Q-TOF LC/MS identification and UHPLC-online ABTS antioxidant activity guided mapping of barley polyphenols. Food Chem. 2018, 266, 323–328. [Google Scholar] [CrossRef]
- Lomakool, S.; Ruangrit, K.; Jeerapan, I.; Tragoolpua, Y.; Pumas, C.; Srinuanpan, S.; Pekkoh, J.; Duangjan, K. Biological activities and phytochemicals profiling of different cyanobacterial and microalgal biomass. Biomass Convers. Biorefinery 2023, 13, 4195–4211. [Google Scholar] [CrossRef]
- Sang, J.; Li, B.; Huang, Y.Y.; Ma, Q.; Liu, K.; Li, C.Q. Deep eutectic solvent-based extraction coupled with green two-dimensional HPLC-DAD-ESI-MS/MS for the determination of anthocyanins from Lycium ruthenicum Murr. Fruit. Anal. Methods 2018, 10, 1247–1257. [Google Scholar] [CrossRef]
- Brinda, B.J.; Zhu, H.J.; Markowitz, J.S. A sensitive LC-MS/MS assay for the simultaneous analysis of the major active components of silymarin in human plasma. J. Chromatogr. B 2012, 902, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Sciammaro, L.; Siano, F.; Volpe, M.G.; Puppo, M.C.; Mamone, G. Comparative analysis of C-glycosidic flavonoids from Prosopis spp. and Ceratonia siliqua seed germ flour. Food Res. Int. 2017, 99, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, Q.; Xu, J. Chemical composition of roots Flemingia philippinensis and their inhibitory kinetics on aromatase. Chem. Biodivers. 2017, 14, e1600193. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Guo, P.M.; Pang, W.H.; Zhang, Y.H.; Zhao, Q.Y.; Jiao, B.N.; Kilmartin, P.A. A rapid UHPLC-QqQ-MS/MS method for the simultaneous qualitation and quantitation of coumarins, furocoumarins, flavonoids, phenolic acids in pummelo fruits. Food Chem. 2020, 325, 126835. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Chang, Y.S.; Wang, J.P.; Chen, S.C.; Kuo, S.C. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med. 1998, 64, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.S.; Håkansson, A.; Linares-Pastén, J.A.; Nilsson, L. Interaction between myricetin aggregates and lipase under simplified intestinal conditions. Foods 2020, 9, 777. [Google Scholar] [CrossRef]
- Yang, T.T.; Zhou, W.T.; Xu, W.Q.; Ran, L.W.; Yan, Y.M.; Lu, L.; Mi, J.; Zeng, X.X.; Cao, Y. Modulation of gut microbiota and hypoglycemic/hypolipidemic activity of flavonoids from the fruits of Lycium barbarum on high-fat diet/streptozotocin-induced type 2 diabetic mice. Food Funct. 2022, 13, 11169–11184. [Google Scholar] [CrossRef]
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Lee, Y.S. Recent molecular mechanisms and beneficial effects of phytochemicals and plant-based whole foods in reducing LDL-C and preventing cardiovascular disease. Antioxidants 2021, 10, 784. [Google Scholar] [CrossRef]
- Wu, P.; Feng, Q.P.; Kerchberger, V.E.; Nelson, S.D.; Chen, Q.X.; Li, B.S.; Edwards, T.L.; Cox, N.J.; Phillips, E.J.; Stein, C.M.; et al. Integrating gene expression and clinical data to identify drug repurposing candidates for hyperlipidemia and hypertension. Nat. Commun. 2022, 13, 46. [Google Scholar] [CrossRef]
- Rozman, D.; Monostory, K. Perspectives of the non-statin hypolipidemic agents. Pharmacol. Therapeut. 2010, 127, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Zhang, B.; Wang, B.X.; Li, C.; Fu, X.; Huang, Q. In-vitro inhibitory effects of flavonoids in Rosa roxburghii and R. sterilis fruits on α-glucosidase: Effect of stomach digestion on flavonoids alone and in combination with acarbose. J. Funct. Foods 2019, 54, 13–21. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, W.B.; Cai, X.H.; Lu, D.D.; He, X.Y.; Qiu, P.Y.; Wu, J. Flavonoids of Rosa roxburghii Tratt act as radioprotectors. Asian Pac. J. Cancer Prev. 2014, 15, 8171–8175. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- McClure-Begley, T.D.; Roth, B.L. The promises and perils of psychedelic pharmacology for psychiatry. Nat. Rev. Drug Discov. 2022, 21, 463–473. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Wang, C.; Pan, Y.X.; Gao, X.D.; Chen, H.X. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food Funct. 2018, 9, 426–439. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The influence of supplementation of anthocyanins on obesity-associated comorbidities: A concise review. Foods 2020, 9, 687. [Google Scholar] [CrossRef]
- Gobalakrishnan, S.; Asirvatham, S.S.; Janarthanam, V. Effect of silybin on lipid profile in hypercholesterolaemic rats. J. Clin. Diagn. Res. 2016, 10, FF01–FF05. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.F.; Chen, J.L.; Wei, H.; Xiong, C.M.; Zhang, Y.H.; Ruan, J.L. Hypolipidemic and anti-inflammatory properties of Abacopterin A from Abacopteris penangiana in high-fat diet-induced hyperlipidemia mice. Food Chem. Toxicol. 2011, 49, 3206–3210. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Huang, Z.Q.; Huang, W.J.; Chen, X.M.; Shan, P.R.; Zhong, P.; Khan, Z.; Wang, J.Y.; Fang, Q.; Liang, G.; et al. Inhibition of epidermal growth factor receptor attenuates atherosclerosis via decreasing inflammation and oxidative stress. Sci. Rep. 2017, 8, 45917. [Google Scholar] [CrossRef] [PubMed]
- Jászai, J.; Schmidt, M.H.H. Trends and challenges in tumor anti-angiogenic therapies. Cells 2019, 8, 1102. [Google Scholar] [CrossRef] [PubMed]
- Motojima, K. A metabolic switching hypothesis for the first step in the hypolipidemic effects of fibrates. Biol. Pharm. Bull. 2002, 25, 1509–1511. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.M.; Howard, T.D.; Hawkins, G.A.; Reboussin, D.M.; Xu, J.; Zheng, S.L.; Brosnihan, K.B.; Meyers, D.A.; Bleecker, E.R. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N. Engl. J. Med. 2002, 346, 967–974. [Google Scholar] [CrossRef]
- Shuai, X.X.; Dai, T.T.; McClements, D.J.; Ruan, R.; Du, L.Q.; Liu, Y.H.; Chen, J. Hypolipidemic effects of macadamia oil are related to AMPK activation and oxidative stress relief: In vitro and in vivo studies. Food Res. Int. 2023, 168, 112772. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Wang, B.; Zhang, J.D.; Jiang, H.; Liu, F.Y. Total panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: Role of downregulation of CD40 and MMP-9 expression. J. Ethnopharmacol. 2009, 126, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Van de Wiele, T.; Verstraete, W.; Bracke, M.; Vanhoecke, B. Angiopoietin-like protein 4: Health effects, modulating agents and structure-function relationships. Expert Rev. Proteom. 2012, 9, 181–199. [Google Scholar] [CrossRef]
- Sierra-Johnson, J.; Romero-Corral, A.; Somers, V.K.; Lopez-Jimenez, F.; Mälarstig, A.; Brismar, K.; Hamsten, A.; Fisher, R.M.; Hellénius, M.L. IGF-I/IGFBP-3 ratio: A mechanistic insight into the metabolic syndrome. Clin. Sci. 2009, 116, 507–512. [Google Scholar] [CrossRef]
- Velasco-Santamaría, Y.M.; Korsgaard, B.; Madsen, S.S.; Bjerregaard, P. Bezafibrate, a lipid-lowering pharmaceutical, as a potential endocrine disruptor in male zebrafish (Danio rerio). Aquat. Toxicol. 2011, 105, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Wang, N.; Long, Y.Q.; Wu, Z.; Zhou, S.H. Zinc homeostasis: An emerging therapeutic target for neuroinflammation related diseases. Biomolecules 2023, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. Biofactors 2021, 47, 218–231. [Google Scholar] [CrossRef]
- Berstein, L.M. Clinical usage of hypolipidemic and antidiabetic drugs in the prevention and treatment of cancer. Cancer Lett. 2005, 224, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]


| Groups | Concentration (μg/mL) | IOD (Mean ± SE) | The Rate of Lipid Reduction Rate (%) |
|---|---|---|---|
| Model control group | - | 1981 ± 57 | - |
| Lovastatin | 0.081 | 1588 ± 125 # | 20 |
| RS | 3 | 1867 ± 46 | 6 *** |
| 10 | 1395 ± 78 ### | 30 *** | |
| 30 | 1159 ± 69 ### | 41 *** |
| No. | R.T. (min) | Mode | Diff (ppm) | Molecular | Fragment Ions (m/z) | Identification | Relative Content (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.466 | [M-H]+ | −2.00 | C20H16O5 | 171.0426; 185.0428; 333.0946 | glabrone | 2.58 | [21] |
| 2 | 2.232 | [M-H]− | −1.56 | C15H14O7 | 125.0233; 137.0245; 179.0346 | gallocatechin | 8.42 | [22] |
| 3 | 3.574 | [M-H]− | −1.19 | C19H18O8 | 151.0381; 216.0075; 300.9950 | quercetagetin-6,7-3′,4′-tetramethyl ether | 1.72 | [23] |
| 4 | 3.910 | [M-H]− | −1.93 | C21H16O7 | 169.0869; 205.1222; 280.1247 | chinensinaphthol | 5.75 | [24] |
| 5 | 3.910 | [M-H]− | 2.80 | C27H28O18 | 577.1340; 578.1369; 425.0870 | nelumboside | 21.26 | [25] |
| 6 | 4.326 | [M-H]− | −0.74 | C45H38O18 | 311.0505; 601.1306; 602.1335 | robinetinidol-(4α->8)-catechin-(6->4α)-robinetinidol | 3.27 | [26] |
| 7 | 4.494 | [M-H]− | 2.60 | C15H14O6 | 127.0396; 163.0383; 271.0590 | (-)-epicatechin | 23.06 | [26] |
| 8 | 7.015 | [M-H]− | 0.10 | C21H21O12 | 125.0259; 285.0365; 303.0500 | delphinidin 3-galactoside | 1.41 | [27] |
| 9 | 7.015 | [M-H]− | 0.06 | C21H22O12 | 61.9884; 250.9457; 303.0507 | plantagoside | 1.29 | [26] |
| 10 | 7.770 | [M-H]− | −1.20 | C15H12O6 | 125.0246; 259.0608; 269.0455 | fustin | 1.67 | [28] |
| 11 | 7.770 | [M-H]− | 4.40 | C15H14O5 | 175.0245; 193.0861; 273.0967 | (-)-epiafzelechin | 0.44 | [29] |
| 12 | 8.102 | [M-H]+ | 2.38 | C31H36O14 | 449.1781; 467.1881; 629.2414 | ikarisoside F | 1.62 | [30] |
| 13 | 8.693 | [M-H]− | −0.48 | C20H20O9 | 135.1172; 179.1081; 223.0969 | 3′-demethylnobiletin | 4.68 | [31] |
| 14 | 8.693 | [M-H]− | −0.21 | C21H21O11 | 287.0556; 299.9896; 447.0561 | cyanidin 3-O-β-D-galactoside | 4.74 | [32] |
| 15 | 9.281 | [M-H]− | −0.63 | C27H31O15 | 173.0069; 315.049; 441.0825 | pelargonidin-3,5-diglucoside | 0.35 | [33] |
| 16 | 11.211 | [M-H]− | −0.61 | C15H11O7 | 125.0235; 152.0093; 285.0393 | delphinidin | 0.93 | [34] |
| 17 | 11.462 | [M-H]− | −3.24 | C17H14O7 | 167.0352; 191.0351; 209.0460 | 3,3′-dimethylquercetin | 0.33 | [35] |
| 18 | 11.462 | [M-H]− | −4.66 | C16H12O5 | 125.0232; 151.0391; 175.0235 | 3′-methoxydaidzein | 0.28 | [36] |
| 19 | 13.057 | [M-H]− | 0.16 | C20H20O8 | 75.0080; 89.0240; 387.1144 | 3′-hydroxy-4′,5′,6,7,8-pentamethoxyflavone | 0.67 | [37] |
| 20 | 13.644 | [M-H]− | −0.12 | C18H14O8 | 163.0037; 285.0767; 329.0640 | 3,8-dimethoxy-5,7-dihydroxy-3′,4′-methylenedioxyflavone | 3.62 | [38] |
| 21 | 13.644 | [M-H]− | −0.23 | C21H24O11 | 101.0235; 179.0552; 405.2113 | (+)-catechin-5-O-glucoside | 1.47 | [39] |
| 22 | 14.064 | [M-H]− | −0.28 | C30H26O10 | 151.0065; 271.0614; 433.1087 | guibourtinidol-(4α-->6)-catechin | 2.39 | [40] |
| 23 | 15.323 | [M-H]− | −0.72 | C16H13O7 | 109.0293; 221.0790; 257.0444 | petunidin | 0.87 | [41] |
| 24 | 17.420 | [M-H]− | 0.26 | C25H22O10 | 112.9861; 149.0440; 293.0869 | silybin | 0.39 | [42] |
| 25 | 17.840 | [M-H]− | −0.93 | C26H28O14 | 174.0163; 340.1416; 563.1515 | isoschaftoside | 1.22 | [43] |
| 26 | 22.791 | [M-H]− | −0.84 | C23H26O10 | 61.9884; 89.0213; 292.8688 | andrographidine A | 0.11 | [44] |
| 27 | 23.546 | [M-H]− | −1.33 | C15H11O5 | 59.0138; 163.0749; 164.0784 | pelargonidin | 0.67 | [45] |
| 28 | 24.301 | [M-H]− | −1.32 | C22H24O9 | 61.9884; 269.0410; 341.0663 | 3′,4′,5,5′,6,7-heptamethoxyflavone | 0.24 | [46] |
| 29 | 25.976 | [M-H]− | −0.90 | C16H14O6 | 89.0601; 133.0866; 283.1752 | (3R)-4′-methoxy-2′,3,7-trihydroxyisoflavanone | 0.60 | [47] |
| 30 | 26.064 | [M-H]− | −1.47 | C22H23O11 | 82.8591; 165.0146; 331.6164 | malvidin-3-arabinoside | 3.85 | [48] |
| 31 | 29.336 | [M-H]− | −3.33 | C26H26O6 | 61.9885; 69.9166; 322.2770 | flemiphilippinin C | 0.10 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Li, C.; Luo, X.; Kan, H.; Li, Y.; Zhang, Y.; Rao, X.; Zhao, P.; Liu, Y. Identification of Key Hypolipidemic Components and Exploration of the Potential Mechanism of Total Flavonoids from Rosa sterilis Based on Network Pharmacology, Molecular Docking, and Zebrafish Experiment. Curr. Issues Mol. Biol. 2024, 46, 5131-5146. https://doi.org/10.3390/cimb46060308
Wu B, Li C, Luo X, Kan H, Li Y, Zhang Y, Rao X, Zhao P, Liu Y. Identification of Key Hypolipidemic Components and Exploration of the Potential Mechanism of Total Flavonoids from Rosa sterilis Based on Network Pharmacology, Molecular Docking, and Zebrafish Experiment. Current Issues in Molecular Biology. 2024; 46(6):5131-5146. https://doi.org/10.3390/cimb46060308
Chicago/Turabian StyleWu, Boxiao, Churan Li, Xulu Luo, Huan Kan, Yonghe Li, Yingjun Zhang, Xiaoping Rao, Ping Zhao, and Yun Liu. 2024. "Identification of Key Hypolipidemic Components and Exploration of the Potential Mechanism of Total Flavonoids from Rosa sterilis Based on Network Pharmacology, Molecular Docking, and Zebrafish Experiment" Current Issues in Molecular Biology 46, no. 6: 5131-5146. https://doi.org/10.3390/cimb46060308
APA StyleWu, B., Li, C., Luo, X., Kan, H., Li, Y., Zhang, Y., Rao, X., Zhao, P., & Liu, Y. (2024). Identification of Key Hypolipidemic Components and Exploration of the Potential Mechanism of Total Flavonoids from Rosa sterilis Based on Network Pharmacology, Molecular Docking, and Zebrafish Experiment. Current Issues in Molecular Biology, 46(6), 5131-5146. https://doi.org/10.3390/cimb46060308






