ADAR Family Proteins: A Structural Review
Abstract
1. Background
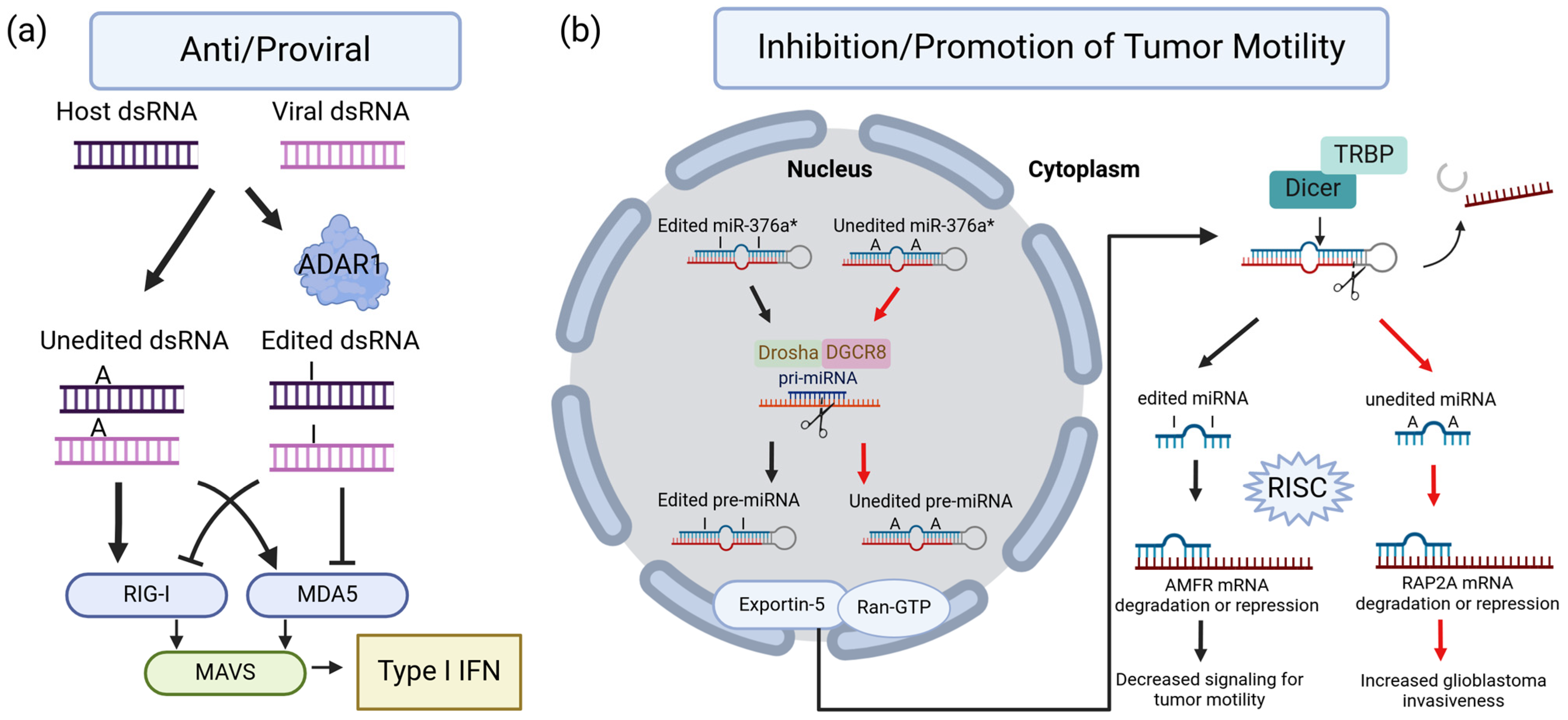
2. Overview of the Biological Impacts of ADAR
3. ADAR Evolution
4. The Base Flipping Mechanism of ADARs and the Inactive ADAR3
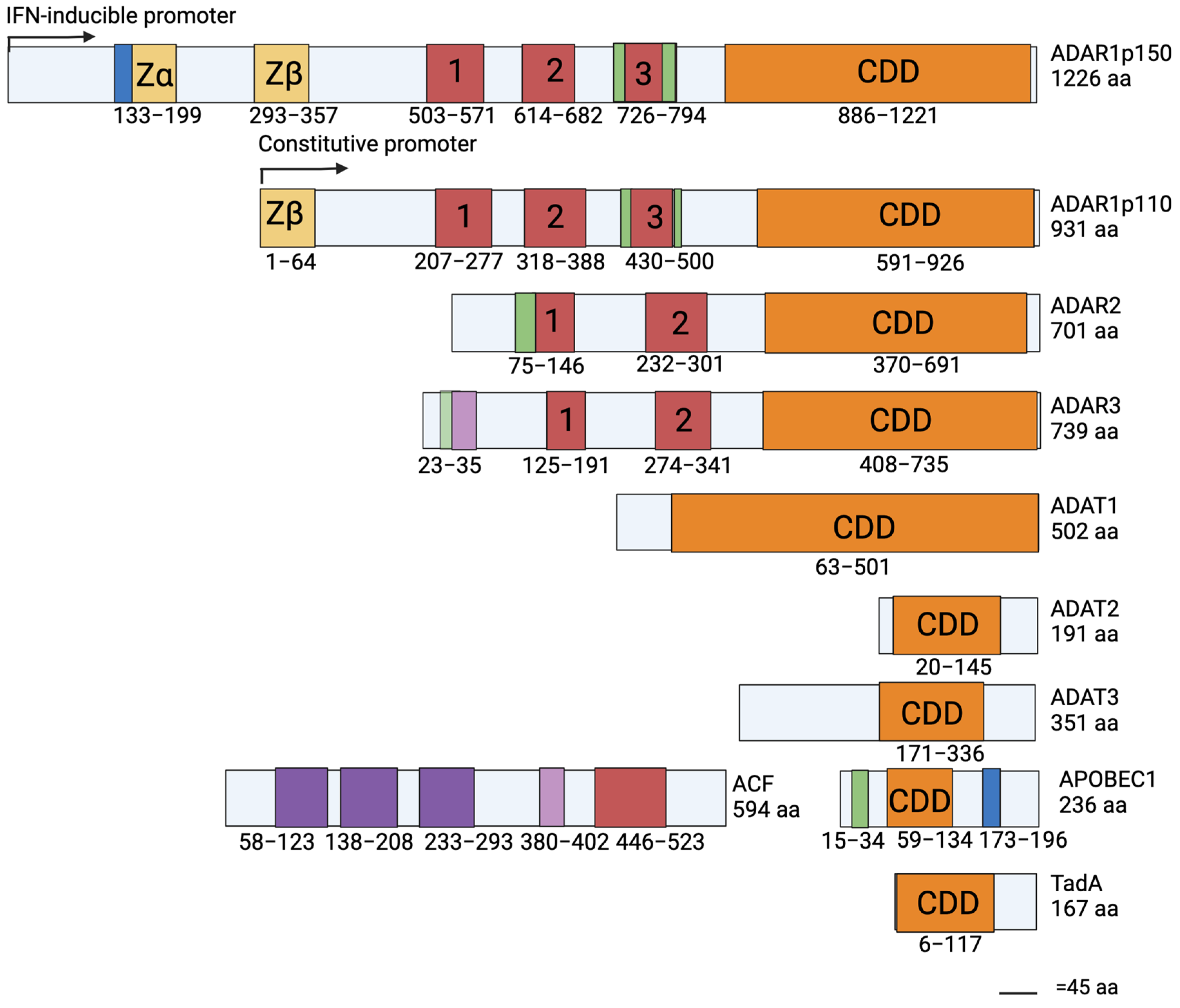
5. Conserved Structures and Their Effects on Substrate Specificity
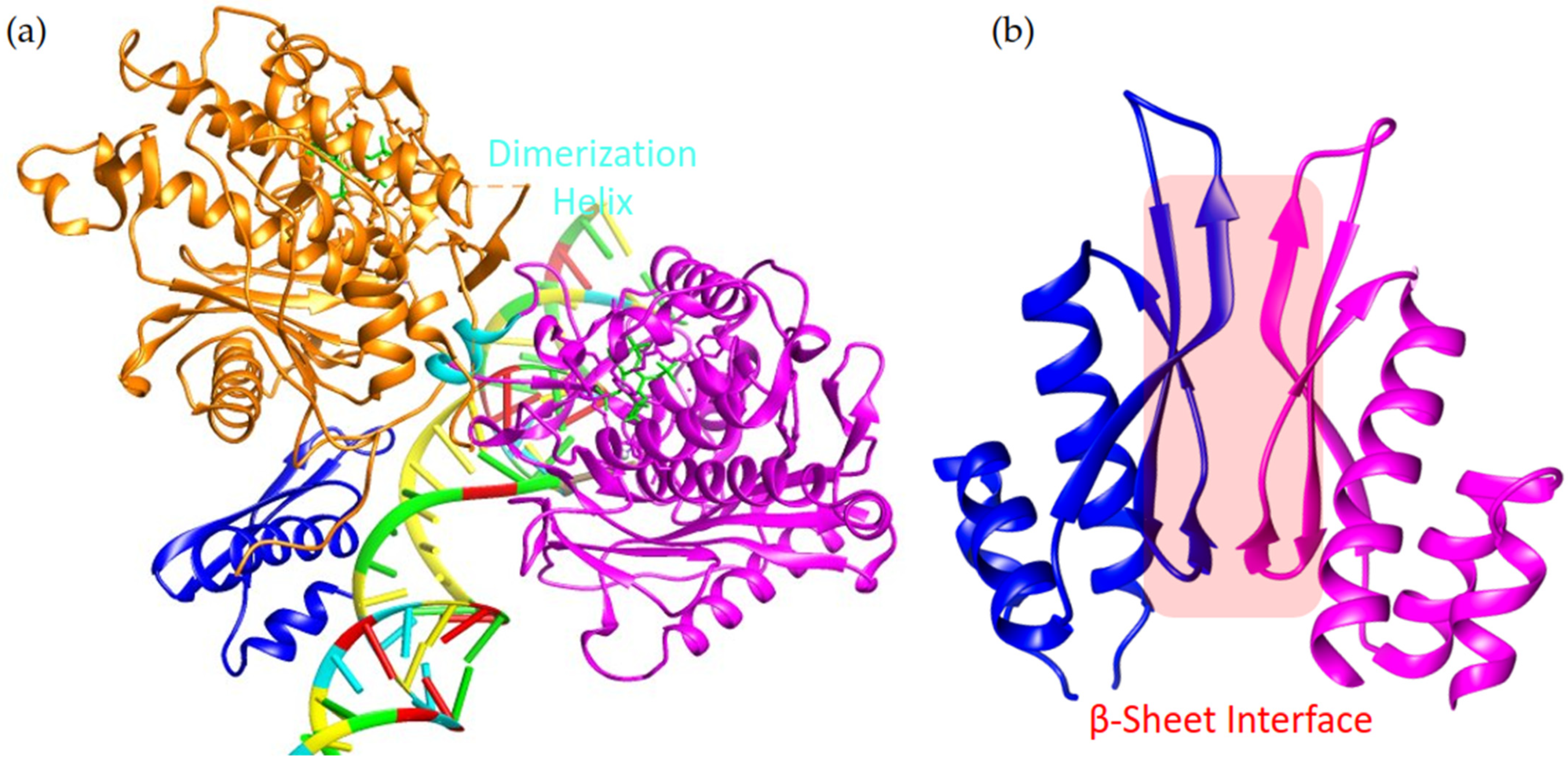
6. The Family Member Specific Structures
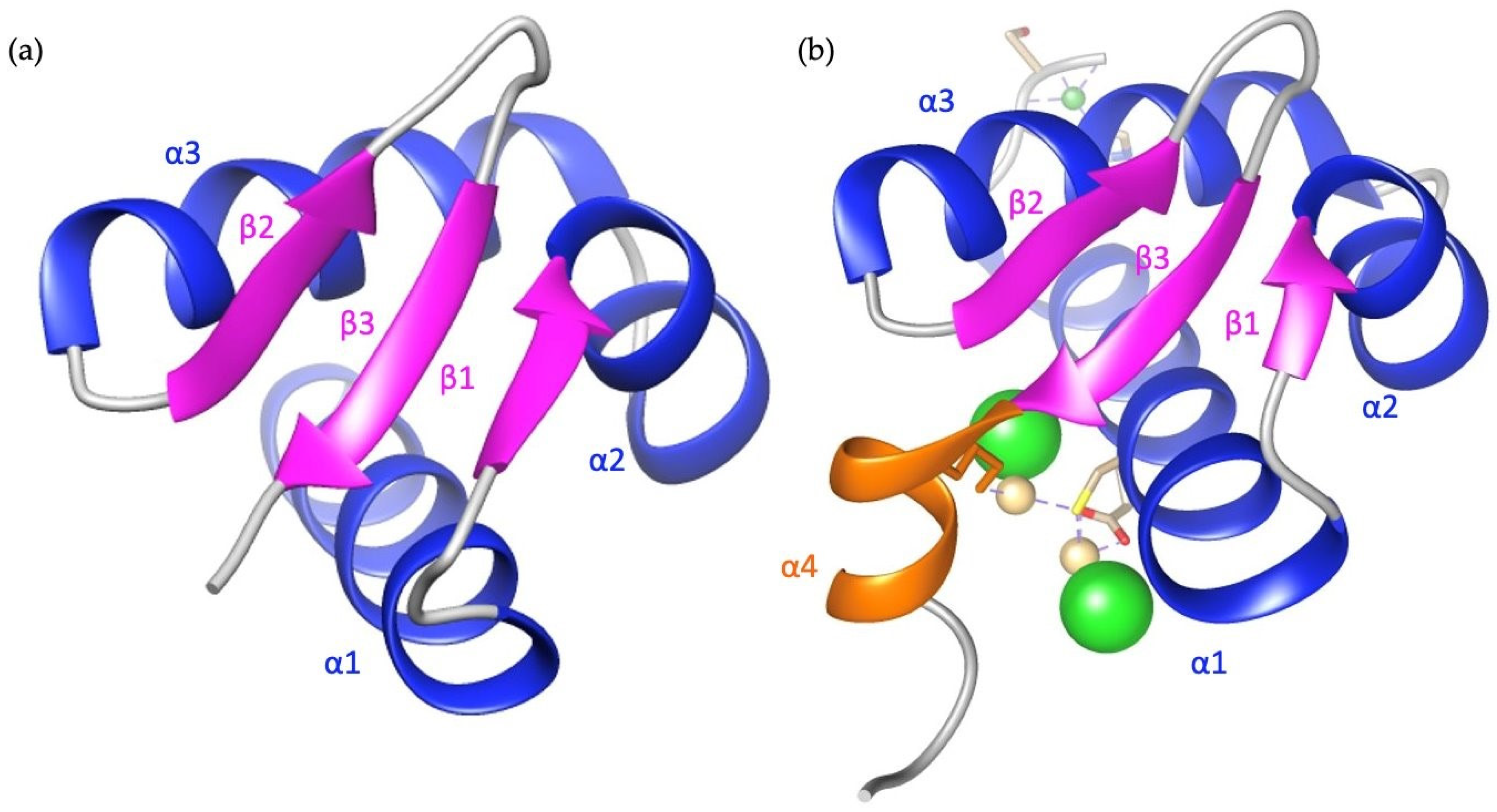
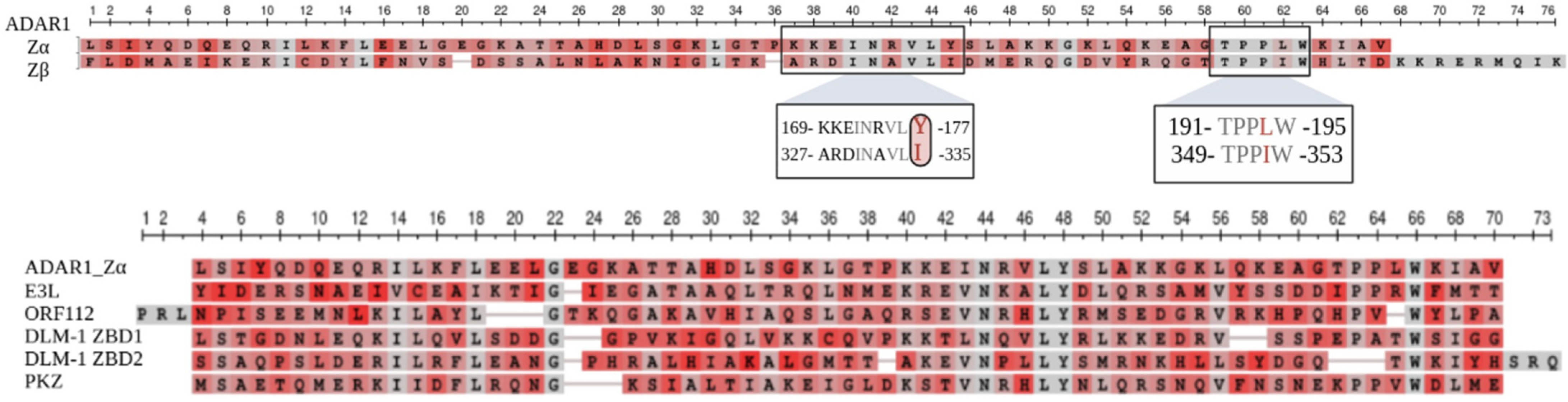
6.1. Z-DNA Binding Domains of ADAR1
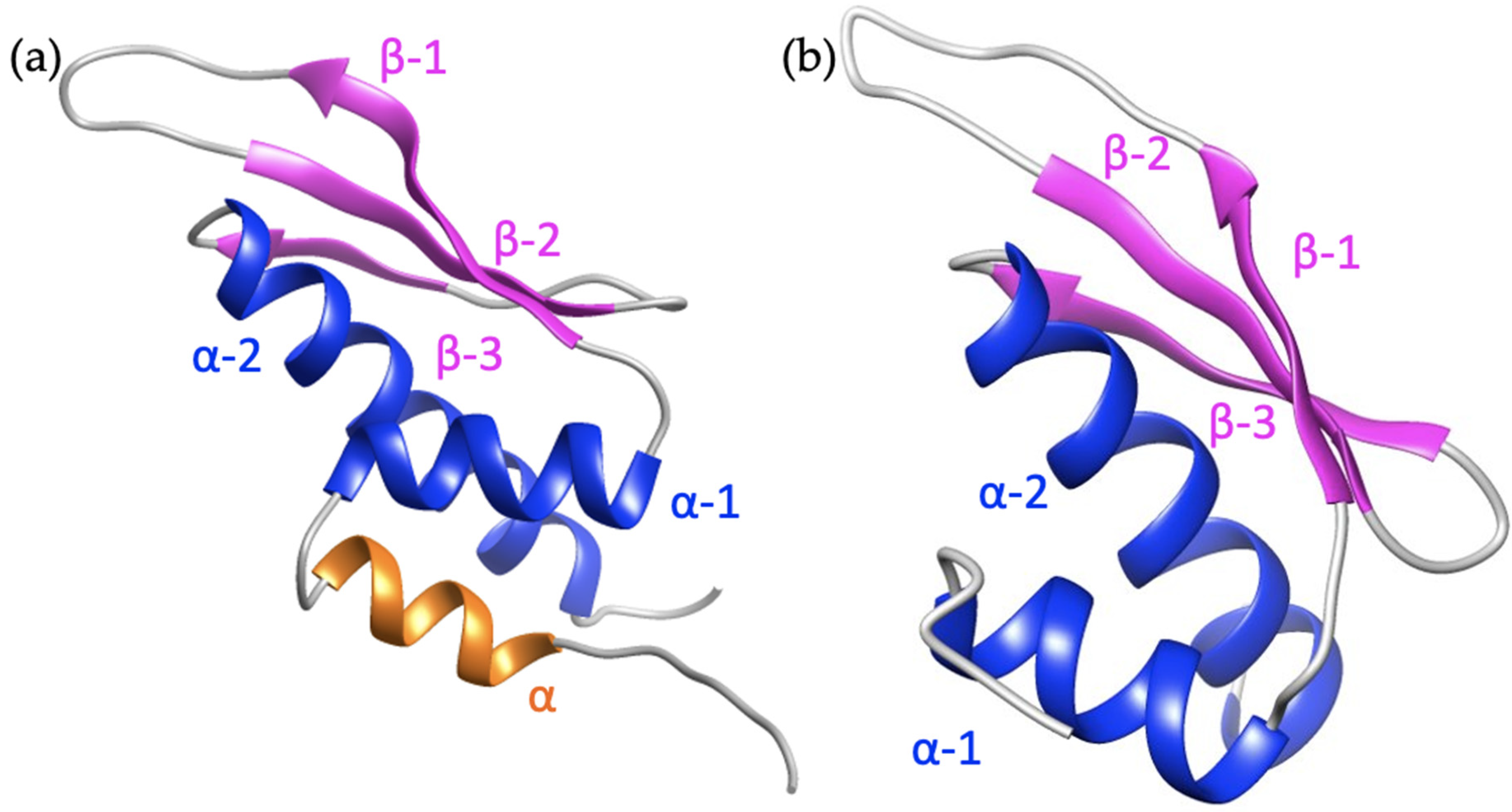
6.2. Structural Comparison between the Zα and Zβ Domain
6.3. The Arginine-Rich Domain of ADAR3
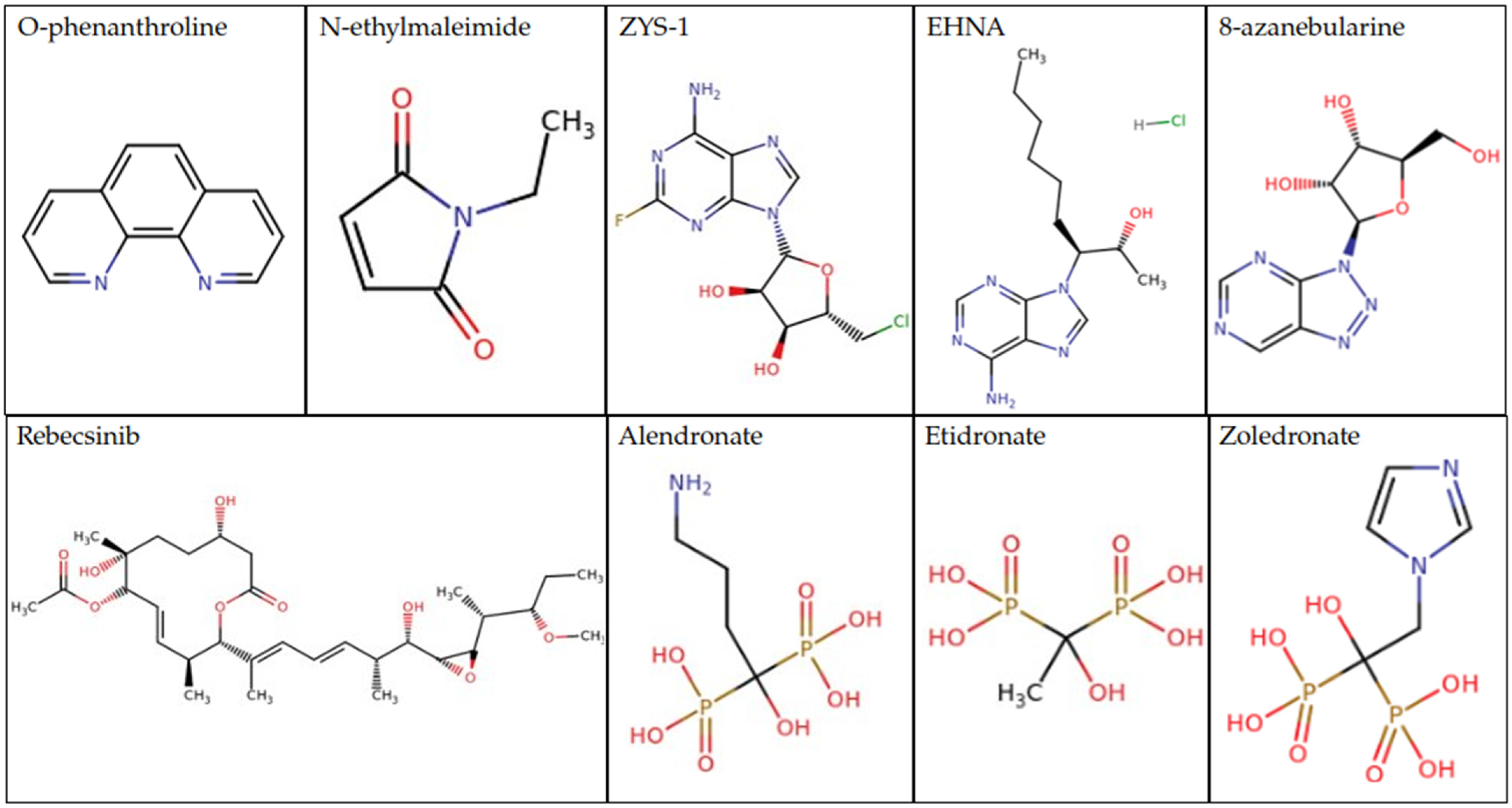
7. Future Explorations for Therapeutics and Drug Design
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gott, J.M.; Emeson, R.B. Functions and Mechanisms of RNA Editing. Annu. Rev. Genet. 2000, 34, 499–531. [Google Scholar] [CrossRef] [PubMed]
- Christofi, T.; Zaravinos, A. RNA Editing in the Forefront of Epitranscriptomics and Human Health. J. Transl. Med. 2019, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Benne, R.; Van Den Burg, J.; Brakenhoff, J.P.J.; Sloof, P.; Van Boom, J.H.; Tromp, M.C. Major Transcript of the Frameshifted CoxII Gene from Trypanosome Mitochondria Contains Four Nucleotides That Are Not Encoded in the DNA. Cell 1986, 46, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Cuddleston, W.H.; Fan, X.; Sloofman, L.; Liang, L.; Mossotto, E.; Moore, K.; Zipkowitz, S.; Wang, M.; Zhang, B.; Wang, J.; et al. Spatiotemporal and Genetic Regulation of A-to-I Editing throughout Human Brain Development. Cell Rep. 2022, 41, 111585. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.L. RNA Editing by Adenosine Deaminases That Act on RNA. Annu. Rev. Biochem. 2002, 71, 817–846. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.L.; Nishikura, K.; Keller, W.; Seeburg, P.H.; Emeson, R.B.; O’connell, M.A.; Samuel, C.E.; Herbert, A. A Standardized Nomenclature for Adenosine Deaminases That Act on RNA. RNA 1997, 3, 947–949. [Google Scholar]
- Zinshteyn, B.; Nishikura, K. Adenosine-to-Inosine RNA Editing. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 209. [Google Scholar] [CrossRef] [PubMed]
- Rebagliati, M.R.; Melton, D.A. Antisense RNA Injections in Fertilized Frog Eggs Reveal an RNA Duplex Unwinding Activity. Cell 1987, 48, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.L.; Weintraub, H. A Developmentally Regulated Activity That Unwinds RNA Duplexes. Cell 1987, 48, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.B.; Samuel, C.E. Expression and Regulation by Interferon of a Double-Stranded-RNA-Specific Adenosine Deaminase from Human Cells: Evidence for Two Forms of the Deaminase. Mol. Cell. Biol. 1995, 15, 5376–5388. [Google Scholar] [CrossRef] [PubMed]
- Raghava Kurup, R.; Oakes, E.K.; Manning, A.C.; Mukherjee, P.; Vadlamani, P.; Hundley, H.A. RNA Binding by ADAR3 Inhibits Adenosine-to-Inosine Editing and Promotes Expression of Immune Response Protein MAVS. J. Biol. Chem. 2022, 298, 102267. [Google Scholar] [CrossRef]
- Ota, H.; Sakurai, M.; Gupta, R.; Valente, L.; Wulff, B.E.; Ariyoshi, K.; Iizasa, H.; Davuluri, R.V.; Nishikura, K. ADAR1 Forms a Complex with Dicer to Promote MicroRNA Processing and RNA-Induced Gene Silencing. Cell 2013, 153, 589. [Google Scholar] [CrossRef]
- Duan, Y.; Dou, S.; Zhang, H.; Wu, C.; Wu, M.; Lu, J. Linkage of A-to-I RNA Editing in Metazoans and the Impact on Genome Evolution. Mol. Biol. Evol. 2018, 35, 148. [Google Scholar] [CrossRef] [PubMed]
- Nishikura, K. A-to-I Editing of Coding and Non-Coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2015, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Nishikura, K. Functions and Regulation of RNA Editing by ADAR Deaminases. Annu. Rev. Biochem. 2010, 79, 321–349. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, V.; Cerboni, C.; Soriani, A. Self or Non-Self? It Is Also a Matter of RNA Recognition and Editing by ADAR1. Biology 2022, 11, 568. [Google Scholar] [CrossRef]
- Alon, S.; Mor, E.; Vigneault, F.; Church, G.M.; Locatelli, F.; Galeano, F.; Gallo, A.; Shomron, N.; Eisenberg, E. Systematic Identification of Edited MicroRNAs in the Human Brain. Genome Res. 2012, 22, 1540. [Google Scholar] [CrossRef]
- Sansam, C.L.; Wells, K.S.; Emeson, R.B. Modulation of RNA Editing by Functional Nucleolar Sequestration of ADAR2. Proc. Natl. Acad. Sci. USA 2003, 100, 14018–14023. [Google Scholar] [CrossRef] [PubMed]
- Hideyama, T.; Yamashita, T.; Aizawa, H.; Tsuji, S.; Kakita, A.; Takahashi, H.; Kwak, S. Profound Downregulation of the RNA Editing Enzyme ADAR2 in ALS Spinal Motor Neurons. Neurobiol. Dis. 2012, 45, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kwak, S. The Molecular Link between Inefficient GluA2 Q/R Site-RNA Editing and TDP-43 Pathology in Motor Neurons of Sporadic Amyotrophic Lateral Sclerosis Patients. Brain Res. 2014, 10, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Deininger, P. Alu Elements: Know the SINEs. Genome Biol. 2011, 12, 236. [Google Scholar] [CrossRef]
- Athanasiadis, A.; Rich, A.; Maas, S. Widespread A-to-I RNA Editing of Alu-Containing MRNAs in the Human Transcriptome. PLoS Biol. 2004, 2, e391. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; DeCerbo, J.N.; Carmichael, G.G. Alu Element-Mediated Gene Silencing. EMBO J. 2008, 27, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Maas, S.; Single, F.N.; Hartner, J.; Rozov, A.; Burnashev, N.; Feldmeyer, D.; Sprengel, R.; Seeburg, P.H. Point Mutation in an AMPA Receptor Gene Rescues Lethality in Mice Deficient in the RNA-Editing Enzyme ADAR2. Nature 2000, 406, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Miyakoda, M.; Yang, W.; Khillan, J.; Stachura, D.L.; Weiss, M.J.; Nishikura, K. Stress-Induced Apoptosis Associated with Null Mutation of ADAR1 RNA Editing Deaminase Gene. J. Biol. Chem. 2004, 279, 4952–4961. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, D.; Barry, G.; Konen, L.M.; Pineda, S.S.; Guennewig, B.; Avesson, L.; Zinn, R.; Schonrock, N.; Bitar, M.; Jonkhout, N.; et al. Adar3 Is Involved in Learning and Memory in Mice. Front. Neurosci. 2018, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Bellingrath, J.-S.; McClements, M.E.; Fischer, M.D.; MacLaren, R.E. Programmable RNA Editing with Endogenous ADAR Enzymes—A Feasible Option for the Treatment of Inherited Retinal Disease? Front. Mol. Neurosci. 2023, 16, 1092913. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, L.A.; Saccomanno, L.; Morse, D.P.; Brodigan, T.; Krause, M.; Bass, B.L. RNA Editing by ADARs Is Important for Normal Behavior in Caenorhabditis Elegans. EMBO J. 2002, 21, 6035. [Google Scholar] [CrossRef] [PubMed]
- Palladino, M.J.; Keegan, L.P.; O’Connell, M.A.; Reenan, R.A. A-to-I Pre-MRNA Editing in Drosophila Is Primarily Involved in Adult Nervous System Function and Integrity. Cell 2000, 102, 437–449. [Google Scholar] [CrossRef]
- Zhai, J.; Koh, J.H.; Soong, T.W. RNA Editing of Ion Channels and Receptors in Physiology and Neurological Disorders. Oxford Open Neurosci. 2022, 1, kvac010. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Okada, S.; Sakurai, M. Adenosine-to-Inosine RNA Editing in Neurological Development and Disease. RNA Biol. 2021, 18, 1013. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, W.; Nishikura, K. Adenosine-to-Inosine RNA Editing and Human Disease. Genome Med. 2013, 5, 105. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, H.; Xu, J.; Gao, W. ADAR, the Carcinogenesis Mechanisms of ADAR and Related Clinical Applications. Ann. Transl. Med. 2019, 7, 686. [Google Scholar] [CrossRef] [PubMed]
- Piontkivska, H.; Wales-McGrath, B.; Miyamoto, M.; Wayne, M.L. ADAR Editing in Viruses: An Evolutionary Force to Reckon With. Genome Biol. Evol. 2021, 13, evab240. [Google Scholar] [CrossRef]
- Kung, C.P.; Maggi, L.B.; Weber, J.D. The Role of RNA Editing in Cancer Development and Metabolic Disorders. Front. Endocrinol. 2018, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.I.; Kasher, P.R.; Forte, G.M.A.; Mannion, N.M.; Greenwood, S.M.; Szynkiewicz, M.; Dickerson, J.E.; Bhaskar, S.S.; Zampini, M.; Briggs, T.A.; et al. Mutations in ADAR1 Cause Aicardi-Goutières Syndrome Associated with a Type I Interferon Signature. Nat. Genet. 2012, 44, 1248. [Google Scholar] [CrossRef] [PubMed]
- Piekutowska-Abramczuk, D.; Mierzewska, H.; Bekiesinska-Figatowska, M.; Ciara, E.; Trubicka, J.; Pronicki, M.; Rokicki, D.; Rydzanicz, M.; Płoski, R.; Pronicka, E. Bilateral Striatal Necrosis Caused by ADAR Mutations in Two Siblings with Dystonia and Freckles-like Skin Changes That Should Be Differentiated from Leigh Syndrome. Folia Neuropathol. 2016, 54, 405–409. [Google Scholar] [CrossRef]
- Tan, T.Y.; Sedmík, J.; Fitzgerald, M.P.; Halevy, R.S.; Keegan, L.P.; Helbig, I.; Basel-Salmon, L.; Cohen, L.; Straussberg, R.; Chung, W.K.; et al. Bi-Allelic ADARB1 Variants Associated with Microcephaly, Intellectual Disability, and Seizures. Am. J. Hum. Genet. 2020, 106, 483. [Google Scholar] [CrossRef] [PubMed]
- Barbon, A.; Magri, C. RNA Editing and Modifications in Mood Disorders. Genes 2020, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.S.; Dobbyn, A.; Li, Q.; Roussos, P.; Hoffman, G.E.; Stahl, E.; Chess, A.; Sklar, P.; Li, J.B.; Devlin, B.; et al. Global Landscape and Genetic Regulation of RNA Editing in Cortical Samples from Individuals with Schizophrenia. Nat. Neurosci. 2019, 22, 1412. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.A.; Mannion, N.M.; Keegan, L.P. The Epitranscriptome and Innate Immunity. PLoS Genet. 2015, 11, e1005687. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Song, X.; Wang, Z.; Zhang, Y.; Zhao, Y.; Peng, X.; Zhang, X.; Li, C.; Gao, C.; et al. Hepatitis B Virus Evades Immune Recognition via RNA Adenosine Deaminase ADAR1-Mediated Viral RNA Editing in Hepatocytes. Cell. Mol. Immunol. 2021, 18, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Zinshteyn, B.; Sethupathy, P.; Iizasa, H.; Hatzigeorgiou, A.G.; Nishikura, K. Redirection of Silencing Targets by Adenosine-to-Inosine Editing of MiRNAs. Science 2007, 315, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Iizasa, H.; Wulff, B.-E.; Alla, N.R.; Maragkakis, M.; Megraw, M.; Hatzigeorgiou, A.; Iwakiri, D.; Takada, K.; Wiedmer, A.; Showe, L.; et al. Editing of Epstein-Barr Virus-Encoded BART6 MicroRNAs Controls Their Dicer Targeting and Consequently Affects Viral Latency*. J. Biol. Chem. 2010, 285, 33358–33370. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Megraw, M.; Kreider, E.; Iizasa, H.; Valente, L.; Hatzigeorgiou, A.G.; Nishikura, K. Frequency and Fate of MicroRNA Editing in Human Brain. Nucleic Acids Res. 2008, 36, 5270–5280. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, Y.; Tay, F.C.; Lam, D.H.; Sandanaraj, E.; Tang, C.; Ang, B.-T.; Wang, S. Attenuated Adenosine-to-Inosine Editing of MicroRNA-376a* Promotes Invasiveness of Glioblastoma Cells. J. Clin. Investig. 2012, 122, 4059–4076. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; van den Hoogen, B.G.; Haagmans, B.L. ADAR1: “Editor-in-Chief” of Cytoplasmic Innate Immunity. Front. Immunol. 2019, 10, 1763. [Google Scholar] [CrossRef]
- Hong, H.; Lin, J.S.; Chen, L. Regulatory Factors Governing Adenosine-to-Inosine (A-to-I) RNA Editing. Biosci. Rep. 2015, 35, e00182. [Google Scholar] [CrossRef] [PubMed]
- Shallev, L.; Kopel, E.; Feiglin, A.; Leichner, G.S.; Avni, D.; Sidi, Y.; Eisenberg, E.; Barzilai, A.; Levanon, E.Y.; Greenberger, S. Decreased A-to-I RNA Editing as a Source of Keratinocytes DsRNA in Psoriasis. RNA 2018, 24, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Wedekind, E.J.; Dance, S.C.G.; Sowden, P.M.; Smith, C.H. Erratum: Messenger RNA Editing in Mammals: New Members of the APOBEC Family Seeking Roles in the Family Business: Wedekind, J.E. et al. (2003) Trends in Genetics 19, 207–216. Trends Genet. 2003, 19, 369. [Google Scholar] [CrossRef]
- Chester, A.; Somasekaram, A.; Tzimina, M.; Jarmuz, A.; Gisbourne, J.; O’Keefe, R.; Scott, J.; Navaratnam, N. The Apolipoprotein B MRNA Editing Complex Performs a Multifunctional Cycle and Suppresses Nonsense-Mediated Decay. EMBO J. 2003, 22, 3971–3982. [Google Scholar] [CrossRef] [PubMed]
- Maris, C.; Masse, J.; Chester, A.; Navaratnam, N.; Allain, F.H.-T. NMR Structure of the ApoB MRNA Stem–Loop and Its Interaction with the C to U Editing APOBEC1 Complementary Factor. RNA 2005, 11, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Grice, L.F.; Degnan, B.M. The Origin of the ADAR Gene Family and Animal RNA Editing. BMC Evol. Biol. 2015, 15, 4. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, W.; Li, Q. Origins and Evolution of ADAR-Mediated RNA Editing. IUBMB Life 2009, 61, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Gerber, A.P.; Keller, W. TadA, an Essential TRNA-Specific Adenosine Deaminase from Escherichia Coli. EMBO J. 2002, 21, 3841–3851. [Google Scholar] [CrossRef]
- Torres, A.G.; Piñeyro, D.; Filonava, L.; Stracker, T.H.; Batlle, E.; Ribas De Pouplana, L. A-to-I Editing on TRNAs: Biochemical, Biological and Evolutionary Implications. FEBS Lett. 2014, 588, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Morales, E.; Bayam, E.; Del-Pozo-Rodríguez, J.; Salinas-Giegé, T.; Marek, M.; Tilly, P.; Wolff, P.; Troesch, E.; Ennifar, E.; Drouard, L.; et al. The Structure of the Mouse ADAT2/ADAT3 Complex Reveals the Molecular Basis for Mammalian TRNA Wobble Adenosine-to-Inosine Deamination. Nucleic Acids Res. 2021, 49, 6529–6548. [Google Scholar] [CrossRef] [PubMed]
- Macbeth, M.R.; Schubert, H.L.; VanDemark, A.F.; Lingam, A.T.; Hill, C.P.; Bass, B.L. Structural Biology: Inositol Hexakisphosphate Is Bound in the ADAR2 Core and Required for RNA Editing. Science 2005, 309, 1534–1539. [Google Scholar] [CrossRef]
- Kim, U.; Wang, Y.; Sanford, T.; Zeng, Y.; Nishikura, K. Molecular Cloning of CDNA for Double-Stranded RNA Adenosine Deaminase, a Candidate Enzyme for Nuclear RNA Editing. Proc. Natl. Acad. Sci. USA 1994, 91, 11457–11461. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.P.; Keller, W. RNA Editing by Base Deamination: More Enzymes, More Targets, New Mysteries. Trends Biochem. Sci. 2001, 26, 376–384. [Google Scholar] [CrossRef]
- Broni, E.; Striegel, A.; Ashley, C.; Sakyi, P.O.; Peracha, S.; Velazquez, M.; Bebla, K.; Sodhi, M.; Kwofie, S.K.; Ademokunwa, A.; et al. Molecular Docking and Dynamics Simulation Studies Predict Potential Anti-ADAR2 Inhibitors: Implications for the Treatment of Cancer, Neurological, Immunological and Infectious Diseases. Int. J. Mol. Sci. 2023, 24, 6795. [Google Scholar] [CrossRef] [PubMed]
- Blanc, V.; Xie, Y.; Kennedy, S.; Riordan, J.D.; Rubin, D.C.; Madison, B.B.; Mills, J.C.; Nadeau, J.H.; Davidson, N.O. Apobec1 Complementation Factor (A1CF) and RBM47 Interact in Tissue-Specific Regulation of C to U RNA Editing in Mouse Intestine and Liver. RNA 2019, 25, 81. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Cho, D.S.C.; Wang, Q.; Lai, F.; Carter, K.C.; Nishikura, K. A Third Member of the RNA-Specific Adenosine Deaminase Gene Family, ADAR3, Contains Both Single- and Double-Stranded RNA Binding Domains. RNA 2000, 6, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Polson, A.G.; McCloskey, J.A.; Bass, B.L.; Crain, P.F.; Pomerantz, S.C.; McCloskey, J.A. The Mechanism of Adenosine to Inosine Conversion by the Double-Stranded Rna Unwinding/Modifying Activity: A High-Performance Liquid Chromatography-Mass Spectrometry Analysis. Biochemistry 1991, 30, 11507–11514. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.M.; Thomas, J.M.; Zheng, Y. Structures of Human ADAR2 Bound to DsRNA Reveal Base-Flipping Mechanism and Basis for Site Selectivity. Publication Date. Nat. Struct. Mol. Biol. 2016, 23, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Cheng, X. DNA Base Flipping: A General Mechanism for Writing, Reading, and Erasing DNA Modifications. Adv. Exp. Med. Biol. 2016, 945, 341. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.S.C.; Yang, W.; Lee, J.T.; Shiekhattar, R.; Murray, J.M.; Nishikura, K. Requirement of Dimerization for RNA Editing Activity of Adenosine Deaminases Acting on RNA. J. Biol. Chem. 2003, 278, 17093–17102. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, D.H.; Monteleone, L.R.; Li, J.; Chiang, Y.; Toney, M.D.; Beal, P.A. RNA Binding Candidates for Human ADAR3 from Substrates of a Gain of Function Mutant Expressed in Neuronal Cells. Nucleic Acids Res. 2019, 47, 10814. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.; Patt, S.; Schrey, M.; Rich, A. Underediting of Glutamate Receptor GluR-B MRNA in Malignant Gliomas. Proc. Natl. Acad. Sci. USA 2001, 98, 14692. [Google Scholar] [CrossRef] [PubMed]
- Oakes, E.; Anderson, A.; Cohen-Gadol, A.; Hundley, H.A. Adenosine Deaminase That Acts on RNA 3 (ADAR3) Binding to Glutamate Receptor Subunit B Pre-MRNA Inhibits RNA Editing in Glioblastoma. J. Biol. Chem. 2017, 292, 4335. [Google Scholar] [CrossRef]
- Lehmann, K.A.; Bass, B.L. The Importance of Internal Loops within RNA Substrates of ADAR1. J. Mol. Biol. 1999, 291, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Polson, A.G.; Bass, B.L. Preferential Selection of Adenosines for Modification by Double-Stranded RNA Adenosine Deaminase. EMBO J. 1994, 13, 5711. [Google Scholar] [CrossRef] [PubMed]
- Nishikura, K.; Yoo, C.; Kim, U.; Murray, J.M.; Estes, P.A.; Cash, F.E.; Liebhaber, S.A. Substrate Specificity of the DsRNA Unwinding/Modifying Activity. EMBO J. 1991, 10, 3532. [Google Scholar] [CrossRef] [PubMed]
- Källman, A.M.; Sahlin, M.; Öhman, M. ADAR2 A→I Editing: Site Selectivity and Editing Efficiency Are Separate Events. Nucleic Acids Res. 2003, 31, 4881. [Google Scholar] [CrossRef]
- Wong, S.K.; Sato, S.; Lazinski, D.W. Substrate Recognition by ADAR1 and ADAR2. RNA 2001, 7, 858. [Google Scholar] [CrossRef] [PubMed]
- Eggington, J.M.; Greene, T.; Bass, B.L. Predicting Sites of ADAR Editing in Double-Stranded RNA. Nat. Commun. 2011, 2, 319. [Google Scholar] [CrossRef] [PubMed]
- Stefl, R.; Oberstrass, F.C.; Hood, J.L.; Jourdan, M.; Zimmermann, M.; Skrisovska, L.; Maris, C.; Peng, L.; Hofr, C.; Emeson, R.B.; et al. The Solution Structure of the ADAR2 DsRBM-RNA Complex Reveals a Sequence-Specific Readout of the Minor Groove. Cell 2010, 143, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Stefl, R.; Xu, M.; Skrisovska, L.; Emeson, R.B.; Allain, F.H.T. Structure and Specific RNA Binding of ADAR2 Double-Stranded RNA Binding Motifs. Structure 2006, 14, 345–355. [Google Scholar] [CrossRef]
- Ryter, J.M.; Schultz, S.C. Molecular Basis of Double-Stranded RNA-Protein Interactions: Structure of a DsRNA-Binding Domain Complexed with DsRNA. EMBO J. 1998, 17, 7513. [Google Scholar] [CrossRef]
- Thomas, J.M.; Beal, P.A. How Do ADARs Bind RNA? New Protein-RNA Structures Illuminate Substrate Recognition by the RNA Editing ADARs. Bioessays 2017, 39, 4. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.J.; Beal, P.A. Effects of Aicardi-Goutières Syndrome Mutations Predicted from ADAR-RNA Structures. RNA Biol. 2017, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Doherty, E.E.; Xie, Y.; Padyana, A.K.; Fang, F.; Zhang, Y.; Karki, A.; Lebrilla, C.B.; Siegel, J.B.; Beal, P.A. High-Throughput Mutagenesis Reveals Unique Structural Features of Human ADAR1. Nat. Commun. 2020, 11, 5130. [Google Scholar] [CrossRef] [PubMed]
- Stephens, O.M.; Haudenschild, B.L.; Beal, P.A. The Binding Selectivity of ADAR2′s DsRBMs Contributes to RNA-Editing Selectivity. Chem. Biol. 2004, 11, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, M.; Samuel, C.E. Chimeric Double-Stranded RNA-Specific Adenosine Deaminase ADAR1 Proteins Reveal Functional Selectivity of Double-Stranded RNA-Binding Domains from ADAR1 and Protein Kinase PKR. Proc. Natl. Acad. Sci. USA 2000, 97, 12541–12546. [Google Scholar] [CrossRef]
- Tian, B.; Bevilacqua, P.C.; Diegelman-Parente, A.; Mathews, M.B. The Double-Stranded-RNA-Binding Motif: Interference and Much More. Nat. Rev. Mol. Cell Biol. 2004, 5, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Fierro-Monti, I.; Mathews, M.B. Proteins Binding to Duplexed RNA: One Motif, Multiple Functions. Trends Biochem. Sci. 2000, 25, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Grünert, S.; Adams, J.; Micklem, D.R.; Proctor, M.R.; Freund, S.; Bycroft, M.; Johnston, D.S.; Varani, G. RNA Recognition by a Staufen Double-Stranded RNA-Binding Domain. EMBO J. 2000, 19, 1009. [Google Scholar] [CrossRef] [PubMed]
- St Johnston, D.; Brown, N.H.; Gall, J.G.; Jantsch, M. A Conserved Double-Stranded RNA-Binding Domain. Proc. Natl. Acad. Sci. USA 1992, 89, 10983. [Google Scholar] [CrossRef] [PubMed]
- Dlakić, M. DUF283 Domain of Dicer Proteins Has a Double-Stranded RNA-Binding Fold. Bioinformatics 2006, 22, 2711–2714. [Google Scholar] [CrossRef] [PubMed]
- Saunders, L.R.; Barber, G.N. The DsRNA Binding Protein Family: Critical Roles, Diverse Cellular Functions. FASEB J. 2003, 17, 961–983. [Google Scholar] [CrossRef] [PubMed]
- Palavicini, J.P.; Correa-Rojas, R.A.; Rosenthal, J.J.C. Extra Double-Stranded RNA Binding Domain (DsRBD) in a Squid RNA Editing Enzyme Confers Resistance to High Salt Environment. J. Biol. Chem. 2012, 287, 17764. [Google Scholar] [CrossRef] [PubMed]
- Barraud, P.; Banerjee, S.; Mohamed, W.I.; Jantsch, M.F.; Allain, F.H.T. A Bimodular Nuclear Localization Signal Assembled via an Extended Double-Stranded RNA-Binding Domain Acts as an RNA-Sensing Signal for Transportin 1. Proc. Natl. Acad. Sci. USA 2014, 111, E1852–E1861. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Drakas, R.; Nishikura, K. Mutagenic Analysis of Double-Stranded RNA Adenosine Deaminase, a Candidate Enzyme for RNA Editing of Glutamate-Gated Ion Channel Transcripts. J. Biol. Chem. 1995, 270, 17098–17105. [Google Scholar] [CrossRef]
- Poulsen, H.; Nilsson, J.; Damgaard, C.K.; Egebjerg, J.; Kjems, J. CRM1 Mediates the Export of ADAR1 through a Nuclear Export Signal within the Z-DNA Binding Domain. Mol. Cell. Biol. 2001, 21, 7871. [Google Scholar] [CrossRef] [PubMed]
- Savva, Y.A.; Rieder, L.E.; Reenan, R.A. The ADAR Protein Family. Genome Biol. 2012, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- George, C.X.; Samuel, C.E. Human RNA-Specific Adenosine Deaminase ADAR1 Transcripts Possess Alternative Exon 1 Structures That Initiate from Different Promoters, One Constitutively Active and the Other Interferon Inducible. Proc. Natl. Acad. Sci. USA 1999, 96, 4621–4626. [Google Scholar] [CrossRef]
- Nie, Y.; Zhao, Q.; Su, Y.; Yang, J.H. Subcellular Distribution of ADAR1 Isoforms Is Synergistically Determined by Three Nuclear Discrimination Signals and a Regulatory Motif. J. Biol. Chem. 2004, 279, 13249–13255. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Strehblow, A.; Taschner, A.; Schopoff, S.; Pasierbek, P.; Jantsch, M.F. RNA-Regulated Interaction of Transportin-1 and Exportin-5 with the Double-Stranded RNA-Binding Domain Regulates Nucleocytoplasmic Shuttling of ADAR1. Mol. Cell. Biol. 2009, 29, 1497. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Barraud, P. Functions of Double-Stranded RNA-Binding Domains in Nucleocytoplasmic Transport. RNA Biol. 2014, 11, 1232. [Google Scholar] [CrossRef]
- Doyle, M.; Jantsch, M.F. Distinct in Vivo Roles for Double-Stranded RNA-Binding Domains of the Xenopus RNA-Editing Enzyme ADAR1 in Chromosomal Targeting. J. Cell Biol. 2003, 161, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.; Gommans, W.M. Identification of a Selective Nuclear Import Signal in Adenosine Deaminases Acting on RNA. Nucleic Acids Res. 2009, 37, 5829. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.P.; Zhu, H.J.; Baldini, A.; Charnsangavej, C.; Chan, L. Dimeric Structure of a Human Apolipoprotein B MRNA Editing Protein and Cloning and Chromosomal Localization of Its Gene. Proc. Natl. Acad. Sci. USA 1994, 91, 8522–8526. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.P.; Keller, W. An Adenosine Deaminase That Generates Inosine at the Wobble Position of TRNAs. Science 1999, 286, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Betts, L.; Xiang, S.; Short, S.A.; Wolfenden, R.; Carter, C.W. Cytidine Deaminase. The 2.3 A Crystal Structure of an Enzyme: Transition-State Analog Complex. J. Mol. Biol. 1994, 235, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Mann, B.R.; Anshu, A.; Mannan, M.A.U. Activation of Protein Kinase PKR Requires Dimerization-Induced Cis-Phosphorylation within the Activation Loop. J. Biol. Chem. 2014, 289, 5747–5757. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, D.; Zenno, S.; Lee, W.C.; Nagata, K.; Saigo, K.; Tanokura, M. Homodimeric Structure and Double-Stranded RNA Cleavage Activity of the C-Terminal RNase III Domain of Human Dicer. J. Mol. Biol. 2007, 374, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Gleghorn, M.L.; Gong, C.; Kielkopf, C.L.; Maquat, L.E. Staufen1 Dimerizes through a Conserved Motif and a Degenerate DsRNA-Binding Domain to Promote MRNA Decay. Nat. Struct. Mol. Biol. 2013, 20, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Jaikaran, D.C.J.; Collins, C.H.; MacMillan, A.M. Adenosine to Inosine Editing by ADAR2 Requires Formation of a Ternary Complex on the GluR-B R/G Site. J. Biol. Chem. 2002, 277, 37624–37629. [Google Scholar] [CrossRef]
- Poulsen, H.; Jorgensen, R.; Heding, A.; Nielsen, F.C.; Bonven, B.; Egebjerg, J. Dimerization of ADAR2 Is Mediated by the Double-Stranded RNA Binding Domain. RNA 2006, 12, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Keegan, L.P.; Ring, G.M.; O’Connell, M.A. An ADAR That Edits Transcripts Encoding Ion Channel Subunits Functions as a Dimer. EMBO J. 2003, 22, 3430. [Google Scholar] [CrossRef] [PubMed]
- Chilibeck, K.A.; Wu, T.; Liang, C.; Schellenberg, M.J.; Gesner, E.M.; Lynch, J.M.; MacMillan, A.M. FRET Analysis of in Vivo Dimerization by RNA-Editing Enzymes. J. Biol. Chem. 2006, 281, 16530–16535. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.; Nishikura, K. RNA Binding-Independent Dimerization of Adenosine Deaminases Acting on RNA and Dominant Negative Effects of Nonfunctional Subunits on Dimer Functions. J. Biol. Chem. 2007, 282, 16061. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, X.; Li, Z.; Wei, J.; Tian, Y. Splicing Variants of ADAR2 and ADAR2-Mediated RNA Editing in Glioma. Oncol. Lett. 2016, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; O‘Connell, M.A.; Keller, W. Two Forms of Human Double-Stranded RNA-Specific Editase 1 (HRED1) Generated by the Insertion of an Alu Cassette. RNA 1997, 3, 463. [Google Scholar]
- Rueter, S.M.; Dawson, T.R.; Emeson, R.B. Regulation of Alternative Splicing by RNA Editing. Nature 1999, 399, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Hajji, K.; Sedmík, J.; Cherian, A.; Amoruso, D.; Keegan, L.P.; O’connell, M.A. ADAR2 Enzymes: Efficient Site-Specific RNA Editors with Gene Therapy Aspirations. RNA 2022, 28, 1297. [Google Scholar] [CrossRef] [PubMed]
- Huntley, M.A.; Lou, M.; Goldstein, L.D.; Lawrence, M.; Dijkgraaf, G.J.P.; Kaminker, J.S.; Gentleman, R. Complex Regulation of ADAR-Mediated RNA-Editing across Tissues. BMC Genom. 2016, 17, 61. [Google Scholar] [CrossRef]
- Maas, S.; Gommans, W.M. Novel Exon of Mammalian ADAR2 Extends Open Reading Frame. PLoS ONE 2009, 4, e4225. [Google Scholar] [CrossRef] [PubMed]
- Thuy-Boun, A.S.; Thomas, J.M.; Grajo, H.L.; Palumbo, C.M.; Park, S.; Nguyen, L.T.; Fisher, A.J.; Beal, P.A. Asymmetric Dimerization of Adenosine Deaminase Acting on RNA Facilitates Substrate Recognition. Nucleic Acids Res. 2020, 48, 7958–7972. [Google Scholar] [CrossRef] [PubMed]
- Mboukou, A.; Rajendra, V.; Messmer, S.; Catala, M.; Tisné, C.; Jantsch, M.F.; Barraud, P. Dimerization of ADAR1 Modulates Site-Specificity of RNA Editing. bioRxiv 2023, 570066. [Google Scholar] [CrossRef]
- Wang, A.H.J.; Quigley, G.J.; Kolpak, F.J.; Crawford, J.L.; Van Boom, J.H.; Van Der Marel, G.; Rich, A. Molecular Structure of a Left-Handed Double Helical DNA Fragment at Atomic Resolution. Nature 1979, 282, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, A.; Placido, D.; Maas, S.; Brown, B.A.; Lowenhaupt, K.; Rich, A. The Crystal Structure of the Zβ Domain of the RNA-Editing Enzyme ADAR1 Reveals Distinct Conserved Surfaces Among Z-Domains. J. Mol. Biol. 2005, 351, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Lowenhaupt, K.; Spitzner, J.; Rich, A. Chicken Double-Stranded RNA Adenosine Deaminase Has Apparent Specificity for Z-DNA. Proc. Natl. Acad. Sci. USA 1995, 92, 7554. [Google Scholar] [CrossRef]
- Brown, B.A.; Lowenhaupt, K.; Wilbert, C.M.; Hanlon, E.B.; Rich, A. The Zα Domain of the Editing Enzyme DsRNA Adenosine Deaminase Binds Left-Handed Z-RNA as Well as Z-DNA. Proc. Natl. Acad. Sci. USA 2000, 97, 13536. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Rich, A. The Role of Binding Domains for DsRNA and Z-DNA in the in Vivo Editing of Minimal Substrates by ADAR1. Proc. Natl. Acad. Sci. USA 2001, 98, 12137. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Rigby, R.E.; Young, G.R.; Hvidt, A.K.; Davis, T.; Tan, T.K.; Bridgeman, A.; Townsend, A.R.; Kassiotis, G.; Rehwinkel, J. Adenosine-to-Inosine Editing of Endogenous Z-Form RNA by the Deaminase ADAR1 Prevents Spontaneous MAVS-Dependent Type I Interferon Responses. Immunity 2021, 54, 1961–1975.e5. [Google Scholar] [CrossRef] [PubMed]
- Koeris, M.; Funke, L.; Shrestha, J.; Rich, A.; Maas, S. Modulation of ADAR1 Editing Activity by Z-RNA in Vitro. Nucleic Acids Res. 2005, 33, 5362–5370. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Wang, J.C. Supercoiling of the DNA Template during Transcription. Proc. Natl. Acad. Sci. USA 1987, 84, 7027. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Rich, A. Left-Handed Z-DNA: Structure and Function. Genetica 1999, 106, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Li, H.; Zhao, J.; Pervushin, K.; Lowenhaupt, K.; Schwartz, T.U.; Dröge, P. Alternate RRNA Secondary Structures as Regulators of Translation. Nat. Struct. Mol. Biol. 2011, 18, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.B.; Kim, Y.G.; Rich, A. Z-DNA-Binding Proteins Can Act as Potent Effectors of Gene Expression in Vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 16671. [Google Scholar] [CrossRef]
- Song, B.; Shiromoto, Y.; Minakuchi, M.; Nishikura, K. The Role of RNA Editing Enzyme ADAR1 in Human Disease. Wiley Interdiscip. Rev. RNA 2022, 13, e1665. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Adenosine Deaminase Acting on RNA (ADAR1), a Suppressor of Double-Stranded RNA–Triggered Innate Immune Responses. J. Biol. Chem. 2019, 294, 1720. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A. Z-DNA and Z-RNA in Human Disease. Commun. Biol. 2019, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.C.; Li, Y.; Ng, S.K. The Role of the Z-DNA Binding Domain in Innate Immunity and Stress Granules. Front. Immunol. 2021, 11, 625504. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Adenosine Deaminases Acting on RNA (ADARs) Are Both Antiviral and Proviral Dependent upon the Virus. Virology 2011, 411, 193. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Langland, J.O. When Two Strands Are Better than One: The Mediators and Modulators of the Cellular Responses to Double-Stranded RNA. Virology 1996, 219, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, R.; Schmid, A.; Eschle, D.; Baczko, K.; ter Meulen, V.; Billeter, M.A. Biased Hypermutation and Other Genetic Changes in Defective Measles Viruses in Human Brain Infections. Cell 1988, 55, 265. [Google Scholar] [CrossRef] [PubMed]
- Barraud, P.; Allain, F.H.T. ADAR Proteins: Double-Stranded RNA and Z-DNA Binding Domains. Curr. Top. Microbiol. Immunol. 2012, 353, 60. [Google Scholar] [CrossRef] [PubMed]
- Schade, M.; Turner, C.J.; Kühne, R.; Schmieder, P.; Lowenhaupt, K.; Herbert, A.; Rich, A.; Oschkinat, H. The Solution Structure of the Za Domain of the Human RNA Editing Enzyme ADAR1 Reveals a Prepositioned Binding Surface for Z-DNA. Proc. Natl. Acad. Sci. USA 1999, 96, 12465–12470. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.; Rould, M.A.; Lowenhaupt, K.; Herbert, A.; Rich, A. Crystal Structure of the Zα Domain of the Human Editing Enzyme ADAR1 Bound to Left-Handed Z-DNA. Science 1999, 284, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Bhuiya, M.W.; Rittery, E.M.; Brown, B.A. Structural Features of the ADAR Family of Enzymes and Their Substrates. In RNA and DNA Editing: Molecular Mechanisms and Their Integration into Biological Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 340–368. [Google Scholar] [CrossRef]
- Placido, D.; Brown, B.A.; Lowenhaupt, K.; Rich, A.; Athanasiadis, A. A Left-Handed RNA Double Helix Bound by the Zα Domain of the RNA-Editing Enzyme ADAR1. Structure 2007, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.H.; Lowenhaupt, K.; Rich, A.; Kim, Y.G.; Kyeong, K.K. Crystal Structure of a Junction between B-DNA and Z-DNA Reveals Two Extruded Bases. Nature 2005, 437, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Garvie, C.W.; Wolberger, C. Recognition of Specific DNA Sequences. Mol. Cell 2001, 8, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.C.; Choi, J.; Hwang, H.Y.; Rich, A.; Kim, Y.G.; Kim, K.K. The Structures of Non-CG-Repeat Z-DNAs Co-Crystallized with the Z-DNA-Binding Domain, HZαADAR1. Nucleic Acids Res. 2009, 37, 637. [Google Scholar] [CrossRef]
- Zheng, X.; Park, C.Y.; Park, S.-Y.; Choi, J.; Kim, Y. Structure and Function of Zalpha, a Z Conformation-Specific Nucleic Acid Binding Domain. BioDesign 2015, 3, 143–153. [Google Scholar]
- Schade, M.; Turner, C.J.; Lowenhaupt, K.; Rich, A.; Herbert, A. Structure–Function Analysis of the Z-DNA-Binding Domain Zα of DsRNA Adenosine Deaminase Type I Reveals Similarity to the (α + β) Family of Helix–Turn–Helix Proteins. EMBO J. 1999, 18, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Muralinath, M.; Brandt, T.; Pearcy, M.; Hauns, K.; Lowenhaupt, K.; Jacobs, B.L.; Rich, A. A Role for Z-DNA Binding in Vaccinia Virus Pathogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 6974–6979. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, I.; Englander, M.T.; Adlersberg, M.; Siegal, N.B.; Schmauss, C. Modulation of Serotonin 2C Receptor Editing by Sustained Changes in Serotonergic Neurotransmission. J. Neurosci. 2002, 22, 10532. [Google Scholar] [CrossRef] [PubMed]
- Tifft, C.J.; Adams, D.R. The National Institutes of Health Undiagnosed Diseases Program. Curr. Opin. Pediatr. 2014, 26, 633. [Google Scholar] [CrossRef]
- Canna, M.; Seligman, R. Dealing with the Unknown. Functional Neurological Disorder (FND) and the Conversion of Cultural Meaning. Soc. Sci. Med. 2020, 246, 112725. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar Disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Altevogt, B.M.; Dunlop, J.; Gage, F.H.; Hyman, S.E. Improving and Accelerating Drug Development for Nervous System Disorders. Neuron 2014, 84, 553. [Google Scholar] [CrossRef]
- O’Connell, M.A.; Gerber, A.; Keller, W. Purification of Human Double-Stranded RNA-Specific Editase 1 (HRED1) Involved in Editing of Brain Glutamate Receptor B Pre-MRNA. J. Biol. Chem. 1997, 272, 473–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Li, J.; Zhu, Y.; Zeng, T.; Ding, J.; Min, W.; Huang, F.; Liang, S.; Yuan, K.; Kuang, W.; et al. Targeting ADAR1 with a Novel Small-Molecule for the Treatment of Prostate Cancer. Res. Sq. 2021, 1–39. [Google Scholar] [CrossRef]
- Crews, L.A.; Ma, W.; Ladel, L.; Pham, J.; Balaian, L.; Steel, S.K.; Mondala, P.K.; Diep, R.H.; Wu, C.N.; Mason, C.N.; et al. Reversal of Malignant ADAR1 Splice Isoform Switching with Rebecsinib. Cell Stem Cell 2023, 30, 250–263.e6. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, H.G.; Matos, V.J.; Park, S.; Pham, K.M.; Beal, P.A. Selective Inhibition of ADAR1 Using 8-Azanebularine-Modified RNA Duplexes. Biochemistry 2023, 62, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.N.T.; Tohda, M.; Tezuka, Y.; Matsumoto, K. Influence of an Adenosine Deaminase Inhibitor, Erythro-9-(2-Hydroxy-3-Nonyl) Adenine Hydrochloride, on 5-HT2CR MRNA Editing in Primary Cultured Cortical Cells. Biol. Pharm. Bull. 2010, 33, 527–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choudhry, H. High-Throughput Screening to Identify Potential Inhibitors of the Zα Domain of the Adenosine Deaminase 1 (ADAR1). Saudi J. Biol. Sci. 2021, 28, 6304. [Google Scholar] [CrossRef] [PubMed]
- Broni, E.; Ashley, C.; Velazquez, M.; Khan, S.; Striegel, A.; Sakyi, P.O.; Peracha, S.; Bebla, K.; Sodhi, M.; Kwofie, S.K.; et al. In Silico Discovery of Potential Inhibitors Targeting the RNA Binding Loop of ADAR2 and 5-HT2CR from Traditional Chinese Natural Compounds. Int. J. Mol. Sci. 2023, 24, 12612. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Jing, Q.; Li, Y.; Han, J. RNA Modification: Mechanisms and Therapeutic Targets. Mol. Biomed. 2023, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Sapiro, A.L.; Freund, E.C.; Restrepo, L.; Qiao, H.H.; Bhate, A.; Li, Q.; Ni, J.Q.; Mosca, T.J.; Li, J.B. Zinc Finger RNA-Binding Protein Zn72D Regulates ADAR-Mediated RNA Editing in Neurons. Cell Rep. 2020, 31, 107654. [Google Scholar] [CrossRef] [PubMed]
- Quinones-Valdez, G.; Tran, S.S.; Jun, H.-I.; Bahn, J.H.; Yang, E.-W.; Zhan, L.; Brümmer, A.; Wei, X.; Van Nostrand, E.L.; Pratt, G.A.; et al. Regulation of RNA Editing by RNA-Binding Proteins in Human Cells. Commun. Biol. 2019, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, J.J.; Manguso, R.T.; Cheruiyot, C.K.; Bi, K.; Panda, A.; Iracheta-Vellve, A.; Miller, B.C.; Du, P.P.; Yates, K.B.; Dubrot, J.; et al. Loss of ADAR1 in Tumours Overcomes Resistance to Immune Checkpoint Blockade. Nature 2019, 565, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Zhang, Y.; Liu, J.; Zhao, B. ADAR1-Mediated RNA Editing and Its Role in Cancer. Front. Cell Dev. Biol. 2022, 10, 956649. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Wei, J.; Song, G.; Zhao, Y. A-to-I RNA Editing in Cancer: From Evaluating the Editing Level to Exploring the Editing Effects. Front. Oncol. 2021, 10, 632187. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A. ADAR and Immune Silencing in Cancer. Trends Cancer 2019, 5, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Stafforst, T.; Schneider, M.F. An RNA-Deaminase Conjugate Selectively Repairs Point Mutations. Angew. Chem. Int. Engl. 2012, 51, 11166–11169. [Google Scholar] [CrossRef]
- Woolf, T.M.; Chase, J.M.; Stinchcomb, D.T. Toward the Therapeutic Editing of Mutated RNA Sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 8302. [Google Scholar] [CrossRef]
- Vogel, P.; Moschref, M.; Li, Q.; Merkle, T.; Selvasaravanan, K.D.; Li, J.B.; Stafforst, T. Efficient and Precise Editing of Endogenous Transcripts with SNAP-Tagged ADARs. Nat. Methods 2018, 15, 538. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Gonzalez, M.F.; Vallecillo-Viejo, I.; Yudowski, G.A.; Rosenthal, J.J.C. Correction of Mutations within the Cystic Fibrosis Transmembrane Conductance Regulator by Site-Directed RNA Editing. Proc. Natl. Acad. Sci. USA 2013, 110, 18285–18290. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA Editing with CRISPR-Cas13. Science 2017, 358, 1027. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Gonzalez, M.F.; Diaz Quiroz, J.F.; Rosenthal, J.J.C. Current Strategies for Site-Directed RNA Editing Using ADARs. Methods 2019, 156, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, H.M.; Jantsch, M.F. Site-Directed RNA Editing: Recent Advances and Open Challenges. RNA Biol. 2021, 18, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Attwaters, M. In Vivo RNA Base Editing with Circular RNAs. Nat. Rev. Genet. 2022, 23, 196–197. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.J.; Nourreddine, S.; Katrekar, D.; Savva, Y.; Bose, D.; Long, T.J.; Huss, D.J.; Mali, P. RNA Editing: Expanding the Potential of RNA Therapeutics. Mol. Ther. 2023, 31, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Hanewich-Hollatz, M.H.; Chen, Z.; Hochrein, L.M.; Huang, J.; Pierce, N.A. Conditional Guide RNAs: Programmable Conditional Regulation of CRISPR/Cas Function in Bacterial and Mammalian Cells via Dynamic RNA Nanotechnology. ACS Cent. Sci. 2019, 5, 1241–1249. [Google Scholar] [CrossRef]

| Key Structural Points for ADAR2 Catalytic Domain | Residues |
|---|---|
| Zinc Coordination | H394, E396, C451, and C516. |
| IHP Coordination | Coordination Residues: N391, R400, R401, K518, R522, W523, K629, Y658, K662, Y668, Q669, K672, W687, E689, and D695. |
| Side Chain Contacts: D392, I397, L511, M514, V688, and K690. | |
| Hydrogen Bond Involvement: K483. | |
| RNA Interface/Contact Residues | Coordination Residues: R348, V351, T375, K376, E396, Q488, C451, E455, R481, S486, T490, S495, R510, and K594. |
| Coordination Residues in RNA Binding Loop: N473, R474, K475, and R477. | |
| RNA Binding Loop | A454, R455, I456, F457, S458, P459, H460, E461, P462, I463, L464, E465, E466, P467, A468, D469, R470, H471, P472, N473, R474, K475, A476, and R477. |
| Base Flipping Loop | G487, Q488, and G489. |
| Key Structural Points in Homodimer Structure | Residues |
|---|---|
| Contacts between CDD 1 and dsRNA | Backbone Contacts: T375, C451, K475, S486, T490, and K594. |
| Side Chain Contacts: T375, K376, E396, R455, H460, R474, R477, R481, S486, Q488, R510, and K594. | |
| Contacts between dsRBD-2 and dsRNA | Backbone Contacts: N235, S258, and K281. |
| Side Chain Contacts: N235, E242, S258, H259, R279, N280, K281, and K282. | |
| Dimerization Helix | T501, W502, D503, G504, V505, L506, Q507, G508, and E509. |
| Protein–Protein Contacts in CDD 2 | G452, R455, F457, S458, H460, R481, Q488, T490, R590, and G593. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashley, C.N.; Broni, E.; Miller, W.A., III. ADAR Family Proteins: A Structural Review. Curr. Issues Mol. Biol. 2024, 46, 3919-3945. https://doi.org/10.3390/cimb46050243
Ashley CN, Broni E, Miller WA III. ADAR Family Proteins: A Structural Review. Current Issues in Molecular Biology. 2024; 46(5):3919-3945. https://doi.org/10.3390/cimb46050243
Chicago/Turabian StyleAshley, Carolyn N., Emmanuel Broni, and Whelton A. Miller, III. 2024. "ADAR Family Proteins: A Structural Review" Current Issues in Molecular Biology 46, no. 5: 3919-3945. https://doi.org/10.3390/cimb46050243
APA StyleAshley, C. N., Broni, E., & Miller, W. A., III. (2024). ADAR Family Proteins: A Structural Review. Current Issues in Molecular Biology, 46(5), 3919-3945. https://doi.org/10.3390/cimb46050243