N-3-Methylbutyl-benzisoselenazol-3(2H)-one Exerts Antifungal Activity In Vitro and in a Mouse Model of Vulvovaginal Candidiasis
Abstract
1. Introduction
2. Materials and Methods
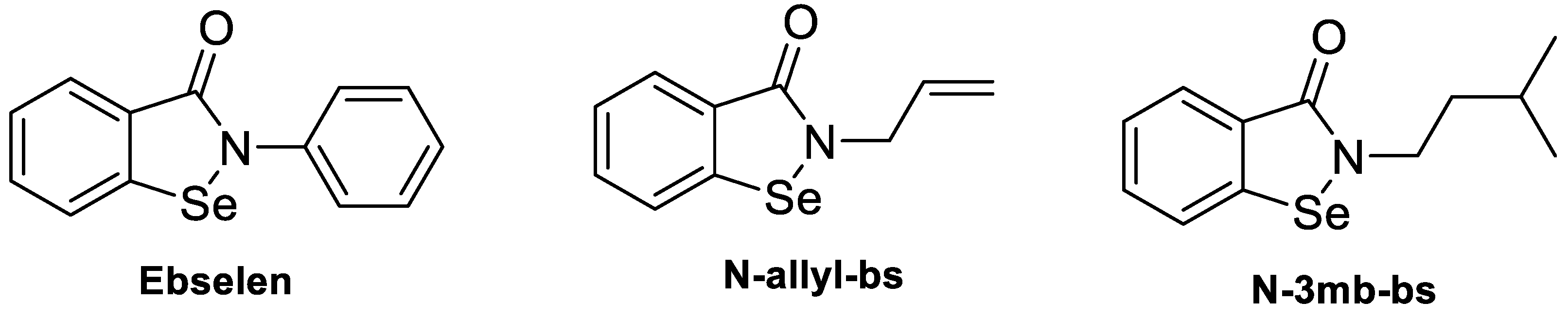
2.1. Chemicals and Cells
2.2. Determination of Minimum Inhibitory Concentration (MIC)
2.3. Medium Acidification Assay
2.4. Cell Viability Assay
2.5. Validation of the Mouse Model of VVC and Intervention Study
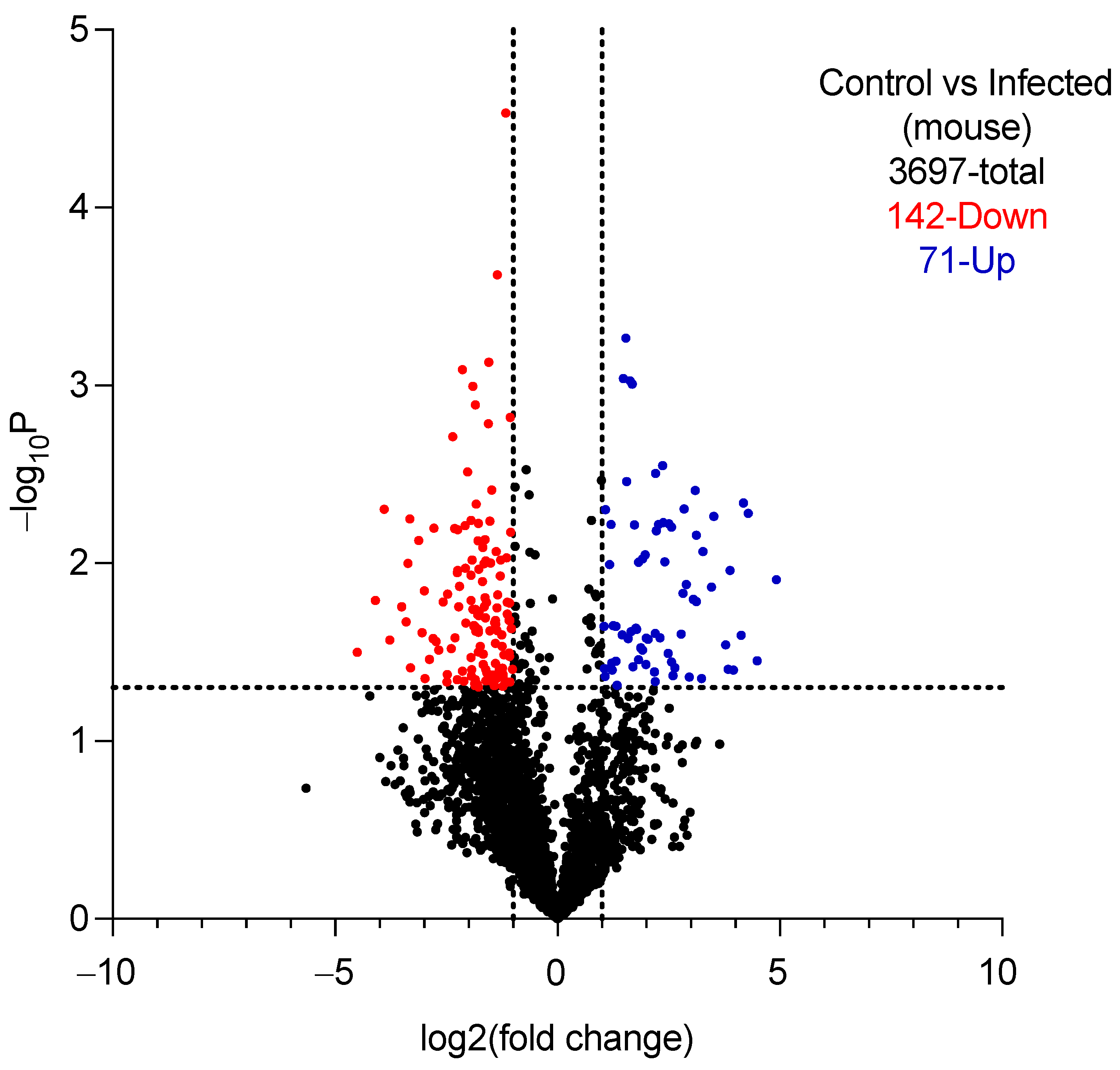
2.5.1. Proteomic Analysis of Vaginal Lavage Fluid
2.5.2. Intervention Treatments
2.6. Immunohistochemistry Analysis
2.7. In Vitro Skin Irritation Test
2.8. Statistical Analysis
3. Results
3.1. Chemical Properties and Determination of MIC
3.2. Half Maximal Inhibitory Concentration (IC50 Values) in KB-3-1 Cells and C. albicans
3.3. Effect on Medium Acidification in C. albicans S1
3.4. Validation of the Mouse Model of VVC and Intervention Study
3.4.1. Validation
3.4.2. Efficacy of Intervention Treatments
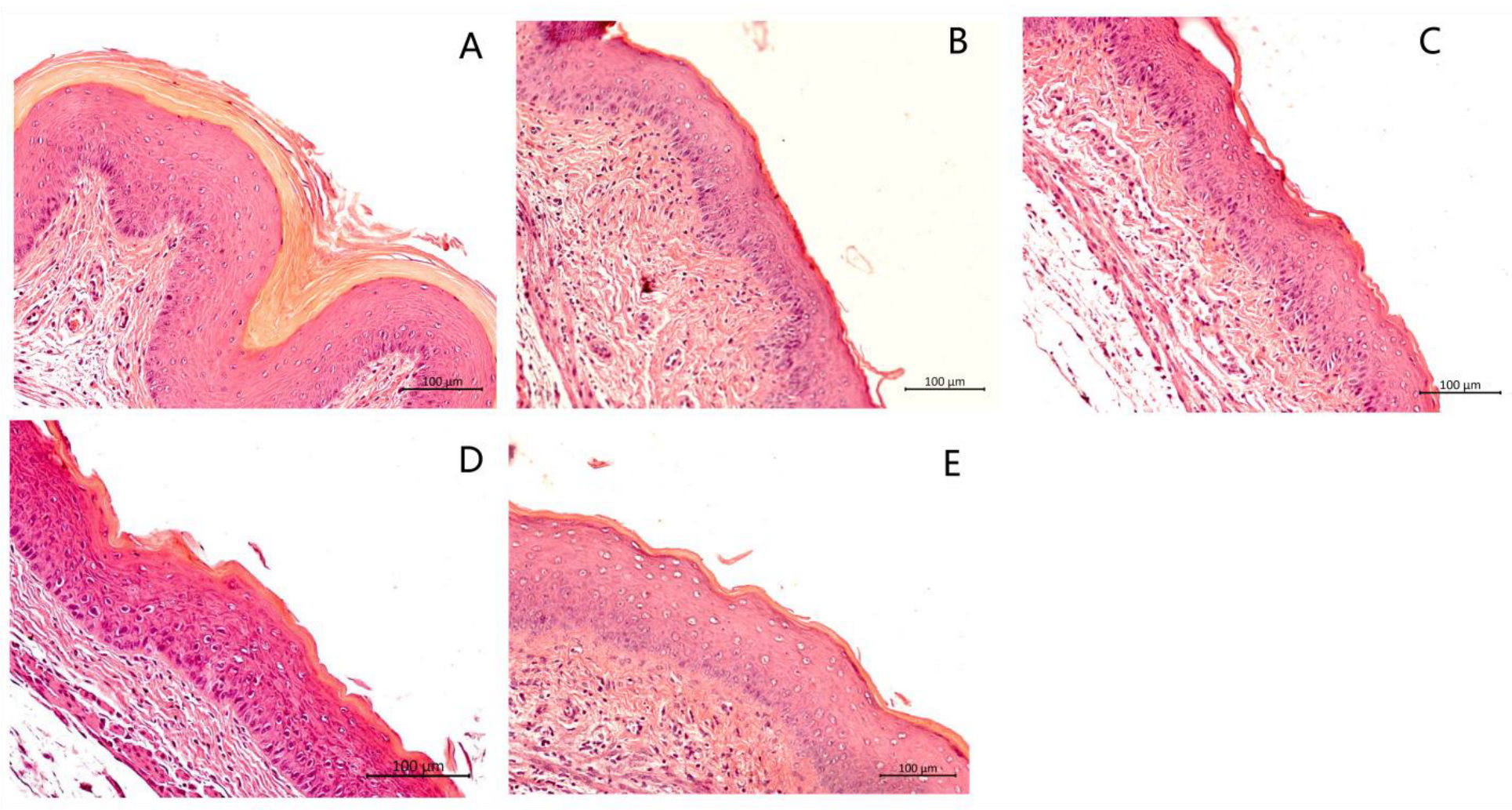
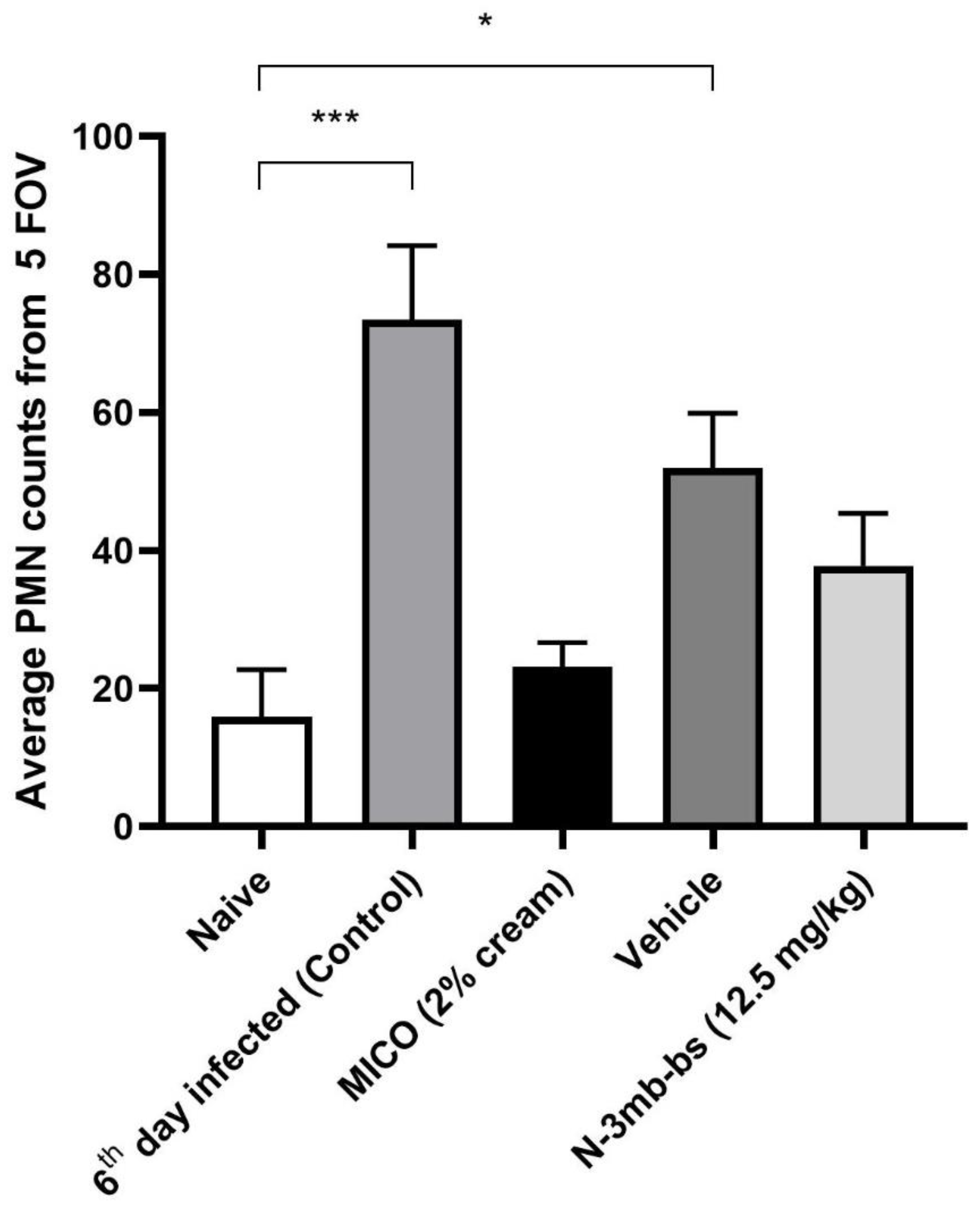
3.5. Histological Analysis of the Vaginal Tissue
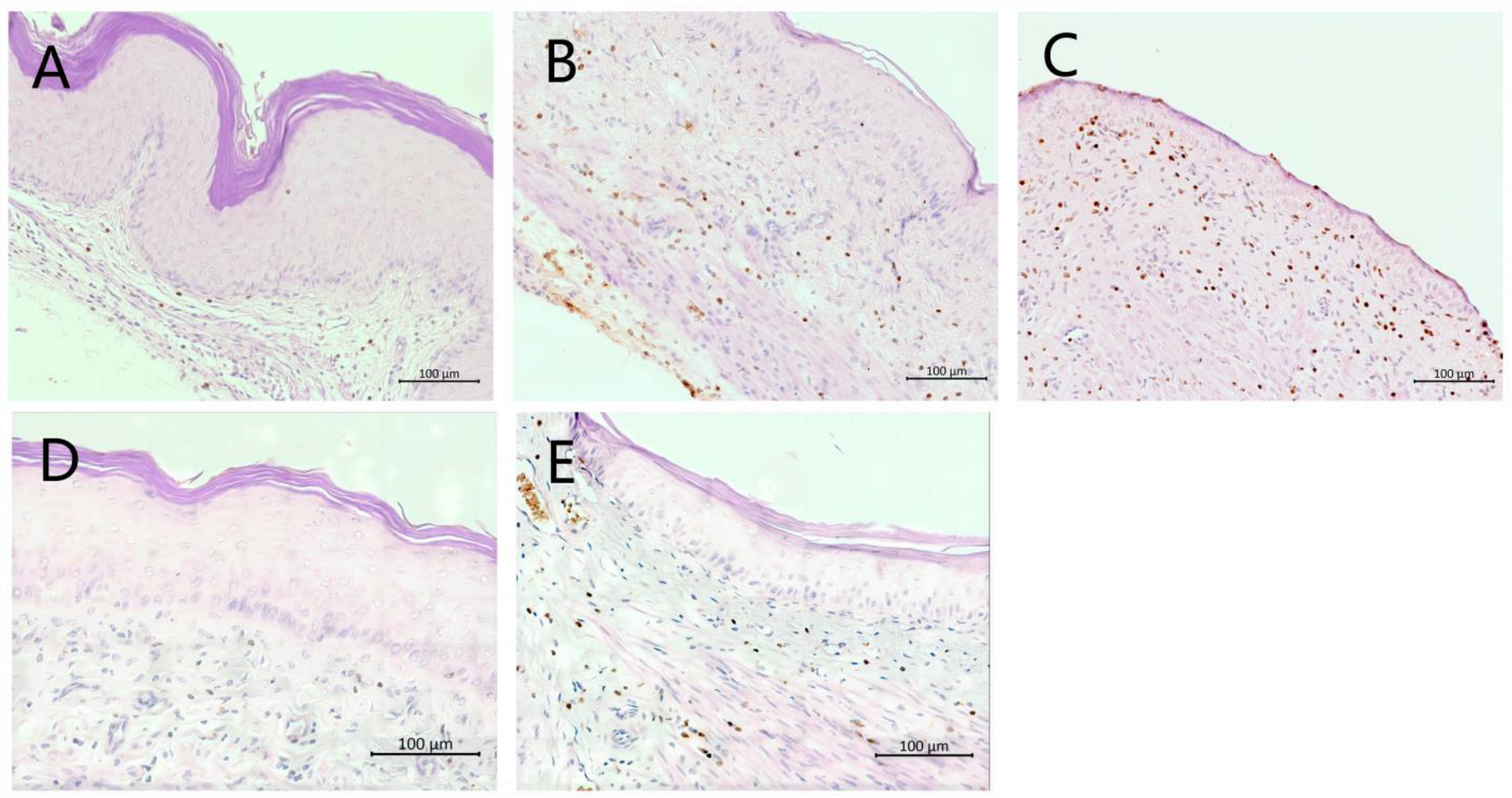
3.6. Immunohistochemical Analysis
3.7. Skin Irritation Test
4. Discussion
5. Conclusions
- N-3mb-bs inhibits the growth of S1 (FLU-sensitive) and S2 (FLU-resistant) strains of C. albicans in vitro (strength);
- N-3mb-bs inhibits Pma1p activity in the C. albicans test strain S1 in vitro (strength);
- N-3mb-bs inhibits the Na+, K+-ATPase obtained from pig (weakness, suggests “off-target” effects may occur in humans);
- C. albicans clinical isolate S1 is an effective inducer of infection and causes a robust vaginal yeast infection in the mouse model of VVC (strength);
- N-3mb-bs reduces vaginal colonization by S1 and relieves inflammation and damage to the vaginal mucosa (strength);
- N-3mb-bs reduces MPO expression and PMN infiltration in the infected vaginal tissues (strength).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hani, U.; Shivakumar, H.G.; Vaghela, R.; Osmani, R.A.; Shrivastava, A. Candidiasis: A Fungal Infection—Current Challenges and Progress in Prevention and Treatment. Infect. Disord.-Drug Targets 2015, 15, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kaur, S. Recent Advances on Vaginal delivery for the Treatment of Vulvovaginal Candidiasis. Curr. Mol. Pharmacol. 2020, 14, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.R.; Bose, S.; Singh, M.; Kumari, P.; Dutta, A.; Utkalaja, B.G.; Patel, S.K.; Acharya, N. Vaccines against Candidiasis: Status, Challenges and Emerging Opportunity. Front. Cell. Infect. Microbiol. 2022, 12, 1002406. [Google Scholar] [CrossRef] [PubMed]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced Topical Drug Delivery System for the Management of Vaginal Candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Lirio, J.; Giraldo, P.C.; Amaral, R.L.; Sarmento, A.C.A.; Costa, A.P.F.; Goncalves, A.K. Antifungal (Oral and Vaginal) Therapy for Recurrent Vulvovaginal Candidiasis: A Systematic Review Protocol. BMJ Open 2019, 9, e027489. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A. Vulvovaginal Candida albicans Infections: Pathogenesis, Immunity and Vaccine Prospects. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, E.D.; Young, T.; Holmes, H. Interventions for the Prevention and Management of Oropharyngeal Candidiasis Associated with HIV infection in Adults and Children. Cochrane Database Syst. Rev. 2010, CD003940. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Ray, S.; George, A.T.; Swaminathan, N. Interventions for Prevention and Treatment of Vulvovaginal Candidiasis in Women with HIV Infection. Cochrane Database Syst. Rev. 2011, CD008739. [Google Scholar] [CrossRef]
- Menon, S.; Vartak, R.; Patel, K.; Billack, B. Evaluation of the Antifungal Activity of an Ebselen-Loaded Nanoemulsion in a Mouse Model of Vulvovaginal Candidiasis. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102428. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Dong, C.; Zhao, Y.; Zhou, J.; Yuan, C.; Zou, L. Mechanisms of Ebselen as a Therapeutic and its Pharmacology Applications. Future Med. Chem. 2020, 12, 2141–2160. [Google Scholar] [CrossRef]
- Chan, G.; Hardej, D.; Santoro, M.; Lau-Cam, C.; Billack, B. Evaluation of the Antimicrobial Activity of Ebselen: Role of the Yeast Plasma Membrane H+-ATPase. J. Biochem. Mol. Toxicol. 2007, 21, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Soteropoulos, P.; Vaz, T.; Santangelo, R.; Paderu, P.; Huang, D.Y.; Tamás, M.J.; Perlin, D.S. Molecular characterization of the plasma membrane H(+)-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob. Agents Chemother. 2000, 44, 2349–2355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orie, N.N.; Warren, A.R.; Basaric, J.; Lau-Cam, C.; Pietka-Ottlik, M.; Mlochowski, J.; Billack, B. In Vitro Assessment of the Growth and Plasma Membrane H(+)-ATPase Inhibitory Activity of Ebselen and Structurally Related Selenium- and Sulfur-Containing Compounds in Candida albicans. J. Biochem. Mol. Toxicol. 2017, 31, 6. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Patki, M.; Menon, S.; Jablonski, J.; Mediouni, S.; Fu, Y.; Valente, S.T.; Billack, B.; Patel, K. Beta-Cyclodextrin Polymer/Soluplus(R) Encapsulated Ebselen Ternary Complex (EbetapolySol) as a Potential Therapy for Vaginal Candidiasis and Pre-exposure Prophylactic for HIV. Int. J. Pharmaceut. 2020, 589, 119863. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Liang, X.; Vartak, R.; Patel, K.; Di Stefano, A.; Cacciatore, I.; Marinelli, L.; Billack, B. Antifungal Activity of Novel Formulations Based on Terpenoid Prodrugs against C. albicans in a Mouse Model. Pharmaceutics 2021, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef] [PubMed]
- Obieziurska, M.; Pacula, A.J.; Dlugosz-Pokorska, A.; Krzeminski, M.; Janecka, A.; Scianowski, J. Bioselectivity Induced by Chirality of New Terpenyl Organoselenium Compounds. Materials 2019, 12, 3579. [Google Scholar] [CrossRef]
- Kaczor-Keller, K.B.; Pawlik, A.; Scianowski, J.; Pacuła, A.; Obieziurska, M.; Marcheggiani, F.; Cirilli, I.; Tiano, L.; Antosiewicz, J. In Vitro Anti-Prostate Cancer Activity of Two Ebselen Analogues. Pharmaceuticals 2020, 13, 47. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Wojtowicz, A.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Ścianowski, J. New Glutathione Peroxidase Mimetics-Insights into Antioxidant and Cytotoxic Activity. Bioorganic Med. Chem. 2017, 25, 126–131. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Yano, J.; Fidel, P.L., Jr. Protocols for Vaginal Inoculation and Sample Collection in the Experimental Mouse Model of Candida vaginitis. J. Visual Exp. 2011, 58, e3382. [Google Scholar] [CrossRef]
- Barth, V.C.; Chauhan, U.; Zeng, J.; Su, X.; Zheng, H.; Husson, R.N.; Woychik, N.A. Mycobacterium tuberculosis VapC4 Toxin Engages Small ORFs to Initiate an Integrated Oxidative and Copper Stress Response. Proc. Natl. Acad. Sci. USA 2021, 118, e2022136118. [Google Scholar] [CrossRef] [PubMed]
- Beavis, R.C. Using the Global Proteome Machine for Protein Identification. Methods Mol. Biol. 2006, 328, 217–228. [Google Scholar] [PubMed]
- Brown, K.E.; Brunt, E.M.; Heinecke, J.W. Immunohistochemical Detection of Myeloperoxidase and its Oxidation Products in Kupffer Cells of Human Liver. Am. J. Pathol. 2001, 159, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development (OECD). Test Guideline No. 439. In Vitro Skin Irritation (Reconstructed Human Epidermis Test Method); Organisation for Economic Cooperation and Development: Paris, France, 2021. [Google Scholar] [CrossRef]
- Liang, X.; Pacuła-Miszewska, A.J.; Obieziurska-Fabisiak, M.; Vartak, R.; Mao, G.; Patel, K.; Fedosova, N.U.; Ścianowski, J.; Billack, B. Selected N-Terpenyl Organoselenium Compounds Possess Antimycotic Activity in Vitro and in a Mouse Model of Vulvovaginal Candidiasis. Molecules 2023, 28, 7377. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Brown, C.L.; Haber, J.E. Membrane Potential Defect in Hygromycin B-Resistant pma1 Mutants of Saccharomyces cerevisiae. J. Biol. Chem. 1988, 263, 18118–18122. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.; Morsomme, P. The Plasma Membrane H(+)-ATPase, a Simple Polypeptide with a Long History. Yeast 2019, 36, 201–210. [Google Scholar] [CrossRef]
- Klodos, I.; Esmann, M.; Post, R.L. Large-Scale Preparation of Sodium-Potassium ATPase from Kidney Outer Medulla. Kidney Int. 2002, 62, 2097–2100. [Google Scholar] [CrossRef]
- Esmann, M. ATPase and Phosphatase Activity of Na+, K+-ATPase: Molar and Specific Activity, Protein Determination. In Methods in Enzymology; Academic Press, Inc.: London, UK, 1988; Volume 156, pp. 105–115. [Google Scholar]
- Gil-Bona, A.; Parra-Giraldo, C.M.; Hernáez, M.L.; Reales-Calderon, J.A.; Solis, N.V.; Filler, S.G.; Monteoliva, L.; Gil, C. Candida albicans Cell Shaving Uncovers New Proteins Involved in Cell Wall Integrity, Yeast to Hypha Transition, Stress Response and Host-Pathogen interaction. J. Proteom. 2015, 127, 340–351. [Google Scholar] [CrossRef]
- Alloush, H.M.; López-Ribot, J.L.; Masten, B.J.; Chaffin, W.L. 3-Phosphoglycerate Kinase: A Glycolytic Enzyme Protein Present in the Cell Wall of Candida albicans. Microbiology 1997, 43, 321–330. [Google Scholar] [CrossRef]
- Angiolella, L.; Facchin, M.; Stringaro, A.; Maras, B.; Simonetti, N.; Cassone, A. Identification of a Glucan-Associated Enolase as a Main Cell Wall Protein of Candida albicans and an Indirect Target of Lipopeptide Antimycotics. J. Infect. Dis. 1996, 173, 684–690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gil, M.L.; Villamón, E.; Monteagudo, C.; Gozalbo, D.; Martínez, J.P. Clinical Strains of Candida albicans Express the Surface Antigen Glyceraldehyde 3-Phosphate Dehydrogenase in vitro and in Infected Tissues. FEMS Immunol. Med. Microbiol. 1999, 23, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Wu, C.C.; Chung, W.L.; Lee, F.S. Differential Secretion of Sap4-6 Proteins in Candida albicans during Hyphae Formation. Microbiology 2002, 148, 3743–3754. [Google Scholar] [CrossRef] [PubMed]
- Aoki, W.; Kitahara, N.; Miura, N.; Morisaka, H.; Yamamoto, Y.; Kuroda, K.; Ueda, M. Comprehensive Characterization of Secreted Aspartic Proteases Encoded by a Virulence Gene Family in Candida albicans. J. Biochem. 2011, 150, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Pitarch, A.; Díez-Orejas, R.; Molero, G.; Pardo, M.; Sánchez, M.; Gil, C.; Nombela, C. Analysis of the Serologic Response to Systemic Candida albicans Infection in a Murine Model. Proteomics 2001, 1, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Ma, C.F.; Shi, L.N.; Lu, J.F.; Wang, Y.; Huang, M.; Kong, Q.Q. Diagnostic Value of Immunoglobulin G Antibodies Against Candida Enolase and Fructose-Bisphosphate Aldolase for Candidemia. BMC Infect. Dis. 2013, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.N.; Solis, N.V.; Phan, Q.T.; Bajwa, J.S.; Kashleva, H.; Thompson, A.; Liu, Y.; Dongari-Bagtzoglou, A.; Edgerton, M.; Filler, S.G. Host Cell Invasion and Virulence Mediated by Candida albicans Ssa1. PLoS Pathog. 2010, 6, e1001181. [Google Scholar] [CrossRef]
- Qiu, X.R.; Shen, C.R.; Jiang, L.W.; Ji, P.; Zhang, Y.; Hou, W.T.; Zhang, W.; Shen, H.; An, M.M. Ssa1-Targeted Antibody Prevents Host Invasion by Candida albicans. Front. Microbiol. 2023, 14, 1182914. [Google Scholar] [CrossRef]
- Schaller, M.; Januschke, E.; Schackert, C.; Woerle, B.; Korting, H.C. Different Isoforms of Secreted Aspartyl Proteinases (Sap) are Expressed by Candida albicans During Oral and Cutaneous Candidosis in vivo. J. Med. Microbiol. 2001, 50, 743–747. [Google Scholar] [CrossRef]
- Felk, A.; Kretschmar, M.; Albrecht, A.; Schaller, M.; Beinhauer, S.; Nichterlein, T.; Sanglard, D.; Korting, H.C.; Schäfer, W.; Hube, B. Candida albicans Hyphal Formation and the Expression of the Efg1-Regulated Proteinases Sap4 to Sap6 Are Required for the Invasion of Parenchymal Organs. Infect. Immun. 2002, 70, 3689–3700. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wilhelmus, K.R.; Hube, B. The Role of Secreted Aspartyl Proteinases in Candida albicans Keratitis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3559–3565. [Google Scholar] [CrossRef] [PubMed]
- Röhm, M.; Lindemann, E.; Hiller, E.; Ermert, D.; Lemuth, K.; Trkulja, D.; Sogukpinar, O.; Brunner, H.; Rupp, S.; Urban, C.F.; et al. A Family of Secreted Pathogenesis-Related Proteins in Candida albicans. Mol. Microbiol. 2013, 87, 132–151. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.; Petre, A.; Youhnovski, N.; Prömm, F.; Schirle, M.; Schumm, M.; Pero, R.S.; Doyle, A.; Checkel, J.; Kita, H.; et al. Post-Translational Tyrosine Nitration of Eosinophil Granule Toxins Mediated by Eosinophil Peroxidase. J. Biol. Chem. 2008, 283, 28629–28640. [Google Scholar] [CrossRef] [PubMed]
- Mii, S.; Murakumo, Y.; Asai, N.; Jijiwa, M.; Hagiwara, S.; Kato, T.; Asai, M.; Enomoto, A.; Ushida, K.; Sobue, S.; et al. Epidermal Hyperplasia and Appendage Abnormalities in Mice Lacking CD109. Am. J. Pathol. 2012, 181, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Vluggens, A.; Andreoletti, P.; Viswakarma, N.; Jia, Y.; Matsumoto, K.; Kulik, W.; Khan, M.; Huang, J.; Guo, D.; Yu, S.; et al. Reversal of Mouse Acyl-CoA oxidase 1 (ACOX1) Null Phenotype by Human ACOX1b isoform [corrected]. Lab. Investig. 2010, 90, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, N.; Zhao, S.; Chen, Z.; Zhang, W.; Yan, F.; Liao, H.; Chi, K. Carboxypeptidase A4 Promotes Cardiomyocyte Hypertrophy Through Activating PI3K-AKT-mTOR Signaling. Biosci. Rep. 2020, 40, BSR20200669. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Mihindukulasuriya, K.; Den, Z.; Kowalczyk, A.P.; Calkins, C.C.; Ishiko, A.; Shimizu, A.; Koch, P.J. Assessment of Splice Variant-Specific Functions of Desmocollin 1 in the Skin. Mol. Cell Biol. 2004, 24, 154–163. [Google Scholar] [CrossRef]
- Zhang, J.; Zhi, H.Y.; Ding, F.; Luo, A.P.; Liu, Z.H. Transglutaminase 3 Expression in C57BL/6J Mouse Embryo Epidermis and the Correlation with its Differentiation. Cell Res. 2005, 15, 105–110. [Google Scholar] [CrossRef]
- Grochot-Przeczek, A.; Lach, R.; Mis, J.; Skrzypek, K.; Gozdecka, M.; Sroczynska, P.; Dubiel, M.; Rutkowski, A.; Kozakowska, M.; Zagorska, A.; et al. Heme Oxygenase-1 Accelerates Cutaneous Wound Healing in Mice. PLoS ONE 2009, 4, e5803. [Google Scholar] [CrossRef]
- Chen, X.S.; Kurre, U.; Jenkins, N.A.; Copeland, N.G.; Funk, C.D. cDNA Cloning, Expression, Mutagenesis of C-terminal Isoleucine, Genomic Structure, and Chromosomal Localizations of Murine 12-Lipoxygenases. J. Biol. Chem. 1994, 269, 13979–13987. [Google Scholar] [CrossRef]
- Deng, W.; Bai, Y.; Deng, F.; Pan, Y.; Mei, S.; Zheng, Z.; Min, R.; Wu, Z.; Li, W.; Miao, R.; et al. Streptococcal Pyrogenic Exotoxin B Cleaves GSDMA and Triggers Pyroptosis. Nature 2022, 602, 496–502, Erratum in Nature 2022, 608, E28. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium, UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [CrossRef] [PubMed]
- Rehring, J.F.; Bui, T.M.; Galan-Enriquez, C.S.; Urbanczyk, J.M.; Ren, X.; Wiesolek, H.L.; Sullivan, D.; Sumagin, R. Released Myeloperoxidase Attenuates Neutrophil Migration and Accumulation in Inflamed Tissue. Front. Immunol. 2021, 12, 654259. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Tyagri, S.; Seth, T.; Pati, H.P.; Gahlot, G.; Tripathi, P.; Somasundaram, V.; Saxena, R. Comparison of Immunohistochemistry, Cytochemistry, and Flow Cytometry in AML for Myeloperoxidase Detection. Indian J. Hematol. Blood Transfus. 2018, 34, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal Candidiasis: Epidemiology, Microbiology and Risk Factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Menon, S.; Patki, M.; Liang, X.; Billack, B.; Patel, K. Nanomedicine for the Treatment of Vaginal Candidiasis. In Nanomedicines for the Prevention and Treatment of Infectious Diseases; Patravale, V.B., Date, A.A., Jindal, A.B., Eds.; Springer: Cham, Switzerland, 2023; Volume 56, pp. 125–147. [Google Scholar] [CrossRef]
- Vartak, R.; Menon, S.; Patki, M.; Billack, B.; Patel, K. Ebselen Nanoemulgel for the Treatment of Topical Fungal Infection. Eur. J. Pharm. Sci. 2020, 148, 105323. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Ścianowski, J.; Aleksandrzak, K.B. Highly Efficient Synthesis and Antioxidant Capacity of N-Substituted Benzisoselenazol-3(2H)-ones. RSC Adv. 2014, 4, 48959–48962. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Sinha, R.; Cooper, A.J.; Pinto, J.T. The Role of Alliums and their Sulfur and Selenium Constituents in Cancer Prevention. In Vegetables, Whole Grains, and Their Derivatives in Cancer Prevention. Diet and Cancer; Mutanen, M., Pajari, A.M., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 2. [Google Scholar] [CrossRef]
- Astrain-Redin, N.; Sanmartin, C.; Sharma, A.K.; Plano, D. From Natural Sources to Synthetic Derivatives: The Allyl Motif as a Powerful Tool for Fragment-Based Design in Cancer Treatment. J. Med. Chem. 2023, 66, 3703–3731. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Kaczor, K.B.; Antosiewicz, J.; Janecka, A.; Długosz, A.; Janecki, T.; Wojtczak, A.; Ścianowski, J. New Chiral Ebselen Analogues with Antioxidant and Cytotoxic Potential. Molecules 2017, 22, 492. [Google Scholar] [CrossRef]
- Franz, R.; Ruhnke, M.; Morschhäuser, J. Molecular Aspects of Fluconazole Resistance Development in Candida albicans. Mycoses 1999, 42, 453–458. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and Pharmacology of Synthetic Organoselenium Compounds: An Update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef]

| Compounds | Molecular Weight (g/mol) | Water Solubility (µg/mL) |
|---|---|---|
| N-allyl-bs | 238.14 | 202 |
| N-3mb-bs | 268.21 | 70.2 |
| EB | 274.18 | 33.0 |
| Compounds | N-allyl-bs | N-3mb-bs | EB ** | FLU ** | ||
|---|---|---|---|---|---|---|
| Colorimetric assay | C. albicans S1 (µM) | 24 h | 50 | 25 | 25 | >100 |
| 48 h | 100 | 25 | 25 | >100 | ||
| C. albicans S2 (µM) | 24 h | 100 | 25 | 25 | >100 | |
| 48 h | 50 | 25 | 25 | >100 | ||
| Turbidity assay | C. albicans S1 (µM) | 24 h | 50 | 25 | 25 | 25 |
| 48 h | 50 | 25 | 25 | 25 | ||
| C. albicans S2 (µM) | 24 h | 50 | 50 | 25 | >100 | |
| 48 h | 50 | 25 | 25 | >100 |
| Compounds | Log P # | MTT Assay IC50 (µM) KB-3-1 Cells | Turbidity Assay IC50 (µM) | |
|---|---|---|---|---|
| C. albicans S1 | C. albicans S2 | |||
| N-allyl-bs | 2.29 | 45.81 ± 4.60 | 29.78 ± 0.13 | 28.05 ± 0.47 |
| N-3mb-bs | 3.29 | 54.92 ± 7.00 | 18.21 ± 0.05 | 5.35 ± 0.07 |
| EB | 2.92 | 89.74 ± 3.42 ** | 17.06 ± 1.01 ** | 14.28 ± 0.46 ** |
| FLU | −0.12 | >1000 ** | 6.01 ± 0.10 ** | >100 ** |
| Compounds | IC50MA, µM |
|---|---|
| N-allyl-bs | 6.42 ± 0.81 |
| N-3mb-bs | 19.61 ± 1.29 |
| EB b | 12.50 ± 1.10 |
| FLU b | >30 |
| Spectral Counts (DDM) | ||||
|---|---|---|---|---|
| UniProt ID | Name | Uninfected Mice N = 4 Mean ± SD, [95% CI] | Infected Mice N = 4 Mean ± SD, [95% CI] | Function |
| A0A1D8PP43 | Aldehyde dehydrogenase (Adh1p) | ND * | 34.50 ± 14.55 [11.35–57.65] | Oxidoreductase, found in yeast and hyphal forms of C. albicans [31]. |
| P46273 | Phosphoglycerate kinase (Pgk) | ND | 34.25 ± 15.65 [9.34–59.15] | Glycolytic enzyme, found in the cell wall of C. albicans [32]. |
| P30575 | Enolase 1 | ND | 31.00 ± 11.17 [13.23–48.77] | Glycolytic enzyme, found in the cell wall of C. albicans [33]. |
| Q5ADM7 | Glyceraldehyde-3-phosphate dehydrogenase | ND | 23.00 ± 17.38 [−4.65–50.65] | Glycolytic enzyme, found in the cell wall of C. albicans [34]. |
| P43094 | Candidapepsin-5 | ND | 21.50 ± 9.47 [6.43–36.57] | Secreted aspartic protease involved in the virulence of C. albicans [35,36]. |
| Q9URB4 | Fructose-bisphosphate aldolase | ND | 21.00 ± 10.23 [4.72–37.28] | Glycolytic enzyme, antibodies to which are found in sera of patients with candidemia [37,38]. |
| P41797 | Heat shock protein SSA1 | ND | 17.50 ± 10.47 [0.84–34.16] | Member of Hsp70 family, involved In virulence of C. albicans [39,40]. |
| Q5A8N2 | Candidapepsin-4 | ND | 16.50 ± 10.47 [−0.16–33.16] | Secreted aspartic protease involved in the virulence of C. albicans [41,42]. |
| Q5AC08 | Candidapepsin-6 | ND | 12.00 ± 5.60 [3.09–20.91] | Secreted aspartic protease involved in the virulence of C. albicans [41,43]. |
| Q5AB48 | RBT4 | ND | 10.00 ± 4.24 [3.25–16.75] | Secreted protein that acts as a virulence factor during infections [43,44]. |
| Groups | Log CFU/100 μL | Remaining C. albicans S1 Compared to Control (100%) |
|---|---|---|
| 6th day infected (control) | 5.57 | 100 |
| Miconazole | 4.36 | 6.17 |
| Vehicle | 5.35 | 60.26 |
| N-3mb-bs | 4.63 | 11.48 |
| Naive | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Pacuła-Miszewska, A.J.; Vartak, R.; Prajapati, M.; Zheng, H.; Zhao, C.; Mao, G.; Patel, K.; Fedosova, N.U.; Ścianowski, J.; et al. N-3-Methylbutyl-benzisoselenazol-3(2H)-one Exerts Antifungal Activity In Vitro and in a Mouse Model of Vulvovaginal Candidiasis. Curr. Issues Mol. Biol. 2024, 46, 2480-2496. https://doi.org/10.3390/cimb46030157
Liang X, Pacuła-Miszewska AJ, Vartak R, Prajapati M, Zheng H, Zhao C, Mao G, Patel K, Fedosova NU, Ścianowski J, et al. N-3-Methylbutyl-benzisoselenazol-3(2H)-one Exerts Antifungal Activity In Vitro and in a Mouse Model of Vulvovaginal Candidiasis. Current Issues in Molecular Biology. 2024; 46(3):2480-2496. https://doi.org/10.3390/cimb46030157
Chicago/Turabian StyleLiang, Xiuyi, Agata J. Pacuła-Miszewska, Richa Vartak, Milankumar Prajapati, Haiyan Zheng, Caifeng Zhao, Ganming Mao, Ketankumar Patel, Natalya U. Fedosova, Jacek Ścianowski, and et al. 2024. "N-3-Methylbutyl-benzisoselenazol-3(2H)-one Exerts Antifungal Activity In Vitro and in a Mouse Model of Vulvovaginal Candidiasis" Current Issues in Molecular Biology 46, no. 3: 2480-2496. https://doi.org/10.3390/cimb46030157
APA StyleLiang, X., Pacuła-Miszewska, A. J., Vartak, R., Prajapati, M., Zheng, H., Zhao, C., Mao, G., Patel, K., Fedosova, N. U., Ścianowski, J., & Billack, B. (2024). N-3-Methylbutyl-benzisoselenazol-3(2H)-one Exerts Antifungal Activity In Vitro and in a Mouse Model of Vulvovaginal Candidiasis. Current Issues in Molecular Biology, 46(3), 2480-2496. https://doi.org/10.3390/cimb46030157









