Low-Salt Diet Regulates the Metabolic and Signal Transduction Genomic Fabrics, and Remodels the Cardiac Normal and Chronic Pathological Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Data
2.2. Primary Independent Characteristics of Individual Genes and Functional Pathways
2.3. Derived Characteristics of Individual Genes
2.4. Quantification of Transcriptomic Changes
2.4.1. Significant Regulation of the Average Expression Value
2.4.2. Weighted Individual (Gene) Regulation (WIR) and Weighted Pathway Regulation (WPR)
2.4.3. Regulation of the Expression Control and Expression Coordination
2.5. Functional Pathways
- (i)
- Carbohydrate metabolism:
- (ii)
- Energy metabolism:
- -
- (OXP) mmu00190 Oxidative phosphorylation [35].
- (iii)
- Lipid metabolism:
- (iv)
- Nucleotide metabolism:
- (v)
- Amino acid metabolism:
- (vi)
- Glycan biosynthesis and metabolism:
- -
- (NGL) mmu00510 N-Glycan biosynthesis [47].
- (vii)
- Xenobiotics biodegradation and metabolism:
3. Results
3.1. The Global Picture
3.2. Independence of the Three Types of Primary Expression Characteristics of Individual Genes
3.3. Important Derived Characteristics of the Individual Genes
3.4. Measures of Transcriptomic Regulation
3.5. Correcting the False Hits of the Traditional Significant Regulation Analysis
3.6. Overall Regulation of Expression Level and Transcription Control within Selected Metabolic, Circulatory System, and Cardiac Chronic Diseases’ Pathways
3.7. Regulated Genes within Selected Metabolic Pathways
3.8. Regulation of Selected Signaling Pathways
3.9. Regulated Genes within Pathways of Selected Cardiac Diseases
3.10. Remodeling of the Gene Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Independent Primary Expression Characteristics of Individual Gene and Functional Pathways
Appendix B. Derived Characteristics of Individual Genes and Their Averages Over Functional Pathways
Appendix C. Measures of Transcriptomic Regulation
References
- Aliasgharzadeh, S.; Tabrizi, J.S.; Nikniaz, L.; Ebrahimi-Mameghani, M.; Lotfi Yagin, N. Effect of salt reduction interventions in lowering blood pressure: A comprehensive systematic review and meta-analysis of controlled clinical trials. PLoS ONE 2022, 17, e0277929. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Li, X.; Heianza, Y.; Qi, L. Adding Salt to Foods and Risk of Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 80, 2157–2167. [Google Scholar] [CrossRef]
- Padilla-Moseley, J.; Blanco-Metzler, A.; L’Abbé, M.R.; Arcand, J. A Program Evaluation of a Dietary Sodium Reduction Research Consortium of Five Low- and Middle-Income Countries in Latin America. Nutrients 2022, 14, 4311. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, R.; Worsley, A. Current levels of salt knowledge: A review of the literature. Nutrients 2014, 6, 5534–5559. [Google Scholar] [CrossRef]
- Kotchen, T.A.; Cowley, A.W., Jr.; Frohlich, E.D. Salt in health and disease—A delicate balance. N. Engl. J. Med. 2013, 368, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/23658998 (accessed on 6 March 2024).
- Aksentijević, D.; Shattock, M.J. With a grain of salt: Sodium elevation and metabolic remodeling in heart failure. J. Mol. Cell Cardiol. 2021, 161, 106–115. [Google Scholar] [CrossRef]
- Ertuglu, L.A.; Kirabo, A. Dendritic Cell Epithelial Sodium Channel in Inflammation, Salt-Sensitive Hypertension, and Kidney Damage. Kidney360 2022, 3, 1620–1629. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, H.; Xue, Y.; Jia, Z.; Dai, M.; He, K.; Zhao, R. Deleterious effect in endothelin receptor-mediated coronary artery smooth muscle contractility in high-salt diet rats. Nutr. Metab. Cardiovasc. Dis. 2022, 33, 234–244. [Google Scholar] [CrossRef]
- Hunter, R.W.; Dhaun, N.; Bailey, M.A. The impact of excessive salt intake on human health. Nat. Rev. Nephrol. 2022, 18, 321–335. [Google Scholar] [CrossRef]
- Jaques, D.A.; Wuerzner, G.; Ponte, B. Sodium Intake as a Cardiovascular Risk Factor: A Narrative Review. Nutrients 2021, 13, 3177. [Google Scholar] [CrossRef]
- Dumančić, D.; Stupin, A.; Kožul, M.; Šerić, V.; Kibel, A.; Goswami, N.; Brix, B.; Debeljak, Ž.; Scitovski, R.; Drenjančević, I. Increased cerebral vascular resistance underlies preserved cerebral blood flow in response to orthostasis in humans on a high-salt diet. Eur. J. Appl. Physiol. 2023, 123, 923–933. [Google Scholar] [CrossRef]
- Carll, A.P.; Haykal-Coates, N.; Winsett, D.W.; Hazari, M.S.; Nyska, A.; Richards, J.H.; Willis, M.S.; Costa, D.L.; Farraj, A.K. Dietary salt exacerbates isoproterenol-induced cardiomyopathy in rats. Toxicol. Pathol. 2011, 39, 925–937. [Google Scholar] [CrossRef]
- Xu, H.; Qing, T.; Shen, Y.; Huang, J.; Liu, Y.; Li, J.; Zhen, T.; Xing, K.; Zhu, S.; Luo, M. RNA-seq analyses the effect of a high-salt diet on hypertension. Gene 2018, 677, 245–250. [Google Scholar] [CrossRef]
- Yim, J.; Cho, H.; Rabkin, S.W. Gene expression and gene associations during the development of heart failure with preserved ejection fraction in the Dahl salt-sensitive model of hypertension. Clin. Exp. Hypertens. 2018, 40, 155–166. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Huang, S. Excessive Sodium Intake Leads to Cardiovascular Disease by Promoting Sex-Specific Dysfunction of Murine Heart. Front. Nutr. 2022, 9, 830738. [Google Scholar] [CrossRef]
- Corona, G.; Giuliani, C.; Parenti, G.; Norello, D.; Verbalis, J.G.; Forti, G.; Maggi, M.; Peri, A. Moderate Hyponatremia Is Associated with Increased Risk of Mortality: Evidence from a Meta-Analysis. PLoS ONE 2013, 8, e80451. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Xi, L. Theory and Applications of the (Cardio) Genomic Fabric Approach to Post-Ischemic and Hypoxia-Induced Heart Failure. J. Pers. Med. 2022, 12, 1246. [Google Scholar] [CrossRef]
- Adesse, D.; Goldenberg, R.C.; Fortes, F.S.; Iacobas, D.A.; Iacobas, S.; de Carvalho, A.C.C. Gap junctions and Chagas disease. Adv. Parasitol. 2011, 76, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Adesse, D.; Iacobas, D.A.; Iacobas, S.; Garzoni, L.R.; de Nazareth Meirelles, M.; Tanowitz, H.B.; Spray, D.C. Transcriptomic signatures of alterations in a myoblast cell line infected with four distinct strains of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2010, 82, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes, and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, C.; Xiong, J.; Yu, J. Cardiovascular aspects of the (pro)renin receptor: Function and significance. FASEB J. 2022, 36, e22237. [Google Scholar] [CrossRef]
- Luk, A.; Ahn, E.; Soor, G.S.; Butany, J. Dilated cardiomyopathy: A review. J. Clin. Pathol. 2009, 62, 219–225. [Google Scholar] [CrossRef]
- Fatkin, D.; Graham, R.M. Molecular mechanisms of inherited cardiomyopathies. Physiol. Rev. 2002, 82, 945–980. [Google Scholar] [CrossRef]
- Akhtar, H.; Al Sudani, H.; Hussein, M.; Farhan, M.U.N.; Elkholy, K. Effects of Renin-Angiotensin-Aldosterone System Inhibition on Left Ventricular Hypertrophy, Diastolic Function, and Functional Status in Patients with Hypertrophic Cardiomyopathy: A Systematic Review. Cureus 2022, 14, e26642. [Google Scholar] [CrossRef]
- Kyoto Encyclopedia of Genes and Genomes. Wiring Diagrams of Molecular Interactions, Reactions and Relations. Available online: https://www.genome.jp/kegg/pathway.html (accessed on 7 January 2024).
- Transcriptomic Effects of Law Salt Diet on the Mouse Left Ventricle. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72561 (accessed on 27 January 2023).
- P-Value from Pearson (R) Calculator. Available online: https://www.socscistatistics.com/pvalues/pearsondistribution.aspx (accessed on 27 December 2023).
- Iacobas, S.; Ede, N.; Iacobas, D.A. The Gene Master Regulators (GMR) Approach Provides Legitimate Targets for Personalized, Time-Sensitive Cancer Gene Therapy. Genes 2019, 10, 560. [Google Scholar] [CrossRef]
- Iacobas, S.; Iacobas, D.A. A Personalized Genomics Approach of the Prostate Cancer. Cells 2021, 10, 1644. [Google Scholar] [CrossRef]
- Fructose and Manose Metabolism. Available online: https://www.genome.jp/pathway/mmu00051 (accessed on 11 January 2024).
- Galactose Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00052 (accessed on 11 January 2024).
- Glycolysis/Glucogenesis. Available online: https://www.genome.jp/pathway/mmu00010 (accessed on 11 January 2024).
- Inositol Phosphate Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00562 (accessed on 11 January 2024).
- Oxidative Phosphorylation. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00190 (accessed on 11 January 2024).
- Fatty acid Biosynthesis. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00061 (accessed on 11 January 2024).
- Glycerolipid Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00561 (accessed on 11 January 2024).
- Glycerophospholipid Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00564 (accessed on 11 January 2024).
- Steroid Biosynthesis. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00100 (accessed on 11 January 2024).
- Steroid Hormone Biosynthesis. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00140 (accessed on 11 January 2024).
- Purine Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00230 (accessed on 11 January 2024).
- Pyrimidine Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00240 (accessed on 11 January 2024).
- Cysteine and Methionine Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00270 (accessed on 11 January 2024).
- Glutathione Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00480 (accessed on 11 January 2024).
- Thyrosine Metabolism. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00350 (accessed on 11 January 2024).
- Valine, Leucine and Isoleucine Degradation. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00280 (accessed on 11 January 2024).
- N-Glycan Biosynthesis. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00510 (accessed on 11 January 2024).
- Drug Metabolism—Cytochrome P450. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00982 (accessed on 11 January 2024).
- Drug Metabolism—Other Enzymes. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu00983 (accessed on 11 January 2024).
- Adrenergic Signaling in Cardiomyocytes. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu04261 (accessed on 11 January 2024).
- Cardiac Muscle Contraction. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu04260 (accessed on 11 January 2024).
- Chagas Disease. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu05142 (accessed on 11 January 2024).
- Diabetic Cardiomyopathy. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu05415 (accessed on 11 January 2024).
- Dilated Cardiomyopathy. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu05414 (accessed on 11 January 2024).
- Hypertrophic Cardiomyopathy. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu05410 (accessed on 11 January 2024).
- MAPK Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04010 (accessed on 11 January 2024).
- PIK3-Akt Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04151 (accessed on 11 January 2024).
- Rap1 Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04015 (accessed on 11 January 2024).
- Ras Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04014 (accessed on 11 January 2024).
- Chemokine Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04062. (accessed on 11 January 2023).
- Calcium Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04020 (accessed on 11 January 2024).
- CAMP Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04024 (accessed on 11 January 2024).
- CGMP-PKG Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04022 (accessed on 11 January 2024).
- MTOR Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04150 (accessed on 11 January 2024).
- Wnt Signaling Pathway. Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa04310 (accessed on 11 January 2024).
- Central Carbon Metabolism in Cancer. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu05230 (accessed on 11 January 2024).
- Choline Metabolism in Cancer. Available online: https://www.genome.jp/kegg-bin/show_pathway?mmu05231 (accessed on 11 January 2024).
- Bayne, E.F.; Rossler, K.J.; Gregorich, Z.R.; Aballo, T.J.; Roberts, D.S.; Chapman, E.A.; Guo, W.; Palecek, S.P.; Ralphe, J.C.; Kamp, T.J.; et al. Top-down proteomics of myosin light chain isoforms define chamber-specific expression in the human heart. J. Mol. Cell. Cardiol. 2023, 181, 89–97. [Google Scholar] [CrossRef]
- Iacobas, S.; Amuzescu, B.; Iacobas, D.A. Transcriptomic uniqueness and commonality of the ion channels and transporters in the four heart chambers. Sci. Rep. 2021, 11, 2743. [Google Scholar] [CrossRef]
- Sun, J.H.; Liu, X.K.; Xing, X.W.; Yang, Y.; Xuan, H.H.; Fu, B.B. Value of Cardiac Troponin, Myoglobin Combined with Heart-type Fatty Acid-binding Protein Detection in Diagnosis of Early Acute Myocardial Infarction. Pak. J. Med. Sci. 2023, 39, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Tcheandjieu, C.; Georges, A.; Xiao, K.; Tejeda, H.; Dina, C.; Le Tourneau, T.; Fiterau, M.; Judy, R.; Tsao, N.L.; et al. Computational estimates of annular diameter reveal genetic determinants of mitral valve function and disease. JCI Insight 2022, 7, e146580. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Huang, Y.; Lu, H.; Zhou, Y.; Tu, T.; Ouyang, F.; Liu, Y.; Liu, Q. Novel Drug Targets for Atrial Fibrillation Identified Through Mendelian Randomization Analysis of the Blood Proteome. Cardiovasc. Drugs Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Iacobas, D.A.; Iacobas, S.; Chachua, T.; Goletiani, C.; Sidyelyeva, G.; Velíšková, J.; Velíšek, L. Prenatal corticosteroids modify glutamatergic and GABAergic synapse genomic fabric: Insights from a novel animal model of infantile spasms. J. Neuroendocrinol. 2013, 25, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, G.; Yu, L.; Wang, X.; Meng, Y.; Mao, J.; Fu, Z.; Yin, Y.; Li, J.; Wang, X.; et al. Heat-shock protein 90α protects NME1 against degradation and suppresses metastasis of breast cancer. Br. J. Cancer 2023, 129, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, Y.; Zihlif, M.; Al Bawab, A.Q.; Sharab, A. Effects of Intermittent Hypoxia on Expression of Glucose Metabolism Genes in MCF7 Breast Cancer Cell Line. Curr. Cancer Drug Targets 2020, 20, 216–222. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Lee, P.R.; Cohen, J.E.; Fields, R.D. Coordinated Activity of Transcriptional Networks Responding to the Pattern of Action Potential Firing in Neurons. Genes 2019, 10, 754. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Obiomon, E.A.; Iacobas, S. Genomic Fabrics of the Excretory System’s Functional Pathways Remodeled in Clear Cell Renal Cell Carcinoma. Curr. Issues Mol. Biol. 2023, 45, 9471–9499. [Google Scholar] [CrossRef]
- Guo, G.L.; Sun, L.Q.; Sun, M.H.; Xu, H.M. LncRNA SLC8A1-AS1 protects against myocardial damage through activation of the cGMP-PKG signaling pathway by inhibiting SLC8A1 in mice models of myocardial infarction. J. Cell. Physiol. 2019, 234, 9019–9032. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Hüttemann, M.; Pak, O.; Scheibe, S.; Knoepp, F.; Sinkler, C.; Malczyk, M.; Gierhardt, M.; Esfandiary, A.; Kraut, S.; et al. Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing. Circ. Res. 2017, 121, 424–438. [Google Scholar] [CrossRef]
- Bayer, A.L.; Alcaide, P. MyD88: At the heart of inflammatory signaling and cardiovascular disease. J. Mol. Cell. Cardiol. 2021, 161, 75–85. [Google Scholar] [CrossRef]
- Geering, K. Function of FXYD proteins, regulators of Na, K-ATPase. J. Bioenerg. Biomembr. 2005, 37, 387–392. [Google Scholar] [CrossRef]
- Nordin, H.; Nakagawa, R.; Wallin, M.; Pernow, J.; Kass, D.A.; Ståhlberg, M. Regional protein expression changes within the left ventricle in a mouse model of dyssynchronous and resynchronized heart failure. ESC Heart. Fail. 2020, 7, 4438–4442. [Google Scholar] [CrossRef]
- Yusuf, A.M.; Qaisar, R.; Al-Tamimi, A.O.; Jayakumar, M.N.; Woodgett, J.R.; Koch, W.J.; Ahmad, F. Cardiomyocyte-GSK-3β deficiency induces cardiac progenitor cell proliferation in the ischemic heart through paracrine mechanisms. J. Cell. Physiol. 2022, 37, 1804–1817. [Google Scholar] [CrossRef]
- Munasinghe, P.E.; Saw, E.L.; Reily-Bell, M.; Tonkin, D.; Kakinuma, Y.; Fronius, M.; Katare, R. Non-neuronal cholinergic system delays cardiac remodeling in type 1 diabetes. Heliyon 2023, 9, e17434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, H.; Huang, P.; Wu, G.; Wang, Q.; Luan, X.; Zhang, H.; Yu, D.; Wang, H.; Lu, D.; et al. Dracorhodin targeting CMPK2 attenuates inflammation: A novel approach to sepsis therapy. Clin. Transl. Med. 2023, 13, e1449. [Google Scholar] [CrossRef] [PubMed]
- Gérus, M.; Bonnart, C.; Caizergues-Ferrer, M.; Henry, Y.; Henras, A.K. Evolutionarily conserved function of RRP36 in early cleavages of the pre-rRNA and production of the 40S ribosomal subunit. Mol. Cell Biol. 2010, 30, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Drouard, G.; Hagenbeek, F.A.; Whipp, A.; Pool, R.; Hottenga, J.J.; Jansen, R.; Hubers, N.; Afonin, A.; BIOS Consortium; BBMRI-NL Metabolomics Consortium; et al. Longitudinal multi-omics study reveals common etiology underlying association between plasma proteome and BMI trajectories in adolescent and young adult twins. BMC Med. 2023, 21, 508. [Google Scholar] [CrossRef] [PubMed]
- Bassaganyas, L.; Popa, S.J.; Horlbeck, M.; Puri, C.; Stewart, S.E.; Campelo, F.; Ashok, A.; Butnaru, C.M.; Brouwers, N.; Heydari, K.; et al. New factors for protein transport identified by a genome-wide CRISPRi screen in mammalian cells. J. Cell Biol. 2019, 218, 3861–3879. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, G.; Jiang, C.; Guo, T.; Tang, H.; Yuan, Z.; Wang, Y.; Tan, X.; Chen, J.; Zhang, E.; et al. USP31 serves as a potential biomarker for predicting prognosis and immune responses for clear cell renal cell carcinoma via single-cell and bulk RNA-sequencing. J. Gene Med. 2023, 26, e3594. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Mgbemena, V.E.; Iacobas, S.; Menezes, K.M.; Wang, H.; Saganti, P.B. Genomic Fabric Remodeling in Metastatic Clear Cell Renal Cell Carcinoma (ccRCC): A New Paradigm and Proposal for a Personalized Gene Therapy Approach. Cancers 2020, 12, 3678. [Google Scholar] [CrossRef]
- Kristofova, M.; Ori, A.; Wang, Z.Q. Multifaceted Microcephaly-Related Gene MCPH1. Cells 2022, 11, 275. [Google Scholar] [CrossRef]
- Martínez-Moreno, R.; Carreras, D.; Sarquella-Brugada, G.; Pérez, G.J.; Selga, E.; Scornik, F.S.; Brugada, R. Loss of sodium current caused by a Brugada syndrome-associated variant is determined by patient-specific genetic background. Heart. Rhythm. 2023, 21, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, S.; Mikati, M.A.; Álvarez-García-Rovés, R.; Carboni, M.; Hunanyan, A.S.; Kherallah, B.; McLean, M.; Prange, L.; De Grandis, E.; Gagliardi, A.; et al. Cardiac phenotype in ATP1A3-related syndromes: A multicenter cohort study. Neurology 2020, 95, e2866–e2879. [Google Scholar] [CrossRef] [PubMed]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef]
- Song, Z.; Tian, X.; Shi, Q. Fas, Caspase-8, and Caspase-9 pathway-mediated bile acid-induced fetal cardiomyocyte apoptosis in intrahepatic cholestasis pregnant rat models. J. Obstet. Gynaecol. Res. 2021, 47, 2298–2306. [Google Scholar] [CrossRef]
- Pahlavani, H.A. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front. Cell. Dev. Biol. 2022, 10, 950927. [Google Scholar] [CrossRef]
- Iacobas, D.A.; Iacobas, S.; Tanowitz, H.B.; Campos de Carvalho, A.; Spray, D.C. Functional genomic fabrics are remodeled in a mouse model of Chagasic cardiomyopathy and restored following cell therapy. Microbes Infect. 2018, 20, 185–195. [Google Scholar] [CrossRef]
- Nisimura, L.M.; Coelho, L.L.; de Melo, T.G.; Vieira, P.C.; Victorino, P.H.; Garzoni, L.R.; Spray, D.C.; Iacobas, D.A.; Iacobas, S.; Tanowitz, H.B.; et al. Trypanosoma cruzi Promotes Transcriptomic Remodeling of the JAK/STAT Signaling and Cell Cycle Pathways in Myoblasts. Front. Cell. Infect. Microbiol. 2020, 10, 255. [Google Scholar] [CrossRef]
- Kolobarić, N.; Mihalj, M.; Kozina, N.; Matić, A.; Mihaljević, Z.; Jukić, I.; Drenjančević, I. Tff3−/− Knock-Out Mice with Altered Lipid Metabolism Exhibit a Lower Level of Inflammation following the Dietary Intake of Sodium Chloride for One Week. Int. J. Mol. Sci. 2023, 24, 7315. [Google Scholar] [CrossRef]
- Bochi, A.P.G.; Ferreira, G.d.S.; Del Bianco, V.; Pinto, P.R.; Rodrigues, L.G.; Trevisani, M.d.S.; Furukawa, L.N.S.; Bispo, K.C.S.; da Silva, A.A.; Velosa, A.P.P.; et al. Aerobic Exercise Training Reduces Atherogenesis Induced by Low-Sodium Diet in LDL Receptor Knockout Mice. Antioxidants 2022, 11, 2023. [Google Scholar] [CrossRef] [PubMed]
- Launonen, H.; Pang, Z.; Linden, J.; Siltari, A.; Korpela, R.; Vapaatalo, H. Evidence for local aldosterone synthesis in the large intestine of the mouse. J. Physiol. Pharmacol. 2021, 72, 807–815. [Google Scholar] [CrossRef]
- Thomas, N.M.; Jasmin, J.F.; Lisanti, M.P.; Iacobas, D.A. Sex differences in expression and subcellular localization of heart rhythm determinant proteins. Biochem. Biophys. Res. Commun. 2011, 406, 117–122. [Google Scholar] [CrossRef] [PubMed]
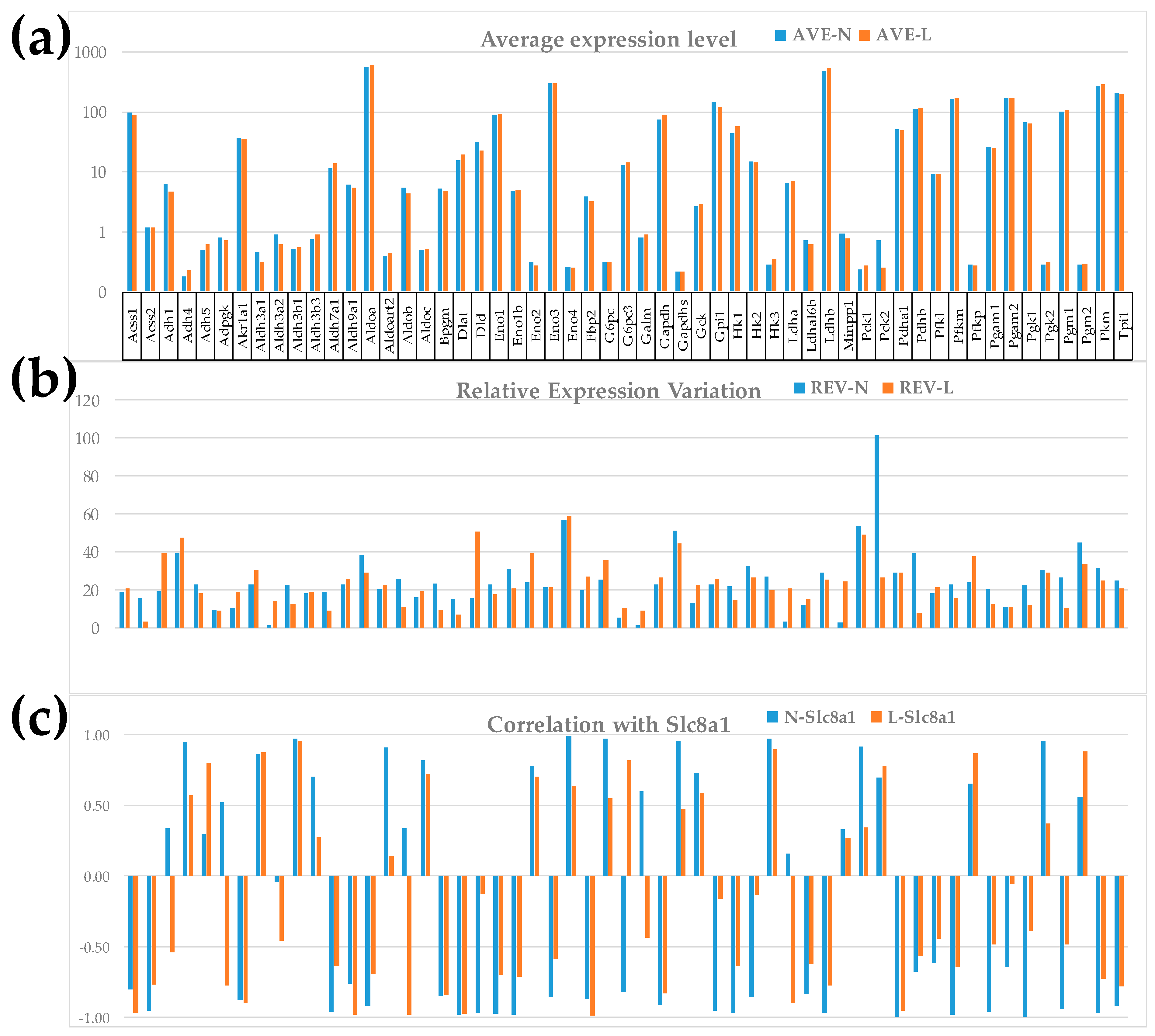
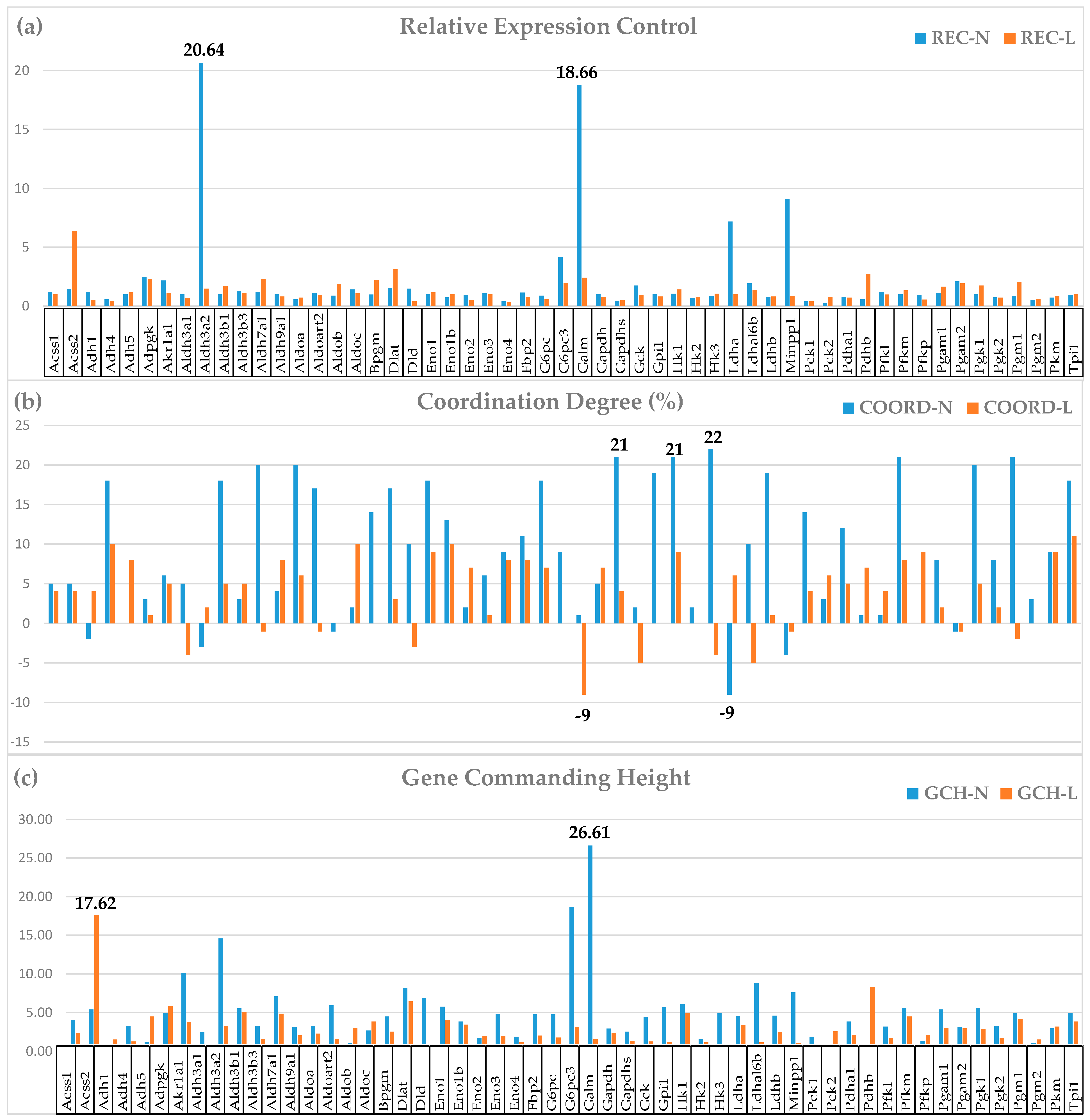
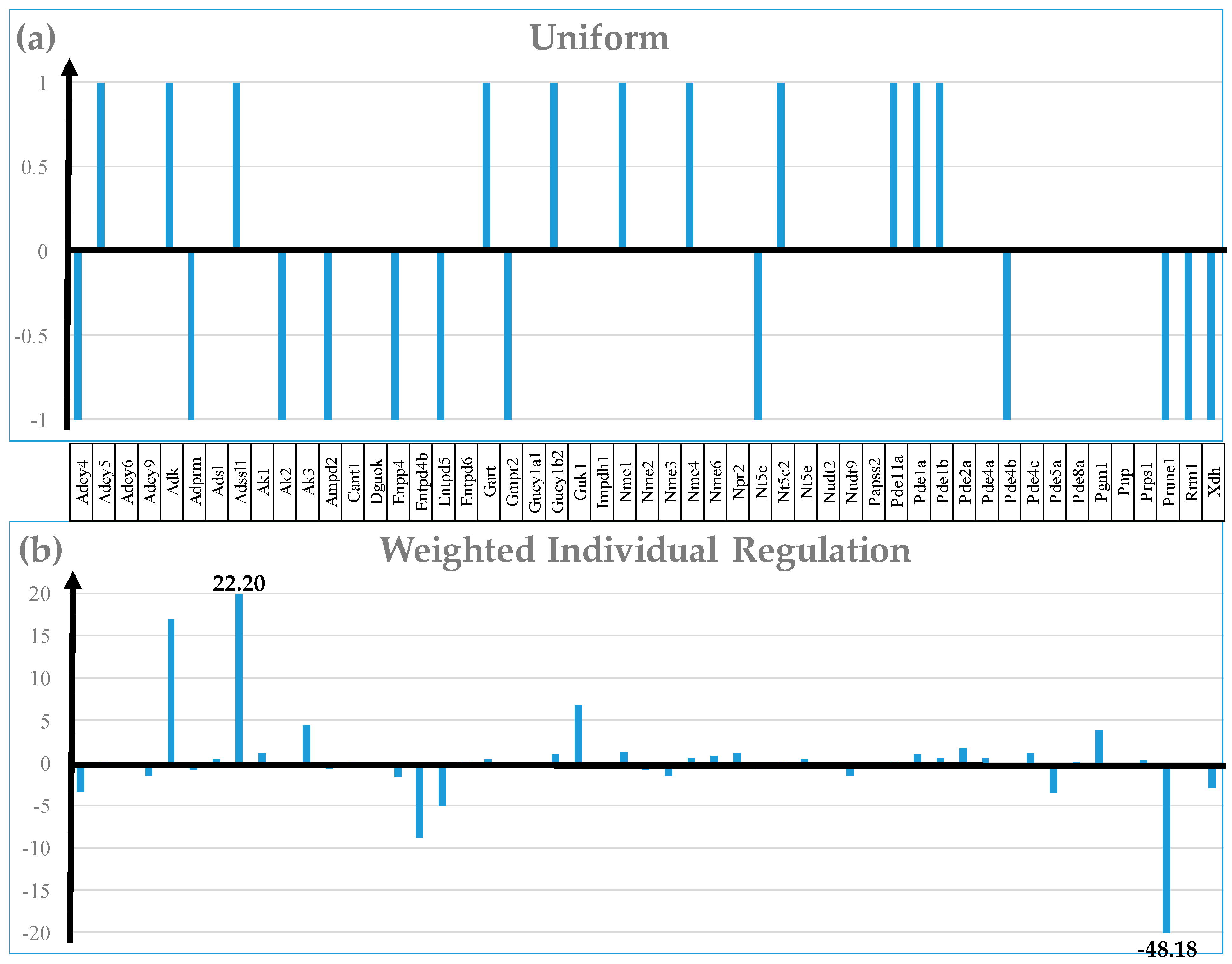
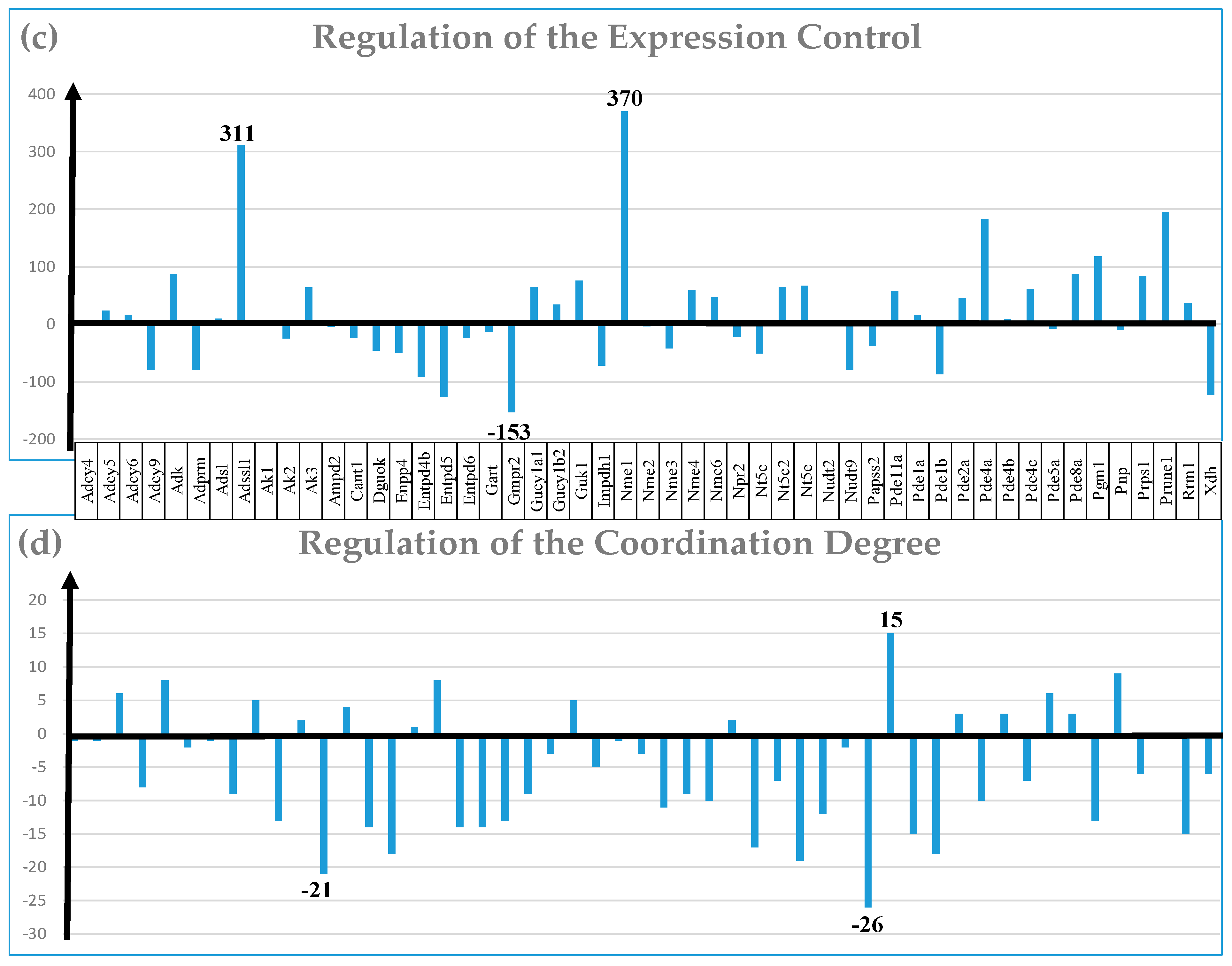
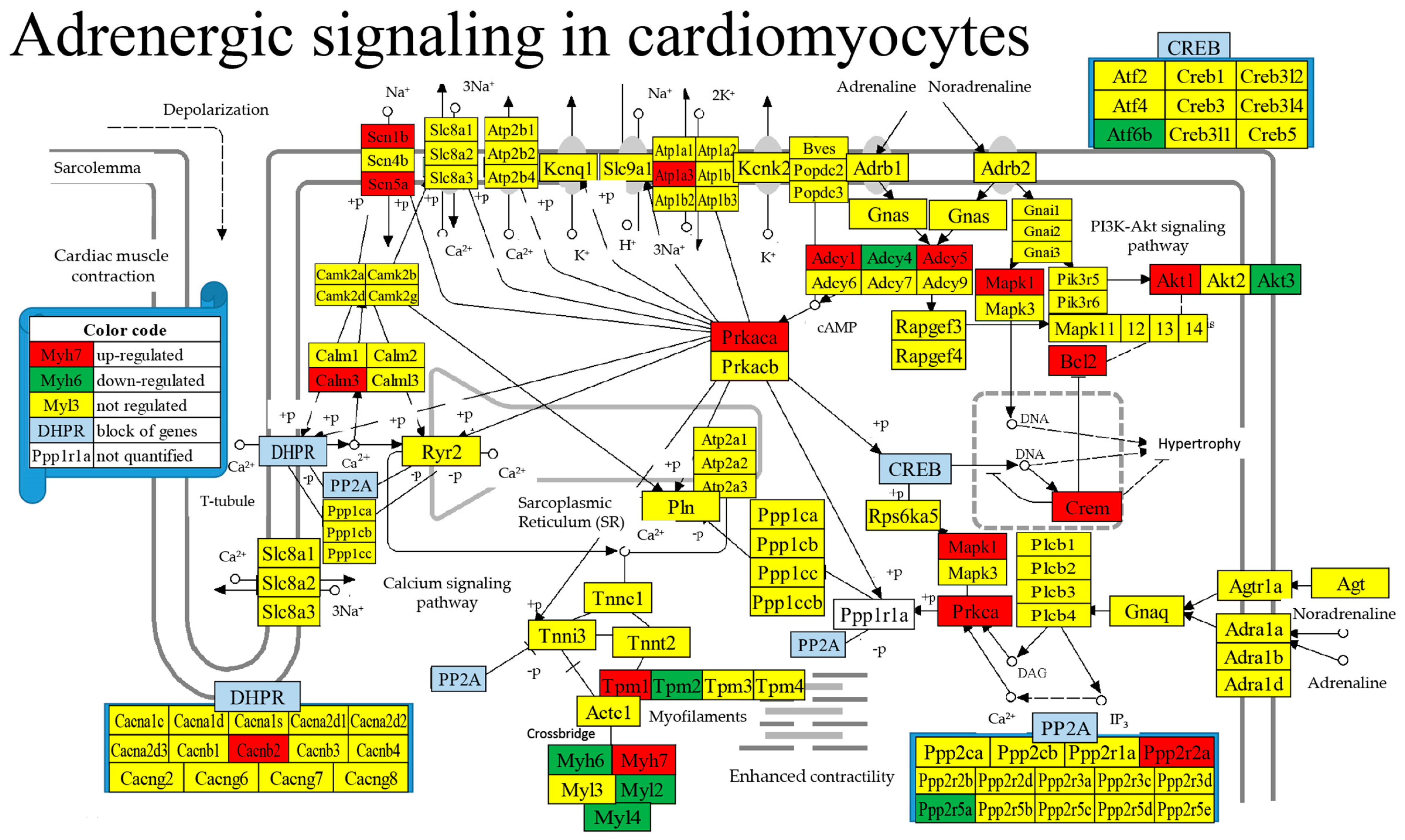
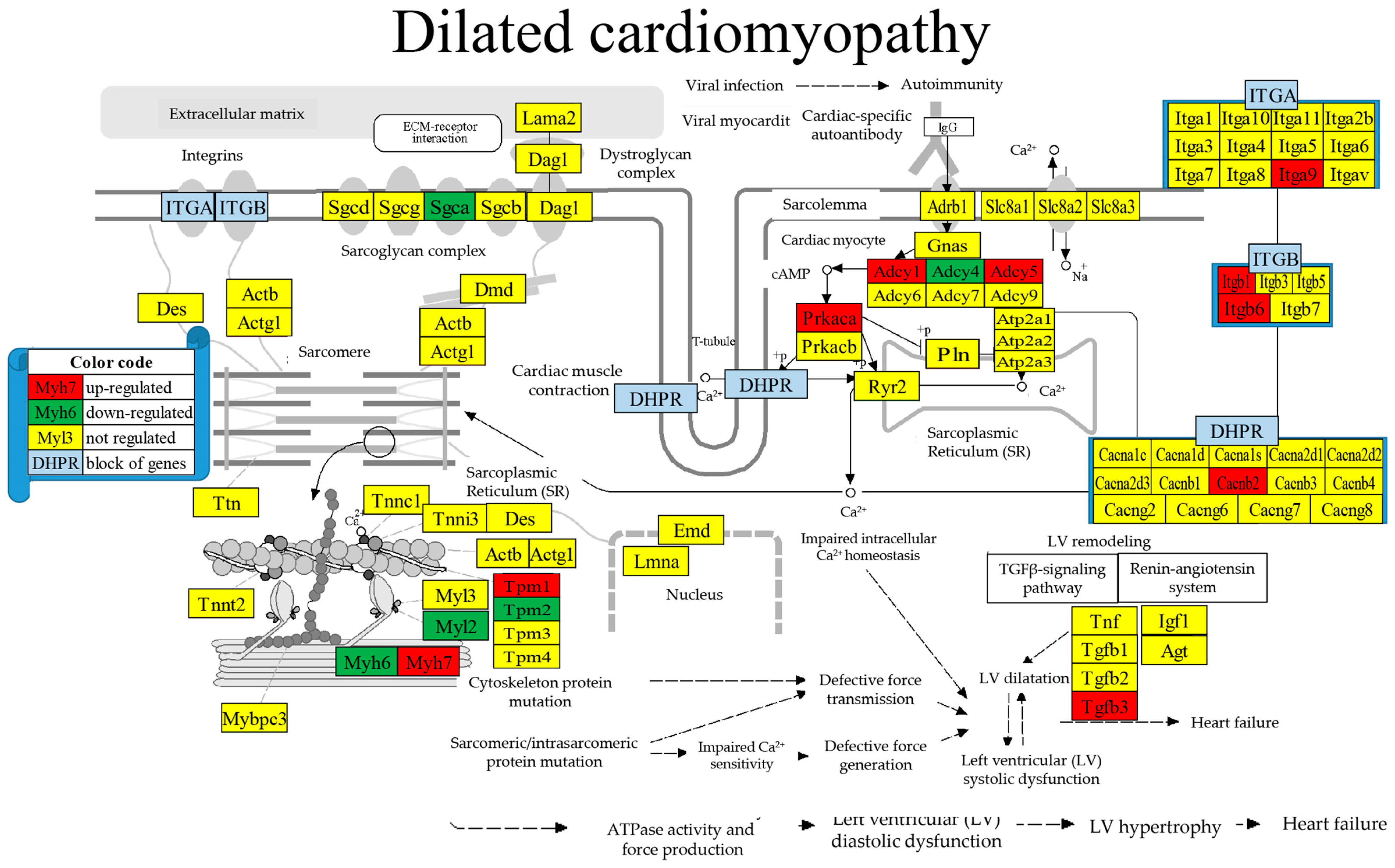

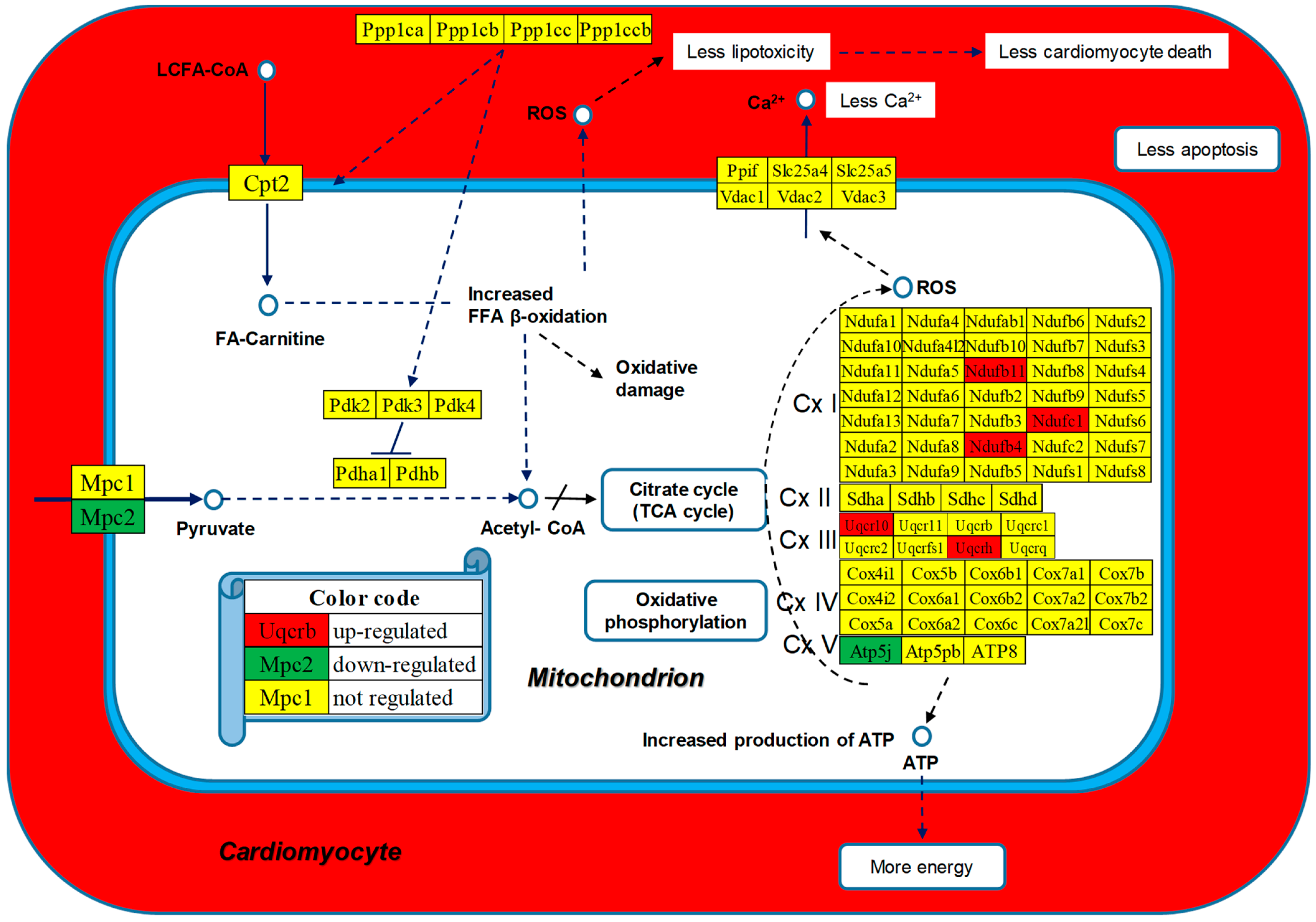



| GENE | DESCRIPTION | X | P | CUT | WIR |
|---|---|---|---|---|---|
| Falsely down-regulated genes | |||||
| Ifitm5 | interferon-induced transmembrane protein 5 | −2.350 | 0.030 | 2.427 | −0.428 |
| Hinfp | histone H4 transcription factor | −2.164 | 0.039 | 2.639 | −0.263 |
| Prdm11 | PR domain containing 11 | −2.000 | 0.026 | 2.170 | −0.376 |
| Myl7 | myosin, light polypeptide 7, regulatory | −1.887 | 0.022 | 2.468 | −4.566 |
| Trim71 | tripartite motif-containing 71 | −1.852 | 0.036 | 2.285 | 0.173 |
| Usf1 | upstream transcription factor 1 | −1.837 | 0.023 | 1.928 | −0.341 |
| Chkb | choline kinase beta | −1.829 | 0.025 | 2.633 | −5.056 |
| Cntnap5c | contactin-associated protein-like 5C | −1.824 | 0.025 | 1.922 | −5.270 |
| Dnajb1 | DnaJ heat shock protein family | −1.812 | 0.034 | 2.129 | −9.529 |
| Csrnp2 | cysteine-serine-rich nuclear protein 2 | −1.797 | 0.032 | 2.176 | −0.228 |
| Missed down-regulated genes | |||||
| Gsk3b | glycogen synthase kinase 3 beta | −1.490 | 0.017 | 1.341 | −4.025 |
| Aldh3a2 | aldehyde dehydrogenase family 3, subfamily A2 | −1.462 | 0.007 | 1.198 | −0.422 |
| Mapk10 | mitogen-activated protein kinase 10 | −1.455 | 0.028 | 1.306 | −2.712 |
| Myl2 | myosin, light polypeptide 2, regulatory, cardiac, slow | −1.431 | 0.007 | 1.329 | −0.868 |
| Tpm2 | tropomyosin 2, beta | −1.421 | 0.027 | 1.359 | −1.751 |
| Atp5j | ATP synthase H+ transporting mitochondrial F0 complex subunit F | −1.401 | 0.013 | 1.272 | −0.171 |
| Gmpr2 | guanosine monophosphate reductase 2 | −1.371 | 0.009 | 1.238 | −0.350 |
| Enpp4 | ectonucleotide pyrophosphatase/phosphodiesterase 4 | −1.362 | 0.028 | 1.316 | −1.748 |
| Chat | choline acetyltransferase | −1.353 | 0.024 | 1.292 | −0.253 |
| Dbt | dihydrolipoamide branched chain transacylase E2 | −1.323 | 0.024 | 1.274 | −1.046 |
| Missed up-regulated genes | |||||
| Lpin3 | lipin 3 | 1.372 | 0.004 | 1.146 | 0.366 |
| Pde1a | phosphodiesterase 1A, calmodulin-dependent | 1.374 | 0.008 | 1.219 | 0.974 |
| Gpam | glycerol-3-phosphate acyltransferase, mitochondrial | 1.374 | 0.019 | 1.214 | 0.427 |
| B4galt1 | UDP-Gal:betaGlcNAc beta 1,4- galactosyltransferase, polypeptide 1 | 1.391 | 0.005 | 1.334 | 3.184 |
| Ncf4 | neutrophil cytosolic factor 4 | 1.397 | 0.046 | 1.320 | 0.273 |
| Bcl2 | B cell leukemia/lymphoma 2 | 1.401 | 0.005 | 1.164 | 0.392 |
| Ndufc1 | NADH: ubiquinone oxidoreductase subunit C1 | 1.410 | 0.018 | 1.303 | 58.827 |
| Ikbkg | inhibitor of kappaB kinase gamma | 1.424 | 0.005 | 1.233 | 0.260 |
| Atp6v1b2 | ATPase, H+ transporting, lysosomal V1 subunit B2 | 1.438 | 0.045 | 1.381 | 0.265 |
| Gucy1b2 | guanylate cyclase 1, soluble, beta 2 | 1.490 | 0.034 | 1.426 | 0.943 |
| Falsely up-regulated genes | |||||
| Kif3c | kinesin family member 3C | 1.706 | 0.009 | 1.832 | 3.179 |
| Nt5el | 5′ nucleotidase, ecto-like | 1.720 | 0.028 | 2.153 | 0.097 |
| Zfp362 | zinc finger protein 362 | 1.758 | 0.024 | 1.852 | 0.637 |
| Ctsg | cathepsin G | 1.887 | 0.018 | 1.890 | 0.192 |
| Tmem231 | transmembrane protein 231 | 1.912 | 0.027 | 2.196 | 0.128 |
| Adam12 | a disintegrin and metallopeptidase domain 12 | 1.966 | 0.036 | 2.313 | 0.423 |
| Ftcd | formiminotransferase cyclodeaminase | 1.979 | 0.033 | 2.214 | 0.163 |
| Ap1m1 | adaptor-related protein complex AP-1, mu subunit 1 | 2.063 | 0.006 | 2.079 | 11.060 |
| Lrrc71 | leucine-rich repeat containing 71 | 2.153 | 0.034 | 2.559 | 0.138 |
| Gclc | glutamate-cysteine ligase, catalytic subunit | 2.330 | 0.028 | 2.456 | 1.332 |
| mmu | PATH | Description | GENES | D% | U% | WPR | ΔREC (%) |
|---|---|---|---|---|---|---|---|
| 04261 | ASC | Adrenergic signaling in cardiomyocytes | 130/156 | 6.15 | 13.08 | 19.97 | −3.71 |
| 04260 | CMC | Cardiac muscle contraction | 75/87 | 5.33 | 10.67 | 45.30 | −1.38 |
| 05142 | CHA | Chagas disease | 85/103 | 3.61 | 12.05 | 3.31 | −6.71 |
| 05415 | DIA | Diabetic cardiomyopathy | 184/211 | 3.80 | 7.07 | 29.55 | 0.40 |
| 05414 | DIL | Dilated cardiomyopathy | 81/94 | 6.17 | 12.35 | 7.05 | −0.45 |
| 00061 | FAB | Fatty acids biosynthesis | 18/19 | 0.00 | 5.56 | 2.49 | 17.10 |
| 00561 | GLM | Glycerolipid metabolism | 52/63 | 3.85 | 15.38 | 4.63 | −5.88 |
| 00564 | GPL | Glycerophospholipid metabolism | 83/98 | 4.82 | 9.64 | 1.54 | 2.27 |
| 00010 | GLY | Glycolysis/glucogenesis | 55/64 | 3.64 | 1.82 | 5.51 | 6.18 |
| 05410 | HCM | Hypertrophic cardiomyopathy | 78/91 | 6.41 | 8.97 | 6.73 | 2.23 |
| 00510 | NGL | N-Glycan biosynthesis | 50/53 | 4.00 | 4.00 | 14.18 | 14.63 |
| 00190 | OXP | Oxidative phosphorylation | 110/135 | 1.82 | 6.36 | 37.42 | 12.39 |
| 00230 | PUM | Purine metabolism | 114/134 | 10.53 | 11.40 | 5.42 | 4.19 |
| 00240 | PYR | Pyrimidine metabolism | 47/56 | 8.51 | 10.64 | 1.64 | −5.83 |
| 00100 | STB | Steroid biosynthesis | 17/20 | 0.00 | 5.88 | 0.69 | −11.37 |
| 00140 | SHB | Steroid hormone biosynthesis | 42/93 | 7.14 | 9.52 | 8.27 | −18.74 |
| 00280 | VLI | Valine, leucine, and isoleucine degradation | 48/57 | 6.25 | 2.08 | 9.28 | 5.72 |
| ALL | All quantified genes | 19,605 | 3.65 | 5.96 | 15.67 | 0.30 |
| PATHWAY | R | GENES |
|---|---|---|
| Purine metabolism | D | Adcy4; Adprm; Ak2; Ampd2; Enpp4; Entpd5; Gmpr2; Nt5c; Pde4b; Prune1; Rrm1; Xdh |
| U | Adcy1; Adcy5; Adk; Adssl1; Gart; Gucy1b2; Nme1; Nme4; Nt5c2; Pde11a; Pde1a; Pde1b; Prps2 | |
| Choline metabolism in cancer | D | Akt3; Gpcpd1; Mapk10; Pdgfd; Pdgfra; Pdgfrb; Rac2 |
| U | Akt1; Egfr; Hif1a; Kras; Mapk1; Pdpk1; Pip5k1a; Plpp1; Plpp2; Plpp3; Prkca; Prkcb; Rac1; Slc44a1 | |
| Drug metabolism—other enzymes | D | Ces1d; Gsta3; Gstt1; Gstt2; Rrm1; Xdh |
| U | Cmpk1; Gsta4; Gstm1; Gstm6; Gstm7; Gstp1; Gusb; Nat2; Nme1; Nme4; Upp1 | |
| Glycerophospholipid metabolism | D | Adprm; Chat; Gpcpd1; Selenoi |
| U | Etnk2; Gpam; Lpin3; Mboat1; Pla1a; Plpp1; Plpp2; Plpp3 | |
| Glutathione metabolism | D | Gsta3; Gstt1; Gstt2; Rrm1 |
| U | Chac1; Gsta4; Gstm1; Gstm6; Gstm7; Gstp1; Odc1; Srm | |
| Central carbon metabolism in cancer | D | Akt3; Fgfr3; Pdgfra; Pdgfrb; Slc1a5 |
| U | Akt1; Egfr; Hif1a; Kras; Mapk1; Sco2 | |
| Drug metabolism—cytochrome P450 | D | Fmo1; Gsta3; Gstt1; Gstt2 |
| U | Fmo5; Gsta4; Gstm1; Gstm6; Gstm7; Gstp1 | |
| Glycerolipid metabolism | D | Aldh3a2; Mgll |
| U | Akr1b8; Aldh1b1; Gpam; Lpin3; Mboat1; Plpp1; Plpp2; Plpp3 | |
| Pyrimidine metabolism | D | Cmpk2; Entpd5; Nt5c; Rrm1 |
| U | Cmpk1; Nme1; Nme4; Nt5c2; Upp1 | |
| Cysteine and methionine metabolism | D | Agxt2; Amd2; Mpst |
| U | Adi1; Apip; Mtap; Srm; Tst | |
| Inositol phosphate metabolism | D | Inpp1; Isyna1 |
| U | Pi4k2a; Pik3c2b; Pip5k1a; Plcd3; Synj2 | |
| Fructose and mannose metabolism | D | Pfkfb1 |
| U | Akr1b8; Gmds; Khk; Pfkfb3; Pfkfb4 | |
| Galactose metabolism | U | Akr1b8; B4galt1; Gaa; Ugp2 |
| Tyrosine metabolism | U | Comt; Dct; Mif; Th |
| MAPK | PI3K-Akt | Rap1 | Ras | Chemokine | |||||
|---|---|---|---|---|---|---|---|---|---|
| 36U | 14D | 28U | 17D | 28U | 13D | 27U | 11D | 21U | 10D |
| Akt1 | Akt3 | Akt1 | Akt3 | Adcy1 | Adcy4 | Abl2 | Akt3 | Adcy1 | Adcy4 |
| Cacnb2 | Cacna1g | Bcl2 | Atf6b | Adcy5 | Adora2a | Akt1 | Fgfr3 | Adcy5 | Akt3 |
| Crk | Fgfr3 | Cdkn1a | Ddit4 | Adora2b | Akt3 | Calm3 | Igf2 | Akt1 | Cxcl11 |
| Csf1 | Hspa1a | Col4a1 | Epor | Akt1 | Fgfr3 | Csf1 | Mapk10 | Ccl21b | Cxcl14 |
| Dusp6 | Igf2 | Col4a2 | Fgfr3 | Calm3 | Map2k6 | Efna3 | Pdgfd | Ccl6 | Dock2 |
| Dusp8 | Map2k6 | Col4a5 | Foxo3 | Crk | P2ry1 | Egfr | Pdgfra | Ccr7 | Foxo3 |
| Efna3 | Map3k11 | Csf1 | Gsk3b | Csf1 | Pdgfd | Ets1 | Pdgfrb | Crk | Gsk3b |
| Egfr | Map3k2 | Efna3 | Igf2 | Efna3 | Pdgfra | Exoc2 | Rac2 | Cx3cr1 | Rac2 |
| Fgf18 | Mapk10 | Egfr | Mlst8 | Egfr | Pdgfrb | Fgf18 | Rapgef5 | Gnb3 | Rhoa |
| Gadd45b | Max | Eif4e | Pck2 | Enah | Prkd2 | Gnb3 | Rgl1 | Gng7 | Stat2 |
| Gna12 | Pdgfd | Fgf18 | Pdgfd | Fgf18 | Rac2 | Gng7 | Rhoa | Grk3 | |
| Ikbkg | Pdgfra | Gnb3 | Pdgfra | Itgal | Rapgef5 | Ikbkg | Ikbkg | ||
| Irak1 | Pdgfrb | Gng7 | Pdgfrb | Itgb1 | Rhoa | Kras | Kras | ||
| Kras | Rac2 | Ikbkg | Ppp2r5a | Itgb2 | Mapk1 | Mapk1 | |||
| Lamtor3 | Il4ra | Sgk1 | Kras | Mras | Prkaca | ||||
| Map3k3 | Itga9 | Thbs2 | Krit1 | Nf1 | Prkcb | ||||
| Map3k7 | Itgb1 | Tnxb | Mapk1 | Ngf | Prkcd | ||||
| Mapk1 | Itgb6 | Mras | Pla1a | Ptk2b | |||||
| Mapt | Kras | Ngf | Prkaca | Rac1 | |||||
| Mknk2 | Mapk1 | Pard6a | Prkca | Stat5b | |||||
| Mras | Ngf | Pfn1 | Prkcb | Tiam1 | |||||
| Myd88 | Pdpk1 | Prkca | Rab5a | ||||||
| Nf1 | Ppp2r2a | Prkcb | Rab5b | ||||||
| Ngf | Prkca | Rac1 | Rac1 | ||||||
| Ppp3ca | Rac1 | Rap1gap | Ralgapa2 | ||||||
| Prkaca | Thbs1 | Sipa1l2 | Stk4 | ||||||
| Prkca | Thbs4 | Thbs1 | Tiam1 | ||||||
| Prkcb | Tlr2 | Tiam1 | |||||||
| Ptpn5 | |||||||||
| Rac1 | |||||||||
| Relb | |||||||||
| Srf | |||||||||
| Stk3 | |||||||||
| Stk4 | |||||||||
| Tgfb3 | |||||||||
| Traf2 | |||||||||
| Calcium | cAMP | cGMP-PKG | mTOR | Wnt | |||||
|---|---|---|---|---|---|---|---|---|---|
| 15U | 14D | 14U | 11D | 15U | 10D | 16U | 9D | 13U | 12D |
| Adcy1 | Adcy4 | Adcy1 | Adcy4 | Adcy1 | Adcy4 | Akt1 | Akt3 | Crebbp | Fzd4 |
| Adora2b | Adora2a | Adcy5 | Adora2a | Adcy5 | Akt3 | Atp6v1b2 | Castor2 | Csnk2a1 | Gpc4 |
| Asph | Cacna1g | Akt1 | Akt3 | Adra2b | Atf6b | Clip1 | Ddit4 | Dvl1 | Gsk3b |
| Calm3 | Fgfr3 | Atp1a3 | Edn1 | Akt1 | Itpr2 | Dvl1 | Fzd4 | Map3k7 | Mapk10 |
| Egfr | Grm1 | Calm3 | Mapk10 | Atp1a3 | Itpr3 | Eif4e | Gsk3b | Notum | Porcn |
| Fgf18 | Itpr2 | Crebbp | Myl9 | Calm3 | Myh6 | Kras | Mlst8 | Ppp3ca | Prickle1 |
| Ngf | Itpr3 | Fxyd2 | Pde4b | Fxyd2 | Myl9 | Lamtor3 | Rhoa | Prkaca | Rac2 |
| Pde1a | Mst1r | Hcn2 | Ppp1r12a | Gna12 | Mylk4 | Lpin3 | Rictor | Prkca | Rhoa |
| Pde1b | Mylk4 | Mapk1 | Ppp1r1b | Gtf2ird1 | Ppp1r12a | Mapk1 | Sgk1 | Prkcb | Sfrp5 |
| Plcd3 | P2rx1 | Prkaca | Rac2 | Gucy1b2 | Rhoa | Pdpk1 | Rac1 | Sox17 | |
| Ppp3ca | Pdgfd | Rac1 | Rhoa | Mapk1 | Prkca | Smad3 | Tle2 | ||
| Prkaca | Pdgfra | Sst | Myh7 | Prkcb | Wnt1 | Tle3 | |||
| Prkca | Pdgfrb | Sstr5 | Nppb | Stradb | Wnt5b | ||||
| Prkcb | Phkg1 | Tiam1 | Ppp3ca | Wdr59 | |||||
| Ptk2b | Srf | Wnt1 | |||||||
| Wnt5b | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobas, D.A.; Allen, H.; Iacobas, S. Low-Salt Diet Regulates the Metabolic and Signal Transduction Genomic Fabrics, and Remodels the Cardiac Normal and Chronic Pathological Pathways. Curr. Issues Mol. Biol. 2024, 46, 2355-2385. https://doi.org/10.3390/cimb46030150
Iacobas DA, Allen H, Iacobas S. Low-Salt Diet Regulates the Metabolic and Signal Transduction Genomic Fabrics, and Remodels the Cardiac Normal and Chronic Pathological Pathways. Current Issues in Molecular Biology. 2024; 46(3):2355-2385. https://doi.org/10.3390/cimb46030150
Chicago/Turabian StyleIacobas, Dumitru A., Haile Allen, and Sanda Iacobas. 2024. "Low-Salt Diet Regulates the Metabolic and Signal Transduction Genomic Fabrics, and Remodels the Cardiac Normal and Chronic Pathological Pathways" Current Issues in Molecular Biology 46, no. 3: 2355-2385. https://doi.org/10.3390/cimb46030150
APA StyleIacobas, D. A., Allen, H., & Iacobas, S. (2024). Low-Salt Diet Regulates the Metabolic and Signal Transduction Genomic Fabrics, and Remodels the Cardiac Normal and Chronic Pathological Pathways. Current Issues in Molecular Biology, 46(3), 2355-2385. https://doi.org/10.3390/cimb46030150







